Abstract
The first step in sexual differentiation of the unicellular green alga Chlamydomonas reinhardtii is the formation of gametes. Three genes, GAS28, GAS30, and GAS31, encoding Hyp-rich glycoproteins that presumably are cell wall constituents, are expressed in the late phase of gametogenesis. These genes, in addition, are activated by zygote formation and cell wall removal and by the application of osmotic stress. The induction by zygote formation could be traced to cell wall shedding prior to gamete fusion since it was seen in mutants defective in cell fusion. However, it was absent in mutants defective in the initial steps of mating, i.e. in flagellar agglutination and in accumulation of adenosine 3′,5′-cyclic monophosphate in response to this agglutination. Induction of the three GAS genes was also observed when cultures were exposed to hypoosmotic or hyperosmotic stress. To address the question whether the induction seen upon cell wall removal from both gametes and vegetative cells was elicited by osmotic stress, cell wall removal was performed under isosmotic conditions. Also under such conditions an activation of the genes was observed, suggesting that the signaling pathway(s) is (are) activated by wall removal itself.
Environmental signals control differentiation and development in plants. In the unicellular green alga Chlamydomonas reinhardtii, a plant model organism, starvation for a utilizable nitrogen source initiates the sexual life cycle that results in the formation of zygotes (Sager and Granick, 1954; Kates and Jones, 1964; Beck and Acker, 1992; Matsuda et al., 1992). For completion of gamete formation, blue light as a second extrinsic cue is required. This signal becomes effective in the late phase of sexual differentiation (Treier et al., 1989; Weissig and Beck, 1991; Beck and Acker, 1992). Irradiation with blue light also is required to maintain the gametes' mating competence (Pan et al., 1997).
The initial contact between gametes of mating types plus and minus is mediated by the mating-type-specific agglutinins, high Mr glycoproteins on the flagellar surface (Adair et al., 1983; Ferris et al., 2005). The resulting flagellar adhesion generates signaling pathways that activate the interacting cells for cell-cell fusion. A consequence is the activation of flagellar adenylyl cyclase, resulting in a nearly 10-fold increase of intracellular cAMP in each of the interacting gametes (Pasquale and Goodenough, 1987; Saito et al., 1993). This triggers dramatic alterations in the mating-competent cells (for review, see Pan and Snell, 2000a), among them the release of a matrix-degrading protease named gamete lytic enzyme (GLE) that degrades the gametes' cell wall (Matsuda et al., 1987; Snell et al., 1989; Kinoshita et al., 1992) and the erection of apically localized mating structures (Cavalier-Smith, 1974; Goodenough and Weiss, 1978).
Fusion of gametes after initial contact of the mating structures triggers another signaling cascade that, among other events (reviewed in Pan and Snell, 2000a), manifests itself in the up-regulation of genes. Some of these genes expressed in young zygotes encode cell wall proteins (Woessner and Goodenough, 1989; Matters and Goodenough, 1992; Suzuki et al., 2000).
Zygotes elaborate a thick wall that ensures the cells' survival under adverse environmental conditions, such as desiccation and low temperatures (Minami and Goodenough, 1978; Harris, 1989; Beck and Haring, 1996). This wall is structurally and biochemically distinct from that of vegetative cells or gametes to which it bears little resemblance (Grief et al., 1987; Woessner and Goodenough, 1989). The major constituent of both proteinaceous walls, though, are Hyp-rich glycoproteins (HRGPs; Catt, 1979; Goodenough and Heuser, 1985; Goodenough et al., 1986). Different sets of genes that encode HRGPs are expressed in vegetative cells and zygotes, accounting for the differences observed (Ferris and Goodenough, 1987; Woessner and Goodenough, 1989; Uchida et al., 1993; Woessner et al., 1994). Repetitions of Pro residues are characteristic for HRGPs both in vegetative cells and zygotes (Woessner and Goodenough, 1989; Suzuki et al., 2000) as documented by an antibody directed against a (serpro)10 epitope. This motif was also observed in cell wall glycoproteins of C. eugametos and Volvox carteri, other members of the volvocales family (Woessner et al., 1994).
In activated gametes, shedding of the cell wall is a dramatic event, resulting in the exposure of naked protoplasts to the environment. This, under hypoosmotic conditions, will result in water influx due to turgor reduction. A crucial role for maintenance of cell integrity under these conditions may be assigned to the contractile vacuole that primarily appears to serve as a pump to remove excess water entering the cells by osmosis (Luykx et al., 1997a, 1997b).
In Chlamydomonas, hyperosmotic conditions induce the intracellular accumulation and excretion of glycerol. This was traced to an activation of enzymes involved in glycerol production (León and Galván, 1995). Most prominent are results showing that osmotic stress in Chlamydomonas rapidly activates several phospholipid-based signaling pathways that result in the accumulation of phospholipids that may serve as second messengers. Among these compounds are phosphatidyl-d-inositol 3,5-bisphosphate, lysophosphatidic acid, phosphatidic acid, and diacyl glycerol (Meijer et al., 1999, 2001, 2002; Munnik et al., 2000). How osmotic stress induces the formation of these compounds in Chlamydomonas has not yet been elucidated, primarily due to the fact that osmosensors have not been defined in this organism. In higher plants, the first osmotic stress receptor identified is the hybrid-type His kinase Arabidopsis thaliana histidine kinase 1 (ATHK1). The function of this protein was demonstrated using transformants of a yeast mutant defective in osmosensor kinase synthetic lethal of N-end rule 1 (SLN1) with an ATHK1 expressing clone that lead to suppression of the mutant phenotype (Urao et al., 1999).
In higher plants, similar to the situation in yeast, hyperosmotic and hypoosmotic stress were shown to activate mitogen-activated protein (MAP) kinases (Cazalé et al., 1999; Munnik et al., 1999). In the signaling of the osmotic stress response, a link between the accumulation of lipid-derived secondary messengers and the activation of MAP kinases is still missing (Munnik and Meijer, 2001). In algae, an analysis of osmo-controlled signaling pathways was hampered by a lack of genes that respond to changes in the osmotic environment.
In this study, we report on three osmo-regulated genes of C. reinhardtii. These genes, encoding HRGPs, are activated in the late phase of sexual differentiation. They experience a second activation that is triggered by wall shedding upon gamete activation. This latter activation appears to be mediated by wall removal since it is also observed when the walls are lost under isosmotic conditions.
RESULTS
Characterization of Three Genes Expressed Late during Gametogenesis
We previously reported on genes that are expressed during sexual differentiation of C. reinhardtii (von Gromoff and Beck, 1993; Rodriguez et al., 1999). In those studies, based on cDNA clones, we identified two genes, GAS28 and GAS29, that are up-regulated in the late phase of gametogenesis. Here, we characterized the gene structure of GAS28 (GenBank accession no. DQ017908) and, in addition, report on two new genes named GAS30 (GenBank accession no. DQ017907) and GAS31 (GenBank accession no. DQ017909; Fig. 1). GAS30 exhibits distinct homology to GAS28: with the exception of parts of its 5′ untranslated region (UTR), the first 160 bp after the translation start codon, and its 3′ UTR, GAS30 is nearly identical to GAS28 (Fig. 1B). A comparison of GAS28 and GAS30 genomic sequences with sequence information from GAS28 cDNAs as well as RT-PCR experiments revealed that GAS28 and GAS30 each harbor three exons and two introns, with the exon-intron borders typical for C. reinhardtii (Silflow, 1998; Fig. 1B). In both GAS28 and GAS30, the 5′ UTRs are interrupted by introns. The location of the exon-intron borders of the second intron in both genes is identical. Except for an insertion of 772 bp in the center of the second intron of GAS30, the sequences of both introns are nearly identical. Genes GAS28 and GAS30 are arranged as an inverted tandem located on scaffold 48 of version 2 of the Chlamydomonas genome sequence (http://genome.jgi-psf.org/chlre2/chlre2.home.html): the 3′ ends of both genes face each other, separated by about 12 kb (Fig. 1A).
Figure 1.
GAS28, GAS30, and GAS31 gene structures and homology between predicted gene products. A, Arrangement of GAS28 and GAS30 within the C. reinhardtii genome. B, Structures of the GAS28, GAS30, and GAS31 genes. Solid lines indicate 5′ and 3′ UTRs. Black boxes indicate deduced protein coding exons; hatched boxes indicate sequences encoding ser(pro)x-rich domains; dotted lines indicate introns. Homology between segments of GAS28 and GAS30 sequences is given in percentage of identity. C, The deduced amino acid sequences of GAS28, GAS30, and GAS31 are grouped in four domains. The boxes indicate the different predicted protein domains (numbered one to four): the secretion signals (dark gray boxes), the N-terminal globular domain, the ser(pro)x-rich domain (S/P), and the C-terminal globular domain. The numbers of amino acids in each domain are given within the boxes. Asterisks indicate potential sites for N-linked glycosylation. Homology between GAS28 and GAS30 domains is given in percentage of amino acid identity.
GAS31 was identified in a bacterial artificial chromosome (BAC) clone that hybridized with a probe derived from the 3′ end of a cDNA named GAS29 (Rodriguez et al., 1999). The 3′ end of GAS31, starting at the 3′ side of the sequence encoding a ser(pro)x-rich domain, is identical to GAS29 cDNA. Its 5′ end clearly differs from the GAS29 sequence. GAS31 is located on scaffold 320 (genome version 2.0). Comparison of the GAS31 genomic sequence with GAS31 cDNA sequences revealed that this gene is composed of three exons and two introns, with the exon-intron borders typical for C. reinhardtii (Silflow, 1998). Both introns of GAS31 are located within the coding region in front of the region encoding the ser(pro)x-rich domain (Fig. 1B).
We previously identified a cDNA named GAS29 (Rodriguez et al., 1999). The 5′ end of the GAS29 cDNA (5′ UTR and the first 135 bp after the start of translation) is identical to that of GAS30, while the 3′ end corresponds to GAS31. A GAS29 gene, however, could not be identified, neither by screening different genomic libraries nor by inspection of C. reinhardtii genomic sequences. Rehybridization of the cDNA library used earlier (Rodriguez et al., 1999) with the 3′ end of GAS29 cDNA revealed an additional GAS29 cDNA clone with the same sequence but a poly(A) tail that is six bases shorter. To test whether this cDNA arose by trans-splicing, we attempted to amplify a GAS29 mRNA using RT-PCR with primers specific for GAS29. Since no PCR products were obtained, we cannot decide whether trans-splicing or artificial joining of cDNAs from the 5′ end of GAS30 and the 3′ end from GAS31 during the generation of the cDNA library may have generated the GAS29 cDNAs.
Analysis of Predicted Gene Products
The amino acid sequences of the deduced gene products revealed that each harbors a ser(pro)x-rich domain, indicative for cell wall proteins (Fig. 1C). The Pro residues of such domains of extracellular proteins usually are hydroxylated and, via O-glycosylation, a substrate for the attachment of sugar residues (O'Neill and Roberts, 1981; Woessner and Goodenough, 1989; Vallon and Wollman, 1995). Computer predictions using the BCM program (http://searchlauncher.bcm.tmc.edu/seq-util/seq-util.html) indicated that the three proteins are made up of four regions typical for cell wall glycoproteins of C. reinhardtii (Goodenough et al., 1986; Woessner and Goodenough, 1992): (1) a leader sequence that encodes a secretion signal (predicted with TargetP), (2) an N-terminal region, (3) a ser(pro)x-rich domain, and (4) a C-terminal region (Fig. 1C). Computer-predicted secondary structures for domains 2 and 4 using the algorithm HNN (http://npsa-pbil.ibcp.fr) by Guermeur et al. (1999) revealed that both domains of all three gene products contain α-helices, β-strands, and a high percentage (55% to 65%) of random coils. This suggests that domains 2 and 4 of the three proteins may adopt globular configurations. All three proteins also harbor potential sites for N-linked glycosylation (Fig. 1C). GAS28 and GAS30 have two sites each, one in domain 2 and one in domain 4. GAS31 has two sites in domain 2 and three sites in domain 4. The deduced gene products of GAS28 and GAS30 are nearly identical in size and sequence (423 amino acids with a molecular mass of 46.2 kD and 451 amino acids with a molecular mass of 47.8 kD, respectively), whereas the GAS31 protein is clearly different in size (618 amino acids with a molecular mass of 68.8 kD) and sequence, although it shares the four protein domains (Fig. 1C).
Presence of Additional Genes with Homology to GAS28, GAS30, and GAS31 in the C. reinhardtii Genome
We investigated whether additional genes that may encode proteins related to GAS28 and GAS30 might be present in the C. reinhardtii genome. For this purpose, we blasted the deduced amino acid sequences of GAS28 and GAS30 against version 2.0 of the genomic sequence using the tBLASTn program. Four different predicted genes that may encode proteins with similarity to domains 2 and 4 of GAS28 and GAS30 (Fig. 1B) were observed in version 2 of the genome sequence: gene models C_ 70040, C_170162, C_700008, and C_700009 (representing a single gene) and C_1550010 (Table I). The gaps in the genomic sequence from two gene models (C_170162 and C_700008/C_700009) are located in regions that have a high GC content (Grossman et al., 2003) and may encode Pro-rich domains. The alignments of the deduced amino acid sequences (prediction with BCM) from these gene models and GAS28 and GAS30 are given in Figure 2 for the N-terminal part (corresponding to domain 2 in Fig. 1B) and the C-terminal part (corresponding to domain 4 in Fig. 1B), respectively. Regions that show a high frequency of conserved residues were numbered one to nine (Fig. 2). These regions are observed in both, the N-terminal and C-terminal parts of the predicted proteins and may represent important motifs for the formation of three-dimensional structures. Except for region four in the C-terminal part and region five in both parts, all conserved motifs have one or two Cys residues. Because the conserved motifs occur in both parts of the proteins, the three-dimensional structures of the N-terminal and C-terminal parts are envisioned to be similar. Additional conserved regions were observed that only occur either in the N-terminal (motifs a and b) or in the C-terminal (motifs c and d) domains (Fig. 2).
Table I.
GAS28 and GAS30 homologous genes within the C. reinhardtii genome
GAS28 and GAS30 proteins were aligned against predicted products of gene models from the C. reinhardtii draft genome (http://genome.jgi-psf.org/chlre2/chlre2.home.html; version 2.0) using the tBLASTn program. Gene models with homology to GAS28 and GAS30 are listed, and their locations within the scaffolds are given. Gene models C_700008 and C_700009 refer to a single predicted gene and therefore are listed together: C_700008 represents its 5′ end and C_700009 represents its 3′ end. Sequences of the gene models were aligned against sequences from expressed sequence tag (EST) libraries (www.kazusa.or.jp/en/plant/chlamy/EST and www.biology.duke.edu/chlamy_genome/). The sequences of expressed sequence tag clones allowed the localization of introns and their borders. Sequence gaps within the regions homologous to GAS28 and GAS30 are listed. n.d., not determined.
| Gene Model | Scaffold | Location of Homologous Regions within Scaffolds | Gaps within the Genomic Sequence | Predicted Introns | Region Covered by ESTs |
|---|---|---|---|---|---|
| C_70040 | 7 | 266899–268219 | None | I: 267215–267400 | Yes |
| II: 267711–268029 | |||||
| C_170162 | 17 | 65789–63307 | 65095–63955 | I: 65782–65614 | Yes |
| II: 63518–63336 | |||||
| C_700008/C_700009 | 70 | 191460–192908 | 191957–192631 | n.d. | No |
| C_1550010 | 155 | 64052–65091 | 64799–64863 | I: 64715–64897 | Yes, starting from 64271 |
Figure 2.
Predicted gene models within the C. reinhardtii genome that may encode proteins with homology to GAS28 and GAS30. N-terminal and C-terminal domains from GAS28 and GAS30 were BLAST searched against sequences of the current draft release, version 2.0, of the C. reinhardtii genome (http://genome.jgi-psf.org/chlre2/chlre2.home.html) using the program tBLASTn. Gene models listed in Table I were used for the alignment. A, Alignment of domain 2 (Fig. 1B) of GAS28 and GAS30 against products of predicted genes of the C. reinhardtii genome. B, Alignment of domain 4 (Fig. 1B) of GAS28 and GAS30 against products of predicted genes of the C. reinhardtii genome. Sequences of gene models obtained from the BLAST search were translated with BCM six frame translation utilities, aligned using ClustalW, and refined manually. Sequences used for translation are described in Table I and the text. Residues highlighted in black are conserved in all proteins; those highlighted in gray are conserved in three or more of the proteins. Conserved amino acids are N/Q, D/E, R/K, S/T, F/W/Y, and A/G/L/M/V. Regions of high homology are marked with solid lines and numbered. Conserved Cys residues are marked with arrowheads. Conserved regions or amino acids that are specific for either domain 2 or domain 4 are indicated by letters.
No genes homologous to GAS31 were detected within the C. reinhardtii genome. We next tested whether GAS28, GAS30, and GAS31 may exhibit similarity to C. reinhardtii cell wall glycoproteins GP1, GP2 (Goodenough et al., 1986; Ferris et al., 2001; GP2 GenBank accession no. AY596305), VSP3 (Woessner et al., 1994), class IV cDNA coding for a 34-kD polypeptide (Ferris and Goodenough, 1987; Woessner and Goodenough, 1989), and ZSP-2 (Suzuki et al., 2000). No similarities in the amino acid sequences of the N-terminal or the C-terminal domain were detected, although these proteins are known to be composed of globular domains with Pro-rich stretches (Goodenough et al., 1986; Woessner et al., 1994; Ferris et al., 2001).
Additionally, we tested whether GAS28, GAS30, and GAS31 may be related to known glycoproteins of the extracellular matrix from Volvox. The N-terminal parts (Fig. 1B, domain 2) of these genes revealed a significant similarity to the N-terminal parts of extracellular matrix glycoproteins from Volvox: Pherophorin I, Pherophorin II, and the sulfated surface glycoprotein 185 (SSG 185; Fig. 3). Pherophorins at their C-terminal ends show homology to the sexual pheromone from Volvox (Sumper et al., 1993; Godl et al., 1995), while glycoprotein SSG 185 forms an insoluble structure within the extracellular matrix (Ertl et al., 1989). All three proteins are localized in the cellular zone of the spheroids (Ertl et al., 1989; Godl et al., 1995). The alignment indicates that the proteins exhibit similarities at six positions in their N-terminal regions (Fig. 3). These conserved motifs not only occur in the GAS28, GAS30, and GAS31 proteins but also in those of the gene models analyzed in Fig. 2. New regions with similarity between these proteins could not be detected. The results indicate that GAS28, GAS30, GAS31, and the extracellular matrix proteins from Volvox, Pherophorin I, Pherophorin II, and SSG 185 may share similar three-dimensional structures at their N-terminal domains.
Figure 3.
Homologies of GAS28, GAS30, and GAS31 to extracellular matrix proteins of the volvocales family. Alignment of the N-terminal domains of GAS28, GAS30, and GAS31 (domain 2 in Fig. 1B) and the N-terminal regions of three proteins encoded by the Volvox pherophorin gene family: Pherophorin I precursor (Per1; accession no. P81131), Pherophorin II (Per2; accession no. P81132), and Sulfated surface glycoprotein 185 precursor (SSG_185; accession no. P21997). Conserved residues are highlighted and grouped as described in the legend of Figure 2. Lines mark conserved regions, and numbers refer to those used in Figure 2. Conserved Cys residues are marked with arrowheads.
Expression Pattern of GAS28, GAS30, and GAS31 during Gametogenesis and in the Early Phase of Zygote Formation
An accumulation of GAS28 mRNA in the late phase of gametogenesis has been described previously (von Gromoff and Beck, 1993; Rodriguez et al., 1999; Abe et al., 2004). GAS30 and GAS31 showed kinetics of mRNA accumulation similar although not identical to those of GAS28 (Fig. 4). The mRNA levels of all three genes increased in the late phase of gametogenesis. The expression patterns were compared to those of FUS1, which encodes a sex recognition protein expressed in the middle phase of gametogenesis (Ferris et al., 1996; Abe et al., 2004). The strong accumulation of the FUS1 mRNA in cells 6 h after start of nitrogen starvation correlated with the appearance of gametes. Compared to FUS1, the expression of GAS28, GAS30, and GAS31 was delayed.
Figure 4.
Changes in the amounts of GAS28, GAS30, and GAS31 mRNAs during gametogenesis and after zygote formation. At time 0, the nitrogen source was removed from vegetatively growing cells. Incubation was continued in continuous light. Zygotes were generated by mixing mating-competent gametes of opposite mating types. Samples for RNA isolation were removed at the times indicated (h). Ten micrograms of total RNA per lane were loaded from gametes and 5 μg from zygotes. Northern-blot hybridizations were performed using probes specific for GAS28, GAS30, and GAS31 mRNAs as outlined in “Materials and Methods.” FUS1 served as a control for a gene expressed during gametogenesis but turned off upon zygote formation. A probe for HSP70B encoding a plastidic chaperone (Drzymalla et al., 1996) served as a loading control. The percentage of gametes or zygotes at each time point was determined as described in “Material and Methods” and is given below the autoradiograms. The percentage of zygotes was calculated from the number of quadriflagellate and biflagellate cells (Beck and Acker, 1992).
The analysis of changes in GAS28, GAS30, and GAS31 mRNA levels upon zygote formation revealed an additional mRNA accumulation in young zygotes (Fig. 4). Only 5 μg/lane of total RNA from young zygotes was applied per lane in contrast to 10 μg/lane of total RNA from mature gametes. GAS31 mRNA levels increased rapidly and strongly within 1 h after fusion, followed by a gradual decrease. The increase in GAS28 and GAS30 mRNA levels was lower and delayed when compared to the GAS31 transcript. An mRNA accumulation up to 3 h after cell fusion was observed for these genes. The FUS1 mRNA, in agreement with previous reports, disappeared in young zygotes (Ferris et al., 1996), an indication that zygotes were formed. We conclude that the mRNAs of all three GAS genes accumulate not only during the late phase of gametogenesis but also strongly in young zygotes.
Accumulation of mRNAs Observed in Young Zygotes Is Dependent on Agglutination But Not on Cell Fusion
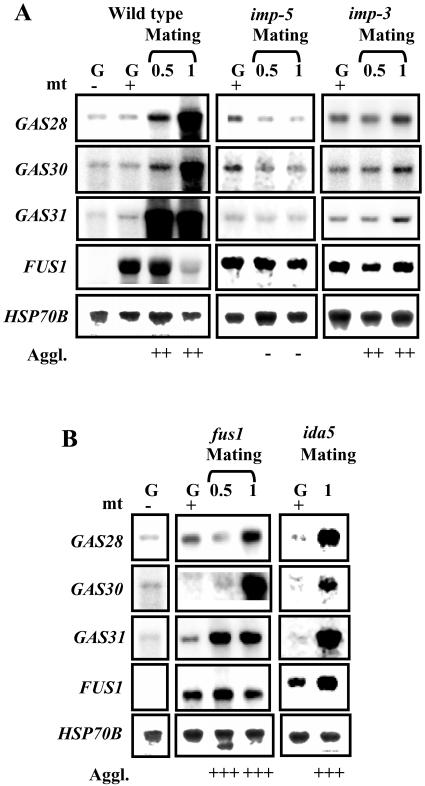
The formation of zygotes is a multistep process, elicited by the interaction of flagella from mt+ and mt− gametes (Pan and Snell, 2000a). We therefore tested which step in the mating reaction induces the accumulation of GAS28, GAS30, and GAS31 mRNAs seen in young zygotes. For this test, we employed Chlamydomonas mutants that are blocked in different steps of zygote formation. No accumulation of GAS28, GAS30, and GAS31 mRNAs was observed in mutant imp-5 after mixing with wild-type gametes (Fig. 5A). Mutant imp-5 lacks mt+-specific agglutinin and thus shows no agglutination reaction (Goodenough et al., 1976; Ferris et al., 2005). In wild-type gametes, an increase in GAS31 mRNA levels was observed already 30 min after mixing of competent gametes; a significant increase in GAS28 and GAS30 mRNAs was seen only 1 h after start of mating. The imp-3 mutant, defective in the production of intracellular cAMP in response to the adhesion reaction (Saito et al., 1993), showed, at most, a very weak accumulation of GAS28, GAS30, and GAS31 mRNAs 1 h after the mixing of gametes (Fig. 5A).
Figure 5.
Analysis of GAS28, GAS30, and GAS31 expression in mutants unable to form zygotes. Gametes (G) of mating type + from the wild type and mutants were generated as described in “Materials and Methods” and mixed for 0.5 or 1 h with wild-type gametes of opposite mating type. Mating type + gametes from the wild type served as a control. Samples were taken for RNA isolation, and northern-blot hybridizations were performed using probes that are specific for GAS28, GAS30, and GAS31 transcripts. FUS1 expression was used as a molecular marker to monitor zygote formation since its mRNA disappears in young zygotes (Ferris et al., 1996). A probe for HSP70B served as a loading control. The agglutination reaction (Aggl.) was monitored in a semiquantitative manner: −, no agglutination; +++, strong agglutination. The mating type (mt) is indicated by + and −. A, Assay of a mutant defective in mating type + agglutinin (imp-5) and of a mutant deficient in the accumulation of cAMP (imp-3). B, Assay of fusion-defective mutants fus1 and ida5, both of which are mt+. The fus1 mutant has a deletion in the FUS1 gene, resulting in a smaller transcript (Ferris et al., 1996).
We next tested whether cell fusion is a prerequisite for induction by using two mutants shown previously to be defective in zygote formation. Mutant fus1 is defective in a protein named fringe that, at the tip of the mating-type structure, is responsible for the contact with mt− gametes (Ferris et al., 1996; Misamore et al., 2003). In mutant ida5, the actin gene is affected that, in mt+ gametes, leads to a defect in the assembly of mating tubules (Kato-Minoura et al., 1997). After the flagella-mediated adhesion reaction of wild-type gametes with either of the fusion-defective mutants fus1 or ida5 mt+, we observed mRNA accumulation for all three GAS genes that was similar to that seen after the mixing of wild-type gametes (Fig. 5B). These results indicate that the agglutination reaction and an increase in intracellular cAMP levels are required for increased gene expression; gamete fusion, however, apparently is not needed.
GAS28, GAS30, and GAS31 Expression in Activated Gametes
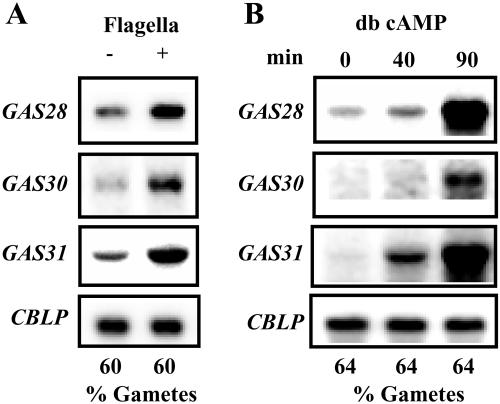
The interaction of flagella from gametes of mating types + and − in the early phase of mating is known to elicit a rise in intracellular cAMP levels (Pasquale and Goodenough, 1987; Zhang et al., 1991; Saito et al., 1993). We therefore tested whether the accumulation of GAS mRNAs is mediated by the agglutination reaction itself or via an increase in intracellular cAMP levels. Here, we made use of observations showing that gametes may be activated either by adding flagella isolated from mature gametes or by addition of dibutyryl cAMP (db cAMP) to gametes (Snell and Moore, 1980; Goodenough, 1986; Kurvari et al., 1995; Pan and Snell, 2000a, 2000b). When isolated flagella from gametes were incubated with gametes of opposite mating type for 1 h, we observed a pronounced increase in GAS28, GAS30, and GAS31 mRNA levels (Fig. 6A).
Figure 6.
Changes in GAS28, GAS30, and GAS31 mRNA levels after gamete activation. Mature gametes were generated as described in “Materials and Methods.” The percentage of gametes is given below the autoradiograms. Northern-blot hybridizations were performed using probes for GAS28, GAS30, and GAS31 transcripts. CBLP encoding a Gβ-like polypeptide served as a loading control. A, Wild-type gametes of mating type plus were either not treated with isolated flagella (−) or treated for 1 h with isolated flagella from gametes of mating type minus (+). B, Wild-type gametes were treated with 10 mm db cAMP for the time indicated.
To answer the question whether cAMP addition also elicits an increase in mRNA levels, we incubated mature gametes with 10 mm db cAMP for 40 and 90 min (Fig. 6B). GAS31 mRNA accumulated already 40 min after adding db cAMP. After 90 min, the mRNA showed a further increase. GAS28 and GAS30 mRNAs clearly have accumulated after 90 min. We conclude that gamete activation, elicited by an increase in intracellular cAMP levels, caused either by the agglutination reaction or the exogenous addition of db cAMP, induces the accumulation of GAS28, GAS30, and GAS31 mRNAs.
Accumulation of GAS mRNAs after Cell Wall Removal
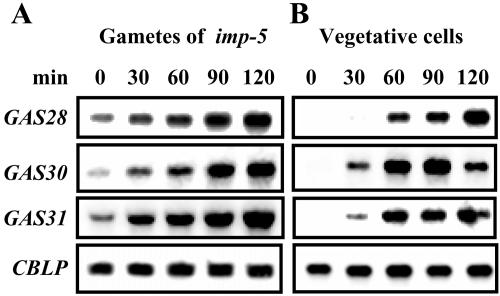
One cellular response caused by rising cAMP levels in gametes is the GLE-mediated removal of the gametes' cell wall (Buchanan et al., 1989; Pan and Snell, 2000a). To test whether the cAMP-induced cell wall removal may cause an enhanced expression of GAS28, GAS30, and GAS31, we incubated gametes from the agglutinin-defective mutant imp-5 (Goodenough et al., 1976; Ferris et al., 2005) with an autolysin-containing supernatant from zygotes. The active component in autolysin is a metalloprotease named GLE. The mutant imp-5 was used to avoid the activation of gametes by agglutinins (see Figs. 5A and 6A) that might be present in the autolysin suspension. The GAS28, GAS30, and GAS31 mRNA levels increased in a time-dependent manner after treatment with autolysin (Fig. 7A).
Figure 7.
Effect of autolysin treatment on GAS28, GAS30, and GAS31 mRNA levels in gametes and in vegetative cells. At time 0, cells were treated with autolysin for different durations, and samples were taken for RNA isolation at the time points indicated. Northern-blot hybridizations were performed using probes that detect the GAS28, GAS30, and GAS31 transcripts. CBLP was used as a loading control. A, Gametes of the agglutinin + defective mutant imp-5, generated as described in “Materials and Methods.” B, Vegetative cells of the wild type (CC-124) grown in TAP medium.
We next treated vegetative cells with autolysin. The mRNA levels of GAS28, GAS30, and GAS31, which in vegetative cells are very low, increased strongly as a consequence of autolysin treatment (Fig. 7B). The removal of cell walls by this treatment was assured by inspection of cells during treatment with Triton X-100. Treatment with the detergent results in a rapid lysis of cells lacking a wall; those with a wall are not destroyed.
Was this induction caused by cell wall removal or, possibly, by compounds present in the autolysin-containing suspension obtained from mating gametes? This question was first approached by inactivation of GLE with either EDTA or by heat treatment (Matsuda et al., 1984, 1987). Vegetative cells treated with autolysin for 2 h either without EDTA or with 1 or 2 mm EDTA were compared (Fig. 8A). The addition of 1 mm EDTA strongly reduced the accumulation of GAS28, GAS30, and GAS31 mRNAs. The increases in mRNA levels were completely abolished by 2 mm EDTA.
Figure 8.
Treatments that affect the autolysin preparations. Vegetative cells were treated with autolysin preparations for 2 h before samples were removed for RNA isolation and northern-blot hybridizations performed using probes specific for the GAS28, GAS30, and GAS31 transcripts. CBLP was used as a loading control. A, Effect of autolysin in the presence or absence of EDTA. Wild-type cells grown in TAP medium were either treated with autolysin (+) or not (−). EDTA was added at the same time at the final concentrations indicated. B, Effect of autolysin that has been heat treated at 50°C for 10 min (A50). Expression levels were compared with nontreated autolysin (A0). Cells without autolysin treatment were used as control (−). C, Effect of dialyzes of autolysin. Autolysin was dialyzed overnight against TAP medium. Wild-type cells (CC-621) and flagellaless mutant bld2 (CC-479) grown in TAP medium were treated with dialyzed autolysin (AD), nondialyzed autolysin (A), or not treated (−).
Since EDTA may block other active ingredients contained in the autolysin suspension, we investigated whether the autolysin-mediated induction of GAS28, GAS30, and GAS31 gene expression may be abolished by heat inactivation of GLE. It was shown previously that the activity of purified GLE is lost completely by a treatment at 50°C for 5 min (Matsuda et al., 1984). The autolysin suspension was incubated at 50°C for 10 min prior to addition to vegetative cells. The induction of GAS28, GAS30, and GAS31 expression was completely abolished in cells incubated with the heat-inactivated autolysin (Fig. 8B).
We also investigated whether small molecules present in the autolysin suspension might be responsible for the induction of GAS28, GAS30, and GAS31 expression. For this purpose, we dialyzed the autolysin suspension against Tris-acetate phosphate (TAP) medium. Wild-type cells and flagellaless mutant bld2 (Goodenough and St. Clair, 1975) were incubated with nondialyzed and dialyzed autolysin for 2 h, and the gene expression patterns were compared (Fig. 8C). A comparison between wild-type cells and the flagellaless mutant should reveal possible influences of the flagella on the induction of the GAS genes. No differences in the expression patterns of GAS28, GAS30, and GAS31 could be observed between cells treated with nondialyzed and dialyzed autolysin. These results indicate that a dialyzable component in the autolysin suspension is not involved in the activation of GAS28, GAS30, and GAS31. There was also no difference in mRNA accumulations of GAS28, GAS30, and GAS31 between wild-type cells and the flagellaless mutant bld2, indicating that signaling via the flagella is not involved. In summary, these results suggest that the GLE-mediated cell wall removal elicits the signal that activates gene expression.
GAS Gene Activation in Response to Changes in Osmotic Conditions
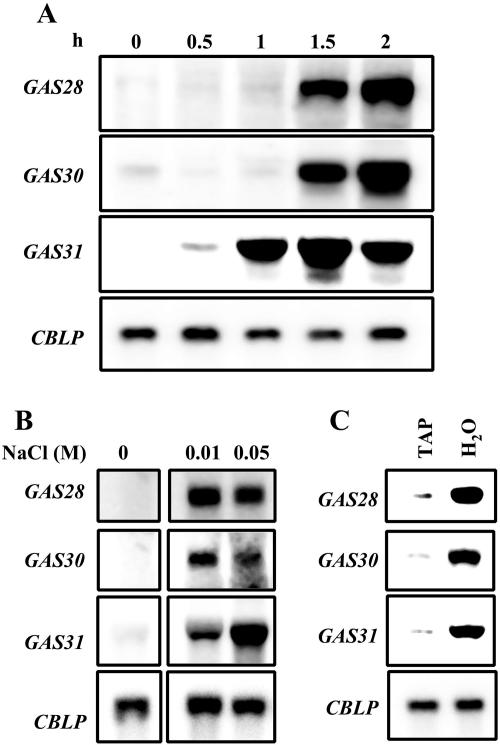
Cell wall removal in yeast was shown to activate a branch of the high osmolarity glycerol MAP kinase signaling pathway, resulting in the expression of genes necessary for cell integrity (Alonso-Monge et al., 2001; Reiser et al., 2003). It was postulated that activation is caused by a reduction in turgor. To test whether changes in osmotic conditions may induce the activation of GAS28, GAS30, and GAS31, vegetative cells grown in TAP medium were shifted to TAP medium containing 0.2 m sorbitol. The three genes were activated by this shift to hyperosmotic conditions although with different kinetics (Fig. 9A). GAS31 mRNA accumulated weakly already 0.5 h after shift and reached a maximal level about 1.5 h later. GAS28 and GAS30 mRNAs started to accumulate with a delay of about 1.5 h after shift to TAP medium containing 0.2 m sorbitol. The dependence of this induction on the solute employed was tested by shifting vegetative cells to hyperosmotic conditions employing different concentrations of NaCl in TAP medium. We used only low concentrations of NaCl because cells started to die after the addition of NaCl to a final concentration of >0.1 m. As seen with sorbitol, a strong increase in GAS28, GAS30, and GAS31 mRNA levels was noted after a shift of cells to 0.01 m NaCl (Fig. 9B). The GAS31 transcript accumulated to even higher levels in cells cultured in medium with 0.05 m NaCl, whereas the mRNA levels of GAS28 and GAS30 mRNA tended to decline at the elevated NaCl concentration. These results indicate that GAS28, GAS30, and GAS31 are induced when cells encounter hyperosmotic conditions.
Figure 9.
Changes in GAS28, GAS30, and GAS31 mRNA levels after a shift of vegetative cells to hyperosmotic and hypoosmotic conditions. Wild-type cells grown in TAP medium under continuous light were sedimented by centrifugation and resuspended in medium of different osmolarities. Northern-blot hybridizations were performed using probes that detected the GAS28, GAS30, and GAS31 transcripts. CBLP was used as a loading control. A, Kinetics of GAS28, GAS30, and GAS31 mRNA accumulation in cells shifted to TAP medium containing 0.2 m sorbitol (time 0). Samples for RNA isolation were taken at the time points indicated. B, Cells were shifted to TAP medium containing the NaCl concentrations indicated, and probes were taken after incubation for 2 h. C, Cells were resuspended in water and compared with cells resuspended in TAP medium. Samples for RNA isolation were taken after a treatment for 2 h.
For yeast, it has been described that the cell wall integrity pathway becomes activated as well by shifting cells to hypoosmotic conditions (Davenport et al., 1995). To test these conditions for C. reinhardtii, cells grown in TAP medium of 53 osmol (mOsM) were shifted to distilled water (0 osmol). After incubation for 2 h, GAS28, GAS30, and GAS31 mRNA levels of water-incubated cells were compared with untreated cells (Fig. 9C). Since the transcripts of all three GAS genes accumulated in cells shifted to water, GAS28, GAS30, and GAS31 expression clearly can be activated by both hyperosmotic and hypoosmotic conditions.
The removal of the cell wall in plant cells results in a reduction of turgor pressure, which leads to an influx of water and cell expansion. We next wanted to find out whether gene expression of GAS28, GAS30, and GAS31 observed upon cell wall removal (Fig. 7) is elicited by changes in osmotic conditions within the cell (water influx) or by a signal that is generated by the detachment of the cell wall from the cell membrane.
For the cytosol of C. reinhardtii, a value of 120 osmol has been determined (Luykx et al., 1997a). Removal of the cell wall in TAP medium (53 osmol) is expected to change the osmotic conditions within the cells due to water influx. Removal of the wall under isosmotic conditions (medium with 120 osmol) is expected not to change the intracellular osmotic conditions. We performed the autolysin-mediated cell wall removal in medium with osmolarities of 93, 120, and 150 osmol achieved by adding sorbitol to TAP medium. Vegetative cells were incubated for 2 h in media of different osmotic values in the presence or absence of autolysin. Analysis of GAS28, GAS30, and GAS31 expression levels revealed an induction of all three GAS genes after cell wall removal not only under slightly hypoosmotic conditions (93 osmol) but also under isoosmotic and slightly hyperosmotic conditions (120 and 150 osmol, respectively; Fig. 10). The degree of mRNA accumulation observed after cell wall removal was about equal under all three osmotic conditions. These results suggest that the autolysin-mediated activation of GAS28, GAS30, and GAS31 is not caused by osmotic changes within the cell but by cell wall removal.
Figure 10.
Effect of autolysin treatment on GAS28, GAS30, and GAS31 mRNA levels under isosmotic conditions. Vegetative cells were treated either with autolysin (+) or without autolysin (−) for 2 h in TAP medium with different concentrations of sorbitol. Osmolarity of the medium is given in osmol (mOsM). Northern-blot hybridizations were performed using probes that detect the GAS28, GAS30, and GAS31 transcripts. CBLP was used as a loading control.
Influence of Inhibition of Cytoplasmic Protein Synthesis on GAS28, GAS30, and GAS31 mRNA Accumulation
To gain first indications on the signaling pathways involved in the activation of these genes, we tested whether, upon zygote formation, cell wall removal, or the application of osmotic stress, and inhibition of cytoplasmic protein synthesis would affect the accumulation of GAS28, GAS30, and GAS31 mRNAs. It has been shown before that this inhibition does not prevent gametes to agglutinate and to form zygotes (Ferris and Goodenough, 1987). We treated mature gametes of both mating types with 10 μg/mL cycloheximide (CHI) for 30 min and then mixed them for 0.5 or 1 h (Fig. 11A). GAS31 mRNA levels increased strongly with kinetics similar to those seen in untreated gametes after fusion (see Figs. 4 and 5A). In contrast, CHI prevented the accumulation of GAS28 and GAS30 mRNAs during zygote formation.
Figure 11.
Consequences of inhibition of cytoplasmatic protein synthesis on GAS28, GAS30, and GAS31 mRNA levels in cells after zygote formation, after autolysin treatment, and after a shift to hyperosmotic conditions. Northern-blot hybridizations were performed using probes specific for the GAS28, GAS30, and GAS31 transcripts. CBLP served as a loading control. A, CHI treatment of gametes and of gametes during mating. Mature gametes of mating types + and − were generated as described in “Materials and Methods” and then suspended in nitrogen-free medium containing 10 μg/mL CHI for 30 min. These gametes were mixed for mating for 0.5 or 1 h, and samples were taken for RNA isolation. B, Influence of CHI on GAS28, GAS30, and GAS31 mRNA levels in vegetative cells treated with autolysin. Wild-type cells were incubated with 10 μg/mL CHI for 30 min prior to autolysin treatment (+) and compared to nontreated cells (−). Autolysin treatment started at time 0, and samples for RNA isolation were taken at the time points indicated. C, Effect of inhibition of cytoplasmatic protein synthesis on GAS gene expression in cells shifted to hyperosmotic conditions. Wild-type cells in TAP medium were either treated with 10 μg/mL CHI for 30 min (+) or not (−) prior to the addition of sorbitol (0.15 m final concentration). Cells were incubated for different durations (h). Sample 8* was treated for 8 h in TAP medium containing 10 μg/mL of CHI without sorbitol. Northern-blot hybridizations were performed as described in “Materials and Methods.”
To analyze the influence of CHI on the mRNA levels of these genes after autolysin treatment, vegetative cells were incubated for 30 min with 10 μg/mL CHI prior to autolysin treatment for 0.5 and 1.5 h. Again, GAS31 mRNA accumulation was not prevented by CHI; rather, an enhanced accumulation was observed when compared to the untreated control (Fig. 11B). CHI, however, completely inhibited the accumulation of GAS28 and GAS30 mRNAs.
To test the influence of CHI on the expression of GAS28, GAS30, and GAS31 upon application of osmotic stress, vegetative cells were preincubated for 30 min with 10 μg/mL CHI prior to transfer to TAP medium containing 0.15 m sorbitol. Here, too, an increase in GAS31 mRNA levels was not prevented by CHI (Fig. 11C). Similar to the observations made after cell wall removal, the accumulation of GAS31 mRNA was distinctly higher in cells treated with CHI when compared to nontreated cells. No GAS31 mRNA accumulation was observed in cells treated with CHI in the absence of osmotic stress, showing that CHI alone did not influence the expression of this gene. GAS28 and GAS30 mRNAs did not accumulate in cells treated with a combination of sorbitol and CHI (Fig. 11C). Upon prolonged incubation of cells in sorbitol (21 h) in the absence of CHI, a reduction in GAS28, GAS30, and GAS31 mRNA levels was observed (Fig. 11C). Microscopy examination of cells kept for 21 h in the presence of 0.2 m sorbitol revealed that most of the cells were dead.
The similarity in response of the three genes, irrespective of the conditions used for their induction, suggests the involvement of similar or identical signaling pathways. However, the accumulation of GAS31 transcripts in the presence of CHI and the absence of GAS28 and GAS30 mRNAs under these conditions may indicate a divergence of these pathways at a later step.
DISCUSSION
The gene products of GAS28, GAS30, and GAS31 exhibit the typical feature of C. reinhardtii cell wall proteins: a ser(pro)x-rich domain. Such protein domains are subject to glycosylation and may form fibrous structures (Woessner and Goodenough, 1992; Ferris et al., 2001, 2005). The predicted fibrous structures of the three GAS proteins are flanked by domains that were predicted to form globular structures. Proteins of this type have been classified as chimeric extensins (Kieliszewski and Lamport, 1994). Although GAS28, GAS30, and GAS31 do not exhibit homology to known C. reinhardtii cell wall proteins, they share the structural features of globular heads and Hyp-rich shafts with the majority of HRGPs from this organism (Ferris et al., 2001).
The presence of four additional genes that may encode proteins with homology to GAS28 and GAS30 was revealed by a search for related genes in draft version 2 of the C. reinhardtii genome (Fig. 2). These results indicate that GAS28 and GAS30 are members of a larger protein family. The N-terminal domains of GAS28 and GAS30 also share conserved sequence motifs with GAS31 and pherophorins I and II as well as SG185, proteins of the extracellular matrix of Volvox (Fig. 3). This supports the hypothesis that different domains of HRGPs are conserved within the order of volvocales and might have evolved from a common ancestor (Woessner et al., 1994).
Successful mating and the subsequent formation of zygotes in C. reinhardtii are based on a program of differentiation involving the activation and inactivation of genes in a predetermined sequence. Among the genes controlled by this program are GAS28, GAS30, and GAS31, which are up-regulated in the late phase of gamete formation, i.e. when mating competent cells already are formed (Fig. 4). Since the gene products appear to be members of the HRGP family of cell wall proteins, a function of these proteins in mature and nondividing gametes is difficult to discern, and we previously speculated that these mRNAs, produced in gametes, may by stored for later translation in the newly formed zygotes (Rodriguez et al., 1999). Preliminary results from studies with GAS31-specific antibodies indicate the presence of this protein in young zygotes, while in gametes or vegetative cells, only a weak signal was detectable (our unpublished data).
The mating of gametes elicited a second increase in the mRNA levels of these GAS genes (Fig. 4). The shedding of the gametes' wall appears to be the ultimate cue that is responsible for their induction (Fig. 12). Since gametes, after shedding of their walls, fuse within minutes after mating (Harris, 1989), these mRNAs, present in gametes and accumulating with accelerated rates in young zygotes, may serve as template for the rapid synthesis of proteins needed for the regeneration of a new wall protecting the young zygotes. While we speculate that these mRNA accumulations are a consequence of increased transcription of the genes, an alternative explanation is a modulation of RNA levels by changes in their stability. These two alternative routes to increases in mRNA levels have not yet been elucidated.
Figure 12.
Model for the control of GAS28, GAS30, and GAS31 expression by endogenous and extrinsic signals. The adhesion reaction between mature gametes elicits an increase in intracellular cAMP levels, which in turn causes the activation of GLE. GLE catalyzes the degradation of the cell wall, which is proposed to lead to a signal that induces GAS28, GAS30, and GAS31.
This induction of the three GAS genes parallels previous findings in which a screening for genes expressed in activated gametes resulted in the identification of a cDNA named G47. The gene for G47 encodes a putative cell wall protein; its mRNA accumulated in gametes upon activation and also in dewalled vegetative cells. Whether its induction in activated gametes is linked to cell wall shedding has not been analyzed but, in view of the results presented here, appears to be possible. In summary, these data point to the important role played by the gametes' cell wall shedding in the generation of a signal that controls the expression of genes during the mating reaction.
Vegetative cells also were shown to be competent for the activation of GAS28, GAS30, and GAS31 by cell wall removal (Fig. 7B), suggesting that the signal is not restricted to cells undergoing gametogenesis. This idea is supported for GAS28, since the gene for this protein is up-regulated after mitosis in dividing cells when new cell walls are being elaborated by the daughter cells (Abe et al., 2004). That alterations, affecting the wall of vegetative cells, generate a signal resulting in the activation of cell wall genes has been demonstrated previously. Three genes, GP1, GP2, and Mrp47, encoding proteins of the HRGP family, in vegetative cells are induced by autolysin treatment (Adair and Apt, 1990; Kurvari, 1997). Since they were not discovered in screens designed to detect genes expressed in young zygotes, they likely are specific for vegetative cells and gametes and thus similar to VSP-3, another cell wall gene induced by removal of the wall that is not expressed in young zygotes (Woessner et al., 1994). Increased expression of genes after cell wall removal possibly may be caused by a general stress response. This, however, appears to be unlikely since the application of heat stress did not induce GAS28 (von Gromoff and Beck, 1993) and GAS30 (data not shown).
How is the activation of the three GAS genes elicited by wall removal? The intracellular osmotic status is expected to be affected by removal of the wall when performed in TAP medium since this medium with 53 osmol has a lower osmotic strength than that of the C. reinhardtii interior, which is isosmotic with a medium of 120 osmol (Luykx et al., 1997a). Cell wall removal is expected to lead to higher activity of the contractile vacuole and/or cell expansion through water influx, possibly causing the activation of osmosensors and linked signaling pathways. However, wall shedding under isosmotic conditions also elicited an induction of the GAS genes (Fig. 10). Thus, the signal appears to be generated by detachment of the cell wall from the plasma membrane. Here, Saccharomyces cerevisiae may provide a model where an activation of the MAP-kinase-mediated high osmolarity glycerol pathway in response to wall removal (seen even in cells adapted to hyperosmotic conditions) is registered by trans-membrane osmosensory His kinase SLN1 possibly via an ectodomain that may form contacts between the plasma membrane and the cell wall (Hohmann, 2002; Reiser et al., 2003). A relationship between the osmo-sensing system in yeast and that in higher plants was deduced from the observation that Arabidopsis (Arabidopsis thaliana) cytokinin response 1 (Cre1) protein, also a His kinase, could complement the defective response to cell wall removal of yeast mutants lacking SLN1 (Reiser et al., 2003). A gene for a His kinase with homology to SLN1 and Cre1 also is present within the C. reinhardtii genome (gene model C_930062). In view of the gene activation observed under isosmotic conditions, also in C. reinhardtii, loss of contact between the plasma membrane and the cell wall is postulated to trigger a signaling pathway that results in the induction of the three GAS genes (Fig. 12). This pathway also may be employed when cells are shifted to hyperosmotic conditions, since shrinking of the cytoplasmic membrane involves its partial detachment from the wall.
The three GAS genes analyzed are also induced by exposing the algal cells to hyperosmotic or hypoosmotic conditions. The addition of either sorbitol or NaCl or a shift of cells from TAP medium to water elicited a pronounced activation of the three genes (Fig. 9). These genes, to our knowledge, are the first described in C. reinhardtii for which an osmoregulation is demonstrated. The osmosensors involved as well as the participating signaling pathways remain to be elucidated as well as the question whether signaling components employed here are the same as those used by the pathway activated by cell wall removal. One argument for the participation of distinct pathways comes from differences in the kinetics of RNA accumulation. Thus, GAS28 and GAS30 mRNA clearly have accumulated 1 h after cell wall removal (Fig. 7B), while this accumulation is delayed upon application of hyperosmotic conditions (Fig. 9A). In contrast, GAS31 responded with similar RNA accumulation kinetics to both sets of conditions. Besides these differences, GAS31 regulation is distinguishable from that of GAS28 and GAS30, since its induction, but not that of the latter genes, under all conditions tested (wall removal and hypoosmotic or hyperosmotic conditions) occurred in the absence of de novo protein synthesis (Fig. 11). This implies that the accumulation of GAS28 and GAS30 mRNAs is dependent on the de novo synthesis of protein factors (Fig. 12). These data are suggestive evidence for the existence of different osmo-regulated signaling pathways in Chlamydomonas. Whether the situation is as complex as in S. cerevisiae, where multiple osmosensors and coupled signaling pathways participate in the regulation of gene expression in response to hypoosmotic and hyperosmotic conditions (Hohmann, 2002), remains to be elucidated.
Another signal that during mating and zygote formation triggers the activation of genes is gamete fusion. Several genes that are expressed after cell fusion (zygote-specific genes) do not show activation in mutants defective in gamete fusion (Ferris and Goodenough, 1987). Two of these zygote-specific genes encode components of the cell wall (Woessner and Goodenough, 1989; Matters and Goodenough, 1992). While additional genes have been classified as zygote specific, their activation by cell fusion has not been demonstrated in a rigorous manner (Uchida et al., 1993). These latter genes may possibly be induced by a signal generated prior to fertilization, i.e. gamete activation and cell wall shedding.
Altered gene expression in response to cell wall alterations and osmotic conditions is a topic of importance in plant physiology (Bray et al., 2000). Osmoregulation in higher plants is due to complex interactions of regulatory metabolites at different levels of organization: organs, tissues, and cells. The basics of this regulation thus are easier to study in unicellular organisms. The characterization of three genes in C. reinhardtii that respond to these cues opens up the route to study the signaling processes involved in this simple plant. Additional attractive aspects are the availability of osmoregulatory mutants (Luykx et al., 1997b) and strains that completely lack a cell wall but are viable even when suspended in water.
MATERIALS AND METHODS
Strains and Cell Culture
We used seven Chlamydomonas reinhardtii strains in this study: wild-type strains CC-620 (mt+) and CC-124 (mt−), the agglutinin + defective mutant imp-5 (CC-469; Adair et al., 1983), a mutant deficient in the accumulation of cAMP (imp-3, CC-465; Goodenough et al., 1976), the fusion-defective mutant fus1 (CC-1158; Ferris et al., 1996), and the flagellarless mutant bld2 (Goodenough and St. Clair, 1975), all obtained from the Chlamydomonas Genetics Center (Duke University, Durham, NC). The fusion-defective mutant ida5 (mt+) was kindly provided by R. Kamiya (Kato-Minoura et al., 1997).
Vegetative cells were grown photomixotrophically at 23°C in TAP medium (Gorman and Levine, 1965) at 23°C on a shaker with continuous irradiation by white light (fluence rate 60 μmol m−2 s−1) to a density of approximately 6 × 106 cells per mL. Fluorescent light (Osram L36W/25) was used for illumination. For induction of gametogenesis, vegetative C. reinhardtii cultures at a density of approximately 5 × 106 cells/mL were concentrated by centrifugation (3000g for 5 min). TAP medium was carefully removed, and the cells were resuspended in nitrogen-free TAP medium at a density of about 5 × 106 cells/mL. Subsequently, cells were incubated with shaking for 5 to 17 h. Mating and determination of the percentage of gametes were performed as described previously (Treier et al., 1989; Beck and Acker, 1992).
Protein synthesis inhibition was performed by incubating cells for 30 min with 10 μg/mL of CHI (obtained from Sigma-Aldrich) prior to additional experimental treatments.
Activation of Gametes
Gametes were activated for mating either by incubation with flagella from gametes of opposite mating type or by the addition of db cAMP. Flagella were isolated from mature gametes (1 L culture, 64% mating) as described by Pan and Snell (2000b). Flagella were resuspended in 500 μL resuspension buffer (20 mm HEPES, pH 7.2, 5 mm MgCl2, 1 mm EDTA, and 1 mm dithiothreitol, containing a 1/100 dilution of the Sigma-Aldrich protease inhibitor mixture for plant cells). Flagella suspension (100 μL) was added to 15 mL of mature gametes and incubated for 1 h prior to harvest on ice. Gametes were also activated by incubating them with 10 mm db cAMP (Fluka) and freshly prepared 0.15 mm paperverine (Sigma-Aldrich) for different durations (Wilson et al., 1997).
Removal of Cell Walls
Crude autolysin preparations were obtained by mixing mature gametes (1 × 107 cells per mL) of opposite mating types and incubating them for 1 h in the dark without shaking. The percentage of zygotes in the mixture was estimated by counting the biflagellated and quadriflagellated (zygotes) cells after fixation in 1% glutaraldehyde. When >80% of the gametes have mated, the autolysin-containing supernatant was collected by spinning down the cells at 2000g at 4°C for 5 min. The supernatant was recentrifuged at 13000g for 10 min at 4°C. The autolysin-containing supernatant was sterilized by filtration using syringe filters (Roth) and frozen in aliquots. For removal of the cell walls, 1 mL of autolysin was added to 10 mL of cells (density 6 × 106 cells per mL). To monitor the removal of the cell walls, 10 μL of treated cells were incubated with 10 μL of 1% Triton X-100 for 5 min. Untreated cells served as a control. Cells were counted under a microscope and compared to cells not treated with Triton X-100. Cells without a cell wall lyse in the presence of Triton X-100.
Osmotic Stress Conditions
For hyperosmotic stress, cells were grown in TAP for 3 d to a density of 6 × 106 cells/mL, harvested by centrifugation, and resuspended in TAP containing a final concentration of 0.15 or 0.2 m sorbitol or a final concentration of 0.01 or 0.05 m NaCl. For hypoosmotic stress, cells were resuspended in destilled water. Osmolarity of the medium was determined by measuring the media used for culturing with an osmometer (OM 801 from Vogel).
RNA Isolation and Northern Blots
For RNA isolation, cells were harvested by centrifugation for 5 min at 4°C, resuspended in 2× lysis buffer (0.6 m NaCl, 10 mm EDTA, 100 mm Tris-HCl, pH 8.0, and 4% SDS), and then frozen in liquid nitrogen. The lysates were extracted with phenol (Sambrook et al., 1989). The RNA was precipitated with 0.33 volume of 8 m LiCl at 4°C overnight (Barlow et al., 1963). The pellet was suspended in diethylpyrocarbonate-treated water, mixed with 0.1 volume of 3 m sodium acetate (pH 6.0), and precipitated with 2.5 volumes of ethanol at −20°C. Total RNA (10 μg/lane) was fractionated on 1.2% (w/v) agarose/formaldehyde gels, transferred to Hybond-n+ membranes (Amersham), using 25 mm sodium phosphate buffer (pH 6.5), and baked at 80°C for 2 h. The probes used for hybridization were as follows: GAS28 (a NheI-XhoI fragment of 690 bp from the 3′ end of GAS28 cDNA), GAS30 (a NruI-XhoI fragment of 1000 bp from the 3′ end of the genomic DNA), GAS31 (a XbaI-XhoI fragment of 1050 bp from the 5′ end of the genomic DNA), FUS1 (an EcoRV-BamHI fragment of 1700 bp from plasmid imp1-17; Ferris et al., 1996), HSP70B (von Gromoff et al., 1989), and CBLP, which encodes a Gβ-like polypeptide (von Kampen et al., 1994). Probes were labeled with 50 μCi [α-32P]dCTP (Amersham) according to the random priming protocol (Feinberg and Vogelstein, 1983). Hybridizations were performed as described previously (Wegener and Beck, 1991). After hybridization, the membranes were washed twice in 2× SSC for 5 min at room temperature, once in 2× SSC, 1% SDS for 30 min at 65°C, and once in 0.1× SSC, 1% SDS for 30 min at 65°C.
Isolation of the GAS28, GAS30, and GAS31 Genes
GAS28 and GAS31 were cloned by screening the membrane-immobilized DNA of a C. reinhardtii BAC library (Lefebvre and Silflow, 1999) obtained from the Clemson University Genomics Institute (Clemson, SC, http://www.genome.clemson.edu/) using a NheI-XhoI fragment from GAS28 cDNA and a SalI-BglII fragment from GAS29 cDNA as probes, respectively. Hybridizations of the membranes were performed using the protocol from Clemson University Genomics Institute. Seven clones were hybridized with the GAS28 probe (21H23, 21K10, 05O05, 29O09, 30L05, 36O14, and 07G16) and three clones with the GAS31 probe (06K23, 22G06, and 34C08). Positive BAC clones (21H23 and 06K23) were obtained from Clemson University Genomics Institute. DNA was isolated using the protocol of the BAC clone providers (http://cbs.umn.edu/∼amundsen/chlamy/methods/bac_med.html). Genes were subcloned in pBluescript SK+/− (Stratagene). GAS31 cDNA clones and GAS29 cDNA clones were obtained by screening a λ-zap cDNA library prepared from mt+ gametes (Kurvari et al., 1995) using the 5′ end of GAS31 (XbaI-XhoI fragment from the genomic subclone) and the 3′ end of GAS29 cDNA (SalI-BglII fragment), respectively, as probes. Inserts of positive clones were excised as pBluescript II SK+/− plasmids using the Stratagene rapid excision kit. The genomic structures of GAS28 and GAS31 were determined by comparison of the cDNA sequences with the genomic sequences.
GAS30 was cloned by screening a cosmid library (Purton and Rochaix, 1994) using an NdeI-XhoI fragment of GAS28 cDNA as a probe. Hybridization was performed as described by Sambrook et al. (1989). The gene was cloned into pBluescript SK+/−. The genomic structure of GAS30 was obtained using information from RT-PCR and 3′ rapid amplification of cDNA ends (RACE) experiments. The first-strand cDNA synthesis for the RT-PCR and 3′ RACE experiments was performed with total RNA from young zygotes using SuperScript III reverse transcriptase (Invitrogen) and an oligo(dT)25 primer. The reaction was performed according to the manual provided. Two microliters of the product were used for PCR reactions to amplify (1) the 5′ UTR (primer pair 5′-GCTCTTCCCTAGCCACCTTAGTAGAC-3′ and 5′-GACTGGATTGAGACCT CATGCTTGC-3′) with an annealing temperature of 60°C, (2) the coding region on both sides of the second intron (primer pair 5′-CAAGCATGAGGTCTCAATCCAGT-3′ and 5′-CCACTTCTTGCCGTCCACAGTCACGTTGC-3′) with an annealing temperature of 62°C, and (3) the 3′ end by a 3′ RACE [primer pair 5′-CTCTAAGTGCCTCCTGATGAG-3′ and oligo(dT)25] with an annealing temperature of 55°C. Reactions were performed in a volume of 50 μL with 1× buffer (500 mm KCl, 100 mm Tris-HCl [pH 8.8], 15 mm MgCl2, 1% Triton X-100, 0.1% gelatin), 10 mm dNTPs-mix, and 2 units Taq polymerase (Amersham). The resulting PCR products were cloned and sequenced.
The coding regions were translated into amino acid sequences using BCM six frame translation utilities at http://searchlauncher.bcm.tmc.edu/seq-util/seq-util.html, and signal sequences were predicted with TargetP (Nielsen et al., 1997; Emanuelsson et al., 2000). Secondary structures of deduced gene products were predicted using the algorithm HNN (Guermeur et al., 1999).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers DQ017908 (GAS28), DQ017907 (GAS30), and DQ017909 (GAS31).
Acknowledgments
We wish to thank Dr. Saul Purton for intellectual input and Dr. Michael Schroda for intellectual input and critical reading of the manuscript.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to C.F.B. X.-K.H. acknowledges the support by the Deutscher Akademischer Austauschdienst for a stay in the laboratory of Dr. Purton.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.065037.
References
- Abe J, Kubo T, Takagi Y, Saito T, Miura K, Fukuzawa H, Matsuda Y (2004) The transcriptional program of synchronous gametogenesis in Chlamydomonas reinhardtii. Curr Genet 46: 304–315 [DOI] [PubMed] [Google Scholar]
- Adair WS, Apt KE (1990) Cell wall regeneration in Chlamydomonas: accumulation of mRNAs encoding cell wall hydroxyproline-rich glycoproteins. Proc Natl Acad Sci USA 87: 7355–7359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair WS, Hwang C, Goodenough UW (1983) Identification and visualization of the sexual agglutinin from the mating-type plus flagellar membrane of Chlamydomonas. Cell 33: 183–193 [DOI] [PubMed] [Google Scholar]
- Alonso-Monge R, Real E, Wojda I, Bebelman J-P, Mager WH, Siderius M (2001) Hyperosmotic stress response and regulation of cell wall integrity in Saccharomyces cerevisiae share common functional aspects. Mol Microbiol 4: 717–730 [DOI] [PubMed] [Google Scholar]
- Barlow JJ, Mathias AP, Williamson R (1963) A simple method for the quantitative isolation of undegraded high molecular weight ribonucleic acid. Biochem Biophys Res Commun 13: 61–66 [DOI] [PubMed] [Google Scholar]
- Beck CF, Acker A (1992) Gametic differentiation of Chlamydomonas reinhardtii. Control by nitrogen and light. Plant Physiol 98: 822–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CF, Haring MA (1996) Gametic differentiation of Chlamydomonas. Int Rev Cytol 168: 259–302 [Google Scholar]
- Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stress. In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 1158–1203
- Buchanan MJ, Imam SH, Eskue WA, Snell WJ (1989) Activation of the cell wall degrading protease, lysin, during sexual signaling in Chlamydomonas: The enzyme is stored as an inactive, higher relative molecular mass precursor in the periplasm. J Cell Biol 108: 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt JW (1979) The isolation and chemical composition of the zygospore cell wall of Chlamydomonas reinhardtii. Plant Sci Lett 15: 69–74 [Google Scholar]
- Cavalier-Smith T (1974) Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardtii. J Cell Sci 16: 529–556 [DOI] [PubMed] [Google Scholar]
- Cazalé A-C, Droillard M-J, Wilson C, Heberle-Bors E, Barbier-Brygoo H, Laurière C (1999) MAP kinase activation by hypoosmotic stress of tobacco cell suspensions: towards the oxidative burst response? Plant J 19: 297–307 [DOI] [PubMed] [Google Scholar]
- Davenport KR, Sohaskey M, Kamada Y, Levin DE (1995) A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J Biol Chem 270: 30157–30161 [DOI] [PubMed] [Google Scholar]
- Drzymalla C, Schroda M, Beck CF (1996) Light-inducible gene HSP70B encodes a chloroplast-localized heat shock protein in Chlamydomonas reinhardtii. Plant Mol Biol 31: 1185–1194 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Ertl H, Mengele R, Wenzl S, Engel J, Sumper M (1989) The extracelluar matrix of Volvox carterii: molecular structure of the cellular compartment. J Cell Biol 109: 3493–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 [DOI] [PubMed] [Google Scholar]
- Ferris PJ, Goodenough UW (1987) Transcription of novel genes, including a gene linked to the mating-type locus, induced by Chlamydomonas fertilization. Mol Cell Biol 7: 2360–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris PJ, Waffenschmidt S, Umen JG, Lin H, Lee J-H, Ishida K, Kubo T, Lau J, Goodenough UW (2005) Plus and minus sexual agglutinins from Chlamydomonas reinhardtii. Plant Cell 17: 597–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris PJ, Woessner JP, Goodenough UW (1996) A sex recognition glycoprotein is encoded by the plus mating-type gene fus1 of Chlamydomonas reinhardtii. Mol Biol Cell 7: 1235–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris PJ, Woessner JP, Waffenschmidt S, Kilz S, Drees J, Goodenough UW (2001) Glycosylated polyproline II rods with kinks as a structural motif in plant hydroxyproline-rich glycoproteins. Biochemistry 40: 2978–2987 [DOI] [PubMed] [Google Scholar]
- Godl K, Hallmann A, Rappel A, Sumper M (1995) Pherophorins: a family of extracellular matrix glycoproteins from Volvox structurally related to sex-inducing pheromone. Planta 196: 781–787 [PubMed] [Google Scholar]
- Goodenough UW (1986) Experimental analysis of the adhesion reaction between isolated Chlamydomonas flagella. Exp Cell Res 166: 237–246 [DOI] [PubMed] [Google Scholar]
- Goodenough UW, Gebhart B, Mecham RP, Heuser JE (1986) Crystals of the Chlamydomonas reinhardtii cell wall: polymerization, depolymerization, and purification of glycoprotein monomers. J Cell Biol 103: 405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW, Heuser JE (1985) The Chlamydomonas cell wall and its constituent glycoproteins analyzed by quick-freez, deep-etch technique. J Cell Biol 101: 1550–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW, Hwang C, Martin H (1976) Isolation and genetic analysis of mutant strains of Chlamydomonas reinhardtii defective in gametic differentiation. Genetics 82: 169–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW, St. Clair HS (1975) Bald-2: a mutation affecting the formation of doublet and triplet sets of microtubules in Chlamydomonas reinhardtii. J Cell Biol 66: 480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW, Weiss RL (1978) Interrelationships between microtubules, a striated fiber, and the gametic mating structure of Chlamydomonas reinhardtii. J Cell Biol 76: 430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in photosynthetic electron transfer chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 54: 1665–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grief C, O'Neill MA, Shaw PJ (1987) The zygote cell wall of Chlamydomonas reinhardtii: a structural, chemical and immunological approach. Planta 170: 433–445 [DOI] [PubMed] [Google Scholar]
- Grossman AR, Harris EE, Hauser C, Lefebvre PA, Martinez D, Rokhsar D, Shrager J, Silflow CD, Stern D, Vallon O, Zhang Z (2003) Chlamydomonas reinhardtii at the crossroads of genomics. Eukaryot Cell 2: 1137–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermeur Y, Geourjon C, Gallinari P, Deleage G (1999) Improved performance in protein secondary structure prediction by inhomogeneous score combination. Bioinformatics 15: 413–421 [DOI] [PubMed] [Google Scholar]
- Harris EH (1989) The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego [DOI] [PubMed]
- Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeast. Microbiol Mol Biol Rev 66: 300–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates JR, Jones RF (1964) Variation in dehygrogenase and glutamate dehydrogenase during the synchronous development of Chlamydomonas. Biochim Biophys Acta 86: 438–447 [DOI] [PubMed] [Google Scholar]
- Kato-Minoura T, Hirono M, Kamiya R (1997) Chlamydomonas inner-arm dynein mutant, ida5, has a mutation in an actin-encoding gene. J Cell Biol 137: 649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszewski MJ, Lamport DTA (1994) Extensin: repetitive motifs, functional sites, post-translational codes and phylogeny. Plant J 5: 157–172 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Fukuzawa H, Shimada T, Saito T, Matsuda Y (1992) Primary structure and expression of a gamete lytic enzyme in Chlamydomonas reinhardtii: similarity of functional domains to matrix metalloproteases. Proc Natl Acad Sci USA 89: 4693–4697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurvari V (1997) Cell wall biogenesis in Chlamydomonas: molecular characterization of a novel protein whose expression is up-regulated during matrix formation. Mol Gen Genet 256: 572–580 [DOI] [PubMed] [Google Scholar]
- Kurvari V, Qian F, Snell WJ (1995) Increased transcript levels of a methionine synthase during adhesion-induced activation of Chlamydomonas reinhardtii gametes. Plant Mol Biol 29: 1235–1252 [DOI] [PubMed] [Google Scholar]
- Lefebvre PA, Silflow CD (1999) Chlamydomonas: the cell and its genomes. Genetics 151: 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León R, Galván F (1995) Metabolic pathway for glycerol synthesis under osmotic stress in freshwater green alga Chlamydomonas reinhardtii. Plant Physiol Biochem 33: 213–218 [Google Scholar]
- Luykx P, Hoppenrath M, Robinson DG (1997. a) Structure and behavior of contractile vacuoles in Chlamydomonas reinhardtii. Protoplasma 198: 73–84 [Google Scholar]
- Luykx P, Hoppenrath M, Robinson DG (1997. b) Osmoregulatory mutants that affect the function of the contractile vacuole in Chlamydomonas reinhardtii. Protoplasma 200: 99–111 [Google Scholar]
- Matsuda Y, Saito T, Yamaguchi T, Koseki M, Hayashi K (1987) Topography of cell wall lytic enzyme in Chlamydomonas reinhardtii: form and location of stored enzyme in vegetative cell and gamete. J Cell Biol 104: 321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Shimada T, Sakamoto Y (1992) Ammonium ions control gametic differentiation in Chlamydomonas reinhardtii. Plant Cell Physiol 33: 909–914 [Google Scholar]
- Matsuda Y, Yamasaki A, Saito T, Yamaguchi T (1984) Purification and characterization of cell wall lytic enzyme released by mating gametes of Chlamydomonas reinhardtii. FEBS Lett 166: 293–297 [Google Scholar]
- Matters GL, Goodenough UW (1992) A gene/pseudogene tandem duplication encodes a cysteine-rich protein expressed during zygote development in Chlamydomonas reinhardtii. Mol Gen Genet 232: 81–88 [DOI] [PubMed] [Google Scholar]
- Meijer HJ, Ter Riet B, van Himbergen JA, Musgrave A, Munnik T (2002) KCl activates phospholipase D at two different concentration ranges: distinguishing between hyperosmotic stress and membrane depolarization. Plant J 31: 51–59 [DOI] [PubMed] [Google Scholar]
- Meijer HJG, Arisz SA, van Himbergen JAJ, Musgrave A, Munnik T (2001) Hyperosmotic stress rapidly generates lyso-phosphatidic acid in Chlamydomonas. Plant J 25: 541–548 [DOI] [PubMed] [Google Scholar]
- Meijer HJG, Divecha N, van den Ende H, Musgrave A, Munnik T (1999) Hyperosmotic stress induces rapid synthesis of phosphatidyl-D-inositol 3,5-bisphosphate in plant cells. Planta 208: 294–298 [Google Scholar]
- Minami S, Goodenough UW (1978) Novel glycopolypeptide synthesis induced by gametic cell fusion in Chlamydomonas reinhardtii. J Cell Biol 77: 165–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misamore MJ, Gupta S, Snell WJ (2003) The Chlamydomonas Fus1 protein is present on the mating type plus fusion organelle and required for a critical membrane adhesion event during fusion with minus gametes. Mol Biol Cell 14: 2530–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Ligterink W, Meskiene I, Calderini O, Beyerly J, Musgrave A, Hirt H (1999) Distinct osmo-sensing protein kinase pathways are involved in signalling moderate and severe hyper-osmotic stress. Plant J 20: 381–388 [DOI] [PubMed] [Google Scholar]
- Munnik T, Meijer HJ, Ter Riet B, Hirt H, Frank W, Bartels D, Musgrave A (2000) Hyperosmotic stress stimulates phospholipase D activity and elevates the levels of phosphatidic acid and diacylglycerol pyrophosphate. Plant J 22: 147–154 [DOI] [PubMed] [Google Scholar]
- Munnik T, Meijer HJG (2001) Osmotic stress activates distinct lipid and MAPK signalling pathways in plants. FEBS Lett 498: 172–178 [DOI] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10: 1–6 [DOI] [PubMed] [Google Scholar]
- O'Neill M, Roberts K (1981) Methylation analysis of cell wall glycoproteins and glycopeptides from Chlamydomonas reinhardtii. Phytochem 20: 25–28 [Google Scholar]
- Pan J, Snell WJ (2000. a) Signal transduction during fertilization in the unicellular green alga, Chlamydomonas. Curr Opin Microbiol 3: 596–602 [DOI] [PubMed] [Google Scholar]
- Pan J, Snell WJ (2000. b) Regulated targeting of a protein kinase into an intact flagellum. J Biol Chem 275: 24106–24114 [DOI] [PubMed] [Google Scholar]
- Pan J-M, Haring MA, Beck CF (1997) Characterization of blue light signal transduction chains that control development and maintenance of sexual competence in Chlamydomonas reinhardtii. Plant Physiol 115: 1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale SM, Goodenough UW (1987) Cyclic AMP functions as a primary sexual signal in gametes of Chlamydomonas reinhardtii. J Cell Biol 105: 2279–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton S, Rochaix J-D (1994) Complementation of a Chlamydomonas reinhardtii mutant using a genomic cosmid library. Plant Mol Biol 24: 533–537 [DOI] [PubMed] [Google Scholar]
- Reiser V, Raitt DC, Saito H (2003) Yeast osmosensor Sln1 and plant cytokinin receptor Cre1 respond to changes in turgor pressure. J Cell Biol 161: 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez H, Haring MA, Beck CF (1999) Molecular characterization of two light-induced, gamete-specific genes from Chlamydomonas reinhardtii that encode hydroxyproline-rich proteins. Mol Gen Genet 261: 267–274 [DOI] [PubMed] [Google Scholar]
- Sager R, Granick S (1954) Nutritional control of sexuality in Chlamydomonas reinhardtii. J Gen Physiol 37: 729–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Small L, Goodenough UW (1993) Activation of adenylyl cyclase in Chlamydomonas reinhardtii by adhesion and heat. J Cell Biol 122: 137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook F, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Silflow CD (1998) Organization of nuclear genome. In J.-D. Rochaix, M. Goldschmidt-Clermont, S. Merchant, eds, The Molecular Biology of Chloroplast and Mitochondria in Chlamydomonas. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 25–40
- Snell WJ, Eskue WA, Buchanan MJ (1989) Regulated secretion of a serine protease that activates an extracellular matrix-degrading metalloprotease during fertilization in Chlamydomonas. J Cell Biol 109: 1689–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell WJ, Moore WS (1980) Aggregation-dependent turnover of flagellar adhesion molecules in Chlamydomonas gametes. J Cell Biol 84: 203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumper M, Berg E, Wenzl S, Godl K (1993) How sex pheromone might act at a concentration below 10−16 M. EMBO J 12: 831–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki L, Woessner JP, Uchida H, Kuroiwa H, Yuasa Y, Waffenschmidt S, Goodenough UW, Kuroiwa T (2000) A zygote-specific protein with hydroxyproline-rich glycoprotein domains and lectin-like domains involved in the assembly of the cell wall of Chlamydomonas reinhardtii (Chlorophyta). J Phycol 36: 571–583 [DOI] [PubMed] [Google Scholar]
- Treier U, Fuchs S, Weber M, Wakarchuk WW, Beck CF (1989) Gametic differentiation in Chlamydomonas reinhardtii: light dependence and gene expression patterns. Arch Microbiol 152: 572–577 [Google Scholar]
- Uchida H, Kawano S, Sato N, Kuroiwa T (1993) Isolation and characterization of novel genes which are expressed during the very early stage of zygote formation in Chlamydomonas reinhardtii. Curr Genet 24: 296–300 [DOI] [PubMed] [Google Scholar]
- Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hirayama T, Shinozaki K (1999) A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell 11: 1743–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon O, Wollman F-A (1995) Mutations affecting o-glycosylation in Chlamydomonas reinhardtii caused delayed cell wall degradation and sex-limited sterility. Plant Physiol 108: 703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gromoff ED, Beck CF (1993) Genes expressed during sexual differentiation of Chlamydomonas reinhardtii. Mol Gen Genet 241: 415–421 [DOI] [PubMed] [Google Scholar]
- von Gromoff ED, Treier U, Beck CF (1989) Three light-inducible heat shock genes of Chlamydomonas reinhardtii. Mol Cell Biol 9: 3911–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kampen J, Nieländer U, Wetter M (1994) Stress-dependent transcription of a gene encoding a Gβ-like polypeptide from Chlamydomonas reinhardtii. J Plant Physiol 143: 756–758 [Google Scholar]
- Wegener D, Beck CF (1991) Identification of novel genes specifically expressed in Chlamydomonas reinhardtii zygotes. Plant Mol Biol 16: 937–946 [DOI] [PubMed] [Google Scholar]
- Weissig H, Beck CF (1991) Action spectrum for the light-dependent step in gametic differentiation of Chlamydomonas reinhardtii. Plant Physiol 97: 118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NF, Foglesong MJ, Snell WJ (1997) The Chlamydomonas mating type plus fertilization tubule, a prototypic cell fusion organelle: isolation, characterization, and in vitro adhesion to mating type minus gametes. J Cell Biol 137: 1537–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner JP, Goodenough UW (1989) Molecular characterization of a zygote wall protein: an extensin-like molecule in Chlamydomonas reinhardtii. Plant Cell 1: 901–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner JP, Goodenough UW (1992) Zygote and vegetative cell wall proteins in Chlamydomonas reinhardtii share a common epitope, (SerPro)x. Plant Sci 83: 65–76 [Google Scholar]
- Woessner JP, Molendijk AJ, van Egmond P, Klis FM, Goodenough UW, Haring MA (1994) Domain conservation in several volvocalean cell wall proteins. Plant Mol Biol 26: 947–960 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ross EM, Snell WJ (1991) ATP-dependent regulation of flagellar adenylylcyclase in gametes of Chlamydomonas reinhardtii. J Biol Chem 266: 22954–22959 [PubMed] [Google Scholar]