Abstract
Early processes underlying plant gravity sensing were investigated in rhizoids of Chara globularis under microgravity conditions provided by parabolic flights of the A300-Zero-G aircraft and of sounding rockets. By applying centrifugal forces during the microgravity phases of sounding rocket flights, lateral accelerations of 0.14g, but not of 0.05g, resulted in a displacement of statoliths. Settling of statoliths onto the subapical plasma membrane initiated the gravitropic response. Since actin controls the positioning of statoliths and restricts sedimentation of statoliths in these cells, it can be calculated that lateral actomyosin forces in a range of 2 × 10−14 n act on statoliths to keep them in place. These forces represent the threshold value that has to be exceeded by any lateral acceleration stimulus for statolith sedimentation and gravisensing to occur. When rhizoids were gravistimulated during parabolic plane flights, the curvature angles of the flight samples, whose sedimented statoliths became weightless for 22 s during the 31 microgravity phases, were not different from those of in-flight 1g controls. However, in ground control experiments, curvature responses were drastically reduced when the contact of statoliths with the plasma membrane was intermittently interrupted by inverting gravistimulated cells for less than 10 s. Increasing the weight of sedimented statoliths by lateral centrifugation did not enhance the gravitropic response. These results provide evidence that graviperception in characean rhizoids requires contact of statoliths with membrane-bound receptor molecules rather than pressure or tension exerted by the weight of statoliths.
Sensing the direction of gravity and orienting their organs with respect to the gravity vector enabled plants to evolve and to explore habitats below and above the surface of the earth. Whereas there is considerable progress in understanding the cellular, physiological, and molecular processes involved in gravitropic responses of shoots and roots (for review, see Boonsirichai et al., 2002; Sievers et al., 2002; Blancaflor and Masson, 2003), very little is known about the cellular and molecular mechanisms underlying the decisive early steps of gravity sensing (for review, see Kiss, 2000; Morita and Tasaka, 2004).
Nĕmec (1900) and Haberlandt (1900) observed that gravitropic responses of plant organs are preceded by the gravity-directed sedimentation of starch-filled amyloplasts, named statoliths, in specialized gravity-sensing cell types, i.e. the central columella cells in the root cap and shoot endodermal cells. Surgical ablation of the root cap eliminated root gravitropism without affecting root growth (Juniper et al., 1966). By destroying individual cells of the root cap using laser or genetic ablation, the central columella cells that exhibit the largest statolith sedimentation velocities were identified as contributing most to the gravitropic response (Blancaflor et al., 1998; Tsugeki and Fedoroff, 1999).
A variety of agravitropic mutants highlights the crucial role of statoliths as primary susceptors of the gravity stimulus in higher plants. Arabidopsis (Arabidopsis thaliana) mutants sgr1 and sgr7, which lack shoot endodermal cells, do not exhibit a gravitropic response of shoots, although they show normal phototropic curvature (Fukaki et al., 1996, 1998). Furthermore, eal1 mutants, whose endodermal cells are devoid of any sedimentable amyloplasts, exhibit strongly impaired gravitropism of hypocotyls and inflorescence stems. Their columella cells, however, contain statoliths and roots exhibit normal gravitropism (Fujihira et al., 2000). In starch-deficient mutants of Arabidopsis and tobacco (Nicotiana tabacum), the gravitropic responses have been reported to correlate with the starch content of amyloplasts in the statocytes of roots and shoots (Kiss et al., 1996; MacCleery and Kiss, 1999; Weise and Kiss, 1999).
The starch statolith theory of gravity sensing in higher plants is substantially supported by the induction of curvature responses in vertically growing plant organs after magnetophoretic displacement of statoliths (Kuznetsov and Hasenstein, 1996, 1997; Kuznetsov et al., 1999; Weise et al., 2000). These experiments provide clear evidence that sedimentation of statoliths is the primary step of gravity sensing (susception) leading to graviperception, the second step that transduces the physical stimulus of gravity-induced statolith sedimentation into a physiological signal.
Early models of graviperception postulated that activation of the gravireceptor depends on a positional effect provided by the weight of sedimented statoliths compressing peripheral endoplasmic reticulum (ER) cisternae at the lower cell flank (Sievers et al., 1991a, and refs. therein). This model was in contradiction to the observations that centrifuged cress roots exhibited gravitropic bending without contact between statoliths and ER (Wendt et al., 1987), and that already small movements of sedimenting statoliths were able to trigger a gravitropic response (Sievers et al., 1991a). Later, kinetic models of graviperception have been developed proposing that cytoskeletal elements, especially the actin microfilaments, are good candidates for playing a role as transducers of tensional forces generated by the gravity-induced sedimentation of statoliths to mechanosensitive receptors in cortical ER membranes or in the plasma membrane (Sievers et al., 1991a; Ding and Pickard, 1993; Yoder et al., 2001; Perbal and Driss-Ecole, 2003). However, experimental evidence for the role of the actin cytoskeleton in susception and perception of gravity remains contradictory since treating statocytes with actin-disrupting drugs increased the sedimentation rate of statoliths (Sievers et al., 1989) and enhanced the gravitropic response (Yamamoto and Kiss, 2002; Hou et al., 2003, 2004). It also remains unclear how statoliths keep gravireceptors activated after they have completed sedimentation and are no longer falling. At least in this phase, continued receptor activation could only be explained by statoliths interacting with membrane-bound receptors (Sievers et al., 1991a) or with hypothetical cortical cytoskeletal arrangements that are insensitive to actin-disrupting drugs (Perbal and Driss-Ecole, 2003).
The experiments presented in this article are not intended to answer these questions for higher plants; however, the results provide insights in the early mechanisms of plant gravity sensing by using single-celled and tip-growing rhizoids of the characean green algae as a gravitropic model system. In positively gravitropic (downward-growing) rhizoids, the gravitropic signaling pathway is short and all steps occur within the tip region of a single cell. Gravity susception is accomplished by the gravity-induced sedimentation of statoliths, small vacuoles containing BaSO4 crystals, onto the lower subapical cell flank (for review, see Sievers et al., 1996; Braun, 1997). There is unambiguous evidence that the actin cytoskeleton plays an essential role in the early processes of gravity sensing. Actomyosin precisely controls statolith positioning (Hejnowicz and Sievers, 1981; Sievers et al., 1991b; Braun et al., 2002) and directs sedimenting statoliths to the confined gravisensitive region of the plasma membrane 10 to 30 μm behind the tip (Braun, 2002). For gravity perception to occur, statoliths have to settle on this narrow belt-like membrane area and interact with membrane-bound receptors. When statoliths are displaced toward the cell flank without reaching the plasma membrane, positively gravitropic reorientation of the cell tip is not initiated (Braun, 2002). These results demonstrate that activation of membrane-bound gravireceptors in rhizoids depends on a positional rather than kinetic effect.
In this study, experiments were conducted under microgravity conditions of sounding rocket (MAXUS) and parabolic plane flights in order to unravel primary processes of gravity sensing in rhizoids of Chara globularis. Determination of the minimum acceleration level required to induce lateral displacement of statoliths allowed a detailed characterization of the cytoskeletal mechanisms underlying gravity susception. The acceleration profile of parabolic flights of the A300 Zero-G aircraft with alternating hyper- and microgravity phases provided good conditions to investigate mechanisms of gravireceptor activation in a gravity-sensing plant cell type.
RESULTS
Threshold Acceleration for Lateral Statolith Displacement
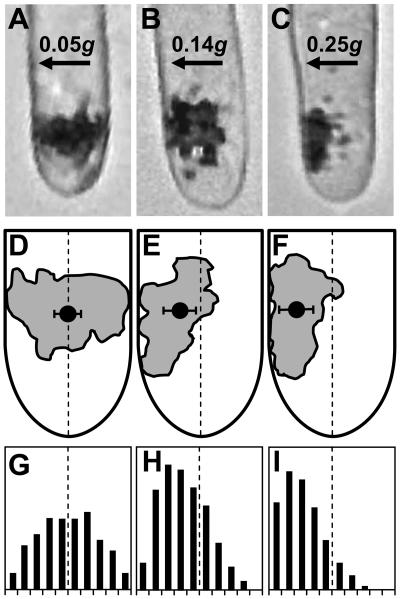
Vertically downward-growing rhizoids were subjected to lateral centrifugation during the 13-min microgravity phases of two MAXUS sounding rocket flights. Analyses of digital images taken from samples that were fixed at the end of the microgravity phases revealed that centrifugation with 0.4g (data not shown), 0.25g, and 0.14g were sufficient to induce lateral displacement of statoliths (Fig. 1, B and C). Several statoliths were sedimented onto the centrifugal cell flank, whereas no statoliths were found to be present at the centripetal plasma membrane (Fig. 1, B and C). At an acceleration level of 0.05g, no redistribution of statoliths was observed (Fig. 1A) and the shape of the statolith complex was similar to those of normal vertically growing cells on ground.
Figure 1.
Position of statoliths in chemically fixed rhizoids that were laterally centrifuged with 0.05g (A, D, and G), 0.14g (B, E, and H), and 0.25g (C, F, and I) for 13 min during the microgravity phase of the MAXUS-5 sounding rocket flight. Centrifugation with 0.25g and 0.14g, but not with 0.05g, induced a displacement of statoliths and settlement of some statoliths onto the centrifugal cell flank. A to C, Micrographs showing the position of statoliths in representative samples of each acceleration level. D to F, Geometric centers of the statolith complexes (black circles represent mean ± se in lateral direction, n ≥ 4). The shape of the statolith complex in one representative rhizoid of each acceleration level (rhizoids are different from those displayed in A–C) is shown in gray. G to I, Distribution of statolith frequency across the cell diameter (n ≥ 4). Arrows indicate direction of centrifugal force. Dashed lines represent median axes of the cells. Diameter of rhizoids, 30 μm.
The distribution of statolith frequency across the cell diameter in the three sets of chemically fixed samples is shown in Figure 1, G to I. Whereas the majority of statoliths was displaced into the centrifugal half of the cells after lateral acceleration with 0.14g and 0.25g (Fig. 1, H and I), statoliths were still symmetrically arranged across the cell diameter after 13 min at 0.05g (Fig. 1G). Accordingly, the geometric center of the statolith complex in 0.14g and 0.25g samples, but not in 0.05g samples, was shifted from a position around the median axis of the cells (Fig. 1, dashed line), the normal position in downward-growing cells, in the direction of the acceleration stimulus (Fig. 1, D–F).
Video microscopic recording allowed tracking of statoliths in four rhizoids during lateral centrifugation at 0.14g under microgravity conditions of the MAXUS-5 sounding rocket flight. A representative example of statolith redistribution induced by 0.14g is shown in Figure 2. Statoliths were symmetrically distributed across the cell diameter of the rhizoid before lift off (Fig. 2; t = −250 s) and shortly after the onset of centrifugation in microgravity (Fig. 2A; t = +130 s). The statolith complex appeared slightly more condensed due to the launch accelerations with peaks of up to 12.8g in the apical direction. During continued lateral acceleration, statoliths were gradually displaced toward the centrifugal flank (Fig. 2; t = +429 s, +692 s, +827 s). Sporadically, some statoliths seemed to settle onto the gravisensitive plasma membrane of the centrifugal flank (Fig. 2; t = +692 s); however, settlement was only transient since the statolith position was not only influenced by centrifugation but also by actomyosin-dependent transport mechanisms. A few individual statoliths were sometimes even observed near the centripetal plasma membrane (Fig. 2; t = +692 s). The image sequence in Figure 2A represents snapshots of the dynamically changing statolith position and is therefore suited to demonstrate the displacement of statoliths toward the centrifugal flank, but the images do not provide clear evidence for the centrifugation-induced settlement of statoliths onto the lateral plasma membrane, which was distinctly visible in chemically fixed samples (Fig. 1B). Nevertheless, microscopic analyses of living rhizoids after retrieval of the payload have shown that lateral acceleration of 0.14g for 13 min resulted in curvature angles of 5 to 9 degrees, whereas centrifugation with 0.05g did not provoke any curvature response (data not shown). It can be concluded that sporadic contact of statoliths with the centrifugal plasma membrane was sufficient to initiate the gravitropic signaling pathway.
Figure 2.
Distribution of statoliths in characean rhizoids before lift off (−250 s) of the MAXUS-5 sounding rocket and during lateral centrifugation in microgravity (indicated in seconds after lift off). A, Series of micrographs of a representative rhizoid exhibiting symmetrical distribution of statoliths across the cell diameter before lift off. Centrifugal displacement of statoliths caused by the 0.14g acceleration in microgravity was first detectable at +429 s. Individual statoliths settled onto the cell flank toward the end of the microgravity phase (+692 s and +827 s). Arrows indicate the direction of gravitational and centrifugal forces. Diameter of rhizoid, 30 μm. B to D, Distribution of statolith frequency across the cell diameter of four rhizoids at indicated times of the rocket flight demonstrating that statoliths were displaced from a symmetrical arrangement before lift off (B) to an asymmetrical distribution during lateral centrifugation (C and D).
In addition to the lateral shift of the statolith position, a basipetal displacement of statoliths during the microgravity phase was observed (Fig. 2A). This effect has already been described earlier and is attributed to net basipetally acting actomyosin forces, which are no longer compensated by the gravity force (Braun et al., 2002). Under normal 1g conditions, both forces work in concert to keep statoliths in a dynamically stable position of balance.
Effects of Increasing the Weight of Sedimented Statoliths on the Gravitropic Response
Ground control experiments were performed to test the impact of the sequence of hypergravity accelerations as they occur during parabolic flights on the gravitropic response of characean rhizoids. The profile of hypergravity phases was mimicked by centrifuging rhizoids 62 times for 22 s at 2g during an overall gravistimulation time of 120 min. The centrifugation stimuli were applied in the direction of the gravistimulus after rhizoids had been placed horizontally for 10 min. Thus, by increasing the weight of fully sedimented statoliths, centrifugation enhanced the mechanical pressure on the plasma membrane of the subapical lateral cell flank, but did not accelerate the statolith sedimentation process. Intermittently centrifuged rhizoids exhibited a mean curvature angle of 45.03° (±8.18), which was in the same range as the curvature angle of control samples (44.62° ± 11.00) that were gravistimulated for 120 min under continuous 1g conditions (Student's t test, P = 0.8136; Fig. 3). These results demonstrate that the graviresponse was not promoted by increasing the pressure of sedimented statoliths on putative gravireceptor molecules located in the plasma membrane.
Figure 3.
Graph showing curvature angles of control cells, which were continuously gravistimulated for 120 min at 90° on ground (white bar; n = 69) and of rhizoids that were horizontally positioned for 120 min on ground and intermittently centrifuged 62 times for 22 s at 2g (gray bar; n = 59). Not significantly different curvature values of controls and centrifuged samples (Student's t test, P = 0.8136) provide evidence that increasing the weight of sedimented statoliths by repeated short-term centrifugation does not affect gravitropic curvature. Data represent means ± se.
Similarly, when rhizoids were prestimulated by tilting them for 10 min by 90° at 1g followed by stimulation at 1g, 2g, 3g, 4g, or 5g for 15 min, the mean curvature angles were all in the same range of 23.09° (±5.33), 24.73° (±6.62), 24.64° (±5.68), 23.71° (±4.61), and 25.92° (±4.65), respectively (pairwise Student's t test, P > 0.05; Fig. 4). No tendency was recognizable toward reduced or enhanced curvature angles that might have been caused by increasing the weight of sedimented statoliths by centrifugation. As mentioned above, prestimulation of all cells at 1g warranted that the statoliths sedimented onto the lateral cell flank under the same conditions so that the graviresponse started at the same time in all samples.
Figure 4.
Curvature angles of rhizoids after 10-min prestimulation at 90° on ground and subsequent stimulation at 1g, 2g, 3g, 4g, or 5g for 15 min in the direction of the initial gravistimulus. 1g controls (white bar) and centrifuged samples (gray bars) exhibited curvature angles that were all in the same range as revealed by pairwise Student's t test (P > 0.05), indicating that enhanced pressure on the gravisensitive site of the plasma membrane due to an increased weight of statoliths had no impact on gravitropic curvature. Data represent means ± se (n ≥ 11).
Effects of Short-Term Removal of Statoliths from the Plasma Membrane on the Gravitropic Response
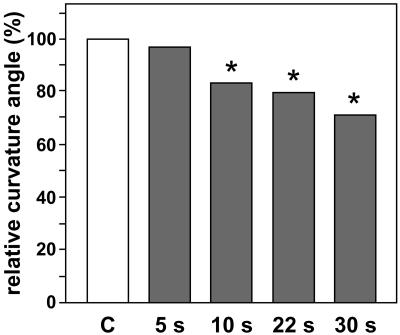
The parabolic plane flight profile provides 31 short microgravity phases within a flight time of approximately 120 min. In order to evaluate whether 22 s, the duration of each of the microgravity phases, would be sufficient to interrupt gravity perception and to alter the gravitropic response, comprehensive inversion experiments were performed on ground. Rhizoids were prestimulated for 10 min by 90° and subsequently inverted 31 times from 90° to 270° within 120 min, according to the sequence of the microgravity phases during parabolic flights. The intermittent removal of statoliths by inverting cells for 30, 22, and 10 s resulted in significantly reduced curvature angles as compared to the corresponding control samples that were continuously gravistimulated (Student's t test, P < 0.01; Fig. 5; Table I). Reduction of curvature was strongest when rhizoids were inverted for 30 s (−28.59%). This effect decreased, but was still significant when the inversion intervals were reduced to 22 s (−15.59% to −19.76%) and 10 s (−13.03% to −20.10%). When rhizoids were inverted 31 times for only 5 s, curvature angles were no longer significantly different from those of continuously gravistimulated control cells (Student's t test, P > 0.05; Fig. 5; Table I). In addition, the gravitropic curvature of rhizoids that were intermittently inverted 31 times for 22 s within 120 min was similar as compared to those that were continuously gravistimulated for only 109 min, matching the total gravistimulation time of 120 min reduced by the total inversion time of 31 × 22 s (data not shown). For inversions and controls of each experiment set, rhizoids of the same age growing under identical conditions were used. The variation in the curvature angles between the different experiment sets (Table I) can be attributed to different growth conditions, e.g. temperature and seasonal conditions.
Figure 5.
Mean curvature angles of rhizoids that were prestimulated on ground for 10 min at 90° and subsequently inverted 31 times to 270° (gray bars) relative to the mean curvature angles of the corresponding control samples that were set to 100% (C, white bar). Rhizoids were repeatedly inverted for 5, 10, 22, or 30 s during a total experiment duration of 120 min, whereas control samples were stimulated at 90° for 120 min under continuous 1g conditions. Significantly reduced curvature angles of the inverted samples (Student's t test, P < 0.01; indicated by an asterisk) were observed when the duration of the inversion phases was longer than 5 s (n ≥ 44 for each sample). Complete data of all inversion experiments, including the absolute curvature values, are summarized in Table I.
Table I.
Curvature angles of intermittently inverted rhizoids and continuously gravistimulated control cells on ground
Inversion profiles are described by the duration of a single inversion phase and by the total duration of 31 inversion events listed as absolute value and as a percentage of the total experiment duration of 120 min. Control rhizoids were continuously gravistimulated for 120 min at 90°. Values of maximally achieved curvature angles are means ± se. The number of cells measured (n) is shown in parentheses. Differences between mean curvature angles of inverted cells and the corresponding control cells are shown in percent. For a description of the experiment, see Figure 5.
| Experiment No.
|
Inversion Profile
|
Curvature Angle (°)
|
Difference
|
|||
|---|---|---|---|---|---|---|
| Single | Total | Total | Control Cells | Inverted Cells | ||
| s | min | % | % | |||
| 1 | 5 | 2.6 | 2.15 | 37.44 ± 8.94 (45) | 36.23 ± 8.23 (44) | −3.25 |
| 2 | 5 | 2.6 | 2.15 | 38.87 ± 8.78 (69) | 36.14 ± 7.23 (63) | −7.02 |
| 3 | 5 | 2.6 | 2.15 | 42.89 ± 6.02 (61) | 44.81 ± 7.37 (53) | +4.49 |
| 4 | 10 | 5.2 | 4.31 | 61.40 ± 8.63 (40) | 53.40 ± 15.00 (25)a | −13.03 |
| 5 | 10 | 5.2 | 4.31 | 39.31 ± 9.36 (55) | 31.41 ± 8.87 (49)a | −20.10 |
| 6 | 10 | 5.2 | 4.31 | 42.89 ± 6.02 (61) | 35.74 ± 7.99 (57)a | −16.67 |
| 7 | 10 | 5.2 | 4.31 | 49.72 ± 10.68 (46) | 41.97 ± 9.63 (34)a | −15.58 |
| 8 | 22 | 10.3 | 8.61 | 48.38 ± 9.60 (50) | 38.82 ± 11.50 (44)a | −19.76 |
| 9 | 22 | 10.3 | 8.61 | 66.23 ± 4.82 (13) | 55.90 ± 6.30 (21)a | −15.59 |
| 10 | 30 | 15.5 | 12.92 | 59.11 ± 11.37 (104) | 42.21 ± 10.24 (77)a | −28.59 |
Curvature angles of inverted and control cells were significantly different as revealed by Student's t test (P < 0.01).
During inversion of gravistimulated rhizoids, movements of statoliths that were initially sedimented on the plasma membrane were tracked by high-magnification video microscopy in order to analyze the time course of statolith sedimentation away from the upper gravisensitive plasma membrane. After 2 s of inversion, individual statoliths had already lost contact with the plasma membrane and, 5 s after inversion, the statoliths were found at a mean distance of approximately 0.5 μm (Fig. 6), indicating that the inversion intervals of the above-described experiments were sufficient to remove statoliths from the plasma membrane. Thus, the inversion experiments demonstrate that gravity perception and the graviresponse are terminated very quickly as soon as the contact of statoliths with the gravisensitive plasma membrane is interrupted.
Figure 6.
Displacement of statoliths after the inversion of gravistimulated rhizoids from 90° to 270° on ground. The graph shows the mean distances (±se, n = 18) of statoliths from the upper cell flank at the indicated times after inversion. Only those statoliths were selected for the measurements that were regarded as fully sedimented on the plasma membrane (PM) after 10 min of gravistimulation at 90°. A dashed line was drawn as a reference to indicate the position of the sedimented statoliths on the upper plasma membrane and the subsequent measurements of the distances were referred to this line. The series of micrographs shows that all statoliths have sedimented away from the upper plasma membrane already 5 s after inverting the representative rhizoid. Bar, 1 μm.
Effects of Short-Term Weightlessness of Sedimented Statoliths on the Gravitropic Response
During the 36th European Space Agency (ESA) and the sixth Deutsches Zentrum für Luft- und Raumfahrt (DLR) parabolic flight campaigns, all in-flight control samples in the onboard centrifuge and flight samples were tilted by 90° 10 min prior to the first parabola. This ensured that the statoliths were properly sedimented on the lateral cell flank in all samples so that the following hypergravity and microgravity phases acted on sedimented statoliths. Immediately after the last parabola, all samples were tilted back into the original orientation. Since the above-mentioned centrifugation experiments have shown that short hypergravity phases do not affect the graviresponse, the comparison of the maximally achieved curvature angles of flight samples and in-flight controls should provide information as to whether sedimented statoliths, which are weightless but still sedimented on the lower plasma membrane during the short microgravity phases, are able to activate the gravireceptor or not. Initially, during the 36th ESA campaign, ground control samples were continuously gravistimulated at 1g conditions for the same time as the flight samples. But, since it was not feasible to provide the same environmental conditions, temperature gradients, and vibrations as for the flight samples, these controls were not regarded as proper controls. In-flight control samples, which were centrifuged at 1g during the microgravity phases, however, experienced the same conditions as flight samples and, therefore, represent the adequate reference system.
Analysis of the statolith position in gravistimulated Chara rhizoids, which were observed by video microscopy during the different flight phases, revealed that the shape of the sedimented statolith complex remained unchanged and that statoliths were not lifted from the plasma membrane during the short-term microgravity conditions (data not shown).
On five out of six flight days, all 31 parabolas were flown in a consecutive sequence, providing a total of 11.4 min of microgravity, which is in a range of 7.73% to 9.88% of the total experiment duration time (Table II). On one flight day, the A300 Zero-G aircraft flew only 18 parabolas in 84 min, followed by 13 parabolas in 59 min, providing microgravity portions of 7.86% and 8.08%, respectively (Table II). In all cases, the curvature angles of the flight samples (n = 32–138 per flight) and the corresponding in-flight controls (n = 38–121 per flight) were almost identical and minor differences were not significant (Student's t test, P > 0.05; Fig. 7; Table II). Even the highest deviations of curvature angles from flight samples and in-flight controls of −1.76% to −4.18% were still small considering the corresponding portions of total microgravity time (7.79%–9.88%; Table II) and compared to the reduction of curvature after intermittent inversion for 10 and 22 s (−13.03% to −20.10%; Table I). These results of the parabolic flight experiments demonstrate that weightless statoliths, which are still present at the graviperception site, but do not exert any pressure, are capable of activating the gravireceptor, which triggers gravity perception and the graviresponse during the microgravity phases as during the hypergravity phases and at 1g.
Table II.
Rhizoid curvature angles of flight samples and in-flight controls of parabolic plane flight experiments
All samples were horizontally positioned for the indicated experiment duration time of seven parabolic plane flights. In-flight control samples were laterally centrifuged at 1g during the microgravity phases. The total duration of all 22-s microgravity phases of each flight is listed as an absolute value and as a percentage of the experiment duration time. Values of maximally achieved curvature angles are means ± se. The number of cells measured (n) is shown in parentheses. Differences between mean curvature angles of flight samples and the corresponding in-flight controls, which are shown in percent, were nonsignificant (Student's t test, P > 0.05). For details of the experiment setup, see Figure 7.
| Flight No.
|
Experiment Duration
|
μg Time
|
Curvature Angle (°)
|
Difference
|
||
|---|---|---|---|---|---|---|
| Total | Total | Control Cells | Flight Cells | |||
| min | min | % | % | |||
| 1 | 121 | 11.4 | 9.39 | 46.76 ± 10.67(62) | 46.65 ± 14.13 (115) | −0.23 |
| 2A | 84 | 6.6 | 7.86 | 40.55 ± 8.69 (67) | 40.44 ± 10.69 (118) | −0.28 |
| 2B | 59 | 4.8 | 8.08 | 36.64 ± 10.20 (80) | 35.78 ± 7.95 (95) | −2.34 |
| 3 | 125 | 11.4 | 9.09 | 47.39 ± 9.27 (121) | 47.22 ± 9.17 (138) | −0.36 |
| 4 | 147 | 11.4 | 7.73 | 50.82 ± 7.53 (38) | 51.21 ± 7.28 (74) | +0.77 |
| 5 | 146 | 11.4 | 7.79 | 51.69 ± 7.73 (81) | 49.53 ± 7.49 (32) | −4.18 |
| 6 | 115 | 11.4 | 9.88 | 53.12 ± 7.68 (58) | 52.18 ± 8.01 (38) | −1.76 |
Figure 7.
Mean curvature angles of flight samples (gray bars) relative to the mean curvature angles of the corresponding in-flight control samples that were set to 100% (C, white bar). The experiments were conducted during the 36th ESA (sample nos. 1–3) and during the sixth DLR parabolic flight campaign (nos. 4–6). On flight day 2, two flights with reduced flight profiles were conducted (nos. 2A and 2B; for details of flight profiles, see Table II). All samples were tilted into a horizontal position 10 min prior to the first parabola and tilted back after the last parabola of the flight profile. In-flight control samples were laterally centrifuged at 1g during the microgravity phases. No significant differences in curvature angles were observed between flight samples and in-flight controls on any of the flights (Student's t test, P > 0.05; n ≥ 32 for each sample), indicating that graviperception was not interrupted when statoliths became weightless during microgravity. For complete data and absolute curvature values of all flight experiments, see Table II.
DISCUSSION
Gravisensitivity Is Determined by Molecular Interactions between Statoliths and the Actin Cytoskeleton
Whereas in most cell types gravity does not affect the position or movement of organelles, gravity-sensing statocytes allow their amyloplast statoliths to sediment along the gravity vector. There are several reports indicating that the actomyosin system is involved in the positioning of statoliths and in the modulation of the sedimentation process in order to meet specific requirements for a most beneficial gravitropic response (Sievers et al., 1991a; Volkmann et al., 1991, 1999; Driss-Ecole et al., 2000; Perbal et al., 2004). Although the kinetics of statolith movements have been analyzed in gravisensing cells of shoots (Sack et al., 1984; Saito et al., 2005) and roots (Sack et al., 1985, 1986; MacCleery and Kiss, 1999; Yoder et al., 2001), interpretation of the results with respect to gravity sensing is difficult because the interaction between statoliths and the cytoskeleton and its role in the graviperception mechanism is far from being understood in these cell types.
In this study, the minimum acceleration level required for lateral statolith displacement was studied in characean rhizoids. Structural and functional details of the actin cytoskeleton are well characterized in this single-celled model system for gravitropism research (Braun and Wasteneys, 1998; Braun et al., 2004) and the specific function of actomyosin forces in the process of gravity sensing is well understood (Braun, 2002; Braun et al., 2002). It has been shown that, whenever statoliths are displaced laterally by sufficiently high accelerations, it is only a matter of time until they settle on the gravisensitive membrane region where perception will inevitably take place followed by the graviresponse (Braun, 2002). Since the gravitropic signaling pathway is short and is not complicated by complex signal transduction and transmission pathways like in higher plant tissues, it is easy to discriminate between susception and perception and interfering with these mechanisms is quickly reflected in a modulation of the response, i.e. the reorientation of the tip.
Based on this knowledge, experimental determination of the threshold acceleration level required for lateral statolith displacement enabled us to describe physical and energetic aspects of molecular interactions between statoliths and the cytoskeleton that underlie the primary phase of gravity sensing. Lateral centrifugation of rhizoids during the microgravity phase of sounding rocket flights was shown to induce statolith displacement at an acceleration level of 0.14g, but not at 0.05g. Given a threshold value of lateral statolith displacement of 0.14g (a = 1.37 m s−2), statolith volume V (statolith diameter 2 μm), statolith density (ρstatolith = ρbarium sulfate = 4.5 g cm−3), and cytoplasmic density (ρcytoplasm = 1.03 g cm−3), the force F that has to be exceeded by any acceleration stimulus in order to move a single statolith toward the cell flank can be calculated to be in a range of:
 |
This value represents the dimension of cytoskeletal forces that restrict lateral displacement of statoliths. Experiments in microgravity (Buchen et al., 1993; Braun et al., 2002) and in simulated weightlessness (Cai et al., 1997; Braun et al., 2002) have shown that in a tip downward-growing rhizoid, actomyosin forces keep statoliths in their dynamically stable resting position by exactly compensating the apically directed gravity force (statolith weight). From this, it follows that the actomyosin forces acting on a statolith in the basal direction to prevent statoliths from settling into the tip are in a range of 1g, which is about 1 order of magnitude higher than the forces that restrict lateral displacement of statoliths. Due to this principle, statoliths are able to sediment toward the lower cell flank upon gravistimulation and, thus, to fulfill their role as susceptors of the gravity vector.
Considering an average distance between a statolith and the plasma membrane (distance between median cell axis and plasma membrane, s = 15 μm), the mechanical work W can be calculated that is necessary to complete sedimentation of a statolith onto the graviperception site at the threshold acceleration level:
 |
This value represents the minimal energy required for gravity susception in characean rhizoids to occur. Although this energy value matches the theoretically calculated minimal energy required for efficient activation of a gravireceptor (Björkman, 1988), it is, however, not relevant for characterizing graviperception in characean rhizoids. The parabolic flight experiments described in this article demonstrate that gravireceptor activation does not depend on the mechanical work that is provided by the gravity-induced statolith sedimentation process, but on direct interaction of sedimented statoliths with the membrane-bound gravireceptor (see below).
Since any acceleration stimuli that exceed the above-mentioned threshold forces and deviate from the cell axis lead to sedimentation of statoliths followed by gravity perception and the gravitropic response, the threshold acceleration gives a good approximation of the general threshold of gravisensitivity in characean rhizoids. A threshold value of 0.14g is in the same range as was determined by microgravity experiments for other gravisensitive cell types, i.e. ciliates (Hemmersbach et al., 1996), flagellates (Häder et al., 1995), and higher plant statocytes (Brown et al., 1995). In conclusion, the mechanisms of gravity sensing might be overbuilt (Björkman, 1988; Sack, 1997); however, only a high sensitivity ensures that the sensing system can operate efficiently and can correct even the smallest deviations from the gravitropic set-point angle.
Upon sedimentation of statoliths onto the specific gravisensitive plasma membrane area, activation of gravireceptors could either be based on the pressure that is exerted by the weight of statoliths or on interactions (contact) with components of the statolith surface that are independent of statolith weight. In this study, the functional mechanism of receptor activation in characean rhizoids was characterized by investigating whether sedimented statoliths were able to activate the gravireceptor even when they were weightless during the microgravity phases of parabolic plane flights. Control experiments on ground revealed that the parabolic flight profile with its alternating acceleration levels was highly suited to study the specific impact of microgravity on graviperception by analyzing the gravitropic curvature responses of rhizoids.
Graviperception Is Rapidly Interrupted upon Removal of Statoliths from Membrane-Bound Receptors But Is Not Affected by Increasing the Weight of Sedimented Statoliths
The results showing that intermittent centrifugation at 2g, as well as extended hypergravity centrifugation of rhizoids, did not alter the gravitropic curvature provide clear evidence that enhancing the pressure on the gravisensitive plasma membrane by increasing the weight of fully sedimented statoliths does not modulate graviperception and does not affect the graviresponse. Consequently, the hypergravity phases of the parabolic flight profile could be neglected when analyzing the effect of microgravity.
Inversion experiments on ground demonstrated that intermittent removal of statoliths from the gravisensitive plasma membrane of gravistimulated rhizoids for only 10 s resulted in decreased curvature angles. Inverting cells for 5 s had no significant effect on gravitropic curvature. However, high-magnification video microscopy confirmed that, 5 s after inversion of gravistimulated cells, statoliths were completely removed from the plasma membrane. It can be concluded from the inversion experiments that the gravireceptor in characean rhizoids is quickly deactivated with a lag time of a few seconds when the contact between statoliths and the plasma membrane is interrupted. Thus, if receptor activation is affected in microgravity, this effect should be detectable by comparing curvature angles of flight samples and in-flight controls, although the duration of a single microgravity phase is only 22 s.
Sedimented Statoliths Activate the Gravireceptor Even When They Are Weightless in Microgravity and Do Not Exert Pressure
Microscopic observation of flight samples indicated that the statolith complex was not lifted from the plasma membrane during the different acceleration levels of the parabolic flight profile. Thus, a removal of statoliths from the graviperception site and a disruption of contact between statoliths and the plasma membrane could be ruled out, and weightlessness of the statoliths was the only parameter analyzed with the experimental setup. Therefore, the results obtained from the parabolic flight experiments provide a stringent line of evidence and interpretation. In none of the parabolic flight experiments was a difference of final curvature angles between flight samples and in-flight controls observed, indicating that gravity perception was not interrupted during the microgravity phases. The experiment demonstrates that even weightless statoliths were capable of activating the gravireceptor. Taking into account that increasing the weight of statoliths by centrifugation did not affect gravitropic curvature, it can be excluded that the gravireceptor in characean rhizoids is a mechanoreceptor, e.g. a stretch-activated ion channel, which is activated by tension or pressure exerted by sedimented statoliths. The inversion experiments on ground and the microgravity experiments confirmed that close contact of statoliths with the gravisensitive plasma membrane is the determinant for graviperception. Even short-term removal of statoliths from the plasma membrane strongly impaired gravitropic curvature. The gravireceptor in characean rhizoids is, therefore, characterized and referred to as a contact receptor.
At the resolution level of microscopic observation during the microgravity experiments, some cellular particles could not unequivocally be identified as statoliths and, due to the three-dimensional shape of the tube-like cells, it was impossible by means of two-dimensional image records to decide whether a statolith was in contact with the plasma membrane or not. Although video microscopy of rhizoids that were laterally centrifuged with 0.14g during the microgravity phase of the MAXUS-5 sounding rocket flight enabled us to spatiotemporally resolve the acceleration-induced lateral displacement of statoliths, it was not intended for identifying contacts of single statoliths with the plasma membrane. However, since these living cells exhibited distinct curvature responses, it is concluded that statoliths have sporadically settled on the gravisensitive membrane site of the centrifugal flank where they initiated the gravitropic signaling pathway. This assumption is confirmed by the analysis of chemically fixed rhizoids underlining the settlement of statoliths onto the lateral cell flank at the end of centrifugation in microgravity. In vertically downward-growing rhizoids on ground, statoliths are symmetrically distributed across the cell diameter and some statoliths are found in close proximity to the plasma membrane at both cell flanks. However, it cannot definitely be decided whether statoliths are in contact with the plasma membrane at both flanks, generating a symmetric gravitropic signal, or whether they are located close to the membrane without being in contact with the membrane-bound gravireceptors so that gravitropic signaling is not initiated at all.
A contact-dependent mechanism of gravireceptor activation in characean rhizoids is supported by previous experiments in which tip reorientation in vertically downward-growing rhizoids could only be induced when statoliths were brought into contact with the gravisensitive area of the plasma membrane by laser tweezer micromanipulation (Braun, 2002). Stretching the gravisensitive membrane from the outside by using a microcapillary does not provoke a curvature response (C. Limbach and M. Braun, unpublished data). Further centrifugation studies have shown that the graviresponse of characean protonemata, a cell type that is very similar to rhizoids but exhibits an opposite gravitropic orientation, also relies on the gravity-induced and actin-mediated settlement of statoliths on a gravisensitive plasma membrane area and cannot be promoted by acceleration forces (Hodick and Sievers, 1998).
Until today, functional mechanisms of graviperception have not been characterized in higher plants. Gravireceptor proteins are commonly addressed as mechanosensitive receptors. Models of gravisensing, including the tensegrity model (Zheng and Staehelin, 2001), postulated that receptor activation depends on mechanical forces (tension, pressure) that are generated by the gravity-driven sedimentation of statoliths and are transferred to stretch-activated ion channels (gravireceptors) via actin-dependent mechanisms (for review, see Boonsirichai et al., 2002; Sievers et al., 2002; Blancaflor and Masson, 2003; Perbal and Driss-Ecole, 2003). Even though a crucial role of actin in the early signal transduction pathway is appealing, physiological and cytological studies, including inhibitor treatments, have so far only given contradictory results (Blancaflor and Hasenstein, 1997; Nick et al., 1997; Yamamoto and Kiss, 2002; Friedman et al., 2003; Hou et al., 2003, 2004), leaving the mechanisms of gravity perception in higher plants enigmatic.
Parabolic flight experiments presented in this study have added greatly to our understanding of the sensitivity and functional characteristics of the decisive early processes of susception and perception of the direction of gravity in characean rhizoids. The results encourage further utilization of the parabolic flight profile with its sequence of short-term microgravity phases as a powerful instrument that complements physiological, biochemical, and genetic approaches, and promise to unravel the mechanisms underlying the early processes of gravity sensing in higher plants.
MATERIALS AND METHODS
Plant Material
Thalli of Chara globularis Thuill. were taken from a pond at the Botanical Garden of the University of Bonn. Young shoots were cut into segments consisting of at least two nodes and one internode. To induce growth of rhizoids, the side branches of the lower node were cut off. The segments were placed in experiment-specific containers and embedded in agar (1.2% [w/v] in distilled water). Rhizoids developed after 4 to 5 d at room temperature under continuous illumination at 150 to 200 μmol m−2 s−1.
Sounding Rocket Flight Hardware and Procedures
The threshold acceleration level that induces lateral displacement of statoliths in characean rhizoids was determined under microgravity conditions provided by the flights of two ESA sounding rockets (MAXUS-3 and MAXUS-5), which were launched from the satellite station Esrange, near Kiruna in northern Sweden. The rockets reached an altitude of about 800 km and provided microgravity conditions (<10−4g) for approximately 13 min. For sample preparation, agar-embedded nodes with bundles of short rhizoids were mounted in two different types of vacuum-tight cuvettes. One type was designed for the analysis of growth responses and the second type allowed chemical fixation of rhizoids at the end of the microgravity phase for analyses of the statolith position on ground.
On MAXUS-3, vertically growing rhizoids were laterally centrifuged at 0.4g during microgravity and the position of statoliths was observed by in vivo video microscopy operated by telecommand from ground. For the MAXUS-5 experiment, one observation cuvette and one fixation cuvette were placed at each of three different positions on the rotating platform of the payload module TEM 06-RO1M constructed by European Aeronautic Defense and Space Company Space Transportion. Vertically downward-growing rhizoids on the three different radii experienced lateral accelerations of 0.05g, 0.14g, and 0.25g during rotation of the platform in microgravity. The temperature in the module was adjusted to 21°C by Peltier elements. Rotation of the platform was started 70 s after lift off, 25 s before the beginning of microgravity, and was stopped at the end of the microgravity phase, shortly before reentry of the payload (840 s after lift off). During the microgravity phase, the movements of statoliths and first gravitropic responses were observed in rhizoids that were accelerated with 0.14g by in vivo video microscopy. Timer-controlled chemical fixation (3% [v/v] glutardialdehyde, 0.1 m PIPES, pH 7.0) of the rhizoids at the end of the microgravity phase (800 s after lift off) allowed documentation and statistical analysis of the redistribution of statoliths caused by the laterally applied accelerations after retrieval of the payload on ground.
The geometric center of the statolith complex of each chemically fixed rhizoid was determined according to Hodick (1994) and averaged for each acceleration level. The microscopic images were overlayed with a Cartesian coordinate system with its origin at the cell vertex and the median cell axis as the x axis. The position of each statolith was determined by its x and y value in the coordinate system. Averaging of coordinate values of all statoliths resulted in the geometric center of the statolith complex.
For further analysis of the acceleration-induced statolith displacement in chemically fixed rhizoids and in the living cells of the observation cuvette of the 0.14g acceleration level, the distribution of statolith frequency across the cell diameter was determined. Microscopic images were overlayed with a lattice (30 × 30 μm, box size 3 × 3 μm) and the number of boxes blackened by the presence of statoliths in all rhizoids of each set of samples was summed up for each column and divided by the number of rhizoids analyzed. This method provided distribution diagrams of statoliths along the lateral cell axis.
Microscopic analysis of the living rhizoids in the observation cuvettes of each acceleration level after retrieval of the payload allowed the detection of any potential curvature responses that were induced by the lateral acceleration stimuli.
Parabolic Plane Flight Hardware and Procedures
Parabolic plane flight experiments were performed during the 36th ESA parabolic flight campaign at Bordeaux airport, France, in March 2004, and during the sixth DLR parabolic flight campaign at Cologne airport, Germany, in September 2004. The flight profile of the aircraft comprised 31 parabolas flown within 120 min on each of three flight days per campaign providing alternating acceleration levels of normal gravity (1g), microgravity (<10−4g for 22 s per parabola), and hypergravity (up to 1.8g for 20 s before and after each microgravity phase). The experiment hardware was mounted on a custom-made aluminum rack and included the specimen containers and a centrifuge for in-flight controls. A modified Biorack microscope equipped with a video camera was used for observations of statolith movements during the flight phases. Video sequences were recorded in MPEG4 format on a laptop computer. In order to reduce vibrations, all specimen containers and the centrifuge were mechanically isolated from the rack by 10-mm silicon foam plates (Castan GmbH).
Flight samples and in-flight controls were tilted by 90° during the flight beginning 10 min prior to the first parabola. This warranted that sedimentation of statoliths onto the lateral cell flank was completed under 1g conditions in all rhizoids and that the following acceleration profile was applied to cells with fully sedimented statoliths. The samples were tilted back to the vertical orientation after the last parabola of the flight profile. In-flight control samples were mounted on the reference centrifuge of the experiment rack and centrifuged at 1g during the microgravity phases. Additional ground control samples were positioned horizontally under continuous 1g conditions for the same total gravistimulation time as the flight samples and in-flight controls. After landing, photographs of the rhizoids in all three sets of samples were recorded by video microscopy. The maximally achieved curvature angles were measured and statistically analyzed.
Preflight Controls
Control experiments at 1g on ground were performed to assess the effect of hypergravity conditions and the effect of short-term removal of sedimented statoliths from the plasma membrane on gravitropic curvature. In all control experiments, rhizoids where initially tilted by 90° for 10 min to allow undisturbed sedimentation of statoliths. In order to test whether increasing the weight of sedimented statoliths alters the gravitropic response, rhizoids were first gravistimulated for 10 min at 1g and then laterally centrifuged parallel to the direction of the gravistimulus for 15 min at 2g, 3g, 4g, or 5g. The maximally achieved growth angles were compared with control cells that were gravistimulated at 90° for 25 min at 1g.
For simulating the short-term hypergravity phases of the parabolic flight profile, rhizoids were intermittently centrifuged 62 times at 2g for 20 s within a total gravistimulation time of 120 min and final curvature angles of these cells were compared with controls cells that were continuously gravistimulated at 1g for 120 min.
The effect of short-term interruption of the contact of sedimented statoliths with the plasma membrane on the gravitropic response was tested by inverting gravistimulated cells from 90° to 270° 31 times for 30, 22, 10, or 5 s within a total of 120 min according to the flight profile. As for the other control experiments, maximally achieved curvature angles of the intermittently inverted rhizoids were measured and compared with control samples that were continuously gravistimulated for 120 min at continuous 1g conditions. For tracking the movements of statoliths following the inversion of gravistimulated cells, high-magnification video microscopy was used. Rhizoids were mounted on the rotatable stage of a vertically positioned Axioskop microscope (Zeiss) and statoliths were observed with a Plan Neofluar 100× oil immersion lens (Zeiss). Rhizoids were gravistimulated for 10 min at 90° and subsequently inverted to 270° by tilting the microscope stage. Digital images of the apical cell regions were recorded before and after inversion at 1-s intervals with an AxioCam HS camera (Zeiss). For statistical analyses of statolith movements, only those statoliths were tracked that were considered to be sedimented on the plasma membrane at the moment of inversion (t = 0). A line was drawn as a reference to indicate the initial position of sedimented statoliths. The distances between statoliths and the reference line were documented and measured using AxioVision software (Carl Zeiss Vision GmbH).
Acknowledgments
We thank Andreas Sievers and Brigitte Buchen for stimulating discussions and critical reading of the manuscript. We are grateful to the teams of DLR, ESA, European Aeronautic Defense and Space Company Space Transportation, Kayser-Threde, Novespace, and Swedish Space Corporation for their dedicated work. The Biorack microscope was kindly provided by ESA.
This work was supported by the Deutsches Zentrum für Luft- und Raumfahrt (DLR) on behalf of the Bundesministerium für Bildung und Forschung (grant no. 50WB9998) and by a fellowship of the Cusanuswerk (to C.L.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.068106.
References
- Björkman T (1988) Perception of gravity by plants. Adv Bot Res 15: 1–41 [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S (1998) Mapping the role of cap cells in root gravitropism. Plant Physiol 116: 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Hasenstein KH (1997) The organization of the actin cytoskeleton in vertical and graviresponding primary roots of maize. Plant Physiol 113: 1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Masson PH (2003) Plant gravitropism: unraveling the ups and downs of a complex process. Plant Physiol 133: 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonsirichai K, Guan C, Chen R, Masson PH (2002) Root gravitropism: an experimental tool to investigate basic cellular and molecular processes underlying mechanosensing and signal transmission in plants. Annu Rev Plant Physiol Plant Mol Biol 53: 421–447 [DOI] [PubMed] [Google Scholar]
- Braun M (1997) Gravitropism in tip-growing cells. Planta 203: S11–S19 [DOI] [PubMed] [Google Scholar]
- Braun M (2002) Gravity perception requires statoliths settled on specific plasma membrane areas in characean rhizoids and protonemata. Protoplasma 219: 150–159 [DOI] [PubMed] [Google Scholar]
- Braun M, Buchen B, Sievers A (2002) Actomyosin-mediated statolith positioning in gravisensing plant cells studied in microgravity. J Plant Growth Regul 21: 137–145 [DOI] [PubMed] [Google Scholar]
- Braun M, Hauslage J, Czogalla A, Limbach C (2004) Tip-localized actin polymerization and remodeling, reflected by the localization of ADF, profilin and villin, are fundamental for gravitropic tip growth in characean rhizoids. Planta 219: 379–388 [DOI] [PubMed] [Google Scholar]
- Braun M, Wasteneys GO (1998) Distribution and dynamics of the cytoskeleton in graviresponding protonemata and rhizoids of characean algae: exclusion of microtubules and a convergence of actin filaments in the apex suggest an actin-mediated gravitropism. Planta 205: 39–50 [DOI] [PubMed] [Google Scholar]
- Brown AH, Chapman DK, Johnsson A, Heathcote D (1995) Gravitropic responses of the Avena coleoptile in space and on clinostats. I. Gravitropic response thresholds. Physiol Plant 95: 27–33 [PubMed] [Google Scholar]
- Buchen B, Braun M, Hejnowicz Z, Sievers A (1993) Statoliths pull on microfilaments. Experiments under microgravity. Protoplasma 172: 38–42 [DOI] [PubMed] [Google Scholar]
- Cai W, Braun M, Sievers A (1997) Displacement of statoliths in Chara rhizoids during horizontal rotation on clinostats. Acta Biol Exp Sin 30: 147–155 [PubMed] [Google Scholar]
- Ding JP, Pickard BG (1993) Mechanosensory calcium-selective cation channels in epidermal cells. Plant J 3: 83–110 [DOI] [PubMed] [Google Scholar]
- Driss-Ecole D, Jeune B, Prouteau M, Julianus P, Perbal G (2000) Lentil root statoliths reach a stable state in microgravity. Planta 211: 396–405 [DOI] [PubMed] [Google Scholar]
- Friedman H, Vos JW, Hepler PK, Meir S, Halevy AH, Philosoph-Hadas S (2003) The role of actin filaments in the gravitropic response of snapdragon flowering shoots. Planta 216: 1034–1042 [DOI] [PubMed] [Google Scholar]
- Fujihira K, Kurata T, Watahiki MK, Karahara I, Yamamoto KT (2000) An agravitropic mutant of Arabidopsis, endodermal-amyloplast less 1, that lacks amyloplasts in hypocotyl endodermal cell layer. Plant Cell Physiol 41: 1193–1199 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Fujisawa H, Tasaka M (1996) SGR1, SGR2, and SGR3: novel genetic loci involved in shoot gravitropism in Arabidopsis thaliana. Plant Physiol 110: 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M (1998) Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J 14: 425–430 [DOI] [PubMed] [Google Scholar]
- Haberlandt G (1900) Über die Perzeption des geotropischen Reizes. Ber Dtsch Bot Ges 18: 261–272 [Google Scholar]
- Häder D-P, Rosum A, Schäfer J, Hemmersbach R (1995) Gravitaxis in the flagellate Euglena gracilis is controlled by an active gravireceptor. J Plant Physiol 146: 474–480 [PubMed] [Google Scholar]
- Hejnowicz Z, Sievers A (1981) Regulation of the position of statoliths in Chara rhizoids. Protoplasma 108: 117–137 [DOI] [PubMed] [Google Scholar]
- Hemmersbach R, Voormanns R, Briegleb W, Rieder N, Häder D-P (1996) Influence of acceleration on the spatial orientation of Loxodes and Paramecium. J Biotechnol 47: 271–278 [DOI] [PubMed] [Google Scholar]
- Hodick D (1994) Negative gravitropism in Chara protonemata: a model integrating the opposite gravitropic responses of protonemata and rhizoids. Planta 195: 43–49 [DOI] [PubMed] [Google Scholar]
- Hodick D, Sievers A (1998) Hypergravity can reduce but not enhance the gravitropic response of Chara globularis protonemata. Protoplasma 204: 145–154 [DOI] [PubMed] [Google Scholar]
- Hou G, Kramer VL, Wang Y-S, Chen R, Perbal G, Gilroy S (2004) The promotion of gravitropism in Arabidopsis roots upon actin disruption is coupled with the extended alkalinization of the columella cytoplasm and a persistent lateral auxin gradient. Plant J 39: 113–125 [DOI] [PubMed] [Google Scholar]
- Hou G, Mohamalawari DR, Blancaflor EB (2003) Enhanced gravitropism of roots with a disrupted cap actin cytoskeleton. Plant Physiol 131: 1360–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper BE, Gorves S, Landau-Schachar B, Audus LJ (1966) Root cap and perception of gravity. Nature 209: 93–94 [Google Scholar]
- Kiss JZ (2000) Mechanisms of the early phases of plant gravitropism. CRC Crit Rev Plant Sci 19: 551–573 [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Wright JB, Caspar T (1996) Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant 97: 237–244 [DOI] [PubMed] [Google Scholar]
- Kuznetsov OA, Hasenstein KH (1996) Magnetophoretic induction of root curvature. Planta 198: 87–94 [DOI] [PubMed] [Google Scholar]
- Kuznetsov OA, Hasenstein KH (1997) Magnetophoretic induction of curvature in coleoptiles and hypocotyls. J Exp Bot 48: 1951–1957 [DOI] [PubMed] [Google Scholar]
- Kuznetsov OA, Schwuchow J, Sack FD, Hasenstein KH (1999) Curvature induced by amyloplast magnetophoresis in protonemata of the moss Ceratodon purpureus. Plant Physiol 119: 645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCleery SA, Kiss JZ (1999) Plastid sedimentation kinetics in roots of wild-type and starch-deficient mutants of Arabidopsis. Plant Physiol 120: 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita MT, Tasaka M (2004) Gravity sensing and signaling. Curr Opin Plant Biol 7: 712–718 [DOI] [PubMed] [Google Scholar]
- Nĕmec B (1900) Ueber die Art der Wahrnehmung des Schwerkraftreizes bei den Pflanzen. Ber Dtsch Bot Ges 18: 241–245 [Google Scholar]
- Nick P, Godbole R, Wang QY (1997) Probing rice gravitropism with cytoskeletal drugs and cytoskeletal mutants. Biol Bull 192: 141–143 [DOI] [PubMed] [Google Scholar]
- Perbal G, Driss-Ecole D (2003) Mechanotransduction in gravisensing cells. Trends Plant Sci 8: 498–504 [DOI] [PubMed] [Google Scholar]
- Perbal G, Lefrance A, Jeune B, Driss-Ecole D (2004) Mechanotransduction in root gravity sensing cells. Physiol Plant 120: 303–311 [DOI] [PubMed] [Google Scholar]
- Sack FD (1997) Plastids and gravitropic sensing. Planta 203: S63–S68 [DOI] [PubMed] [Google Scholar]
- Sack FD, Suyemoto MM, Leopold AC (1984) Kinetics of amyloplast sedimentation in gravistimulated maize coleoptiles. Planta 161: 459–464 [PubMed] [Google Scholar]
- Sack FD, Suyemoto MM, Leopold AC (1985) Amyloplast sedimentation kinetics in gravistimulated maize roots. Planta 165: 295–300 [PubMed] [Google Scholar]
- Sack FD, Suyemoto MM, Leopold AC (1986) Amyloplast sedimentation and organelle saltation in living corn columella cells. Am J Bot 73: 1692–1698 [PubMed] [Google Scholar]
- Saito C, Morita MT, Kato T, Tasaka M (2005) Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell 17: 548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers A, Braun M, Monshausen GB (2002) Root cap: structure and function. In Y Waisel, A Eshel, U Kafkafi, eds, Plant Roots—The Hidden Half, Ed 3. Marcel Dekker, New York, pp 33–47
- Sievers A, Buchen B, Hodick D (1996) Gravity sensing in tip-growing cells. Trends Plant Sci 1: 273–279 [DOI] [PubMed] [Google Scholar]
- Sievers A, Buchen B, Volkmann D, Hejnowicz Z (1991. a) Role of the cytoskeleton in gravity perception. In CW Lloyd, ed, The Cytoskeletal Basis for Plant Growth and Form. Academic Press, London, pp 169–182
- Sievers A, Kramer-Fischer M, Braun M, Buchen B (1991. b) The polar organization of the growing Chara rhizoid and the transport of statoliths are actin-dependent. Bot Acta 104: 103–109 [DOI] [PubMed] [Google Scholar]
- Sievers A, Kruse S, Kuo-Huang L-L, Wendt M (1989) Statoliths and microfilaments in plant cells. Planta 179: 275–278 [DOI] [PubMed] [Google Scholar]
- Tsugeki R, Fedoroff NV (1999) Genetic ablation of root cap cells in Arabidopsis. Proc Natl Acad Sci USA 96: 12941–12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann D, Baluska F, Lichtscheidl I, Driss-Ecole D, Perbal G (1999) Statolith motions in gravity-perceiving plant cells: Does actomyosin counteract gravity? FASEB J 13: S143–S147 [DOI] [PubMed] [Google Scholar]
- Volkmann D, Buchen B, Hejnowicz Z, Tewinkel M, Sievers A (1991) Oriented movement of statoliths studied in a reduced gravitational field during parabolic flights of rockets. Planta 185: 153–161 [PubMed] [Google Scholar]
- Weise SE, Kiss JZ (1999) Gravitropism of inflorescence stems in starch-deficient mutants of Arabidopsis. Int J Plant Sci 160: 521–527 [DOI] [PubMed] [Google Scholar]
- Weise SE, Kuznetsov OA, Hasenstein KH, Kiss JZ (2000) Curvature in Arabidopsis inflorescence stems is limited to the region of amyloplast displacement. Plant Cell Physiol 41: 702–709 [DOI] [PubMed] [Google Scholar]
- Wendt M, Kuo-Huang L-L, Sievers A (1987) Gravitropic bending of cress roots without contact between amyloplasts and complexes of endoplasmic reticulum. Planta 172: 321–329 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Kiss JZ (2002) Disruption of the actin cytoskeleton results in the promotion of gravitropism in inflorescence stems and hypocotyls of Arabidopsis. Plant Physiol 128: 669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder TL, Zheng H-Q, Todd P, Staehelin LA (2001) Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol 125: 1045–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HQ, Staehelin LA (2001) Nodal endoplasmic reticulum, a specialized form of endoplasmic reticulum found in gravity-sensing root tip columella cells. Plant Physiol 125: 252–265 [DOI] [PMC free article] [PubMed] [Google Scholar]