Abstract
Purpose
To present best-corrected visual acuity (BCVA) findings and other clinical outcomes from eyes of patients enrolled in one of the Submacular Surgery Trials (SST) evaluating surgical removal versus observation of predominantly hemorrhagic subfoveal choroidal neovascularization (CNV) associated with age-related macular degeneration.
Design
Randomized clinical trial (SST Group B Trial).
Participants
Eligible patients had subfoveal choroidal neovascular lesions greater than 3.5 disk areas (8.9 mm2) composed of at least 50% blood (either blood or CNV underlying the center of the foveal avascular zone) and BCVA of 20/100 to light perception in the study eye.
Intervention
Patients were assigned randomly at time of enrollment to observation or surgical removal of blood and any associated CNV.
Main Outcome Measure
A successful outcome was defined a priori as either improvement in visual acuity (VA), no change in VA, or a decline in VA of no more than 1 line (7 letters) from baseline to the 24-month examination based on an intent-to-treat analysis.
Results
Of 336 patients enrolled, 168 were assigned to each treatment arm; treatment arms were balanced by baseline characteristics. Of 1501 expected examinations 3 months through 36 months after baseline, 1370 (91%) were performed. Loss of ≥2 lines (≥8 letters) of VA occurred in 56% of surgery eyes, versus 59% of observation eyes examined at 24 months. Although severe loss of VA was not the primary outcome of interest, surgery more often prevented such loss: 36% in the observation arm versus 21% in the surgery arm at the 24-month examination (χ2 P = 0.004). Of initially phakic eyes, the cumulative percentage that had undergone cataract surgery by 24 months was 44% in the surgery arm, compared with 6% in the observation arm. Twenty-seven eyes (16%) in the surgical arm, compared with 3 eyes (2%) in the observation arm, had a rhegmatogenous retinal detachment (RD).
Conclusions
Submacular surgery as performed in the SST Group B Trial did not increase the chance of stable or improved VA (the primary outcome of interest) and was associated with a high risk of rhegmatogenous RD, but did reduce the risk of severe VA loss in comparison with observation.
Projections made in 2000 estimate that by 2005 the advanced stage of age-related macular degeneration (AMD) will develop in 1 million to 1.3 million people in the United States 55 years and older, with the choroidal neovascular form of AMD developing in about two thirds of these individuals.1 Some of the choroidal neovascularization (CNV) from AMD will result in predominantly hemorrhagic lesions.2,3 Reports from retrospective case series suggest that visual acuity (VA) outcomes in eyes with subfoveal hemorrhage are often poor without treatment,4–6 especially when the hemorrhage is relatively thick.7 Large randomized clinical trials of laser photocoagulation,8,9 photodynamic therapy with verteporfin (Visudyne, Novartis Pharma AG, Basel, Switzerland),10–13 and antiangiogenic drugs have not included predominantly hemorrhagic lesions.
At least 3 surgical treatment approaches have been considered in the management of submacular hemorrhage. Pneumatic displacement of subretinal hemorrhage with or without intravitreal tissue plasminogen activator (tPA) has been undertaken to move the blood to a nonfoveal (usually inferior) location.14–17 Successful displacement of the blood may uncover CNV that then may be treated by laser photocoagulation, photodynamic therapy, other locally delivered pharmacological treatments, or macular translocation. Intraoperative subretinal18–20 or preoperative intravitreal21 injection of tPA followed by surgical drainage or removal or displacement of liquefied blood has been another approach. Variable results of surgical removal of the clot (with or without subretinal tPA) and, presumably, any CNV within the clot also have been reported from some case series.22–27 All series involved relatively few patients, with variable, often short follow-up periods after the intervention. In addition, these series usually evaluated treatment of relatively small hemorrhagic lesions and included some lesions that had subfoveal hemorrhage but were not necessarily predominantly hemorrhagic lesions, with short intervals (typically <2 weeks) between sudden vision loss from hemorrhage and intervention. None of these series had a concurrent control group for comparison of outcomes. These limitations make it difficult to translate the results of these case series to treatment recommendations and to depict accurately the benefits and risks of these procedures for patients presenting with predominantly hemorrhagic lesions from AMD.
Since 1997, the Submacular Surgery Trials (SST) Research Group has been conducting 3 randomized trials of surgical removal of subfoveal choroidal neovascular lesions to evaluate the role of this procedure in the treatment of patients with AMD and other conditions.28 In a randomized pilot study of 93 predominantly hemorrhagic subfoveal lesions, changes in VA favored surgery, with a mean loss in VA of 3.7 lines in the observation arm but only 1.1 lines in the surgery arm among 63 eyes followed for 24 months. More eyes in the surgery arm than in the observation arm had improvements in VA of ≥2 lines from baseline.
One of the randomized clinical trials subsequently conducted by the SST Research Group was for patients who had predominantly hemorrhagic subfoveal choroidal neo-vascular lesions associated with AMD (SST Group B [blood] Trial). The SST Planning Committee hypothesized that removal of the blood with associated CNV from eyes with predominantly hemorrhagic choroidal neovascular lesions would halt, minimize, or delay damage to the macula from the CNV and hemorrhage, leaving smaller areas of damage and, thus, better VA than if left untreated. The committee chose not to limit the surgery to removal of liquefied blood only because natural history studies suggested that, as the blood cleared, residual CNV and associated fibrosis often were visible and portended a poor VA outcome. Furthermore, benefits of treatments, such as photodynamic therapy, of the residual neovascular lesion were not considered at that time. Similarly, the committee chose not to limit the trial to relatively recent (1 or 2 week) hemorrhages or relatively small hemorrhagic lesions because the Planning Committee recognized that many predominantly hemorrhagic lesions presenting to an ophthalmologist did not have these features. Furthermore, the suggestion that hemorrhage duration of >7 days might result in a poorer outcome than a shorter duration was derived from only 24 surgically treated patients, 10 of whom were observed <12 months postoperatively.18 Potential confounding factors known to affect VA outcomes, such as initial lesion size or VA, could not be evaluated in that small case series.
The purpose of this report is to present ophthalmic outcomes from the SST Group B Trial. A separate report presents findings regarding general and vision-targeted health-related quality of life for the same group of patients.29 The findings from other trials conducted by the SST Research Group for patients with subfoveal CNV secondary to AMD (SST Group N Trial30,31) and the ocular histoplasmosis syndrome or idiopathic CNV (SST Group H Trial32,33) have been reported separately.
Materials and Methods
The members of an independent Data and Safety Monitoring Committee (DSMC), appointed by the Director of the National Eye Institute (Bethesda, Maryland), reviewed findings from the pilot study for patients similar to those enrolled in the SST Group B Trial. Based on these findings and those of other pilot trials of submacular surgery performed in parallel, the DSMC approved the SST design and methods before initiation of the Group B Trial in July 1998. They hypothesized that, although surgery might not prove useful for the broad range of patients eligible to be enrolled in this trial, the clinical trial should permit identification of subgroups expected to benefit from surgery. Institutional review boards at all participating institutions reviewed and approved the study design and the consent forms to be used locally. All patients gave signed consent before enrollment and random treatment assignment.
The SST Manual of Procedures,34 the SST Forms Book,35 and a report on the ophthalmic findings from the SST Group N Trial30 provide detailed information regarding study design, methods, and policies. Only those pertinent to this report are summarized here.
Patient Eligibility and Enrollment
Eligible patients were identified from referrals to 24 of the 27 clinical centers that participated in the SST.36 Participating ophthalmologists identified patients with eyes that had predominantly hemorrhagic subfoveal lesions (Fig 1) in which at least 50% of the choroidal neovascular lesion was occupied by blood thick enough to limit the examining ophthalmologist’s ability to determine whether the area of blood also was occupied by CNV, using definitions previously published.2,3 SST-certified vision examiners measured best-corrected VA (BCVA),37 contrast threshold,38 and reading speed8,34 of each eye. The details of VA measurements and scoring are described elsewhere.30 Stereoscopic color photographs and a film-based fluorescein angiogram of the disc and macula of each eye were taken by SST-certified photographers applying a standard protocol to document eligibility and baseline status for comparison with photographs taken during follow-up examinations.
Figure 1.
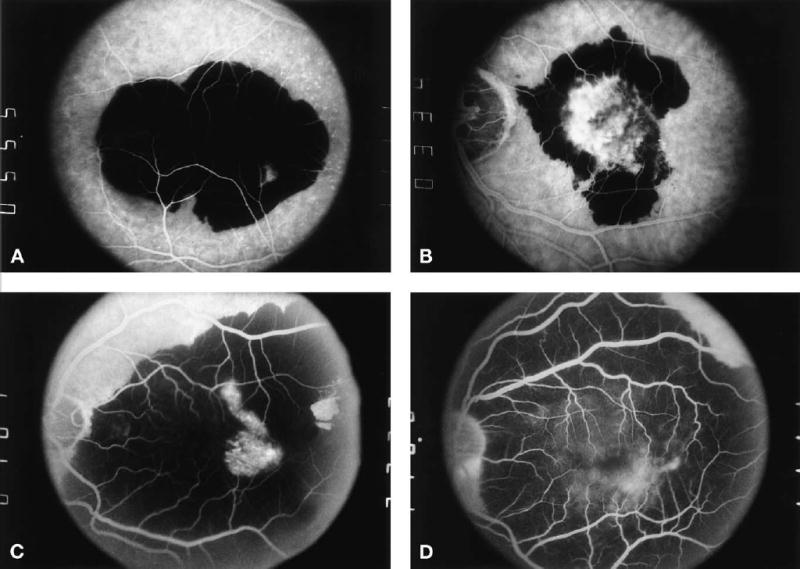
A, Midphase frame from fluorescein angiogram showing an example of a predominantly hemorrhagic lesion (i.e., the area of blood that precludes the ability to determine if fluorescence from choroidal neovascularization [CNV] in that area is at least 50% of the area of the entire lesion), in which the hemorrhage extends under the geometric center of the foveal avascular zone. B, Midphase frame from fluorescein angiogram showing an example of a predominantly hemorrhagic lesion with fluorescence from CNV under the geometric center of the foveal avascular zone. C, Midphase frame from fluorescein angiogram showing an example of a large (>16 disc areas) predominantly hemorrhagic lesion. D, Midphase frame from fluorescein angiogram showing a predominantly hemorrhagic lesion that extends outside of the posterior pole.
To be eligible for enrollment and random treatment assignment in the Group B Trial, the patient had to be 50 years or older. Previous treatment of an extrafoveal or juxtafoveal choroidal neovascular lesion with laser photocoagulation was permitted in otherwise eligible eyes. Either hemorrhage (Fig 1A) or CNV (Fig 1B) had to extend under the geometric center of the foveal avascular zone. As much as 49% and as little as 0% of the lesion could be areas of classic or occult CNV. In all cases, the study ophthalmologist had to be confident that the subretinal hemorrhage was not from other causes such as a macroaneurysm. The total area of any classic and occult CNV visible on the fluorescein angiogram (limited to fields centered on the disc and macula) could be no greater than 9 disc areas (22.9 mm2 on the retina), even though the entire lesion, including all areas of blood considered components of the lesion, had no size limit as long as the entire lesion was larger than 3.5 disc areas (8.9 mm2) and at least 75% of the blood was posterior to the equator. Eligible eyes had BCVA of 20/100 or worse but at least light perception (LP). Visual acuity of the nonstudy eye had to be LP or better. Patients who had evidence of other progressive ocular disease in either eye that could affect VA or assessment of other ophthalmic outcomes were ineligible. In particular, patients who had evidence of angioid streaks, pathologic myopia, or other conditions in either eye that could have accounted for CNV in the eye considered for enrollment were ineligible for the SST Group B Trial. Use of aspirin, warfarin (Coumadin, Bristol-Myers Squibb, Princeton, NJ), or other medications for treatment of systemic conditions did not exclude a patient from participating in the trial.
Study Entry and Randomization
Patients judged eligible by an SST-certified ophthalmologist were invited to participate as described elsewhere,30 with only one eye (study eye) included per patient. After giving written consent, study patients were randomized to surgery or observation by personnel at the SST Coordinating Center (Baltimore, Maryland), as described elsewhere.30 Random assignments were stratified by the VA of the study eye (better than 20/200, or 20/200 or worse) and by clinical center. Surgery was scheduled as soon as possible (but no more than 8 days after randomization) for each patient assigned to that treatment arm. After enrollment, baseline photographs were reviewed at the SST Photograph Reading Center (Baltimore, Maryland), as described elsewhere,39 to provide a final judgment regarding eligibility and to record baseline characteristics of the eyes and the subfoveal lesion.
Masking
All baseline measurements were obtained before randomization. Vision examinations at 24 months, and at 36 and 48 months when feasible, were conducted by traveling vision examiners. Traveling vision examiners were masked to the clinical trial in which the patient was enrolled, the treatment assigned or received, and the study eye. Study patients, coordinators, and ophthalmologists were not masked to treatment assignment, as the risks of intraocular sham surgery were judged not to be warranted. Personnel at the Photograph Reading Center were masked to the treatment assignment when evaluating baseline photographs but not when evaluating follow-up photographs.
Treatment, Follow-up Examinations, and Adverse Events
Whenever surgery could not be performed within 8 days after enrollment, a fluorescein angiogram taken no more than 8 days before surgery was required to document preoperative status of the eye and lesion; the original angiogram at the time of enrollment was retained and used for confirmation of eligibility and baseline characteristics.
Only surgeons who met requirements established by the SST Surgery Subcommittee,34 based on the surgeon’s training, experience, and submission of a video documenting facility with the surgery protocol, were approved to perform submacular surgery in the SST. The goal of surgery in the SST Group B Trial was to remove the entire neovascular lesion, which, in addition to blood, included classic CNV, occult CNV, and scar tissue when present. The surgical protocol was identical to that used in the SST Group N Trial,30 with the following additions. At the discretion of the surgeon, 10 to 12.5 μg of recombinant tPA in 0.1 ml could be injected into the hemorrhagic lesion before removal of the blood. When there was a very large clot, more than one 0.1-ml injection could be used as needed to bathe the entire clot in tPA. If tPA was used, any liquefied blood was to be irrigated from the subretinal space no earlier than 40 minutes after tPA injection. The clot and any associated fibrovascular tissue were extracted mechanically whenever possible, based on the judgment of the surgeon. Whenever blood was encountered beneath the retinal pigment epithelium (RPE), 10 to 12.5 μg of tPA in 0.1 ml could be injected beneath the RPE at the discretion of the surgeon and an attempt made to irrigate liquefied blood no earlier than 40 minutes after injection. If more than a thin layer of blood persisted after initial removal of hemorrhage, clot, and any associated fibrovascular tissue, an additional injection of 10 to 12.5 μg of tPA in 0.1 ml could be used and irrigation repeated no earlier than 40 minutes later. When the tissue mass was too large to remove easily from the eye, the surgeon could cut it into smaller pieces. However, reasonable efforts were made to remove the entire complex, at the discretion of the surgeon, without adversely affecting the eye, so the tissue could be examined histopathologically.
When surgery was performed in an eye that had received prior nonfoveal laser photocoagulation, the surgeon attempted to remove the laser treatment scar along with any recurrent CNV. When any prior laser scar could not be extracted easily, the CNV was disconnected at the interface of the recurrent lesion with the laser treatment scar. Liquid perfluorocarbons could be used at the discretion of the SST surgeon. No endolaser photocoagulation was to be applied to the retinotomy unless it was large (>1 disc diameter). The procedure was completed with a total air–fluid exchange, followed by reinstillation of 50% fluid in phakic patients. A nonexpansile gas could be substituted for air if judged necessary by the surgeon—for example, when the retinotomy was >1 disc diameter. Details of the surgery were recorded at the time of surgery on standard forms. Patients were instructed to maintain a facedown position overnight and until the gas/air bubble was <20%.
Eyes treated surgically were examined as often as judged necessary by the surgeon during the postoperative period for care of either the study eye or the fellow eye. Patients were scheduled for a postsurgical examination and data collection at 1 month after enrollment. Patients assigned to observation were scheduled for a telephone review by the local clinical center coordinator regarding ocular status and history 1 month after enrollment. All patients were scheduled for follow-up examinations for data collection purposes at 3, 6, 12, and 24 months after enrollment. Patients who enrolled by September 2000 were scheduled for a 36-month examination, and those who enrolled by September 1999 also were scheduled for a 48-month examination. Telephone reviews were conducted at the midpoint between scheduled study examinations. The last study examinations for all patients who had not completed a 48-month examination were performed during the final year of patient follow-up, which began on October 1, 2002.
Eyes in the surgery arm were considered for additional treatment whenever fluorescein leakage from CNV was observed by the ophthalmologist either beyond the area of RPE disturbance after surgery (recurrent CNV) or within this area. The protocol for subsequent treatments originally specified laser photocoagulation or surgery for these areas, depending on the location of the CNV causing the fluorescein leakage at follow-up. Beginning in April 2000, photodynamic therapy with verteporfin also could be used to treat CNV during follow-up. Eyes in the observation arm could receive laser photocoagulation or, after April 2000, photodynamic therapy with verteporfin during follow-up if, as blood cleared, a lesion was noted for which one of these treatments was judged to be beneficial, compared with no treatment.2,3,8–13 For study eyes in each arm, cataract surgery was recommended for consideration whenever the ophthalmologist judged that lenticular opacity was in a location and dense enough to cause a loss of ≥2 lines of BCVA in an otherwise healthy phakic eye.
Adverse events were reported to the Coordinating Center and managed as judged appropriate by the local ophthalmologist. At the request of the DSMC, a detailed review of all cases of retinal detachment (RD) was initiated by the Adverse Events Review Subcommittee in March 2002 to investigate this particular complication more completely. Based on review of detailed case histories provided by the ophthalmologists, the subcommittee classified the detachments as rhegmatogenous or nonrhegmatogenous and, when rhegmatogenous, determined whether proliferative vitreoretinopathy had developed. The most recent VA and anatomic outcome were summarized.
Statistical Design and Study Monitoring
The primary successful outcome of interest was improvement or stabilization of BCVA, defined as loss of no more than 1 line (7 letters) from baseline, at the 24-month examination, as long as the baseline BCVA was 20/1600 or better. For eyes with VA worse than 20/1600, a level at which VA was not measurable by SST methods, a successful outcome was defined as improvement to measurable VA (i.e., improvement to VA of 20/1600 or better). Data from the pilot study of patients similar to those eligible for the Group B Trial bracketed the rate of successful outcomes in observed eyes to be 40% to 56%. Sample size was calculated during the planning phase under the assumption that 48% of eyes assigned to observation would have a successful outcome as defined above. The minimum clinically meaningful relative improvement in success rates after surgery, taking account of the cost and inconvenience of surgery, was judged by the members of the Planning Committee to be 50% (i.e., a successful outcome in ≥72% of surgically treated eyes). A conservative type I error (α, 2 tailed) equal to 0.01 and a type II error (β) of 0.10 were chosen. Projecting a loss of outcome information at the 24-month examination due to deaths and missed examinations of 20% (based on experience in the pilot study and other trials involving patients with AMD), a target sample size of 360 patients (360 eyes) was calculated. Although the 24-month examination was deemed the one at which the primary outcome would be assessed and compared between treatment arms, examinations at 36 and 48 months were specified to document whether any treatment effect persisted.
The definition of success adopted by the SST Planning Committee was discussed by the DSMC at the time the Group B Trial was initiated. The committee members agreed that VA outcomes defined as beneficial in other clinical trials of treatments for AMD, such as prevention of 3-line1,10,11 or 6-line8,9 losses of VA from baseline, were appropriate when eligible patients had relatively good (e.g., better than 20/200) baseline VA, but not when baseline VA could be as poor as LP. Thus, these outcomes were considered within the better baseline VA stratum during interim monitoring along with the primary outcome specified in the design assumptions.
Monitoring procedures were as described for the SST Group N Trial.30 At the August 22, 2001 DSMC meeting, with 322 patients enrolled in the SST Group B Trial, the committee recommended that SST accrual halt on September 30, 2001, and that the last patient enrolled be observed for 3 years. Subsequently, due to funding constraints, the follow-up phase of the trial was curtailed so that a minimum of only 2 years of follow-up was permitted for each patient. Thus, patient follow-up continued through September 30, 2003.
Data Analysis and Statistical Methods
Data from masked vision examinations by traveling vision examiners were used in analyses whenever available; otherwise, measurements by local examiners were analyzed. For analysis of change in BCVA, eyes with VA worse than 20/1600 but with at least LP were coded as having VA 3 lines worse than 20/1600. When VA was reported to be no LP, the score assigned was 7 lines worse than the poorest score measurable at 0.5 m (i.e., 20/1600). The statistical methods and tests have been described elsewhere.30,40–43 Risk factors for rhegmatogenous RD were explored using recursive partitioning44 and multivariable logistic regression analyses.45 All pertinent data from each patient received at the SST Coordinating Center by October 31, 2003 were analyzed with the treatment arm to which the patient was assigned randomly at the time of enrollment (intent-to-treat approach). P values were not adjusted for multiple examinations of outcome data. However, only P values of ≤0.01 were deemed statistically significant. Data were analyzed using SAS46 supplemented with custom-written programs.
Results
From July 17, 1998 until accrual ended on September 30, 2001, 336 patients (336 eyes) enrolled in the SST Group B Trial; 168 were assigned to the surgery arm and 168 to the observation arm. Based on central review of baseline photographs, 38 eyes (11%) were judged not to meet 1 or more of the strict criteria for eligibility. Data for ineligible patients were analyzed with data from all other patients in the treatment arm to which they were assigned.
Characteristics of Patients and Eyes at Study Entry
Characteristics of patients, eyes, and subfoveal lesions at time of enrollment are summarized by treatment arm and VA stratum (Tables 1 [online only, available at http://www.ophsource.com/periodicals/ophtha], 2). Treatment arms were well balanced on all characteristics shown, both overall and within baseline VA strata (Table 1, available at http://www.ophsource.com/periodicals/ophtha). The median age of patients was 79 years (range: 57–94). Twenty-nine (17%) of the observation patients and 43 (26%) of the surgery patients had used warfarin; of these 72 patients, 8 of the observation patients and 22 of the surgery patients had used warfarin within 4 weeks of enrollment. Fifty-five patients (33%) in the observation arm took ≥7 aspirin per week, compared with 54 (32%) in the surgery arm. Only 18 (11%) and 15 (9%) of the patients in the observation and surgery arms, respectively, were current smokers, although an additional 83 (49%) and 84 (50%), respectively, had quit smoking. Comorbidities were common, with >90% reporting at least 1 comorbid condition in addition to AMD.47
Table 2.
Status of Eyes at Time of Patient Enrollment in the SST Group B Trial
|
No. (%) of Eyes |
||
|---|---|---|
| Observation (n = 168*) | Surgery (n = 168) | |
| Study eyes | ||
| Visible CNV | ||
| Classic only | 32 (19) | 30 (18) |
| Classic and occult | 48 (29) | 57 (34) |
| Occult only | 50 (30) | 52 (31) |
| None | 38 (23) | 29 (17) |
| CNV in FAZ center | ||
| No | 97 (58) | 94 (56) |
| Yes | 65 (39) | 72 (43) |
| Indeterminate | 5 (3) | 2 (1) |
| Blood > 50% of total lesion | ||
| Yes | 151 (90) | 157 (93) |
| No | 10 (6) | 9 (5) |
| Indeterminate | 6 (4) | 2 (1) |
| Total size of subfoveal lesion (MPS DAs) | ||
| ≤6 | 20 (12) | 21 (12) |
| >6 to ≤9 | 24 (14) | 21 (12) |
| >9 to ≤12 | 23 (14) | 20 (12) |
| >12 to ≤16 | 26 (16) | 25 (15) |
| >16 | 67 (40) | 79 (47) |
| Indeterminate | 7 (4) | 2 (1) |
| Previous laser photocoagulation | ||
| No | 143 (86) | 150 (89) |
| Possible | 11 (7) | 6 (4) |
| Yes | 13 (8) | 12 (7) |
| Lens status | ||
| Phakic | 99 (59) | 97 (58) |
| Pseudophakic or aphakic | 69 (41) | 71 (42) |
| Etiology of neovascular lesion | ||
| Age-related macular degeneration | 163 (98) | 163 (97) |
| Possible ocular histoplasmosis | 0 | 2 (1) |
| Idiopathic or other | 1 (1) | 1 (1) |
| Indeterminate | 3 (2) | 2 (1) |
| Fellow eyes | ||
| Most advanced lesion | ||
| Choroidal neovascular lesion | 74 (44) | 73 (43) |
| Geographic atrophy | 16 (10) | 15 (9) |
| Drusen | 68 (41) | 69 (41) |
| Indeterminate | 8 (5) | 11 (7) |
| None | 1 (1) | 0 |
| Visual acuity | ||
| ≥20/20 | 31 (18) | 24 (14) |
| 20/25–20/40 | 45 (27) | 62 (37) |
| 20/50–20/80 | 17 (10) | 23 (14) |
| 20/100–20/160 | 11 (7) | 14 (8) |
| 20/200–20/320 | 20 (12) | 15 (9) |
| 20/400–20/640 | 19 (11) | 10 (6) |
| 20/800–20/1250 | 15 (9) | 14 (8) |
| ≤20/1600–LP | 10 (6) | 6 (4) |
CNV = choroidal neovascularization; FAZ = foveal avascular zone; LP = light perception; MPS DAs = disc areas (by definitions adapted from a publication by the Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration; guidelines for evaluation and treatment in the Macular Photocoagulation Study. Arch Ophthalmol 1991;109:1242–57). One MPS DA = 2.5 mm2.
Baseline photographs missing for one patient in observation arm.
Treatment arms also were balanced on characteristics of study eyes and fellow eyes (Table 2). Median BCVA scores in the study eye and fellow eye were 48 (20/200) and 77 (20/64), respectively, in the observation arm, compared with 46 (20/250) and 84 (20/40), respectively, in the surgery arm. The subfoveal lesion was larger than 16 Macular Photocoagulation Study disc areas in 67 eyes (40%) in the observation arm and 79 eyes (47%) in the surgery arm. Almost half the patients had advanced AMD in both eyes at time of enrollment.
Surgery Protocol Variations and Intraoperative Complications
Two patients assigned to the observation arm had surgery in the study eye by a non-SST ophthalmologist shortly after enrollment, 3 patients assigned to the surgery arm refused surgery after enrollment, and 6 other patients (4%) in the surgery arm had surgery > 8 days after enrollment.
Recombinant tPA was injected in 62 eyes (38%). Additional procedures judged necessary by the surgeons included use of perfluorocarbon in 10 eyes (6%), endolaser to a retinotomy in 23 eyes (14%), leaving an air bubble that filled >50% of the vitreous cavity in 25 phakic eyes (15%), and use of a gas bubble rather than air in an additional 49 eyes (30%). In only 4 eyes did the surgeon believe that any portion of CNV, a laser treatment scar, or both remained in the eye at the conclusion of the surgical procedure. However, the surgeon noted subretinal or sub-RPE blood remaining in 154 eyes (93%), including 42 (25%) with >4 disc areas of blood; in 26 of these 42 eyes, the blood extended under the center of the fovea. In 15 (9%) of the 168 eyes assigned to surgery, the surgeon judged that blood remaining after surgery was thick enough to elevate the retina.
Intraoperative complications resulting in additional procedures included 36 eyes (22%) with retinal tears treated with cryopexy or laser and 17 eyes (10%) with an area judged at risk for a peripheral tear also treated with cryopexy or laser. A scleral buckle was placed intraoperatively on 1 eye (1%). An additional 12 eyes were reported to have retinal tears observed intraoperatively within the posterior pole (including 6 involving the fovea) or peripherally that were not treated. No intraoperative choroidal hemorrhages were reported.
Completion of Scheduled Examinations
Twenty-six patients (15%) in the observation arm died after enrollment; 14 of these deaths occurred before the 24-month examination could be completed. For the surgery arm, 16 patients (10%) died after enrollment, including 9 before the 24-month examination. The number of patients expected to be examined at each scheduled time after enrollment, based on date of enrollment and vital status, is shown in Table 3. Of 754 examinations scheduled for patients in the surgery arm at 3 months through 36 months after enrollment, 706 (94%) were completed. Patients in the observation arm returned for 664 (89%) of 747 scheduled examinations through 36 months. Follow-up examination rates were consistently somewhat lower in the observation arm beginning at the 3-month examination, although the differences in the expected number of examinations completed never exceeded 6% after the 3-month examination.
Table 3.
Number (%) of Patients Examined at Each Scheduled Examination Time in the SST Group B Trial
|
By Treatment Arm |
By Baseline VA Stratum |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Observation |
Surgery |
VA> 20/200 |
VA≤ 20/200 |
|||||
| Months SinceEnrollment | No. Expected* | No. (%) Examined | No. Expected* | No. (%) Examined | No. Expected* | No. (%) Examined | No Expected* | No. (% ) Examined |
| 0 | 168 | 168 (100) | 168 | 168 (100) | 129 | 129 (100) | 207 | 207 (100) |
| 1 | — | — | 168 | 162 (96) | 61 | 60 (98) | 107 | 102 (95) |
| 3 | 168 | 151 (90) | 167 | 163 (98) | 129 | 125 (97) | 206 | 189 (92) |
| 6 | 165 | 147 (89) | 167 | 154 (92) | 128 | 123 (96) | 204 | 178 (87) |
| 12 | 160 | 139 (87) | 163 | 152 (93) | 125 | 118 (94) | 198 | 173 (87) |
| 24 | 154 | 140 (91) | 159 | 151 (95) | 122 | 116 (95) | 191 | 175 (92) |
| 36 | 100 | 87 (87) | 98 | 86 (88) | 75 | 67 (89) | 123 | 106 (86) |
| 48 | 45 | 39 (87) | 36 | 30 (83) | 29 | 24 (83) | 52 | 45 (87) |
VA = visual acuity.
Based on date of enrollment, vital status, and data analysis date.
Best-Corrected Visual Acuity and Changes in It
The distributions of BCVA of study eyes at the baseline, 3-month, and annual examinations through the 36-month examinations are shown in Table 4. Only 15 (11%) of 140 eyes in the observation arm and 14 (9%) of 150 eyes in the surgery arm had BCVA better than 20/200 at the 24-month examination, in contrast to 38% of eyes at baseline. At the other end of the distribution, 56 eyes (40%) in the observation arm and 46 eyes (31%) in the surgery arm had BCVA of 20/800 or worse at the 24-month examination, even though only 18% of eyes had this level of VA at baseline. Three eyes (2%) in each arm had no LP at the 24-month examination. One additional patient in the observation arm was reported to have no LP in the study eye when examined by a non-SST ophthalmologist 6 months after enrollment; this patient died without any later examination by an SST examiner.
Table 4.
Distribution of Patients by Visual Acuity (VA) of the Study Eye When Examined at Baseline and at Specified Times after Enrollment in the SST Group B Trial
|
0 (Baseline) |
3 Months |
12 Months* |
24 Months |
36 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VA (Snellen Equivalent) | Observation (n = 168) | Surgery (n = 168) | Observation (n = 151) | Surgery(n = 163) | Observation (n = 139) | Surgery (n = 152) | Observation (n = 140†) | Surgery (n = 150‡) | Observation (n = 87) | Surgery (n = 86) |
| ≥20/40 | — | — | 0 | 2 (1) | 2 (1) | 4 (3) | 2 (1) | 4 (3) | 0 | 4 (5) |
| 20/50–20/80 | — | — | 8 (5) | 6 (4) | 3 (2) | 2 (1) | 6 (4) | 0 | 0 | 1 (1) |
| 20/100–20/160 | 67 (40) | 61 (36) | 22 (15) | 10 (6) | 14 (10) | 11 (7) | 7 (5) | 10 (7) | 7 (8) | 7 (8) |
| 20/200–20/320 | 38 (23) | 47 (28) | 36 (24) | 42 (26) | 28 (20) | 46 (30) | 24 (17) | 43 (29) | 15 (17) | 26 (30) |
| 20/400–20/640 | 35 (21) | 27 (16) | 36 (24) | 54 (33) | 41 (29) | 41 (27) | 45 (32) | 47 (31) | 25 (29) | 15 (17) |
| 20/800–20/1280 | 20 (12) | 18 (11) | 27 (18) | 26 (16) | 31 (22) | 26 (17) | 40 (29) | 24 (16) | 25 (29) | 17 (20) |
| ≤20/1600–LP | 8 (5) | 15 (9) | 22 (15) | 22 (13) | 20 (14) | 19 (12) | 13 (9) | 19 (13) | 14 (16) | 15 (17) |
| NLP | — | — | 0 | 1 (1) | 0 | 3 (2) | 3 (2) | 3 (2) | 1 (1) | 1 (1) |
| Median VA | 20/200 | 20/250 | 20/500 | 20/400 | 20/500 | 20/400 | 20/500 | 20/400 | 20/640 | 20/500 |
LP = light perception; NLP = no LP.
Number (%) of study eyes, by time after enrollment.
Six-month measurements omitted.
Measurements of VA were made by masked examiners for 124 eyes (89%).
Measurements of VA were made by masked examiners for 135 eyes (90%).
Distributions of changes in BCVA from baseline are shown in Table 5. For the primary outcome, 57 (41%) of 140 observation eyes, compared with 66 (44%) of 150 surgery eyes (Fisher exact test P = 0.63), had improvement or stabilization of BCVA at the 24-month examination. Only 25 (18%) of 140 observation eyes and 27 (18%) of 150 surgery eyes had BCVA at the 24-month examination that was ≥2 lines better than at baseline; however, at that time, 51 observation eyes (36%) and 31 surgery eyes (21%) had lost ≥6 lines of BCVA from baseline (Fisher exact test P = 0.004). Median loss from baseline VA in the surgery arm remained close to 2 lines from 3 months through 24 months, whereas the median loss in the observation arm increased from 1.2 lines at 3 months to 3.2 lines at 24 months.
Table 5.
Distribution of Patients by Change in Visual Acuity of the Study Eye from Baseline to Examinations at Specified Times after Enrollment, SST Group B Trial
|
3 Months |
6 Months |
12 Months |
24 Months |
36 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change in Visual Acuity (Lines) | Observation (n = 151) | Surgery (n = 163) | Observation (n = 147) | Surgery (n = 154) | Observation (n = 139) | Surgery (n = 152) | Observation (n = 140) | Surgery (n = 150) | Observation (n = 87) | Surgery (n = 86) |
| ≥4 better | 11 (7) | 16 (10) | 15 (10) | 17 (11) | 11 (8) | 14 (9) | 14 (10) | 16 (11) | 6 (7) | 10 (12) |
| 2–3 better | 21 (14) | 15 (9) | 18 (12) | 13 (8) | 15 (11) | 17 (11) | 11 (8) | 11 (7) | 10 (11) | 7 (8) |
| <2-line change* | 46 (30) | 40 (25) | 37 (25) | 45 (29) | 31 (22) | 38 (25) | 32 (23) | 39 (26) | 15 (17) | 25 (29) |
| 2–3 worse | 21 (14) | 36 (22) | 17 (12) | 30 (19) | 22 (16) | 30 (20) | 16 (11) | 33 (22) | 16 (18) | 17 (20) |
| 4–5 worse | 19 (13) | 28 (17) | 18 (12) | 23 (15) | 24 (17) | 23 (15) | 16 (11) | 20 (13) | 12 (14) | 9 (10) |
| 6–7 worse | 12 (8) | 17 (10) | 14 (10) | 13 (8) | 12 (9) | 14 (9) | 26 (19) | 11 (7) | 10 (11) | 7 (8) |
| ≥8 worse | 21 (14) | 11 (7) | 28 (19) | 13 (8) | 24 (17) | 16 (11) | 25 (18) | 20 (13) | 18 (21) | 11 (13) |
| Median change | −1.2 | −2.2 | −1.6 | −1.6 | −3.0 | −1.8 | −3.2 | −2.0 | −3.2 | −1.7 |
Number (%) of patients, by time after enrollment.
This row includes 2 patients in each arm at mos 3 and 6, in the observation arm at mo 24, and in the surgery arm at mo 36; 1 patient in the observation arm at mos 12 and 36 and in the surgery arm at mo 24; and 3 patients in the surgery arm at mo 12 who had no change from light perception.
Figures 2 and 3 display the percentages of study eyes with BCVA at each follow-up examination of 2 lines (8 letters) or more worse than at baseline (failures) and 6 lines (28 letters) or more worse than at baseline, respectively, after taking into account both losses and recoveries of VA and all measurements available for each eye. By the 24-month examination, 60% of the observation eyes, compared with 56% of the surgery eyes, had lost ≥2 lines of BCVA from baseline. In contrast, although a majority and similar proportion of eyes in both the observation and surgery arm lost ≥2 lines of VA from baseline, Figure 3 shows that, by the 24-month examination, 36% of the observation eyes, compared with 20% of the surgery eyes, lost ≥6 lines of VA from baseline.
Figure 2.
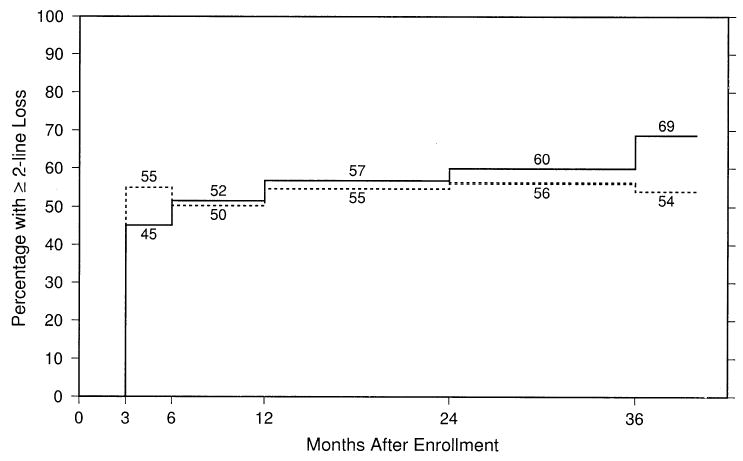
Estimated percentages of study eyes with best-corrected visual acuity at each follow-up examination 2 lines (8 letters) or more worse than at baseline, by treatment arm. Solid line, observation eyes; dashed line, surgery eyes.
Figure 3.
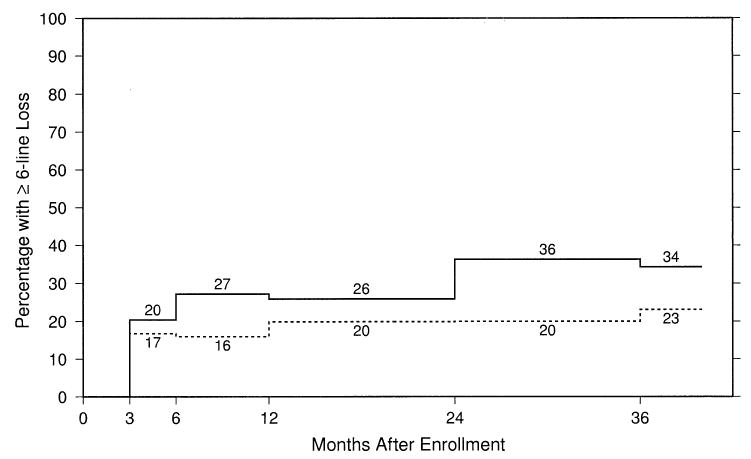
Estimated percentages of study eyes with best-corrected visual acuity at each follow-up examination 6 lines (28 letters) or more worse than at baseline by treatment arm. Solid line, observation eyes; dashed line, surgery eyes.
Outcomes by VA stratum (20/100–20/160, 20/200–LP) de-fined at time of enrollment were evaluated. Eyes in both arms in the better VA stratum showed negligible successes (stabilization or improvement by 24 months): 10 eyes (17%) and 9 eyes (16%) in the observation and surgery arms, respectively. The percentage of patients with a ≥2-line loss in VA (failures) was ≥80% in both arms from 12 months on for the subgroup of patients in the better VA stratum. However, the percentage with ≥6 lines of VA loss in the better VA stratum paralleled the findings for the entire Group B Trial. Specifically, 38 (64%) of 59 observation eyes, compared with 23 (41%) of 56 surgery eyes, lost ≥6 lines of VA from baseline at the 24-month examination. By this time, median losses of VA were 6.4 lines and 4.8 lines in the observation and surgery arms, respectively. Differences between treatment arms for ≥6-line loss were not as large within the subgroup of the lower VA stratum in which a ≥6-line loss could be measured (baseline VA of 20/200–20/400). Specifically, 10 (22%) of 46 observation eyes, compared with 7 (13%) of 52 surgery eyes, lost ≥6 lines of VA from baseline, with a median loss of VA of 2.6 and 1.4 in the observation and surgery arms, respectively. For eyes at baseline with baseline VA of 20/500 to LP, where a ≥6-line loss could not be measured, differences between the observation and surgery arms were even smaller with respect to median change in VA from baseline to the 24-month examination—specifically, no change (0 lines) in the observation arm and a gain of 0.6 lines in the surgery arm.
Findings also were compared between treatment arms in >50 other patient subgroups defined by baseline characteristics. Comparisons by gender and treatment for VA outcomes suggested that losses of ≥2 lines and ≥6 lines of BCVA favored surgery in men but favored observation in women (P = 0.004 for a ≥2-line loss and P = 0.04 for a ≥6-line loss for the surgery–gender interaction), although this difference in response to surgery could be due to chance alone, given the number of subgroups evaluated. Within the surgery arm, the use of tPA (62 eyes), compared with no tPA (103 eyes), revealed no notable difference in VA outcomes. Analysis of VA outcomes by patient’s self-report of duration of vision loss, presumed to be from the hemorrhagic lesion, showed no difference in outcomes within either the surgery or the observation arm.
Reading Speed and Contrast Threshold
Reading speed at baseline was <40 words per minute for more than two thirds of the study eyes. No differences in the distributions of changes in reading speed at baseline or during follow-up were noted in the observation arm relative to the surgery arm (data not shown). In addition, there was little difference in the median change in reading speed (Table 6), with reading speeds in most observation and surgery eyes remaining <40 words per minute throughout follow-up.
Table 6.
Distribution of Patients by Change in Reading Speed from Baseline in the Study Eye to Examinations at Specified Times after Enrollment in the SST Group B Trial
|
3 Months |
6 Months |
12 Months |
24 Months |
36 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change (wpm) | Observation (n = 144) | Surgery (n = 162) | Observation (n = 141) | Surgery (n = 151) | Observation (n = 135) | Surgery (n = 149) | Observation (n = 132) | Surgery (n = 144) | Observation (n = 78) | Surgery (n = 82) |
| >30 faster | 11 (8) | 17 (10) | 15 (11) | 13 (9) | 11 (8) | 17 (11) | 10 (8) | 10 (7) | 5 (6) | 5 (6) |
| 11–30 faster | 16 (11) | 22 (14) | 17 (12) | 19 (13) | 10 (7) | 16 (11) | 11 (8) | 20 (14) | 7 (9) | 13 (16) |
| ≤10 change | 70 (49) | 70 (43) | 58 (41) | 66 (44) | 59 (44) | 67 (45) | 56 (42) | 63 (44) | 31 (40) | 39 (48) |
| 11–30 slower | 26 (18) | 31 (19) | 32 (23) | 28 (19) | 30 (22) | 25 (17) | 29 (22) | 31 (22) | 20 (26) | 19 (23) |
| >30 slower | 21 (15) | 22 (14) | 19 (13) | 25 (17) | 25 (19) | 24 (16) | 26 (20) | 20 (14) | 15 (19) | 6 (7) |
| Median change | −1 | −2 | −1 | −2 | −3 | −1 | −4 | −1 | −4 | −1 |
wpm = words per minute.
Number (%) of patients, by time after enrollment.
The distributions of change in threshold contrast sensitivity (Table 7) were more favorable for surgery eyes than for observation eyes, with 54% of eyes in the surgery arm having improvement by 24 months, versus 45% of observation eyes. Median improvements at the 24-month examination were 1 contrast segment (0.15 log unit) in the surgery arm and no change in the observation arm (Wilcoxon P = 0.01). In addition, fewer surgery eyes lost contrast sensitivity (33%, vs. 45% of observation eyes).
Table 7.
Distribution of Patients by Changes in Contrast Threshold of Study Eyes from Baseline to Examinations at Specified Times after Enrollment in the SST Group B Trial
|
3 Months |
6 Months |
12 Months |
24 Months |
36 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change (No. of Segments) | Observation (n = 144) | Surgery (n = 161) | Observation (n = 141) | Surgery (n = 150) | Observation (n = 134) | Surgery (n = 147) | Observation (n = 128) | Surgery (n = 137) | Observation (n = 76) | Surgery (n = 78) |
| ≥3 improved | 12 (8) | 26 (16) | 15 (11) | 30 (20) | 22 (16) | 27 (18) | 22 (17) | 37 (27) | 15 (20) | 18 (23) |
| 1–2 improved | 38 (26) | 43 (27) | 35 (25) | 44 (29) | 30 (22) | 39 (27) | 36 (28) | 37 (27) | 18 (24) | 22 (28) |
| <1 (no change) | 28 (19) | 32 (20) | 25 (18) | 26 (17) | 23 (17) | 25 (17) | 13 (10) | 18 (13) | 7 (9) | 13 (17) |
| 1–2 worse | 40 (28) | 41 (25) | 38 (27) | 32 (21) | 31 (23) | 36 (24) | 20 (16) | 24 (18) | 16 (21) | 12 (15) |
| ≥3 worse | 26 (18) | 19 (12) | 28 (20) | 18 (12) | 28 (21) | 20 (14) | 37 (29) | 21 (15) | 20 (26) | 13 (17) |
| Median change | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
Number (%) of patients, by time after enrollment. Change of one 3-letter segment on Pelli–Robson chart = change of 0.15 log units.
Change in Size of Subfoveal Lesions
Three months after enrollment, the neovascular lesions of more than one quarter of the eyes in the observation arm and nearly half of the eyes in the surgery arm were smaller than at baseline by at least 1 size category (Table 8). Although the median change was no change in size category in both arms from baseline to most follow-up examinations, the distributions of changes in lesion size differed between treatment arms at all follow-up examinations (χ2 P < 0.001), with more and larger size decreases in the surgery arm than in the observation arm. Only 17 (14%) of 121 eyes in the surgery arm had subfoveal lesions larger at 24 months than at baseline, compared with 43 (38%) of 112 eyes in the observation arm.
Table 8.
Changes in Size of Subfoveal Lesions from Baseline to Follow-up Examinations at Specified Times after Enrollment in the SST Group B Trial
|
3 Months |
6 Months |
12 Months |
24 Months |
36 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change from Baseline (Categories) | Observation (n = 125) | Surgery (n = 148) | Observation (n = 126) | Surgery (n = 135) | Observation (n = 120) | Surgery (n = 127) | Observation (n = 112) | Surgery (n = 121) | Observation (n = 68) | Surgery (n = 66) |
| ≥1 smaller | 32 (26) | 68 (46) | 41 (33) | 70 (52) | 33 (28) | 61 (48) | 34 (30) | 54 (45) | 17 (25) | 30 (45) |
| No change | 61 (49) | 65 (44) | 49 (39) | 53 (39) | 47 (39) | 51 (40) | 35 (31) | 50 (41) | 22 (32) | 28 (42) |
| 1 larger | 20 (16) | 10 (7) | 26 (21) | 11 (8) | 24 (20) | 13 (10) | 25 (22) | 11 (9) | 15 (22) | 2 (3) |
| ≥2 larger | 12 (10) | 5 (3) | 10 (8) | 1 (1) | 16 (13) | 2 (2) | 18 (16) | 6 (5) | 14 (21) | 6 (9) |
| Median change | 0 | 0 | 0 | −1 | 0 | 0 | 0 | 0 | 0 | 0 |
Number (%) of patients, by time after enrollment.
Fluorescein Leakage from Choroidal Neovascularization at Follow-up in Surgery Eyes
By 1 month after surgery, 23% of the surgery eyes already had fluorescein leakage from CNV either within or beyond the area of RPE abnormality after surgery, as determined by the Photograph Reading Center. By the 24-month examination, a cumulative total of 55% of eyes had fluorescein leakage from CNV within or at the margin of the area of RPE abnormality at ≥1 examinations after surgery (51% at the periphery of the area of RPE abnormality after surgery and an additional 4% within the area of RPE abnormality after surgery in the absence of any leakage at the periphery of this area).
Figure 4 shows the prevalence of visible fluorescein leakage from CNV at each examination, as determined at the Photograph Reading Center, for both the observation arm and the surgery arm. Although at baseline almost all eyes in each arm had some visible fluorescein leakage from CNV, the percentage of eyes with fluorescein leakage from CNV during follow-up always was much greater in the observation arm, beginning at the 3-month examination (81% for eyes assigned to observation and 53% for eyes assigned to surgery), through the 24-month examination (78% for eyes assigned to observation and 29% for eyes assigned to surgery), and thereafter.
Figure 4.
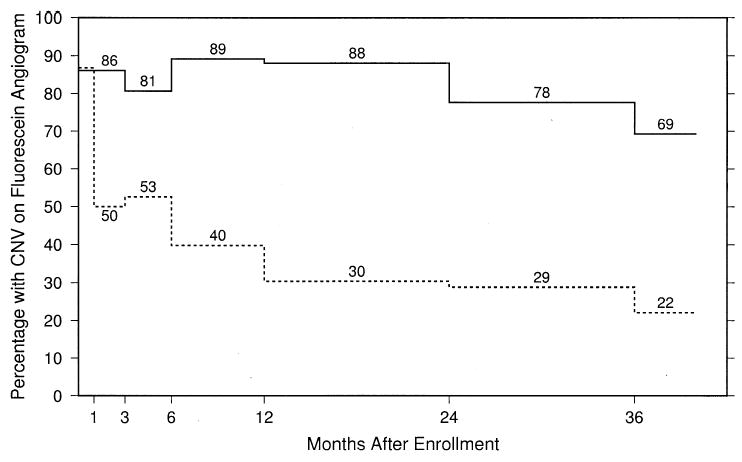
Estimated percentages of study eyes with fluorescein leakage from choroidal neovascularization (CNV) at baseline and at each follow-up examination by treatment arm. Solid line, observation eyes; dashed line, surgery eyes.
The cumulative percentage of eyes treated with laser photocoagulation, photodynamic therapy with verteporfin, or submacular surgery for fluorescein leakage from CNV during follow-up is shown in Figure 5. Only 1 patient in each treatment arm had >1 treatment. Although 55% of the surgery eyes had had fluorescein leakage from CNV observed on at least 1 set of follow-up photographs, only 19% had received additional treatment by the 24-month examination (Fig 5), either because criteria for additional treatment were not satisfied or because of decisions by the patient or surgeon. Laser photocoagulation was applied to 20 of the 32 surgery eyes re-treated; 6 surgery eyes had repeat surgery. By the 24-month examination, 12% of the observation eyes had received treatment prompted by fluorescein leakage from CNV observed as blood cleared.
Figure 5.
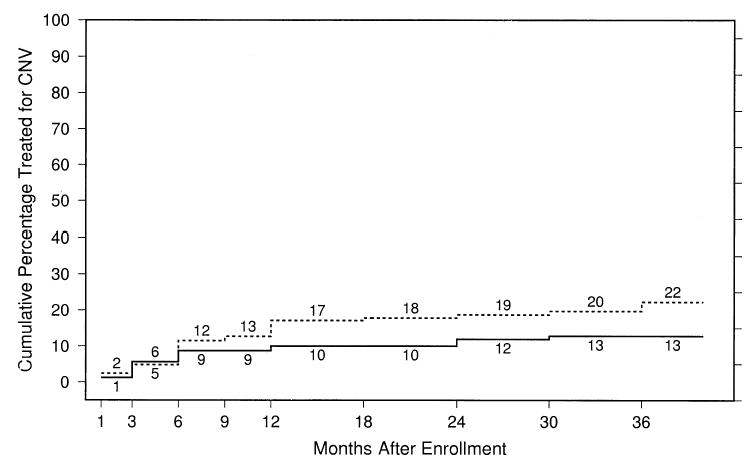
Cumulative percentage of eyes treated after identification by the ophthalmologist of fluorescein dye leakage from choroidal neovascularization (CNV) by specified follow-up examinations. Solid line, observation eyes; dashed line, surgery eyes.
Complications and Risks
A summary of adverse events by treatment arm is shown in Table 9. The cumulative percentage of study eyes that were phakic at baseline and that had cataract surgery after enrollment is shown by treatment arm in Figure 6. By the 24-month examination, the cumulative percentage of study eyes that had had cataract surgery was 6% of observation eyes, compared with 44% of surgery eyes; although these percentages increased with longer follow-up, the increase was at a faster rate in surgery eyes (e.g., nearly 25% per year). Ophthalmologists reported that lenticular opacity sufficient to reduce VA by ≥2 lines in an otherwise normal eye was present in 30 additional observation eyes and 41 additional surgery eyes that had not undergone cataract surgery by the 24-month examination. Among the 35 eyes assigned to surgery in the better VA stratum that were phakic at baseline, 14 (40%) had undergone cataract surgery. Ophthalmologists reported that lenticular opacity sufficient to reduce VA by ≥2 lines in an otherwise normal eye was present in 15 additional surgery eyes (43%) that had not undergone cataract surgery by the 24-month examination within the better VA stratum at baseline. When VA results were stratified by whether the study eye was phakic or not at baseline, the VA findings in both strata were similar to the overall findings.
Table 9.
Adverse Events Reported in Study Eyes in the Submacular Surgery Trials Group B Trial, by Treatment Arm
|
No. (%) of Patients |
||
|---|---|---|
| Adverse Event | Observation (n = 168) | Surgery (n = 168) |
| Retinal detachment | ||
| Rhegmatogenous | 3 (2) | 27 (16) |
| Nonrhegmatogenous | 3 (2) | 4 (2) |
| Vitreous hemorrhage | 28 (17) | 14 (8) |
| Retinal vessel occlusion | 0 | 1 (1) |
| Massive subretinal or choroidal hemorrhage | 7 (4) | 7 (4) |
| No light perception | 3 (2) | 3 (2) |
| Other | ||
| Choroidal detachment | 2 (1) | 1 (1) |
| Angle-closure glaucoma | 2 (1) | 1 (1) |
| Enucleation | 1 (1) | 0 |
| Macroaneurysm | 0 | 1 (1) |
| Pthisis | 0 | 1 (1) |
| Any of the above | 33 (20) | 38 (23) |
Figure 6.
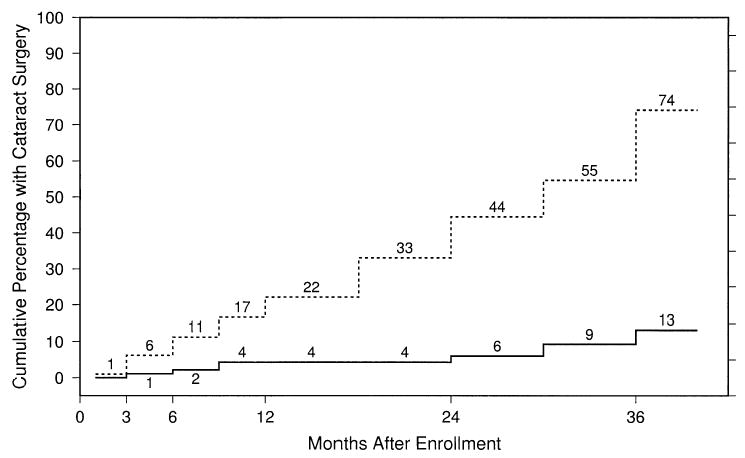
Cumulative percentage of eyes that were phakic at baseline that had cataract surgery by specified follow-up times. Solid line, observation eyes; dashed line, surgery eyes.
There were no cases of endophthalmitis; massive subretinal hemorrhages or choroidal hemorrhages occurred in 4% of the eyes in each arm. Vitreous hemorrhages were more common in the observation arm (28 cases) than in the surgery arm (14 cases), associated with a median BCVA of 20/1000 in the observation arm and 20/1600 in the surgery arm and a median loss from baseline of 5.6 lines in the observation arm and 2.3 lines in the surgery arm at the 24-month examination. However, rhegmatogenous RDs were more common in the surgery arm, occurring in 27 (16%) of 168 surgery eyes, including 14 (8%) with proliferative vitreoretinopa-thy. Among these 27 eyes with rhegmatogenous detachments, at the most recent study examination the retina was attached in 17, with VA of at least 20/1600 in 11, of less than 20/1600 but at least LP in 5, and of no LP in 1. The retina remained detached in 6, with VA of at least 20/1600 in 1 and of less than 20/1600 but at least LP in 5. The retinal status of the remaining 4 eyes was reported by eye care providers outside of the SST Research Group: VA of at least 20/1600 in 2, of counting fingers in 1, and of no LP in 1. Among the 61 eyes assigned to surgery in the better VA stratum (20/100–20/160) at baseline, 6 (10%) had a rhegmatogenous detachment.
Because of the high risk of RD and its adverse effect on VA outcomes, we evaluated likely baseline characteristics of patients, eyes, and lesions as predictors of the rhegmatogenous RDs. Recursive partitioning and logistic regression analysis indicated that the risk of rhegmatogenous detachment after surgery increased with poorer VA or larger lesion size at baseline. The same risk factors were predictive of the vitreous hemorrhages noted at follow-up, which occurred more often in observation eyes. The group at highest risk included eyes with baseline VA worse than 20/1280 or lesion size at baseline of >16 disc areas; 24 of the 30 rhegmatogenous RDs and 32 of the 42 vitreous hemorrhages occurred in the 151 eyes in the subgroup with VA worse than 20/1280 or lesion size at baseline of >16 disc areas, compared with 6 detachments and 10 vitreous hemorrhages among the other 185 study eyes.
Discussion
The SST Research Group was unable to demonstrate any benefit to submacular surgery, as performed in this study, relative to observation with respect to achieving stable or improved BCVA by the 24-month examination among eyes with predominantly hemorrhagic subfoveal choroidal neo-vascular lesions from AMD enrolled and followed in the Group B Trial. Changes in BCVA from baseline tended to favor surgery by 1 year, although never to a degree considered statistically significant. Follow-up of all patients for at least 3 years (or 4 years as originally planned) would have permitted assessment of whether these trends disappeared or became more pronounced.
Although not the primary outcome of interest, fewer surgery eyes lost ≥6 lines of BCVA by the 24-month examination, paralleling the contrast sensitivity outcomes with respect to loss of at least 3 segments of contrast sensitivity. This finding was judged to be important by the SST Research Group. Although the main goal of surgery was to improve the chance of stable or improved VA relative to observation, loss of ≥6 lines of VA, termed severe VA loss, has been of primary interest in trials evaluating laser photocoagulation8,9 and a prespecified important secondary outcome in trials evaluating photodynamic therapy.10,11 Furthermore, in cross-sectional studies, a difference of ≥6 lines of VA correlated with a large change in visual function as measured by the National Eye Institute Visual Function Questionnaire.47 It is of interest, then, that although surgery reduced the risk of severe VA loss, these findings were not accompanied by any large changes in vision-targeted health-related quality of life findings.29 These results also differed from those reported in the SST Group N Trial (with lesions that had subfoveal CNV and some evidence of classic CNV on fluorescein angiography although the lesion was not predominantly hemorrhagic). Surgery did not reduce the risk of severe VA loss compared with observation in the Group N Trial30 (nor were there any differences between observation and surgery for other VA outcomes), even though vision-targeted health-related quality of life findings favored surgery over observation in that trial.31 Clearly, more research is needed to develop a better understanding of longitudinal changes in vision-targeted health-related quality of life findings and their relationship with VA changes.
As the cumulative percentage of observation eyes ever treated with laser photocoagulation, photodynamic therapy with verteporfin, or submacular surgery when a treatable lesion was observed after blood cleared was only 13%, it is unlikely that a beneficial effect of submacular surgery with respect to VA and other outcomes was masked by any benefits of treatment given to observation eyes (Fig 5). It should be noted that the SST Group B Trial was initiated before publication of reports regarding beneficial effects of photodynamic therapy with verteporfin for selected subfoveal choroidal neovascular lesions,10–13 although at time of enrollment, cases eligible for the Group B Trial would not have met criteria for which photodynamic therapy has been recommended.13 Photodynamic therapy was not available until April 2000, when most patients participating in the Group B Trial already had enrolled. Furthermore, although the cumulative percentage of phakic eyes in the surgery arm that underwent cataract surgery within 2 years of enrollment was 44%, there was no evidence to suggest that any additional beneficial effect of submacular surgery with respect to VA and other outcomes was masked by effects of cataract on VA during follow-up examinations.
The lesion at follow-up was defined as any areas of CNV or features that could obscure the ability to identify CNV such as blood, staining scars, and areas of retinal pigment epithelial abnormality after surgery; with this definition, only approximately 50% of the lesions were at least 1 category smaller at follow-up examinations (Table 8). There are numerous factors potentially contributing to a failure of surgery to reduce lesion size relative to baseline. Fluorescein leakage from CNV was noted at the first postoperative evaluation in approximately 50% of eyes in the surgery arm (Fig 4), suggesting that part of the lesion remained in the eye, recurrent neovascularization developed, or both. Furthermore, the surgeon noted subretinal or sub-RPE blood remaining in 154 eyes (93%), including 42 (25%) with >4 disc areas of blood. In 26 of these 42 eyes, this blood elevated the retina and was presumed to be thick enough to obscure one’s ability to determine whether there was CNV at the first postoperative visit, so that the area of blood would be included in the total lesion when judging lesion size. As the blood cleared, additional neovascularization may have developed in its place in some eyes, as suggested by an increasing cumulative percentage of eyes developing new fluorescein leakage from CNV in Figure 4.
The surgical arm of the SST Group B Trial showed a relatively high risk of rhegmatogenous RD (16% of all patients in the surgery arm), often with very poor vision outcomes. The risk increased with worse levels of VA and larger lesion size at baseline. Of note, these factors also were associated with an increased risk of vitreous hemorrhage in the observation eyes. This trial also confirmed a high rate of development or progression of cataract after this surgery. Of the 97 eyes in the surgical arm that were phakic at baseline, 79 either had cataract surgery by the 24-month examination or had lenticular opacity judged by the treating ophthalmologist to be sufficient to account for at least 2 lines of VA loss.
Although the composition of the lesions enrolled in the Group B Trial was predominantly hemorrhagic, the baseline characteristics otherwise were typical of those seen in other trials of patients with subfoveal CNV, even for the use of warfarin or aspirin intake.30 The ophthalmic outcomes in terms of absolute level of VA were much worse in the Group B Trial than in the Group N Trial.30
Although the targeted number of patients (360) was not enrolled, the 336 patients provided at least 90% power to rule out differences in success rates (success defined as improvement or stabilization of BCVA relative to baseline at the 24-month examination) such as 40% versus 60% and 45% versus 65% in the observation and surgery arms, respectively, differences that would yield an approximate 50% relative difference in success rates between arms. These differences are similar to those found in trials evaluating photodynamic therapy with verteporfin for selected cases of subfoveal CNV.10 Thus, it is unlikely that a large beneficial effect of surgery with respect to the prespecified primary outcome of stable or improved VA was missed for the patients enrolled in the SST Group B Trial.
Based on the entire SST Group B Trial, it is possible that surgery as performed in this trial can reduce the risk of additional severe VA loss beyond that already lost when presenting with a predominantly hemorrhagic lesion, as was enrolled in the SST Group B Trial. Because the risk of rhegmatogenous RD after surgery as performed in this trial was greater in eyes presenting with very poor VA or very large lesions, and because the risk of severe VA loss also was reduced with surgery when results were analyzed within the better VA stratum (20/100–20/160) at baseline, some ophthalmologists may conclude that, in the absence of strong evidence from evaluations of other treatments for eyes with such lesions, it is reasonable to consider submacular surgery for similar lesions in eyes that do not have either very poor vision or very large lesions. However, both the patient and the ophthalmologist should weigh this possibility with full awareness of the limitations upon which this conclusion is based, including the recognition that reducing the risk of severe VA loss was not the primary outcome of interest, chances of stable or improved VA were similar with or without surgery, many of the initially phakic patients underwent cataract surgery after submacular surgery, and the risk of RD within the better VA stratum in the surgery arm was 10%.
As published elsewhere,47,48 patients in the SST Group B Trial reported a high degree of impairment due to their vision on entry into the study, and only 10% had VA better than 20/200 at 2 years. Thus, treatments to reduce the magnitude of vision loss from eyes similar to those enrolled in the SST Group B Trial are needed. The information provided by the SST Group B Trial, in which all patients were observed for at least 2 years and many for 3 years, should be useful to researchers who design future trials of treatments for patients with predominantly hemorrhagic choroidal neovascular lesions from AMD. Because the percentage with VA loss seemed to continue to increase in the observation arm through at least 3 years, future trials of alternative surgical techniques (such as displacement or removal of blood without removal of CNV, with subsequent treatment of residual CNV with photodynamic therapy or other treatments) or medical treatments should consider outcomes aimed at reducing the risk of additional severe VA loss beyond that noted at enrollment with follow-up for at least 3 to 4 years.
Table 1.
Sociodemographic and Health Characteristics of Patients at Time of Enrollment in the SST Group B Trial
|
By VA Stratum |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
By Treatment Arm |
VA>20/200 |
VA≤20/200 |
|||||||
| Total (n = 336) | Observation (n = 168) | Surgery (n = 168) | Total (n = 129) | Observation (n = 68) | Surgery (n = 61) | Total (n = 207) | Observation (n = 100) | Surgery (n = 107) | |
| Age (yrs) | |||||||||
| <60 | 3 (1) | 0 | 3 (2) | 1 (1) | 0 | 1 (2) | 2 (1) | 0 | 2 (2) |
| 60–69 | 26 (8) | 14 (8) | 12 (7) | 10 (8) | 8 (12) | 2 (3) | 16 (8) | 6 (6) | 10 (9) |
| 70–79 | 148 (44) | 73 (43) | 75 (45) | 57 (44) | 29 (43) | 28 (46) | 91 (44) | 44 (44) | 47 (44) |
| 80–89 | 148 (44) | 80 (48) | 68 (40) | 56 (43) | 30 (44) | 26 (43) | 92 (44) | 50 (50) | 42 (39) |
| ≥90 | 11 (3) | 1 (1) | 10 (6) | 5 (4) | 1 (1) | 0 | 6 (3) | 0 | 6 (6) |
| Gender | |||||||||
| Woman | 180 (54) | 89 (53) | 91 (54) | 76 (59) | 38 (56) | 38 (62) | 104 (50) | 51 (51) | 53 (50) |
| Man | 156 (46) | 79 (47) | 77 (46) | 53 (41) | 30 (44) | 23 (38) | 103 (50) | 49 (49) | 54 (50) |
| Race/ethnicity* | |||||||||
| White, not Hispanic | 326 (97) | 165 (98) | 161 (96) | 128 (99) | 67 (99) | 61 (100) | 198 (96) | 98 (98) | 100 (93) |
| Hispanic | 4 (1) | 2 (1) | 2 (1) | 1 (1) | 1 (1) | 0 | 3 (1) | 1 (1) | 2 (2) |
| Asian or Pacific Islander | 3 (1) | 0 | 3 (2) | 0 | 0 | 0 | 3 (1) | 0 | 3 (3) |
| American Indian or Alaskan Native | 2 (1) | 0 | 2 (1) | 0 | 0 | 0 | 2 (1) | 0 | 2 (2) |
| Black, not Hispanic | 1 (<1) | 1 (1) | 0 | 0 | 0 | 0 | 1 (<1) | 1 (1) | 0 |
| Occupational status | |||||||||
| Retired | 295 (88) | 145 (86) | 150 (89) | 114 (88) | 57 (84) | 57 (93) | 181 (87) | 88 (88) | 93 (87) |
| Employed | 23 (7) | 12 (7) | 11 (7) | 7 (5) | 6 (9) | 1 (2) | 16 (8) | 6 (6) | 10 (9) |
| Housespouse | 13 (4) | 7 (4) | 6 (4) | 5 (4) | 3 (4) | 2 (3) | 8 (4) | 4 (4) | 4 (4) |
| Disabled due to vision | 4 (1) | 3 (2) | 1 (1) | 3 (2) | 2 (3) | 1 (2) | 1 (<1) | 1 (1) | 0 |
| Disabled, other cause | 1 (<1) | 1 (1) | 0 | 0 | 0 | 0 | 1 (<1) | 1 (1) | 0 |
| Smoking history | |||||||||
| Never smoked | 136 (40) | 67 (40) | 69 (41) | 50 (39) | 26 (38) | 24 (39) | 86 (42) | 41 (41) | 45 (42) |
| Quit smoking | 167 (50) | 83 (49) | 84 (50) | 67 (52) | 38 (56) | 29 (48) | 100 (48) | 45 (45) | 55 (51) |
| Currently smoking | 33 (10) | 18 (11) | 15 (9) | 12 (9) | 4 (6) | 8 (13) | 21 (10) | 14 (14) | 7 (7) |
| Aspirin intake | |||||||||
| <7/wk | 227 (68) | 113 (67) | 114 (68) | 85 (66) | 42 (62) | 43 (70) | 142 (69) | 71 (71) | 71 (66) |
| ≥7/wk | 109 (32) | 55 (33) | 54 (32) | 44 (34) | 26 (38) | 18 (30) | 65 (31) | 29 (29) | 36 (34) |
| Warfarin use | |||||||||
| ≤4 wks ago | 30 (9) | 8 (5) | 22 (13) | 14 (11) | 4 (6) | 10 (16) | 16 (8) | 4 (4) | 12 (11) |
| >4 wks ago | 42 (12) | 21 (12) | 21 (13) | 15 (12) | 8 (12) | 7 (11) | 27 (13) | 13 (13) | 14 (13) |
| Never | 264 (79) | 139 (83) | 125 (74) | 100 (78) | 56 (82) | 44 (72) | 164 (79) | 83 (83) | 81 (76) |
| Chronic conditions present | |||||||||
| Hypertension | 188 (56) | 89 (53) | 99 (59) | 76 (59) | 37 (54) | 39 (64) | 112 (54) | 52 (52) | 60 (56) |
| Diabetes mellitus | 53 (16) | 25 (15) | 28 (17) | 22 (17) | 12 (18) | 10 (16) | 31 (15) | 13 (13) | 18 (17) |
| Any other known† | 264 (89) | 134 (92) | 130 (86) | 107 (93) | 57 (95) | 50 (91) | 157 (86) | 77 (89) | 80 (83) |
VA = visual acuity. Number (%) of patients.
Based on self-reports.
Based on self-reports of 146 and 151 patients in observation and surgery arms, respectively.
Acknowledgments
The Submacular Surgery Trials Research Group gratefully acknowledges the contributions of ophthalmologists who referred patients to the study and, in particular, of patients who enrolled, agreed to random assignment to treatment arm, and returned for scheduled follow-up examinations to benefit future patients.
The following SST Research Group Members take authorship responsibility for SST report no. 13: Neil M. Bressler, MD (writing team chair), Susan B. Bressler, MD, Ashley L. Childs, MS, Julia A. Haller, MD, Barbara S. Hawkins, PhD, Hilel Lewis, MD, Mathew W. MacCumber, MD, PhD, Marta J. Marsh, MS, Maryann Redford, DDS, MPH, Paul Sternberg, Jr, MD, Matthew A. Thomas, MD, George A. Williams, MD.
Footnotes
The Submacular Surgery Trials are sponsored by the National Eye Institute, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland (cooperative agreements U10 EY11547, EY11557, EY11558 with the Johns Hopkins University, Baltimore, Maryland). Relevant disclosures for consulting and research efforts are summarized in a companion article,30 with detailed statements on file at the SST Chairman’s Office, Baltimore, Maryland.
References
- 1.Age-Related Eye Disease Study Research Group. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003;121:1621–4. doi: 10.1001/archopht.121.11.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the Macular Photocoagulation Study. Arch Ophthalmol. 1991;109:1242–57. [PubMed] [Google Scholar]
- 3.Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) and Verteporfin in Photodynamic Therapy (VIP) Study Groups. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment—TAP and VIP report no. 2. Arch Ophthalmol. 2003;121:1253–68. doi: 10.1001/archopht.121.9.1253. [DOI] [PubMed] [Google Scholar]
- 4.Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16:183–9. doi: 10.1097/00006982-199616030-00001. [DOI] [PubMed] [Google Scholar]
- 5.Berrocal MH, Lewis ML, Flynn HW., Jr Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol. 1996;122:486–93. doi: 10.1016/s0002-9394(14)72107-5. [DOI] [PubMed] [Google Scholar]
- 6.Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999;213:97–102. doi: 10.1159/000027400. [DOI] [PubMed] [Google Scholar]
- 7.Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990;109:33–7. doi: 10.1016/s0002-9394(14)75575-8. [DOI] [PubMed] [Google Scholar]
- 8.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1220–31. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- 9.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal recurrent neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1232–41. doi: 10.1001/archopht.1991.01080090056026. [DOI] [PubMed] [Google Scholar]
- 10.Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin. One-year results of 2 randomized clinical trials—TAP report 1. Arch Ophthalmol. 1999;117:1329–45. [PubMed] [Google Scholar]
- 11.Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult but no classic choroidal neovascularization—Verteporfin in Photodynamic Therapy report 2. Am J Ophthalmol. 2001;131:541–60. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Ophthalmology Retina Panel. Preferred practice patterns. Age-related macular degeneration. Available at: http://www.aao.org/aao/education/library/ppp/upload/Age-Related-Macular-Degeneration.pdf Accessed July 15, 2004.
- 13.Verteporfin Roundtable 2000 and 2001 Participants, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Principal Investigators, Verteporfin in Photodynamic Therapy (VIP) Study Group Principal Investigators. Guidelines for using verteporfin (Visudyne) in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina. 2002;22:6–18. doi: 10.1097/00006982-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ohji M, Saito Y, Hayashi A, et al. Pneumatic displacement of subretinal hemorrhage without tissue plasminogen activator. Arch Ophthalmol. 1998;116:1326–32. doi: 10.1001/archopht.116.10.1326. [DOI] [PubMed] [Google Scholar]
- 15.Hesse L, Schmidt J, Kroll P. Management of acute submacular hemorrhage using recombinant tissue plasminogen activator and gas. Graefes Arch Clin Exp Ophthalmol. 1999;237:273–7. doi: 10.1007/s004170050232. [DOI] [PubMed] [Google Scholar]
- 16.Krepler K, Kruger A, Tittl M, et al. Intravitreal injection of tissue plasminogen activator and gas in subretinal hemorrhage caused by age-related macular degeneration. Retina. 2000;20:251–6. [PubMed] [Google Scholar]
- 17.Hassan AS, Johnson MW, Schneiderman TE, et al. Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology. 1999;106:1900–6. doi: 10.1016/S0161-6420(99)90399-8. discussion 1906–7. [DOI] [PubMed] [Google Scholar]
- 18.Lewis H. Intraoperative fibrinolysis of submacular hemorrhage with tissue plasminogen activator and surgical drainage. Am J Ophthalmol. 1994;118:559–68. doi: 10.1016/s0002-9394(14)76571-7. [DOI] [PubMed] [Google Scholar]
- 19.Olivier S, Chow DR, Packo KH, et al. Subretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in age-related macular degeneration. Ophthalmology. 2004;111:1201–8. doi: 10.1016/j.ophtha.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo T, Shiraga F, Takasu I. Planned two-step vitrectomy for extremely large and thick subretinal hematoma. Acta Ophthalmol Scand. 2001;79:533–7. doi: 10.1034/j.1600-0420.2001.790522.x. [DOI] [PubMed] [Google Scholar]
- 21.Kimura AE, Reddy CV, Folk JC, Farmer SG. Removal of subretinal hemorrhage facilitated by preoperative intravitreal tissue plasminogen activator [letter] Retina. 1994;14:83–4. doi: 10.1097/00006982-199401000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Hanscom TA, Diddie KR. Early surgical drainage of subretinal hemorrhage. Arch Ophthalmol. 1987;105:1722–3. doi: 10.1001/archopht.1987.01060120120037. [DOI] [PubMed] [Google Scholar]
- 23.de Juan E, Jr, Machemer R. Vitreous surgery for hemorrhagic and fibrous complications of age-related macular degeneration. Am J Ophthalmol. 1988;105:25–9. doi: 10.1016/0002-9394(88)90116-x. [DOI] [PubMed] [Google Scholar]
- 24.Wade EC, Flynn HW, Jr, Olsen KR, et al. Subretinal hemorrhage management by pars plana vitrectomy and internal drainage. Arch Ophthalmol. 1990;108:973–8. doi: 10.1001/archopht.1990.01070090075043. [DOI] [PubMed] [Google Scholar]
- 25.Vander JF, Federman JL, Greven C, et al. Surgical removal of massive subretinal hemorrhage associated with age-related macular degeneration. Ophthalmology. 1991;98:23–7. doi: 10.1016/s0161-6420(91)32348-0. [DOI] [PubMed] [Google Scholar]
- 26.Ibanez HE, Williams DF, Thomas MA, et al. Surgical management of submacular hemorrhage: a series of 47 consecutive cases. Arch Ophthalmol. 1995;113:62–9. doi: 10.1001/archopht.1995.01100010064022. [DOI] [PubMed] [Google Scholar]
- 27.Kamei M, Tano Y, Maeno T, et al. Surgical removal of sub-macular hemorrhage using tissue plasminogen activator and per-fluorocarbon liquid. Am J Ophthalmol. 1996;121:267–75. doi: 10.1016/s0002-9394(14)70274-0. [DOI] [PubMed] [Google Scholar]
- 28.Bressler NM. Submacular surgery. Are randomized trials necessary? Arch Ophthalmol. 1995;113:1557–60. doi: 10.1001/archopht.1995.01100120087016. [DOI] [PubMed] [Google Scholar]
- 29.Submacular Surgery Trials (SST) Research Group. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: quality-of-life findings. SST report no. 14. Ophthalmology. 2004;111:2007–14. doi: 10.1016/j.ophtha.2004.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings. SST report no. 11. Ophthalmology. 2004;111:1967–80. doi: 10.1016/j.ophtha.2004.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: quality-of-life findings. SST report no. 12. Ophthalmology. 2004;111:1981–92. doi: 10.1016/j.ophtha.2004.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Submacular Surgery Trials Research Group. Surgical removal versus observation for subfoveal choroidal neovascularization, either associated with the ocular histoplasmosis syndrome or idiopathic. I. Ophthalmic findings from a randomized clinical trial: SST Group H Trial. SST report no. 9. Arch Ophthalmol. In press. [DOI] [PMC free article] [PubMed]
- 33.Submacular Surgery Trials Research Group. Surgical removal versus observation for subfoveal choroidal neovascularization, either associated with the ocular histoplasmosis syndrome or idiopathic—II. Quality-of-life findings from a randomized clinical trial: SST Group H Trial. SST report no. 10. Arch Ophthalmol. In press. [DOI] [PMC free article] [PubMed]
- 34.Submacular Surgery Trials (SST). Manual of Procedures. Springfield, VA: National Technical Information Service; 1998. NTIS Publication PB98-166648.
- 35.Submacular Surgery Trials (SST). Forms Book. Springfield, VA: National Technical Information Service; 1998. NTIS Publication PB98-159445.
- 36.Submacular Surgery Trials Research Group. Clinical trial performance of community- versus university-based practices in the Submacular Surgery Trials (SST). SST report no. 2. Arch Ophthalmol. 2004;122:857–63. doi: 10.1001/archopht.122.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferris FL, III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–6. [PubMed] [Google Scholar]
- 38.Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci. 1988;2:187–99. [Google Scholar]
- 39.Submacular Surgery Trials Research Group. Guidelines for grading retinal photographs in the Submacular Surgery Trials (SST): SST report no. 8. Retina. In press. [DOI] [PMC free article] [PubMed]
- 40.Snedecor GW, Cochran WG. Statistical Methods. 7th ed. Ames, IA: Iowa State University Press; 1980:144–5.
- 41.Armitage P, Berry G. Statistical Methods in Medical Research. 2nd ed. Oxford, United Kingdom: Blackwell Scientific; 1987:372– 4.
- 42.Hillis A, Maguire M, Hawkins BS, Newhouse MM. The Markov process as a general method for nonparametric analysis of right-censored medical data. J Chronic Dis. 1986;39:595–604. doi: 10.1016/0021-9681(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 44.Zhang H, Holford T, Bracken MB. A tree-based method of analysis for prospective studies. Stat Med. 1996;15:37–49. doi: 10.1002/(SICI)1097-0258(19960115)15:1<37::AID-SIM144>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 45.Cox DR, Snell EJ. Analysis of Binary Data. 2nd ed. New York: Chapman & Hall; 1989:66–9.
- 46.SAS [computer program]. Version 8.2. Cary, NC: SAS, Inc.; 2001.
- 47.Submacular Surgery Trials Research Group. Health- and vision-related quality of life among patients with choroidal neovascularization secondary to age-related macular degeneration at time of enrollment in randomized trials of submacular surgery: SST report no. 4. Am J Ophthalmol. 2004;138:91–108. doi: 10.1016/j.ajo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Submacular Surgery Trials Research Group. Patients’ perceptions of the value of current vision: assessment of preference values among patients with subfoveal choroidal neovascularization—the Submacular Surgery Trials Vision Preference Value Scale (SST-VPVS): SST report no. 6. Arch Ophthalmol. In press. [DOI] [PMC free article] [PubMed]


