Abstract
Purpose
To describe health-related quality of life (HRQOL), overall and in patients with unilateral or bilateral choroidal neovascularization (CNV), in a clinical trial (Group N Trial) comparing observation and surgical removal of subfoveal CNV secondary to age-related macular degeneration (AMD).
Design
Randomized clinical trial.
Participants
Eligible patients had untreated subfoveal CNV and AMD, best-corrected visual acuity (VA) of 20/100 to 20/800, classic CNV on fluorescein angiography, and a total subfoveal lesion size of ≤9.0 disc areas in the study eye.
Methods
Health-related quality of life data (the National Eye Institute Visual Function Questionnaire [NEI-VFQ], 36-item Short Form Health Survey [SF-36], and Hospital Anxiety and Depression Scale [HADS]) and clinical data were collected at baseline and at 6, 12, 24, 36, and 48 months. Patients were divided into unilateral and bilateral CNV subgroups based on fluorescein angiographic and clinical evidence.
Main Outcome Measure
Two-year change in the NEI-VFQ.
Results
Of 454 patients enrolled, 228 were assigned to observation and 226 to surgery. At baseline, median overall NEI-VFQ scores were 67 in the observation group and 69 in the surgery group; by 2 years, the observation group had lost a median of 3 points (95% confidence interval [CI]: −6 to −2), and the surgery group gained a median of 1 point (CI: −1 to 3). The largest difference was observed for the mental health subscale, where the observation group lost a median of 5 points (CI: −5 to 0), and the surgery group gained a median of 5 points (CI: 0–10) by 2 years. Treatment differences in median 2-year changes in NEI-VFQ scores favored surgery by up to 10 points for unilateral cases and up to 8 points for bilateral cases. No treatment difference in 2-year change was observed for the SF-36 physical component summary; 2-year change in the mental component summary favored surgery by 2 points. Few patients (2%–4%) had HADS definite anxiety or depression at baseline or at 24 months.
Conclusions
Although HRQOL outcomes were better in the submacular surgery arm than in the observation arm, surgery (per protocol) is not recommended because VA outcomes (reported elsewhere) were similar in the treatment arms.
Health-related quality of life (HRQOL) may be an important outcome in ophthalmic clinical trials to confirm that clinical measurements of vision capture outcomes that are important to patients. Health-related quality of life measures also are useful in assessing harm or benefit of treatment in a clinical trial by comparing treatment arms with respect to self-reported ability to perform daily activities and self-reported emotional well-being. From the patient’s perspective, the effect of treatment on clinical measures of visual acuity (VA) may be less important than an effect of treatment on visual function that can be demonstrated by a valid, reliable, responsive HRQOL measure, such as the National Eye Institute Visual Function Questionnaire (NEI-VFQ).1–4 This conclusion may be particularly true in clinical trials of treatments for the advanced stage of age-related macular degeneration (AMD), which often includes choroidal neo-vascularization (CNV) in a subfoveal location, because the loss of vision associated with this stage of the disease may not be reversible, and current treatments offer only a modest clinical benefit.
The Submacular Surgery Trials (SST), a set of 3 randomized multicenter clinical trials conducted in the United States, were sponsored by the National Eye Institute of the National Institutes of Health and designed in the 1990s. The present analysis focuses on one SST clinical trial that compared submacular surgery to observation in patients with previously untreated subfoveal CNV due to AMD (SST Group N Trial). Clinical outcomes from the SST Group N Trial have been reported elsewhere.5 Although submacular surgery was an anatomic success in maintaining a smaller subfoveal lesion, compared with observation, this success did not translate to better VA outcomes in the surgical group by 24 months after enrollment. The purpose of this analysis was to report HRQOL outcomes from the SST Group N Trial overall and in the subgroups of patients with unilateral or bilateral CNV at the time of enrollment, because large HRQOL differences between these 2 subgroups of patients were observed at baseline.6 Although both unilateral and bilateral cases had significantly reduced vision-targeted HRQOL at baseline, the level of disability in the bilateral cases was greater than expected, based solely on the level of baseline VA.6
Patients and Methods
Study Population
The Data and Safety Monitoring Committee and the institutional review boards of the participating clinical centers and resource centers reviewed and approved the study before initiation of patient enrollment in July 1998. Written informed consent was obtained from each patient before enrollment. Patient eligibility criteria and methods used to carry out the study have been described elsewhere.5,7 Briefly, eligible patients were 50 years or older and had untreated subfoveal CNV secondary to AMD, best-corrected VA (BCVA) of 20/100 to 20/800 (Snellen equivalent) inclusive (i.e., VA scores of 67–18), evidence of classic CNV on fluorescein angiography, lesion size of ≤9 Macular Photocoagulation Study disk areas (22.9 mm2), and blood, if any, occupying <50% of the area of the entire lesion in the eye (i.e., study eye) considered for random assignment to submacular surgery or observation.5,7 Patients who enrolled had to be willing and able to return for scheduled clinical examinations and to complete telephone-administered English-language HRQOL interviews as part of the study. A few patients were excluded from enrollment due to a considerable hearing difficulty or a language barrier. Patients judged to be eligible by an examining ophthalmologist reviewed and signed a consent form before enrollment.
Quality of Life Interviews
The NEI-VFQ, the 36-item Short Form Health Survey (SF-36),8 and the Hospital Anxiety and Depression Scale (HADS)9 interviews were conducted at baseline before random treatment assignment to submacular surgery or observation. The follow-up interviews were administered at 6, 12, and 24 months of follow-up. Patients who enrolled early on also were interviewed at 36 months; some also were eligible for 48-month interviews. Interviews were administered by telephone from the SST Coordinating Center and were conducted by trained interviewers who were masked to treatment assignment, study eye, and clinical data. Interviewers used a computer-assisted interview system whereby answers to interview questions were entered into an electronic file during the interview. The NEI-VFQ interview included the 25-item base set of questions and the appendix of additional questions.3 The items on the NEI-VFQ can be divided to form 11 subscales (excluding a 2-item general health subscale) that can be combined to create an overall score. The 11 subscales are denoted as follows: general vision, driving, near activities, distance activities, role difficulties, mental health, dependency, social functioning, peripheral vision, ocular pain, and color vision. Each subscale has 1 to 6 items; each item is scored from 0 to 100. Subscale scores for each patient were calculated by taking an unweighted average of items when at least half of the items that make up the subscale were answered. The overall NEI-VFQ score was calculated by taking an unweighted average of the 11 subscale scores. Subscale scores and the overall score also are on a 0 to 100 scale, where 0 represents perception of visual function to be the worst possible and 100, the best possible. For the purpose of this report, color vision subscale scores will not be reported separately, although the scale was included in the NEI-VFQ overall score. The color vision scale was excluded because color vision changes would not be expected based on existing knowledge of natural history of the disease, distribution of scores at baseline, and previously published data from the SST that showed the color vision scale to be unresponsive to changes in VA.4
The SF-36 physical component summary (PCS) and mental component summary (MCS) scores, but not the 8 individual sub-scale scores, were analyzed for this report.8 The SF-36 summary scores are indicators of self-reported physical and mental health status. The summary scores were standardized by the developers to have a mean value of 50 and a standard deviation of 10 in the general United States population.8 Theoretically, the summary scores can range in value from 0, the worst possible score, to 100, the best, but extreme scores are highly unlikely in an outpatient setting.
The HADS interview was designed to be a clinical screening tool for anxiety and depression in outpatients.9 The interview consists of 14 questions, of which 7 contribute to an anxiety scale and 7 to a depression scale. The anxiety and depression scales were constructed when at least half the items on the scale were answered; the missing items were replaced by the average of non-missing items in the scale. The anxiety and depression scores were calculated by summing the items in the subscales. Hospital Anxiety and Depression Scale scores range in value from 0 to 21, but values typically are presented in categories: a score of ≤7 for the anxiety or depression scale is an indicator of a noncase, a score of 8 to 10 suggests a doubtful case, and a score of >10 is a definite case.9 Because the HADS was designed to be a screening tool, scores at the higher end of the spectrum are not a diagnosis of clinical anxiety or depression but suggest that additional evaluation by a mental health professional is indicated.
The SST Vision Preference Value Scale10 also was part of the interview, but follow-up findings will be discussed in a separate report.
Clinical Data
Information on demographic and clinical characteristics and VA were recorded at baseline and at scheduled follow-up times. Detailed procedures for measuring BCVA have been reported elsewhere.5,7 For each eye, VA was initially measured at a 2-m distance using a modified Bailey–Lovie chart (Early Treatment Diabetic Retinopathy Study).11 Whenever the patient was unable to read at least 15 letters at the 2-m distance with the eye being tested, the testing was continued at 0.5 m with appropriately modified refractive correction. Patients who could read no letters (i.e., VA score of 0) on the chart with the eye being tested were tested for light perception (LP) (LP and no LP were assigned scores of −10 and −30, respectively). Baseline VA was converted to Snellen equivalents for presentation.
For the purpose of this analysis, patients were divided into bilateral and unilateral cases based on central evaluation of baseline stereoscopic fundus photographs and film-based fluorescein angiograms submitted to the SST Photograph Reading Center and treatment history of the fellow eye at baseline. Evidence of CNV, disciform scar, treatment for CNV, or hemorrhage in the fellow eye was considered an indicator of bilateral disease.6 The remaining patients were considered unilateral cases.
Statistical Methods and Data Analysis
The SST Group N Trial was designed and powered to identify treatment arm differences in VA outcomes but not in HRQOL outcomes, which were considered secondary outcomes from a design perspective. Although the purpose of this report was to describe the quality-of-life findings from the SST Group N Trial, change in NEI-VFQ scores from baseline to 24 months was chosen as the main HRQOL outcome to correspond to the main ophthalmic outcomes. Post hoc power calculations indicated that at a 0.01 α level (2 sided) the study had 78% power to identify 5-point differences between treatment arms with respect to 24-month change in the overall NEI-VFQ score and from 18% to 59% power to identify the same difference in subscale scores. Power to identify 10-point differences between treatment arms in NEI-VFQ change scores was ≥91% for the overall NEI-VFQ score and 7 of the 10 subscale scores, with the exception of peripheral vision (85%), dependency (84%), and driving (77%). Data were analyzed using an intent-to-treat approach.
Descriptive statistics were calculated for all variables of interest. Student’s t test or the Wilcoxon rank-sum test was used to compare treatment arms for variables that had a continuous Gaussian or non-Gaussian distribution, respectively. Descriptive statistics and the Shapiro–Wilk statistic12 were used to identify variables with a non-Gaussian distribution. Linear regression models were used to evaluate whether changes in lesion size from baseline to 24 months (independent variable in regression model) could explain the treatment differences (second independent variable) in changes in NEI-VFQ scores from baseline to 24 months (dependent variable). Interquartile ranges or distribution-free 95% confidence intervals (95% CIs) for the median values13 were calculated.
Treatment arm comparisons of HADS anxiety and depression scores were analyzed by calculation of frequencies and by using a 2-state Markov model14 for definite cases. The Markov model was used because it accounts for changes in state over time and incorporates all available data for each patient.
Continuous time mixed linear marginal models (with a first-order autoregressive moving average correlation structure)15,16 were used to estimate the treatment effect on NEI-VFQ scores after adjustment for interview times, baseline NEI-VFQ scores, general health status (using the SF-36 PCS as a time-dependent covariate), and age. Furthermore, these models were used to evaluate for treatment-by-time and treatment-by-age interactions. This method was chosen as a sensitivity analysis because it includes HRQOL outcome data for every follow-up visit for each patient, thus providing a more powerful tool to identify treatment arm differences, as compared with analysis of HRQOL data at a specific follow-up visit.
Because of multiple comparisons of treatment arms on multiple outcomes, P values of ≤0.01 were considered statistically significant in bivariate analyses. Apart from this criterion, no other adjustment for multiple comparisons was made. In multivariate analyses, this criterion was used to decide whether variables were statistically significant in the models. For subgroup analyses, we have noted P values of ≤0.05 as suggestive of a treatment effect. Data were analyzed using SAS17 on a UNIX platform.
Results
Demographic and Clinical Characteristics
Four hundred fifty-four patients were enrolled in the SST Group N Trial; 228 were assigned to observation and 226 to submacular surgery. Treatment arms were balanced with regard to most of the demographic characteristics, VA, contrast threshold, reading speed, and other characteristics of the eyes and neovascular lesions.5 The median age of patients was 77 years at baseline, 53% were women, and 98% were non-Hispanic white. Patients assigned to observation were more often retired (92%) than patients assigned to surgery (85%). More information on demographic characteristics and medical history is available in Table 1 (online only, available at http://www.ophsource.com/periodicals/ophtha). Visual acuity in the better-seeing eye ranged from 20/20 to 20/800 (Snellen equivalent) (Table 2). Thirty-nine percent of patients were aphakic or pseudophakic at baseline.
Table 2.
Clinical Characteristics at Baseline
|
Unilateral Cases |
Bilateral Cases |
|||
|---|---|---|---|---|
| Observation (n = 123) | Surgery (n = 128) | Observation (n = 105) | Surgery (n = 98) | |
| Better-seeing eye [n (%)] | ||||
| Study eye | 5 (4) | 5 (4) | 56 (53) | 58 (59) |
| Fellow eye | 118 (96) | 122 (95) | 46 (44) | 40 (41) |
| Both eyes same visual acuity | 0 | 1 (1) | 3 (3) | 0 |
| Visual acuity* [median (quartiles)] | ||||
| Better-seeing eye | 20/25 (20/40–20/20) | 20/25 (20/32–20/20) | 20/100 (20/200–20/64) | 20/125 (20/200–20/64) |
| Worse-seeing eye | 20/200 (20/400–20/160) | 20/200 (20/400–20/125) | 20/400 (20/640–20/200) | 20/320 (20/640–20/200) |
| Contrast threshold† [median (quartiles)] | ||||
| Better-seeing eye (% contrast required) | 3.2 (4.5–3.2) | 3.2 (4.5–3.2) | 4.5 (6.3–4.5) | 6.3 (6.3–4.5) |
| Worse-seeing eye (% contrast required) | 8.9 (17.8–6.3) | 8.9 (17.8–6.3) | 12.6 (25.1–6.3) | 12.6 (25.1–6.3) |
| Reading speed† [median (quartiles)] | ||||
| Better-seeing eye (words/min) | 109 (86–120) | 105 (84–126) | 72 (43–96) | 69 (39–90) |
| Worse-seeing eye (words/min) | 37 (17–65) | 35 (17–63) | 17 (3–45) | 30 (5–51) |
| Lens status [n (%)] | ||||
| Phakic | 83 (67) | 82 (64) | 52 (50) | 61 (62) |
| Aphakic/pseudophakic | 40 (33) | 46 (36) | 53 (50) | 37 (38) |
Snellen equivalent.
Unilateral cases, observation group: 1 patient did not have contrast threshold measured, and 2 patients did not have reading speed measured at baseline.
Of 454 enrolled patients, 251 were unilateral cases and 203 were bilateral cases. Unilateral cases were on average 2 years younger than bilateral cases and, as expected, had better VA, contrast threshold, and reading speed in both the better-seeing eye and the worse-seeing eye.
Interview Completion
Interview completion rates during follow-up were somewhat higher among patients in the surgery arm (92%) than among those in the observation arm (87%) at 24 months’ follow-up (Table 3), regardless of whether the total group in each treatment arm was compared or only bilateral or unilateral cases (data not shown). At baseline, 90% of NEI-VFQ interviews in both the observation group and the surgery group had all questions on the NEI-VFQ answered completely; at 24 months, 96% of interviews in the observation group and 90% of interviews in the surgery group were complete (data not shown).
Table 3.
Change in National Eye Institute Visual Function Questionnaire (NEI-VFQ) Scores from Baseline to Follow-up Visits*
|
6 Months |
12 Months |
24 Months |
36 Months |
48 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obs (n = 204) | Sur (n = 215) | Obs (n = 199) | Sur (n = 207) | Obs (n = 186) | Sur (n = 198) | Obs (n = 143) | Sur (n = 145) | Obs (n = 82) | Sur (n = 84) | |
| Interview completion | ||||||||||
| Expected (n) | 222 | 225 | 221 | 221 | 213 | 215 | 179 | 166 | 100 | 96 |
| % interviewed | 92 | 96 | 90 | 94 | 87 | 92 | 80 | 87 | 82 | 88 |
| Overall NEI-VFQ [n (%)] | ||||||||||
| ≥17 | 11 (5) | 16 (7) | 10 (5) | 21 (10) | 14 (8) | 21 (11) | 12 (8) | 10 (7) | 9 (11) | 13 (15) |
| 6–16 | 45 (22) | 57 (27) | 46 (23) | 48 (23) | 34 (18) | 49 (25) | 20 (14) | 36 (25) | 16 (20) | 24 (29) |
| −5 to 5 | 81 (40) | 93 (43) | 69 (35) | 83 (40) | 57 (31) | 68 (34) | 43 (30) | 47 (32) | 27 (33) | 19 (23) |
| −6 to −16 | 47 (23) | 39 (18) | 50 (25) | 34 (16) | 46 (25) | 35 (18) | 39 (27) | 26 (18) | 8 (10) | 11 (13) |
| ≤−17 | 20 (10) | 10 (5) | 24 (12) | 21 (10) | 35 (19) | 25 (13) | 29 (20) | 26 (18) | 22 (27) | 17 (20) |
| Median | −1 | 1 | −1 | 1 | −3 | 1 | −5 | −2 | 0 | 2 |
| 95% CI | −2 to 2 | −1 to 3 | −3 to 0 | 0–3 | −6 to −2 | −1 to 3 | −7 to −2 | −4 to 2 | −4 to 2 | −3 to 8 |
| Wilcoxon P value† | — | — | 0.003 | — | — | |||||
| General vision [n (%)] | ||||||||||
| ≥17 | 27 (13) | 34 (16) | 21 (11) | 37 (18) | 20 (11) | 33 (17) | 13 (9) | 18 (12) | 7 (9) | 14 (17) |
| 6–16 | 33 (16) | 45 (21) | 35 (18) | 37 (18) | 30 (16) | 36 (18) | 22 (15) | 25 (17) | 8 (10) | 16 (19) |
| −5 to 5 | 84 (41) | 79 (37) | 84 (42) | 79 (38) | 77 (41) | 71 (36) | 52 (36) | 52 (36) | 31 (38) | 23 (27) |
| −6 to −16 | 30 (15) | 35 (16) | 26 (13) | 32 (15) | 19 (10) | 29 (15) | 21 (15) | 28 (19) | 14 (17) | 17 (20) |
| ≤−17 | 30 (15) | 22 (10) | 33 (17) | 22 (11) | 40 (22) | 29 (15) | 35 (24) | 22 (15) | 22 (27) | 13 (15) |
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −5 | 0 |
| 95% CI | 0–5 | 0–5 | −5 to 0 | 0–5 | −5 to 0 | 0–5 | −5 to 0 | −5 to 5 | −10 to 0 | −5 to 5 |
| Wilcoxon P value† | — | — | 0.10 | — | — | |||||
| Driving, no. (%) | ||||||||||
| ≥17 | 17 (8) | 32 (15) | 26 (13) | 26 (13) | 16 (9) | 20 (10) | 11 (8) | 10 (7) | 9 (11) | 4 (5) |
| 6–16 | 27 (13) | 14 (7) | 13 (7) | 18 (9) | 13 (7) | 17 (9) | 6 (4) | 5 (3) | 1 (1) | 3 (4) |
| −5 to 5 | 75 (37) | 90 (42) | 67 (34) | 77 (37) | 65 (35) | 74 (37) | 51 (36) | 53 (37) | 28 (34) | 30 (36) |
| −6 to −16 | 15 (7) | 13 (6) | 18 (9) | 13 (6) | 10 (5) | 10 (5) | 5 (4) | 9 (6) | 1 (1) | 5 (6) |
| ≤−17 | 36 (18) | 38 (18) | 38 (19) | 48 (23) | 57 (31) | 50 (25) | 51 (36) | 47 (32) | 27 (33) | 27 (32) |
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 95% CI | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | −8 to 0 | −8 to 0 | −33 to 0 | −17 to 0 |
| Wilcoxon P value† | — | — | 0.22 | — | — | |||||
| Near activities [n (%)] | ||||||||||
| ≥17 | 20 (10) | 46 (21) | 27 (14) | 41 (20) | 32 (17) | 42 (21) | 30 (21) | 31 (21) | 18 (22) | 18 (21) |
| 6–16 | 47 (23) | 33 (15) | 35 (18) | 38 (18) | 26 (14) | 29 (15) | 15 (10) | 20 (14) | 15 (18) | 11 (13) |
| −5 to 5 | 64 (31) | 74 (34) | 58 (29) | 68 (33) | 47 (25) | 59 (30) | 31 (22) | 33 (23) | 20 (24) | 27 (32) |
| −6 to −16 | 40 (20) | 33 (15) | 23 (12) | 30 (14) | 30 (16) | 26 (13) | 25 (17) | 24 (17) | 5 (6) | 6 (7) |
| ≤−17 | 33 (16) | 29 (13) | 56 (28) | 30 (14) | 50 (27) | 42 (21) | 42 (29) | 37 (26) | 24 (29) | 22 (26) |
| Median | 0 | 0 | 0 | 0 | −4 | 0 | −4 | −4 | 4 | 0 |
| 95% CI | −4 to 0 | 0–4 | −4 to 4 | 0–4 | −8 to 0 | −4 to 0 | −8 to 0 | −4 to 0 | −4 to 8 | −4 to 4 |
| Wilcoxon P value† | — | — | 0.14 | — | — | |||||
| Distance activities [n (%)] | ||||||||||
| ≥17 | 26 (13) | 44 (20) | 25 (13) | 36 (17) | 32 (17) | 42 (21) | 25 (17) | 22 (15) | 16 (20) | 24 (29) |
| 6–16 | 31 (15) | 38 (18) | 33 (17) | 39 (19) | 22 (12) | 32 (16) | 10 (7) | 22 (15) | 14 (17) | 11 (13) |
| −5 to 5 | 71 (35) | 70 (33) | 58 (29) | 61 (29) | 46 (25) | 52 (26) | 43 (30) | 38 (26) | 15 (18) | 21 (25) |
| −6 to −16 | 38 (19) | 30 (14) | 33 (17) | 32 (15) | 34 (18) | 29 (15) | 23 (16) | 23 (16) | 12 (15) | 3 (4) |
| ≤−17 | 38 (19) | 33 (15) | 50 (25) | 39 (19) | 52 (28) | 43 (22) | 42 (29) | 40 (28) | 25 (30) | 25 (30) |
| Median | −1 | 0 | −4 | 0 | −4 | 0 | −4 | 0 | −4 | 0 |
| 95% CI | −4 to 0 | 0–4 | −4 to 0 | −4 to 4 | −8 to 0 | −4 to 4 | −8 to 0 | −7 to 0 | −8 to 4 | −4 to 7 |
| Wilcoxon P value† | — | — | 0.08 | — | — | |||||
| Role difficulties [n (%)] | ||||||||||
| ≥+17 | 29 (14) | 39 (18) | 26 (13) | 37 (18) | 24 (13) | 42 (21) | 17 (12) | 27 (19) | 11 (13) | 22 (26) |
| 6–16 | 49 (24) | 54 (25) | 46 (23) | 46 (22) | 38 (20) | 39 (20) | 33 (23) | 22 (15) | 15 (18) | 16 (19) |
| −5 to 5 | 30 (15) | 37 (17) | 27 (14) | 39 (19) | 21 (11) | 25 (13) | 14 (10) | 24 (17) | 7 (9) | 8 (10) |
| −6 to −16 | 45 (22) | 40 (19) | 47 (24) | 50 (24) | 38 (20) | 45 (23) | 27 (19) | 31 (21) | 16 (20) | 15 (18) |
| ≤−17 | 51 (25) | 45 (21) | 53 (27) | 35 (17) | 64 (34) | 47 (24) | 52 (36) | 41 (28) | 33 (40) | 23 (27) |
| Median | 0 | 0 | −6 | 0 | −6 | 0 | −6 | 0 | −9 | 0 |
| 95% CI | −6 to 0 | 0–6 | −6 to 0 | 0–0 | −12 to 0 | −6 to 0 | −12 to 0 | −6 to 0 | −19 to 0 | −6 to 6 |
| Wilcoxon P value† | — | — | 0.03 | — | — | |||||
| Mental health [n (%)] | ||||||||||
| ≥17 | 32 (16) | 44 (20) | 32 (16) | 51 (25) | 29 (16) | 56 (28) | 27 (19) | 35 (24) | 18 (22) | 28 (33) |
| 6–16 | 34 (17) | 46 (21) | 31 (16) | 35 (17) | 33 (18) | 32 (16) | 19 (13) | 24 (17) | 15 (18) | 16 (19) |
| −5 to 5 | 71 (35) | 61 (28) | 67 (34) | 72 (35) | 52 (28) | 58 (29) | 46 (32) | 36 (25) | 25 (30) | 17 (20) |
| −6 to −16 | 30 (15) | 32 (15) | 27 (14) | 21 (10) | 23 (12) | 23 (12) | 21 (15) | 19 (13) | 6 (7) | 9 (11) |
| ≤−17 | 37 (18) | 32 (15) | 42 (21) | 28 (14) | 48 (26) | 29 (15) | 30 (21) | 31 (21) | 18 (22) | 14 (17) |
| Median | 0 | 5 | 0 | 5 | −5 | 5 | 0 | 0 | 2 | 10 |
| 95% CI | −5 to 5 | 0–5 | −5 to 5 | 0–5 | −5 to 0 | 0–10 | −5 to 5 | −5 to 5 | −5 to 10 | 0–15 |
| Wilcoxon P value† | — | — | 0.0007 | — | — | |||||
| Dependency [n (%)] | ||||||||||
| ≥17 | 38 (19) | 46 (21) | 41 (21) | 49 (24) | 33 (18) | 48 (24) | 22 (15) | 31 (21) | 17 (21) | 19 (23) |
| 6–16 | 34 (17) | 36 (17) | 35 (18) | 40 (19) | 33 (18) | 24 (12) | 20 (14) | 33 (23) | 13 (16) | 17 (20) |
| −5 to 5 | 48 (24) | 59 (27) | 39 (20) | 53 (26) | 25 (13) | 50 (25) | 24 (17) | 25 (17) | 11 (13) | 12 (14) |
| −6 to −16 | 31 (15) | 34 (16) | 31 (16) | 28 (14) | 33 (18) | 29 (15) | 20 (14) | 19 (13) | 8 (10) | 14 (17) |
| ≤−17 | 53 (26) | 40 (19) | 53 (27) | 37 (18) | 61 (33) | 47 (24) | 57 (40) | 37 (26) | 33 (40) | 22 (26) |
| Median | 0 | 0 | 0 | 0 | −6 | 0 | −6 | 0 | −3 | 0 |
| 95% CI | 0–0 | 0–0 | 0–0 | 0–6 | −6 to 0 | 0–0 | −12 to 0 | 0–6 | −19 to 6 | −6 to 6 |
| Wilcoxon P value† | — | — | 0.03 | — | — | |||||
| Social functioning [n (%)] | ||||||||||
| ≥17 | 34 (17) | 47 (22) | 43 (22) | 51 (25) | 44 (24) | 48 (24) | 30 (21) | 31 (21) | 21 (26) | 26 (31) |
| 6–16 | 31 (15) | 37 (17) | 27 (14) | 30 (14) | 22 (12) | 28 (14) | 26 (18) | 23 (16) | 14 (17) | 10 (12) |
| −5 to 5 | 60 (29) | 63 (29) | 60 (30) | 65 (31) | 51 (27) | 54 (27) | 28 (20) | 32 (22) | 16 (20) | 12 (14) |
| −6 to −16 | 22 (11) | 35 (16) | 26 (13) | 21 (10) | 21 (11) | 26 (13) | 22 (15) | 19 (13) | 10 (12) | 13 (15) |
| ≤−17 | 56 (27) | 33 (15) | 42 (21) | 40 (19) | 48 (26) | 41 (21) | 36 (25) | 40 (28) | 20 (24) | 23 (27) |
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 95% CI | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–8 | −8 to 8 |
| Wilcoxon P value† | — | — | 0.24 | — | — | |||||
| Peripheral vision‡ [n (%)] | ||||||||||
| ≥+25 | 57 (28) | 73 (34) | 49 (25) | 53 (26) | 58 (31) | 60 (30) | 37 (26) | 46 (32) | 31 (38) | 38 (45) |
| 0 | 83 (41) | 89 (41) | 88 (44) | 98 (47) | 74 (40) | 77 (39) | 56 (39) | 56 (39) | 33 (40) | 29 (35) |
| ≤−25 | 62 (30) | 52 (24) | 61 (31) | 55 (27) | 53 (28) | 57 (29) | 48 (34) | 39 (27) | 18 (22) | 16 (19) |
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 95% CI | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–25 |
| Wilcoxon P value† | — | — | 0.82 | — | — | |||||
| Ocular pain§ [n (%)] | ||||||||||
| ≥+25 | 23 (11) | 31 (14) | 35 (18) | 34 (16) | 35 (19) | 35 (18) | 32 (22) | 24 (17) | 13 (16) | 19 (23) |
| 12 | 38 (19) | 38 (18) | 26 (13) | 34 (16) | 29 (16) | 34 (17) | 16 (11) | 23 (16) | 8 (10) | 19 (23) |
| 0 | 88 (43) | 104 (48) | 80 (40) | 95 (46) | 73 (39) | 79 (40) | 58 (41) | 70 (48) | 45 (55) | 28 (33) |
| −12 | 30 (15) | 20 (9) | 23 (12) | 26 (13) | 26 (14) | 33 (17) | 21 (15) | 18 (12) | 5 (6) | 11 (13) |
| ≤−25 | 25 (12) | 22 (10) | 35 (18) | 18 (9) | 23 (12) | 17 (9) | 16 (11) | 10 (7) | 11 (13) | 7 (8) |
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 95% CI | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–12 |
| Wilcoxon P value† | — | — | 0.82 | — | — | |||||
CI = confidence interval; Obs = Observation; Sur = Surgery.
Counts may not add up to the total number of interviews, and percentages may not add up to 100 owing to missing responses.
Reported for treatment arm comparison of change in NEI-VFQ scores from baseline to 24 mos only.
Subscale is a 1-item scale; possible values for the scales are 0, 25, 50, 75, and 100.
Subscale is a 2-item scale; possible values are 0, 12.5, 25, 37.5, etc., up to 100.
National Eye Institute Visual Function Questionnaire Scores at Baseline
Patients enrolled in the SST Group N Trial reported relatively poor visual function at baseline. Median overall NEI-VFQ scores were 67 (interquartile range, 51–80) in the observation arm and 69 (interquartile range, 50–82) in the surgery arm at baseline (Fig 1). Six of the subscales (general vision, driving, near activities, distance activities, role difficulties, mental health) had median scores ranging from 42 to 65. The most affected subscale was driving. As shown in Figure 1, the NEI-VFQ scores in the 2 treatment arms were distributed similarly on all subscales.
Figure 1.
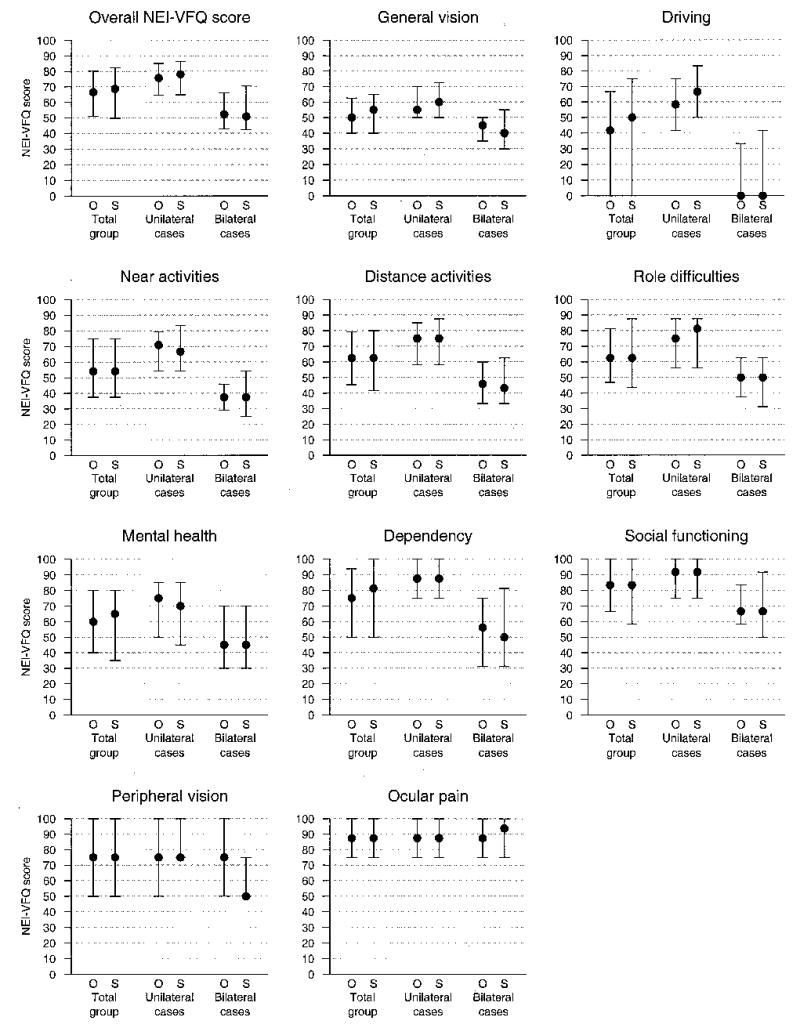
Summaries (medians and interquartile ranges) of distributions of National Eye Institute Visual Function Questionnaire (NEI-VFQ) scores by scale and treatment arm at baseline. The leftmost pair representing the total group is for all patients in each treatment arm, the middle pair is for patients in each treatment arm who were classified as unilateral cases, and the rightmost pair is for patients in each treatment arm who were classified as bilateral cases. O = observation; S = surgery.
Bilateral cases had considerably lower NEI-VFQ scores than unilateral cases at baseline, as previously reported6; however, treatment arms within each subgroup had similar scores (Fig 1).
National Eye Institute Visual Function Questionnaire Scores during Patient Follow-up
Patients in the surgery group had better median NEI-VFQ scores during follow-up than patients in the observation group. At the 24-month interview, median overall NEI-VFQ scores were 61 in the observation arm and 65 in the surgery arm (Wilcoxon P = 0.06 for treatment arm comparison; data not shown). The general vision subscale was the only subscale with a treatment difference judged to be statistically significant at 24 months (median scores, 50 for observation and 55 for surgery; Wilcoxon P = 0.01). In general, median NEI-VFQ scores declined slightly or stayed the same during follow-up in both treatment arms, with the exception of the driving subscale, where median driving scores declined rapidly and reached 0 (i.e., unable to drive because of vision) by 24 months of follow-up in the observation arm and by 36 months of follow-up in the surgery arm. Seventy-one (42%) of 170 patients (with past driving experience) in the observation arm and 65 (35%) of 187 patients in the surgery arm were not driving at baseline because of their vision; by 24 months, 91 (57%) of 161 patients in the observation arm and 81 (47%) of 171 patients in the surgery arm were not driving because of poor vision.
Figure 2 shows median overall NEI-VFQ scores during follow-up by observation versus surgery for unilateral cases and bilateral cases separately. In unilateral cases, median overall NEI-VFQ scores were 72 in the observation arm and 80 in the surgery arm (Wilcoxon P = 0.04) at 24 months of follow-up; in bilateral cases, median overall NEI-VFQ scores were 52 in the observation arm and 54 in the surgery arm (Wilxocon P = 0.19). No statistically significant treatment difference was observed for any of the NEI-VFQ subscales among unilateral cases or bilateral cases at 24 months of follow-up; however, 4 NEI-VFQ subscales suggested a treatment benefit favoring surgery at 24 months in unilateral cases only (data not shown). These subscales were general vision (median scores, 55 for observation and 60 for surgery; Wilcoxon P = 0.03), mental health (median scores, 70 for observation and 80 for surgery; Wilcoxon P = 0.05), dependency (median scores, 84 for observation and 94 for surgery; Wilcoxon P = 0.05), and driving (median scores, 50 for observation and 58 for surgery; Wilcoxon P = 0.05) subscales. For 8 of 10 subscales (with the exception of peripheral vision and ocular pain), unilateral cases had better NEI-VFQ scores during follow-up than bilateral cases.
Figure 2.
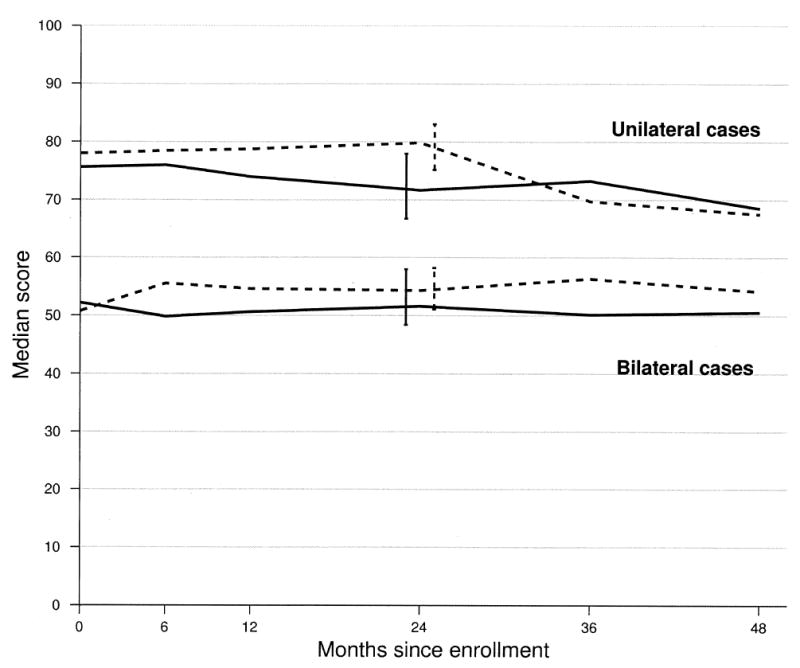
Median overall National Eye Institute Visual Function Questionnaire score at each interview time point and summary by patients who were classified with unilateral disease and with bilateral disease and treatment arm. Solid lines indicate the observation arm, and dashed lines indicate the surgery treatment arm. Error bars indicate 95% confidence intervals.
Change in National Eye Institute Visual Function Questionnaire Scores from Baseline to Follow-up Interviews
Changes in the overall NEI-VFQ scores from baseline to follow-up interviews indicated that patients in the surgery arm had less decline with respect to perceived ability to function visually than patients in the observation arm (Table 3). By 24 months of follow-up, 48 (26%) of 186 patients in the observation group and 70 (35%) of 198 patients in the surgery group reported vision-targeted HRQOL scores that were better than baseline scores (by at least 6 points). Median changes in the overall NEI-VFQ score from baseline to 24 months were a loss of 3 points in the observation group and a gain of 1 point in the surgery group (Wilcoxon P = 0.003). The only subscale statistically favoring surgery by 24 months was the vision-related mental health subscale: 62 (33%) of 186 patients in the observation arm and 88 (44%) of 198 patients in the surgery arm had vision-related mental health scores ≥6 points higher than at baseline. This translated to a 10-point difference—specifically, a median loss of 5 points (median score at baseline, 60) in the observation arm and a median gain of 5 points (median score at baseline, 65) in the surgery arm (Wilcoxon P < 0.001). Changes from baseline that favored surgery early were evident for the distance activities subscale through the 12-month interview, but the early benefit disappeared at later follow-up times. There was no subscale for which the observation arm had a more favorable distribution of change in scores than the surgery arm.
Figure 3 presents a treatment arm comparison of change in the overall NEI-VFQ scores from baseline to each interview separately for unilateral and bilateral cases. Among unilateral cases, a median 24-month change in the overall NEI-VFQ score was a loss of 5 points (median score at baseline, 76) in the observation group and a change of 0 points (median score at baseline, 78) in the surgery group (Wilcoxon P = 0.03). Among bilateral cases, a similar 5-point difference was noted; the observation group lost a median of 3 points (median score at baseline, 52), and the surgery group gained a median of 2 points (median score at baseline, 51) during the 24-month interval (Wilcoxon P = 0.06).
Figure 3.
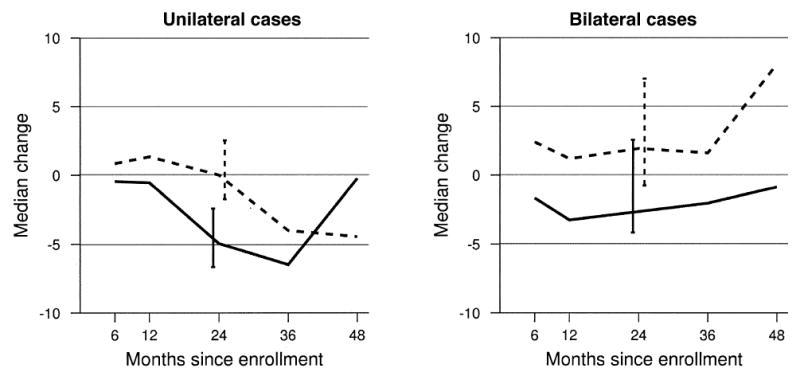
Median change in the overall National Eye Institute Visual Function Questionnaire score from baseline to follow-up interviews by patients who were classified with unilateral disease and with bilateral disease and treatment arm. Solid lines indicate the observation arm, and dashed lines indicate the surgery treatment arm. Error bars indicate 95% confidence intervals.
Treatment arm comparisons of changes from baseline scores for selected NEI-VFQ subscales are presented in Figure 4 for unilateral and bilateral cases separately. Among unilateral cases, changes in scores to 24 months were more favorable after surgery than with observation on the mental health subscale (Wilcoxon P = 0.008); patients assigned to surgery improved 5 points (median score at baseline, 70) by the 24-month interview on the mental health subscale, and patients assigned to observation lost 5 points (median score at baseline, 75) by that time. Differences between treatment arms in change scores from baseline to 24 months suggested a benefit from surgery for dependency (observation arm lost a median of 6 points; surgery, no change; Wilcoxon P = 0.03), distance activities (observation arm lost a median of 4 points; surgery, no change; Wilcoxon P = 0.04), and near activities (observation arm lost a median of 7 points; surgery, no change; Wilcoxon P = 0.05) scores. Among bilateral cases, no statistically significant treatment differences were observed by 24 months; however, changes from baseline to 24 months for mental health (observation arm, no change; surgery arm, median gain of 5 points; Wilcoxon P = 0.03) and role difficulties (observation arm, median 6-point loss; surgery, no change; Wilcoxon P = 0.05) subscales were suggestive of a 24-month benefit after surgery. On average, no changes were observed for the peripheral vision and ocular pain subscales from baseline to 24 months of follow-up (data not shown).
Figure 4.
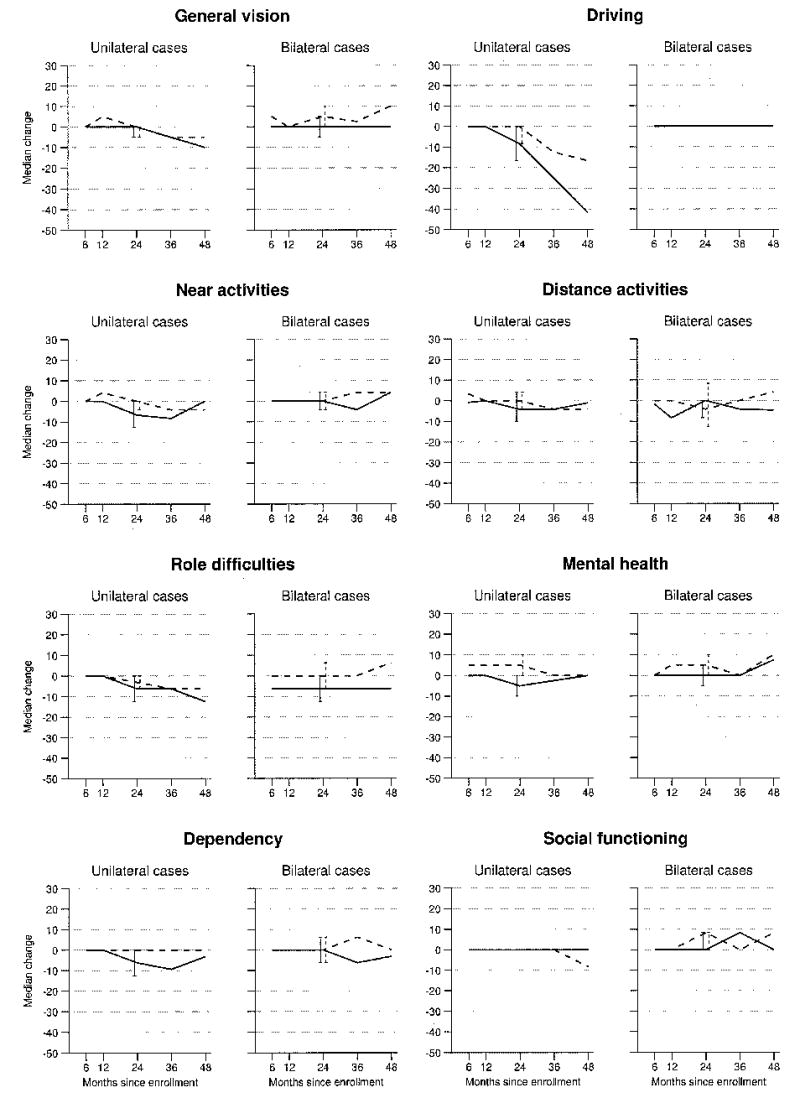
Median changes in National Eye Institute Visual Function Questionnaire subscale scores from baseline to follow-up interviews at each interview time point. Solid lines indicate the observation arm, and dashed lines indicate the surgery treatment arm. Error bars indicate 95% confidence intervals.
Change in subfoveal lesion size statistically significantly differed between treatment arms at the 24-month examination, as reported with other ophthalmic outcomes elsewhere.5 Change in lesion size was evaluated as an explanatory factor for the observed differences in HRQOL outcomes. There was weak evidence of lower NEI-VFQ scores with larger lesion size (data not shown). However, change in lesion size (1–2 categories of disc area sizes smaller, vs. same size or larger) was not a statistically significant explanatory variable in linear regression models with change in NEI-VFQ scores as the dependent variable and an indicator variable for treatment arm as an independent variable.
Sensitivity Analysis of National Eye Institute Visual Function Questionnaire Outcomes
Mixed linear models provided marginal estimates of treatment differences in NEI-VFQ scores after adjustment for baseline NEI-VFQ score, general health status (SF-36 PCS) during follow-up, and interview times (data not shown). The following statistically significant (t test P ≤ 0.01) treatment differences were identified, all favoring surgery over observation: 3.0-point difference in the overall NEI-VFQ score, 5.1-point difference in the dependency scale, 4.4-point difference in the mental health scale, 3.7-point difference in the general vision scale, and 3.4-point difference in the distance activities scale. Age was not a statistically significant covariate. Treatment-by-time and treatment-by-age interactions were not present.
36-Item Short Form Health Survey Summary Scores at Baseline and Change in Scores from Baseline to 24 Months
The median PCS score of patients enrolled in the trial was 47, and the median MCS score was 57 (data not shown). The PCS scores were on average lower than the median U.S. population value of 53 but higher than the median PCS score of 38 for ≥75-year-old individuals.8 The median MCS score also was higher than the median scores of 53 and 54 published for the general U.S population and for ≥75-year-old men and women, respectively.8 At baseline, treatment arms had similar PCS and MCS scores. By 24 months of follow-up, both treatment arms had a median of a 4-point decline in the PCS score (Wilcoxon P = 0.87); however, a median of a 1-point decline was observed in the MCS score in the observation arm, and a median of 1-point improvement in the surgery arm (Wilcoxon P < 0.001).
Among unilateral cases, the median PCS score at baseline was 47, and the median MCS score, 57. Both treatment arms had similar declines in physical function (a median of a 3-point decline) by 24 months; the MCS score declined by a median of 1 point in the observation arm and improved by a median of 1 point in the surgery arm (Wilcoxon P = 0.001).
Among bilateral cases, median PCS and MCS scores were 46 and 56, respectively, at baseline. By 24 months, the PCS score declined by 4 points in the observation arm and by 5 points in the surgery arm; the MCS score changed by a median of 0 points in the observation arm and improved by a median of 1 point in the surgery arm (Wilcoxon P = 0.05).
Hospital Anxiety and Depression Scale Summary Scores
The prevalence of anxiety and depression was low (2%–4%) at baseline and at 24 months of follow-up (data not shown). Based on 2-state stochastic models, 3.1% of the observation arm and 1.5% of the surgery arm had definite anxiety, and 2.6% of the observation arm and 3.7% of the surgery arm had definite depression at 24-months of follow-up. The percentage of definite cases of anxiety among the unilateral cases (observation, 4.7%; surgery, 2.6%) was higher than that in bilateral cases (observation, 1.2%,; surgery, 0%) at 24 months; however, a similar trend was not seen for depression (unilateral cases: observation, 3.0%; surgery, 2.0%; bilateral cases: observation, 2.3%; surgery, 6.0%).
Discussion
Differences between treatment arms (submacular surgery vs. observation without treatment) in HRQOL outcomes 24 months after enrollment in the SST Group N Trial were small but favored submacular surgery over observation (Table 3). The plausibility of the small treatment difference in HRQOL by 24 months that favored surgery was strengthened by consistent evidence of benefit at several follow-up times and the consistency of the findings for the NEI-VFQ mental health subscale with the SF-36 MCS. One possible explanation of why the HRQOL findings were not consistent with the VA findings reported elsewhere5 is that clinical outcomes may not have captured aspects of visual functioning that were important to patients enrolled in the SST Group N Trial, such as the impact of visual disability on emotional well-being or dependency on others. Thus, HRQOL measures may have captured changes in visual function not measurable by standard means. Although patients in the surgery arm maintained smaller lesions and had fewer active lesions during follow-up than patients in the observation arm,5 these findings did not fully explain better vision-targeted functioning in the surgery arm. Adjustment for possible explanatory factors, such as general health status and baseline NEI-VFQ scores, diminished the differences between the surgery arm and the observation arm on NEI-VFQ scores but did not eliminate them. Another possible explanation is that an intervention led patients to respond more favorably to the HRQOL questions than patients observed without treatment (i.e., interventional effect or placebo effect), particularly when the mental health sub-scale was a major contributor to the overall HRQOL outcomes that favored the intervention arm. It is possible that surgeons were more positive when examining patients after submacular surgery than when examining observation patients. This special attention may have resulted in surgery patients feeling better and more optimistic about their vision than observation patients. However, such an interventional effect also would have been expected in the SST Group B Trial and was not reported there.18
The HRQOL portion of the SST Group N Trial was intended to support and amplify VA findings. Although the NEI-VFQ has been reported to be a valid and reliable instrument1–3 and responsive to changes in VA of the better-seeing eye over time,4 it is unknown whether differences of the magnitude identified in the SST Group N Trial are clinically important. A similar magnitude of treatment difference between the submacular surgery arm and the observation arm on NEI-VFQ scores was observed in the SST Group H Trial that compared submacular surgery and observation in patients with subfoveal idiopathic CNV or CNV due to ocular histoplasmosis.19,20 Although underlying diseases that are associated with subfoveal CNV in the SST Group N and Group H Trials differ and affect different age groups, magnitudes of the differences between treatment arms were similar in the 2 trials, and the treatment differences were observed for similar domains of vision-targeted HRQOL.
The SST Group N Trial was designed and powered to identify meaningful differences in VA outcomes but not in HRQOL outcomes, which were considered secondary outcomes to be supportive of any VA benefit from surgery. Nevertheless, post hoc power calculations indicated that the trial had excellent power to identify 10-point differences between treatment arms in NEI-VFQ change scores but moderate to poor power to identify 5-point differences. Because the trial had power to identify 10-point differences in NEI-VFQ scores, we are confident that we did not miss any large differences between treatment arms that are likely to be clinically important based on our previous work.4 However, most treatment differences in NEI-VFQ scores, when identified, were small. The consistency of benefit from surgery across time and across NEI-VFQ scales leads one to suspect that the differences are real, although possibly too small to indicate true benefit to patients.
The SF-36 did not show differences between treatment arms to the same extent as the NEI-VFQ. This observation is not surprising, considering that a condition-specific instrument, such as the NEI-VFQ, would be expected to be more responsive to vision-targeted problems than a generic measure of HRQOL.4,21 Our previous investigations have indicated that the SF-36 is not responsive to changes in VA,21 but the summary scores have proven to be helpful when accounting for the contribution of general health to NEI-VFQ scores.22,23
The strengths of the current study include prospective data collection from a relatively large number of patients with subfoveal CNV due to AMD, use of standardized HRQOL measures, and high interview completion rates among elderly patients. Furthermore, interviewers were masked to the treatment assignment and to study eye to avoid any bias in the data collection. At baseline, HRQOL scores and other patient characteristics, including VAs in study and fellow eyes, were well balanced between the treatment arms and assure a valid comparison of treatment arms on HRQOL scores during follow-up. However, the results may be applicable only to patients who volunteer for research and meet eligibility criteria for the trial, a select group of patients with subfoveal CNV due to AMD who were willing and healthy enough to return for follow-up examinations. Patients were not masked to treatment assignment due to ethical and logistical reasons.
These data show that patients assigned to submacular surgery perceive that they have better vision-targeted HRQOL than patients assigned to observation without treatment. Because it is unclear whether the HRQOL differences between treatment arms are clinically meaningful, sub-macular surgery, as performed in this trial, cannot be recommended in the absence of VA benefit from submacular surgery in the SST Group N Trial. As new treatments are developed and become available for clinical trials, natural history data from the SST Group N Trial observation group may contribute important information in studies designed to include HRQOL measures. Further evaluation of the clinical significance of changes in NEI-VFQ scores over time in this and other prospective studies is warranted.
Table 1.
Demographic Characteristics and Medical History at Baseline
|
Total Group |
Unilateral Cases |
Bilateral Cases |
||||
|---|---|---|---|---|---|---|
| Observation (n = 228) | Surgery (n = 226) | Observation (n = 123) | Surgery (n = 128) | Observation (n = 105) | Surgery (n = 98) | |
| Age (yrs) [n (%)] | ||||||
| < 60 | 1 (< 1) | 4 (2) | 0 | 4 (3) | 1 (1) | 0 |
| 60–69 | 22 (10) | 26 (12) | 13 (11) | 12 (9) | 9 (9) | 14 (14) |
| 70–79 | 120 (53) | 121 (54) | 69 (56) | 75 (59) | 51 (49) | 46 (47) |
| 80–89 | 81 (36) | 73 (32) | 40 (33) | 35 (27) | 41 (39) | 38 (39) |
| ≥90 | 4 (2) | 2 (1) | 1 (1) | 2 (2) | 3 (3) | 0 |
| Gender [n (%)] | ||||||
| Woman | 122 (54) | 119 (53) | 59 (48) | 67 (52) | 63 (60) | 52 (53) |
| Man | 106 (46) | 107 (47) | 64 (52) | 61 (48) | 42 (40) | 46 (47) |
| Race/ethnicity* [n (%)] | ||||||
| White, not Hispanic | 223 (98) | 220 (98) | 121 (98) | 127 (99) | 102 (98) | 93 (96) |
| American Indian or Alaskan Native | 2 (1) | 2 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) |
| Asian or Pacific Islander | 1 (< 1) | 3 (1) | 0 | 0 | 1 (1) | 3 (3) |
| Black, not Hispanic | 1 (< 1) | 0 | 1 (1) | 0 | 0 | 0 |
| Unknown | 1 (<1) | 1 (< 1) | 0 | 0 | 1 (1) | 1 (1) |
| Occupational status [n (%)] | ||||||
| Retired | 210 (92) | 191 (85) | 114 (93) | 108 (84) | 96 (91) | 83 (85) |
| Employed | 10 (4) | 24 (11) | 7 (6) | 13 (10) | 3 (3) | 11 (11) |
| Housespouse | 7 (3) | 8 (4) | 2 (2) | 5 (4) | 5 (5) | 3 (3) |
| Visually disabled | 1 (< 1) | 2 (1) | 0 | 1 (1) | 1 (1) | 1 (1) |
| Unemployed | 0 | 1 (<1) | 0 | 1 (1) | 0 | 0 |
| Smoking history [n (%)] | ||||||
| Never smoked | 75 (33) | 91 (40) | 37 (30) | 48 (38) | 38 (36) | 43 (44) |
| Quit smoking | 124 (54) | 108 (48) | 74 (60) | 65 (51) | 50 (48) | 43 (44) |
| Currently smoking | 29 (13) | 27 (12) | 12 (10) | 15 (12) | 17 (16) | 12 (12) |
| Aspirin Intake, no. (%) | ||||||
| <7/wk | 138 (61) | 145 (64) | 73 (59) | 85 (66) | 65 (62) | 60 (61) |
| ≥7/wk | 90 (39) | 81 (36) | 50 (41) | 43 (34) | 40 (38) | 38 (39) |
| Warfarin use [n (%)] | ||||||
| Never | 197 (86) | 201 (89) | 107 (87) | 114 (90) | 90 (86) | 87 (89) |
| Ever | 31 (14) | 24 (11) | 16 (13) | 13 (10) | 15 (14) | 11 (11) |
| Chronic conditions present [n (%)] | ||||||
| Hypertension | 133 (58) | 127 (56) | 77 (63) | 69 (54) | 56 (53) | 58 (59) |
| Diabetes mellitus | 27 (12) | 21 (9) | 12 (10) | 10 (8) | 15 (14) | 11 (11) |
| Any other known† | 157 (87) | 158 (84) | 85 (87) | 90 (82) | 72 (88) | 68 (87) |
Based on self-reports.
Based on responses from 180 patients in the observation arm and 188 in the surgery arm who were queried.
Acknowledgments
Members of the SST Patient-Centered Outcomes Subcommittee served as the writing team for SST report no. 12 on behalf of the SST Research Group: Päivi H. Miskala, PhD (writing team chair), Eric B. Bass, MD, Neil M. Bressler, MD, Ashley L. Childs, MS, Barbara S. Hawkins, PhD, Carol M. Mangione, MD, MSPH, Marta J. Marsh, MS. Dr Mangione’s participation as Chair of the SST Patient-Centered Outcomes Subcommittee is supported by contract between the David Geffen School of Medicine, University of California, Los Angeles, and the Johns Hopkins University, Baltimore, Maryland.
Footnotes
The Submacular Surgery Trials are sponsored by the National Eye Institute, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland (cooperative agreements U10 EY11547, EY11557, EY11558 with the Johns Hopkins University, Baltimore, Maryland). Participating clinical centers were supported by contracts with the Johns Hopkins University.
References
- 1.Mangione CM, Berry S, Spritzer K, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998;116:227–33. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- 2.Mangione CM, Lee PP, Pitts J, et al. NEI-VFQ Field Test Investigators. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ) Arch Ophthalmol. 1998;116:1496–504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 3.Mangione CM, Lee PP, Gutierrez PR, et al. National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 4.Submacular Surgery Trials Research Group. . Responsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization—SST report no. 1. Arch Ophthalmol. 2003;121:531–9. doi: 10.1001/archopht.121.4.531. [correction: 2003;121:1513] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Submacular Surgery Trials (SST) Research Group. . Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings. SST report no. 11. Ophthalmology. 2004;111:1967–80. doi: 10.1016/j.ophtha.2004.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Submacular Surgery Trials Research Group. . Health- and vision-related quality of life among patients with choroidal neovascularization secondary to age-related macular degeneration at enrollment in randomized trials of submacular surgery. SST report no. 4. Am J Ophthalmol. 2004;138:91–108. doi: 10.1016/j.ajo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Submacular Surgery Trials. Manual of Procedures. Spring-field, VA: National Technical Information Service; 1998. NTIS Publication PB98-16648.
- 8.Ware JEJr, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston: The Health Institute; 1994.
- 9.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 10.Submacular Surgery Trials Research Group. Patients’ perceptions of the value of current vision: assessment of preference values among patients with subfoveal choroidal neovascularization—the Submacular Surgery Trials Vision Preference Value Scale (SST-VPVS): SST report no. 6. Arch Ophthalmol. In press. [DOI] [PMC free article] [PubMed]
- 11.Ferris FL, III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–6. [PubMed] [Google Scholar]
- 12.Royston JP. An extension of Shapiro and Wilk’s W test for normality to large samples. Appl Stat. 1982;31:115–24. [Google Scholar]
- 13.SAS Procedures Guide. Version 8. Vol. 2. Cary, NC: SAS Publishing; 2000:1404–5.
- 14.Hillis A, Maguire M, Hawkins BS, Newhouse MM. The Markov process as a general method for nonparametric analysis of right-censored medical data. J Chronic Dis. 1986;39:595–604. doi: 10.1016/0021-9681(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 15.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 1994:131–2.
- 16.SAS/STAT User’s Guide. Version 8. Vol. 2. Cary, NC: SAS Publishing; 2000:2085–226.
- 17.SAS [computer program]. Version 8.2. Cary, NC: SAS Inc.; 2001.
- 18.Submacular Surgery Trials (SST) Research Group. . Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: quality-of-life findings. SST report no. 14. Ophthalmology. 2004;111:2007–14. doi: 10.1016/j.ophtha.2004.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Submacular Surgery Trials Research Group. Surgical removal versus observation for subfoveal choroidal neovascularization, either associated with the ocular histoplasmosis syndrome or idiopathic. II. Quality-of-life findings from a randomized clinical trial: SST Group H Trial. SST report number 10. Arch Ophthalmol. In press. [DOI] [PMC free article] [PubMed]
- 20.Submacular Surgery Trials Research Group. Surgical removal versus observation for subfoveal choroidal neovascularization, either associated with the ocular histoplasmosis syndrome or idiopathic. I. Ophthalmic findings from a randomized clinical trial: SST Group H Trial. SST report number 9. Arch Ophthalmol. In press. [DOI] [PMC free article] [PubMed]
- 21.Childs AL Submacular Surgery Trials Patient-Centered Outcomes Subcommittee for the Submacular Surgery Trials Pilot Study Investigators. Responsiveness of the SF-36 Health Survey to changes in visual acuity among patients with subfoveal choroidal neovascularization. Am J Ophthalmol. 2004;137:373–5. doi: 10.1016/S0002-9394(03)00911-5. [DOI] [PubMed] [Google Scholar]
- 22.Miskala PH, Bressler NM, Meinert CL. Relative contributions of reduced vision and general health to NEI-VFQ scores in patients with neovascular age-related macular degeneration. Arch Ophthalmol. 2004;122:758–66. doi: 10.1001/archopht.122.5.758. [DOI] [PubMed] [Google Scholar]
- 23.Miskala PH, Bressler NM, Meinert CL. Is adjustment of National Eye Institute Visual Function Questionnaire scores for general health necessary in randomized trials? Am J Ophthalmol. 2004;137:961–3. doi: 10.1016/j.ajo.2003.11.018. [DOI] [PubMed] [Google Scholar]


