Abstract
Purpose
To present visual acuity (VA) and related findings from patients enrolled in one of the Submacular Surgery Trials (SST) evaluating surgical removal versus observation of subfoveal choroidal neovascularization secondary to age-related macular degeneration (SST Group N Trial).
Design
Randomized clinical trial.
Participants
Eligible patients had age-related macular degeneration with subfoveal choroidal neovascularization, some with a classic pattern on fluorescein angiography, and best-corrected VA (BCVA) of 20/100 to 20/800 in one eye (study eye) that had received no treatment in the macula. Any contiguous blood had to account for <50% of the total area occupied by the subfoveal lesion (maximum size, 9.0 disc areas [22.9 mm2]).
Methods
Randomization was stratified by VA and by clinical center. All patients were scheduled for study examinations at 3, 6, 12, and 24 months after enrollment for assessment of study outcomes.
Main Outcome Measure
A successful outcome was defined a priori to be either improvement of BCVA or VA no more than 1 line (7 letters) worse than baseline at the 24-month examination.
Results
Of 454 patients enrolled, 228 study eyes were assigned to observation and 226 to surgery. The percentages of eyes that had successful outcomes were similar in the 2 arms: 44% assigned to observation and 41% assigned to surgery. Median VA losses from baseline to the 24-month examination were 2.1 lines (10.5 letters) in the observation arm and 2.0 lines (10 letters) in the surgery arm. Median VA declined from 20/100 at baseline to 20/400 at 24 months in both arms. No subgroup of patients was identified in which submacular surgery led to better VA outcomes. In the surgery arm, 55 (39%) of 142 initially phakic eyes had cataract surgery by the 24-month examination, compared with 6 (5%) of 133 eyes in the observation arm. Rhegmatogenous retinal detachment occurred in 12 surgery eyes (5%) and 1 observation eye.
Conclusions
Submacular surgery, as performed in this clinical trial, did not improve or preserve VA for 24 months in more eyes than observation and is not recommended for patients with similar lesions.
When choroidal neovascularization develops in a subfoveal location, the effect on visual acuity (VA) and other aspects of vision in the affected eye may be devastating. Because choroidal neovascularization develops in the second eye of patients with age-related macular degeneration at a rate of 5% to 7% per year within the first few years of onset in the first eye,1,2 patients who develop choroidal neovascularization in one eye are at substantial risk of bilateral involvement during their remaining lifespan. The impact on quality of life of subfoveal choroidal neovascularization secondary to age-related macular degeneration in one or both eyes has been documented to be substantial.3 Although laser photocoagulation of extrafoveal and juxtafoveal choroidal neovascularization has been proven to be beneficial with respect to retaining useful VA and avoiding growth into the fovea,4–7 many patients present initially with subfoveal choroidal neovascularization or have subfoveal recurrence after laser photocoagulation.8,9 Laser photocoagulation also has been shown to be effective for delaying or preventing severe loss of VA in eyes with selected subfoveal neovascular lesions, both with and without previous nonfoveal laser photocoagulation, in which VA already is reduced.10–12 More recently, photodynamic therapy with verteporfin (Visudyne, Novartis Pharma AG, Basel, Switzerland) was demonstrated to be effective in delaying or preventing moderate or severe loss of VA in selected cases in which the subfoveal neovascular lesion was composed primarily of classic choroidal neovascularization13,14 and in cases of subfoveal occult choroidal neovascularization and no classic choroidal neovascularization.15 However, fluorescein leakage from choroidal neovascularization requiring additional treatment was observed frequently after both laser photocoagulation16–18 and photodynamic therapy.13–15 To date, treatments such as interferon19 and low-dose radiation20,21 have not proven to be effective.
In the 1980s, retinal surgeons began to develop surgical techniques to remove choroidal neovascularization and associated tissue from the eye.22–24 From 1997 to 2003, the Submacular Surgery Trials (SST) Research Group conducted 3 randomized trials of surgical removal of subfoveal choroidal neovascular lesions to evaluate the role of this procedure in the treatment of patients with age-related macular degeneration and other conditions. These clinical trials were preceded by a pilot study conducted to refine the design and methods to be employed in formal evaluations of submacular surgery.25 One of the randomized clinical trials conducted by the SST Research Group was for patients who had relatively large or poorly demarcated new (never treated) subfoveal choroidal neovascularization associated with age-related macular degeneration (SST Group N Trial). The target group of patients were those with relatively large subfoveal lesions for whom laser photocoagulation was judged to be inappropriate and others with poorly demarcated subfoveal lesions who were ineligible for randomized trials conducted by the Macular Photocoagulation Study Group. No other treatment had been documented to benefit these patients at the time the SST Group N Trial was initiated. The SST Planning Committee hypothesized that removal of subfoveal choroidal neovascular lesions from the eyes of selected patients with age-related macular degeneration would halt or delay expansion of the disruption of photoreceptors and other affected cells in the central macula so that eyes would have smaller subfoveal lesions and, thus, better VA than if left untreated.
The purpose of this report is to present ophthalmic findings from the SST Group N Trial. A separate report presents findings regarding health-related quality of life.26 Findings from another trial conducted by the SST Research Group for patients with a different type of subfoveal neovascular lesions secondary to age-related macular degeneration—that is, hemorrhagic lesions in which the predominant feature is blood (SST Group B Trial)—also have been reported separately.27,28
Materials and Methods
The members of an independent Data and Safety Monitoring Committee, appointed late in 1996 by the Director of the National Eye Institute (National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland), reviewed findings from the SST pilot study for similar patients and approved the SST design and methods before enrollment of patients was initiated in the SST Group N Trial in July 1998. Institutional review boards at all participating institutions reviewed and approved the study design and the consent forms to be used locally. All patients gave signed consent before enrollment and random treatment assignment.
The SST Manual of Procedures29 and the SST Forms Book30 provide detailed information regarding study design, methods, and policies. Only that pertinent to this report is summarized here.
Patient Eligibility and Enrollment
Eligible patients were identified from referrals to 25 clinical centers that participated in the SST Group N Trial, including 12 centers that participated in the pilot study. Participating ophthalmologists were required to meet standard criteria to be SST certified to enroll, treat, and observe study patients.29
After a diagnosis of subfoveal choroidal neovascularization secondary to age-related macular degeneration had been made by the study ophthalmologist, but before enrollment of the patient in the study, an SST-certified vision examiner measured best-corrected VA (BCVA) using modified Bailey–Lovie charts (Early Treatment Diabetic Retinopathy Study charts),31 contrast threshold using Pelli–Robson charts at 0.5 m,32,33 and reading speed with enlarged text using charts and methods developed for the Macular Photocoagulation Study to simulate reading with a magnifier.10 For each measurement, the vision examiner tested the two eyes separately, using a different chart to test each eye, and followed a standard testing protocol. Stereoscopic color photographs of the disc and macula of each eye and a stereoscopic film-based fluorescein angiogram were taken by SST-certified photographers, who followed a standard protocol, to document eligibility and baseline status and to compare with photographs taken during follow-up examinations to monitor changes over time. Usage of warfarin (Coumadin, Bristol-Myers Squibb Co., Princeton, NJ) and other anticoagulants and history of diabetes, hypertension, and other major medical conditions were elicited from the patient before a detailed examination to establish eligibility for the randomized trial.
To be eligible for enrollment and random treatment assignment in the Group N Trial, the patient had to be 50 years or older and have a subfoveal neovascular lesion, some part of which was classic choroidal neovascularization, defined by the pattern of dye leakage during fluorescein angiography, and other evidence of age-related macular degeneration. The subfoveal lesion could contain occult choroidal neovascularization or blood as components. However, the component located under the geometric center of the foveal avascular zone was required to be choroidal neovascularization (either classic or occult). Furthermore, any contiguous blood had to occupy less than half the total area occupied by the subfoveal lesion. Prior treatment for choroidal neovascularization or any other macular condition or earlier vitrectomy for any reason rendered an eye ineligible. The size of the total lesion, including contiguous blood, if any, could not exceed 9.0 disc areas (DAs) (22.9 mm2 on the retina). Eyes with lesions smaller than 3.5 DAs were eligible only when the lesion boundaries were poorly demarcated (i.e., the eye was an unsuitable candidate for laser photocoagulation). Eligible eyes had BCVA corresponding to Snellen fractions of 20/100 to 20/800 inclusive (VA scores of 67–18); VA of the nonstudy eye had to be light perception (LP) or better. Patients who had evidence of other progressive ocular disease in either eye that could affect VA or assessment of other ophthalmic outcomes were ineligible. In particular, patients who had evidence of angioid streaks, pathologic myopia, ocular histoplasmosis, or other conditions in either eye that could have accounted for choroidal neovascularization in the eye considered for enrollment were ineligible. Only one eye of each patient (study eye) was eligible for enrollment and random treatment assignment. Whenever both eyes of a patient were eligible, the study eye was selected by the patient and ophthalmologist before enrollment; the fellow eye was managed as they decided.
Patients judged to be eligible by an SST-certified ophthalmologist were invited to participate and given detailed information about the study. For those who agreed to participate, a study identification number and an alphabetic code were assigned before they completed an interview by telephone with personnel at the SST Coordinating Center (Baltimore, Maryland).3 After the patient signed the consent form for the trial, the identification number and alphabetic code were recorded on the baseline data forms, which were telecopied to the Coordinating Center for preliminary review of eligibility and completeness of baseline data recording. The next treatment arm assignment was selected from the file of random assignments that had been prepared for that clinical center specifically for the SST Group N Trial and for the VA of the study eye (>20/200 [20/100–20/160] or ≤20/200 [20/200–20/800]). The clinical center staff were notified of the assigned treatment, surgery or observation, by means of an automated message returned to them by telecopier. The assignment was communicated to the patient by the enrolling ophthalmologist; surgery was scheduled as soon as possible for each patient assigned to that treatment arm.
Evaluation of Baseline Photographs
After enrollment, baseline photographs were sent to the SST Photograph Reading Center (Baltimore, Maryland), where they were reviewed by trained readers who were masked to the treatment arm to which the study eye had been assigned. The focus of the review was on eligibility for enrollment and documentation of characteristics of eyes and subfoveal lesions. A final judgment regarding eligibility was made after review of baseline photographs and evaluation of pertinent baseline data had been completed. The underlying etiology of the neovascular lesion in the study eye was assigned based on the appearance of both eyes and the presence of other lesions in the two eyes. The total size of the subfoveal lesion on the baseline photographs was categorized using a transparent overlay with printed circles with areas equivalent to 2.0, 3.5, 6.0, 9.0, 12.0, and 16.0 DAs that was adapted from one used in the Macular Photocoagulation Study.34 The approximate areas of the circles on the retina were 5.1 mm2 to 40.6 mm2.
Measurement and Scoring of Visual Acuity
At baseline and each follow-up examination, BCVA of each eye was measured separately by an SST-certified vision examiner. Testing began at 2 m; whenever the patient could read ≥15 letters correctly at 2 m using the eye, the VA score was the number of letters read correctly plus 30. When the patient could not read at least 15 letters at 2 m with the eye being tested, VA was measured at 0.5 m after adjusting the refractive correction for the closer distance. In the latter situation, the VA score was calculated to be the sum of the number of letters read correctly at 2 m and the smaller of 30 or the number of letters read correctly at 0.5 m. Visual acuities from 20/20 (scores of 98–100) to 20/1600 (5/400; scores of 3–7) could be measured using these 2 test distances. Any eye with which a patient could not read any letters correctly at either test distance (VA<20/1600 [<5/400]) was tested for LP following a standard protocol. Visual acuity was recorded for such eyes as at least LP or as no LP after confirmation by the ophthalmologist.
At the 24-month examination, designated the primary outcome assessment examination, and at the 48-month examination for patients enrolled by September 1999, a traveling vision examiner based in the SST Chairman’s Office (Baltimore, Maryland) refracted both eyes and measured BCVA, contrast threshold, and reading speed. Beginning in June 2002, whenever possible, a traveling vision examiner also conducted masked measurements of vision at 36-month examinations for patients who enrolled early enough to be eligible for those examinations. The traveling vision examiner was masked to the SST clinical trial in which the patient was enrolled, the study eye, and the treatment assigned or received.
Treatment, Follow-up Examinations, and Adverse Events
Patients assigned to surgery were scheduled for surgery within 8 days after enrollment. Whenever this time limit could not be met, a fluorescein angiogram taken no more than 8 days before surgery was required to document preoperative status of the eye and lesion. Only surgeons who met defined conditions based on training and experience were approved to perform submacular surgery on study eyes of SST patients.29
The surgery protocol specified a standard 3-port pars plana vitrectomy and surgical detachment of the posterior hyaloid when not already detached preoperatively. The retinotomy site was chosen by the surgeon to provide optimal access to the neovascular lesion and to minimize surgical trauma to the fovea. Surgeons had the option of infusing balanced saline solution to separate the neurosensory retina from the neovascular lesion. Fibrovascular tissue was removed manually from the subretinal space with a microforceps. Intraocular pressure was elevated immediately before removal of the tissue from beneath the retina to minimize hemorrhage from the choroid and was returned to normal before removal of the neovascular tissue from the eye. The peripheral retina was examined with indirect ophthalmoscopy and scleral depression for breaks or tears before the fluid–air exchange. A complete fluid–air exchange was performed before fluid was rein-stilled to create an air bubble of up to 50% in phakic eyes or up to 100% in pseudophakic eyes. Patients were instructed to remain in a facedown position overnight after surgery or until the air bubble was ≤20%. Details of the surgery, deviations from the surgical protocol, and intraoperative complications were reported by the surgeon on a standard form.30
Eyes treated surgically were examined as often as judged necessary by the surgeon during the postoperative period. Patients were scheduled for a postsurgical examination and data collection at 1 month after enrollment. Patients assigned to observation were scheduled for a telephone review of ocular status and history 1 month after enrollment.
All patients were scheduled for follow-up examinations for data collection purposes at 3, 6, 12, and 24 months after enrollment. Patients who enrolled by September 2000 were scheduled for a 36-month examination, and those who enrolled by September 1999 also were scheduled for a 48-month examination. In addition to the examinations prescribed by the study protocol, patients were seen by SST ophthalmologists as often as necessary to evaluate symptoms and the need for treatment of the study eye or fellow eye. For patients not examined at the midpoint between scheduled SST examinations, local clinical personnel elicited an interim medical and ocular history by telephone. The last study examinations for all patients who had not completed a 48-month examination already were performed during the final year of patient follow-up that began on October 1, 2002.
At the 3-month and later examinations, BCVA, contrast threshold, and reading speed were measured by an SST-certified examiner according to the study protocol. A detailed history of systemic and ocular complications and intervening treatments to the study eye was elicited. Color photography and fluorescein angiography were repeated following a standard protocol; photographs were sent to the SST Photograph Reading Center for interpretation and coding. The emphasis of central review of photographs taken during follow-up was on identification of choroidal neovascularization and its location in eyes after surgery, measurement of the current size of the subfoveal lesion (including the area of disturbed retinal pigment epithelium in surgery eyes), and documentation of retinal complications. Eyes in the surgery arm were considered for additional treatment whenever fluorescein leakage from choroidal neovascularization was observed by the ophthalmologist at a follow-up examination. The retreatment protocol specified laser photocoagulation or surgery depending upon the location of fluorescein leakage from choroidal neovascularization; however, no more than one repeat surgery was recommended. After publication of findings from randomized trials that demonstrated that photodynamic therapy with verteporfin was beneficial for selected choroidal neovascular lesions,13–15 this treatment also was permitted by the SST protocol for treatment of subfoveal choroidal neovascularization documented by fluorescein leakage during follow-up in eyes in either treatment arm, including eyes in the observation arm that met eligibility criteria for the verteporfin trials. Regardless of the treatment arm to which assigned, the status of the study eye, or the vision of the fellow eye, the study protocol recommended that cataract surgery be considered whenever the ophthalmologist judged that lenticular opacity was in a location and dense enough to cause a loss of ≥2 lines of VA in an otherwise healthy phakic eye.
Selected adverse events were reported by the ophthalmologist on the basis of findings at scheduled study examinations. Additional adverse events were reported to the Coordinating Center and to local institutional review boards as clinical center personnel became aware of them. An Adverse Event Review Committee, appointed by the Data and Safety Monitoring Committee, classified events and requested additional information when needed to judge whether the event was in any way related to surgery in the study eye. At the request of the Data and Safety Monitoring Committee, a detailed review of all cases of retinal detachment (RD) was initiated in May 2002 to determine whether the retina had been successfully reattached and whether proliferative vitreoretinopathy had developed.
Statistical Design and Study Monitoring
Before the SST Group N Trial was initiated, the SST Planning Committee selected the primary successful outcome of interest to be improvement or stabilization of VA within 2 years after enrollment, with stabilization defined as loss of no more than 7 letters from baseline to the 24-month examination. Examinations at 36 months and 48 months, specified for all patients in the original design, were intended to monitor persistence of findings observed at the 24-month examination. Sample size was calculated during the planning phase under the assumption that 26% of eyes assigned to observation would have a successful outcome, as defined above. This assumption was based on early unpublished data from cases assigned to the observation arm in the SST Group N pilot trial and data from the Macular Photocoagulation Study Group.10,35 Taking account of the cost and inconvenience of surgery and the fact that the only treatment alternative was laser photocoagulation, the members of the Planning Committee judged the minimum clinically meaningful relative improvement in success rates after surgery to be 50% (i.e., a successful outcome in ≥39% of surgically treated eyes). Data from eyes randomly assigned to surgery or observation in the Group N pilot trial and followed through October 1996 suggested that a treatment effect of this magnitude was realistic. Review of data from longer follow-up in the pilot trial in April 1998 suggested that the percentage of eyes in the observation arm that would have successful outcomes at 24 months after enrollment would be between 35% and 40%. Type I (α) and type II (β) errors were set at 0.01 and 0.10, respectively. These parameters and allowances for death and missed 24-month examination (20% projected) yielded a target sample size of 600 patients (600 eyes).
Responsibility for monitoring accumulating data regarding safety and effectiveness of surgery was entrusted to the Data and Safety Monitoring Committee appointed in 1996. Informal statistical monitoring guidelines of the Lan–DeMets type36 were presented to the Data and Safety Monitoring Committee in August 1997; these were based on the assumption that there would be 5 interim analyses of the data on which a recommendation would be made to continue or to halt accrual. The Data and Safety Monitoring Committee met in person approximately every 12 months with interim teleconferences to review adverse events. Their reviews of accumulating outcomes data emphasized comparison of distributions of VA and changes from baseline to each examination by treatment arm as well as comparison of proportions of eyes in the 2 treatment arms with successful outcomes at the 24-month examination, the primary outcome defined in the design. The chair of the Adverse Event Review Committee reported findings from the most recent interim review of potential adverse events to the Data and Safety Monitoring Committee at the beginning of each meeting.
The December 7, 1999 meeting took place shortly after 1-year findings from the trials of photodynamic therapy with verteporfin were published,13 showing that the new treatment was effective for a subset of patients eligible for the Group N Trial. The Data and Safety Monitoring Committee recommended that the SST Research Group revise the consent forms for future patients eligible for the Group N Trial, evaluate all Group N Trial patients for eligibility for photodynamic therapy with verteporfin, and permit the new treatment to be used to manage choroidal neovascularization during follow-up. At the August 25, 2001 meeting, with 447 patients enrolled, the Data and Safety Monitoring Committee recommended that accrual halt on September 30, 2001 and that the last patient enrolled be observed for 3 years. Subsequently, due to funding constraints, the follow-up phase of the trial was curtailed so that a minimum of only 2 years of follow-up was provided for each patient. Thus, patient follow-up for SST purposes ended September 30, 2003. At their meeting on February 20, 2004, the Committee reviewed a detailed summary of findings from the final SST Group N Trial database and recommendations regarding submacular surgery for eyes similar to those eligible for this clinical trial.
Data Analysis and Statistical Methods
Data from masked vision examinations by traveling vision examiners were used in analyses; when not available, measurements by local examiners were analyzed. For analysis of change in VA, eyes that could not identify any letter correctly at either test distance, but had at least LP, were coded as having VA 3 lines worse than 20/1600 (i.e., equivalent to 3 lines worse than the smallest line measurable at 0.5 m). Although VA scores were analyzed, findings have been presented using equivalent Snellen fractions. Because fewer than half the patients in each treatment arm enrolled early enough to be eligible for a 48-month examination, cross-sectional findings at individual examination times have been presented in tables only for examinations at 36 months or earlier. However, 48-month findings have been included in longitudinal data analyses and presented in graphical displays of those findings.
The chi-square test was used to compare the proportions of eyes with successful outcomes, as defined above, between treatment arms at the 24-month examination. Distributions of VA, changes in VA from baseline to follow-up examinations, and other continuous measurements were compared between treatment arms using the Wilcoxon rank-sum test.37 Distributions of variables in quantitatively ordered categories were compared using the chi-square test for trend.38 Confidence limits on estimated success ratios were calculated using the method of Katz et al.39
Subgroups defined by baseline characteristics of patients, eyes, and lesions were examined for consistency of findings by treatment arm. Characteristics evaluated were selected primarily from those identified in earlier clinical trials of laser photocoagulation and photodynamic therapy with verteporfin for subfoveal choroidal neovascularization as influencing VA outcomes.4,5,10,14,35 Subgroups defined by the baseline VA of the study eye during stratification at time of enrollment and randomization were of particular interest.
The proportions of eyes at each examination in which VA had improved or stabilized (successes) and proportions with choroidal neovascularization present were calculated using a 2-state stochastic model that took account of both increases and decreases in VA over time.40 Time to first appearance of fluorescein leakage from choroidal neovascularization after surgery (recurrence) and time to cataract surgery and visually significant cataract in each treatment arm were analyzed using the product-limit method.41 When these methods were used, patients were withdrawn from analysis after the last examination completed. All pertinent data received at the SST Coordinating Center by October 31, 2003 were analyzed. Data from each patient were analyzed with the treatment arm to which he or she was assigned randomly at time of enrollment (intent-to-treat analysis approach). P values were not adjusted for multiplicity of outcomes or comparisons. Although P values close to those that conventionally would be considered to indicate statistical significance (P value < 0.05) have been noted in the text, only P values of <0.01 have been deemed statistically significant to minimize the chance of an incorrect conclusion regarding effectiveness of surgery.
Results
From July 13, 1998 until accrual ended on September 30, 2001, 454 patients (454 eyes) enrolled in the SST Group N Trial; 226 patients were assigned to the surgery arm and 228 patients to the observation arm. Based on central review of baseline photographs and other baseline data, 77 eyes (17%) (41 in the surgery arm and 36 in the observation arm) were judged not to meet 1 or more of the strict criteria for eligibility. One eye in the surgery arm had baseline VA worse than Snellen equivalent 20/800 (5/200); in 36 eyes the neovascular lesion was larger than 9 DAs (22.9 mm2). Data for patients judged centrally to be ineligible were analyzed with those of all other patients in the treatment arm to which assigned.
Characteristics of Patients and Eyes at Study Entry
Treatment arms were well balanced on all patient characteristics recorded at time of enrollment, both overall and within VA subgroups used to stratify random assignments (additional online-only Table 1 available at http://www.ophsource.com/periodicals/ophtha). The median age of patients was 77 years (range, 54–92); 160 (35%) of the patients were 80 years or older. Almost all patients (98%) classified themselves as non-Hispanic whites, consistent with epidemiologic data42,43 and patients enrolled in other clinical trials of treatment for choroidal neovascularization associated with age-related macular degeneration.4,5,10,13 Consistent with their ages, 401 (88%) patients reported that they were retired; only 49 (11%) of all patients were employed either inside or outside the home at time of enrollment. Of the 4 patients who reported that they were unemployed or unable to work, 3 attributed their situation to poor vision. Slightly more than one third of the patients reported that they had never smoked cigarettes; half of the patients had stopped smoking.
The most frequently reported medications used were vitamin E (52% of patients with recent use), antihypertensive agents (52%), and aspirin (38% taking at least 1 tablet per day). Warfarin and other anticoagulants had been used within the 4 weeks before enrollment by only 22 (5%) patients. Hypertension, based on earlier diagnosis, use of antihypertensive agents, or both, was reported at baseline by 260 (57%) of all 454 patients (additional online-only Table 1 available at http://www.ophsource.com/periodicals/ophtha). Only 48 patients (11%) reported having diabetes, due to exclusion of patients with moderate to severe diabetic retinopathy or clinically significant macular edema. Other comorbidity was common; 86% of the 368 patients queried reported having at least 1 other major comorbid condition. Of these 368 patients, for whom information regarding other major comorbidities was collected at baseline, 190 (51%) reported having arthritis or rheumatism, 108 (29%) reported back problems, 101 (27%) reported trouble hearing or deafness, and 70 (19%) reported having angina pectoris or ≥1 earlier myocardial infarctions. Percentages of patients who reported each condition and any comorbidity were similar in the 2 treatment arms.
Treatment arms also were balanced on characteristics of study eyes (Table 2) and fellow eyes (Table 3). The median VA score of study eyes at baseline was 51 letters, equivalent to Snellen VA of 20/200 (interquartile range, 20/125–20/320); the median contrast required to read letters on a Pelli–Robson chart using only the study eye was 8.9%. Mean reading speed with the study eye alone, as measured using cards with enlarged text, was 47 words per minute. Nearly half the subfoveal neovascular lesions (222 [49%]) were larger than 6 DAs (15.1 mm2) but no larger than 9 DAs (22.9 mm2) in total size (Table 2). The median VA score of fellow eyes at baseline was 86, equivalent to Snellen VA of 20/40 (Table 3). Two hundred three (45%) of the patients had a neovascular lesion of some type in the fellow eye; 34 (7%) fellow eyes had geographic atrophy but not a neovascular lesion (Table 3).
Table 2.
Status of Study Eyes at Time of Patient Enrollment in the Submacular Surgery Trials Group N Trial
|
No. (%) of Patients |
||
|---|---|---|
| Observation(n = 228) | Surgery(n = 226) | |
| Total size of subfoveal lesion (DAs) | ||
| ≤3.5 | 13 (6) | 8 (4) |
| >3.5 to ≤6.0 | 90 (39) | 74 (33) |
| >6.0 to ≤9.0 | 105 (46) | 117 (52) |
| >9.0 | 17 (7) | 22 (10) |
| Indeterminate | 3 (1) | 5 (2) |
| Classic CNV; proportion of total lesion | ||
| <50% | 118 (52) | 111 (49) |
| ≥50% | 107 (47) | 112 (50) |
| Indeterminate | 3 (1) | 3 (1) |
| Occult CNV present | ||
| Yes | 186 (82) | 183 (81) |
| No | 40 (18) | 41 (18) |
| Indeterminate | 2 (1) | 2 (1) |
| Visual acuity (Snellen equivalent) | ||
| 20/100–20/160 | 104 (46) | 102 (45) |
| 20/200–20/320 | 70 (31) | 77 (34) |
| 20/400–20/640 | 49 (21) | 38 (17) |
| ≤20/800 | 5 (2) | 9 (4) |
| Contrast threshold (% contrast required) | ||
| ≤3.2 | 18 (8) | 13 (6) |
| >3.2 to ≤6.3 | 87 (38) | 88 (39) |
| >6.3 to 12.6 | 67 (29) | 79 (35) |
| >12.6 to 25.1 | 34 (15) | 30 (13) |
| >25.1 | 22 (10) | 16 (7) |
| Reading speed with enlarged text (wpm) | ||
| ≥100 | 19 (8) | 17 (8) |
| 80–99 | 28 (12) | 18 (8) |
| 60–79 | 39 (17) | 40 (18) |
| 40–59 | 32 (14) | 37 (16) |
| 20–39 | 54 (24) | 61 (27) |
| 0–19 | 56 (25) | 53 (23) |
| Lens status | ||
| Phakic | 135 (59) | 143 (63) |
| Pseudophakic or aphakic | 93 (41) | 83 (37) |
| Etiology of neovascular lesion | ||
| Age-related macular degeneration | 224 (98) | 222 (98) |
| Possibly ocular histoplasmosis | 2 (1) | 2 (1) |
| Idiopathic | 1 (<1) | 1 (<1) |
| Indeterminate | 1 (<1) | 1 (<1) |
CNV = choroidal neovascularization; DAs = standard disc areas adapted from those published by the Macular Photocoagulation Study Group*; wpm = words per minute.
One DA = 2.5 mm2 on the retina.
Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the Macular Photocoagulation Study. Arch Ophthalmol 1991;109:1242–57.
Table 3.
Status of Fellow Eyes at Time of Patient Enrollment in the Submacular Surgery Trials Group N Trial
|
No. (%) of Patients |
||
|---|---|---|
| Observation (n = 228) | Surgery (n = 226) | |
| Most advanced lesion in fellow eye | ||
| Choroidal neovascular lesion | 105 (46) | 98 (43) |
| Geographic atrophy | 20 (9) | 14 (6) |
| Drusen | 91 (40) | 107 (47) |
| Other | 1 (<1) | 4 (2) |
| None | 3 (1) | 0 |
| Indeterminate | 8 (4) | 3 (1) |
| Visual acuity, fellow eye | ||
| ≥20/20 | 35 (15) | 54 (24) |
| 20/25–20/40 | 86 (38) | 75 (33) |
| 20/50–20/80 | 28 (12) | 20 (9) |
| 20/100–20/160 | 13 (6) | 13 (6) |
| 20/200–20/320 | 17 (7) | 25 (11) |
| 20/400–20/640 | 24 (11) | 19 (8) |
| 20/800–20/1250 | 20 (9) | 15 (7) |
| ≤20/1600–LP | 5 (2) | 5 (2) |
| Contrast threshold, fellow eye (% contrast required)* | ||
| ≤3.2 | 80 (35) | 94 (42) |
| >3.2 to ≤6.3 | 82 (36) | 86 (38) |
| >6.3 to ≤12.6 | 27 (12) | 16 (7) |
| >12.6 to ≤25.1 | 16 (7) | 16 (7) |
| >25.1 | 22 (10) | 14 (6) |
| Reading speed with enlarged text, fellow eye (wpm)† | ||
| >120 | 49 (22) | 52 (23) |
| 100–119 | 37 (16) | 36 (16) |
| 80–99 | 30 (13) | 38 (17) |
| 60–79 | 37 (16) | 30 (13) |
| 40–59 | 16 (7) | 18 (8) |
| 20–39 | 12 (5) | 15 (7) |
| 0–19 | 45 (21) | 37 (16) |
LP = light perception; wpm = words per minute.
Not measured for 1 fellow eye in the observation arm.
Not measured for 2 fellow eyes in the observation arm.
Surgery Protocol Variations and Intraoperative Complications
One patient assigned to the observation arm had submacular surgery in the study eye 1 month before the 12-month examination. Of 226 patients assigned to surgery, 6 refused surgery after enrollment; 11 other patients had surgery >8 days after enrollment. In 25 (17%) of 143 phakic eyes that had surgery, the air fill at the end of surgery was >50%. Surgeons elected to use gas fill in 23 eyes and used perfluorocarbons in 3 eyes.
Relatively few intraoperative complications were reported. In 13 eyes, a small peripheral retinal tear was noted or suspected during surgery and treated with cryotherapy or laser photocoagulation; 12 retinal tears in the posterior pole were reported, 4 in a foveal location and 8 in extrafoveal locations. In 153 eyes (70%), some blood remained after surgery; however, in only 11 (7%) of the 155 eyes for which information was available was residual subfoveal blood thick enough to elevate the retina. A choroidal hemorrhage and a macular hole were observed intraoperatively in one eye each; the surgeon reported that choroidal tissue had been extracted along with the fibrovascular lesion from another eye.
Completion of Scheduled Examinations
Thirty patients in the observation arm (13%) died after enrollment; 15 deaths occurred before the 24-month examination. Twenty-eight patients in the surgery arm (12%) died after enrollment, 11 before the 24-month examination. Among the 19 patients in the observation arm who missed the 24-month examination, 8 were examined at 36 or 48 months. Two of 8 surgery patients who missed the 24-month examination were examined at 36 or 48 months.
Of 1148 examinations scheduled for patients in the surgery arm at 3 months or later after enrollment, 1093 (95%) were completed. Except for the 48-month examination, more than 90% of the patients in the surgery arm were examined at each scheduled time. Patients in the observation arm returned for 1056 (91%) of 1158 scheduled examinations. Follow-up examination rates were consistently a little lower in the observation arm than in the surgery arm at each examination time. A similar percentage of patients in each VA stratum completed each scheduled examination.
Visual Acuity and Changes in Visual Acuity
The distributions of VA of study eyes at the 3-month examination through the 36-month examination are shown in Table 4. Of 401 study eyes with VA measured and reported at the 24-month examination, 364 (91%) had the measurements made by a traveling vision examiner masked to the SST clinical trial in which the patient was enrolled, the study eye, and the treatment assigned or received.
Table 4.
Distribution of Patients by Visual Acuity (VA) in the Study Eye When Examined at Specified Times after Enrollment, Submacular Surgery Trials Group N Trial
|
No. (%) of Study Eyes, by Time After Enrollment |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
3 Months |
6 Months |
12 Months |
24 Months |
36 Months |
||||||
| VA (Snellen Equivalent) | Observation (n = 205) | Surgery (n = 217) | Observation (n = 203) | Surgery (n = 215) | Observation (n = 210) | Surgery (n = 214) | Observation (n = 194)* | Surgery (n = 207)† | Observation (n = 154) | Surgery (n = 150) |
| ≥20/40 | 0 | 1 (<1) | 0 | 0 | 1 (<1) | 1 (<1) | 3 (2) | 0 | 2 (1) | 1 (1) |
| 20/50–20/80 | 15 (7) | 5 (2) | 9 (4) | 5 (2) | 9 (4) | 3 (1) | 8 (4) | 3 (1) | 5 (3) | 2 (1) |
| 20/100–20/160 | 43 (21) | 27 (13) | 44 (22) | 28 (13) | 26 (12) | 24 (11) | 16 (8) | 25 (12) | 15 (10) | 15 (10) |
| 20/200–20/320 | 71 (35) | 75 (34) | 62 (31) | 75 (35) | 68 (32) | 78 (36) | 65 (34) | 72 (35) | 47 (31) | 60 (40) |
| 20/400–20/640 | 59 (29) | 78 (36) | 60 (30) | 78 (36) | 64 (30) | 82 (38) | 67 (35) | 73 (35) | 57 (37) | 47 (31) |
| 20/800–20/1280 | 16 (8) | 25 (12) | 23 (11) | 25 (12) | 34 (16) | 23 (11) | 28 (14) | 25 (12) | 20 (13) | 17 (11) |
| ≤20/1600–LP | 1 (<1) | 6 (3) | 5 (2) | 4 (2) | 8 (4) | 3 (1) | 7 (4) | 9 (4) | 8 (5) | 8 (5) |
| Median VA | 20/250 | 20/400 | 20/320 | 20/320 | 20/400 | 20/400 | 20/400 | 20/400 | 20/400 | 20/320 |
LP = light perception.
Measurements of VA were made by masked examiners for 177 eyes (91%).
Measurements of VA were made by masked examiners for 187 eyes (90%).
The median VA of eyes in the observation arm declined from 20/200 at baseline to 20/250 at the 3-month examination (inter-quartile range, 20/160–20/500) and stabilized at 20/400 at the 12-month examination and later (interquartile range, 20/200–20/640). The median VA of study eyes in the surgery arm declined from 20/200 at baseline to 20/400 at the 3-month examination (interquartile range, 20/200–20/500) and remained close to 20/400 at later examinations (interquartile range, 20/250–20/500). The VA of the study eyes of 2 patients in the surgery arm had dropped to worse than 20/1600 (<5/400) by the 3-month examination but was at least LP. Two patients in the observation arm lost VA to this low level by the 24-month examination. The distributions of VA differed between treatment arms to a statistically significant degree only at the 3-month examination (P = 0.002) and favored the observation arm.
Distributions of changes in VA from baseline to annual follow-up examinations through the 36-month examination are summarized in Table 5. Distributions of change in VA to the 48-month examination (data not shown) were nearly identical to the 36-month distributions. In the surgery arm, 113 eyes (52%) lost 2 lines (8 letters) of VA or more from baseline to the 3-month examination, compared with 75 eyes (37%) in the observation arm. At the 12-month examination and each examination thereafter, half or more of the patients in each treatment arm had VA ≥2 lines worse than at baseline. The median change in VA in the observation arm was a ≥2.0-line loss from the 12-month examination through the 48-month examination. In the surgery arm, the median change ranged from a loss of 1.6 lines at the 6-month examination to a loss of 2.6 lines at the 48-month examination. The distributions of change in VA differed between treatment arms to a statistically significant degree only at the 3-month examination (P = 0.0014, Wilcoxon rank-sum test) in favor of observation. Based on 194 study eyes in the observation arm and 207 eyes in the surgery arm that had VA measured at the 24-month examination, the numbers and percentages of eyes with successful outcomes, by the SST definition, were 85 (44%) and 85 (41%), respectively (P = 0.61, chi-square test). The success ratio calculated for surgery eyes versus observation eyes examined at 24 months was 0.94 (95% confidence interval [CI], 0.75–1.18). Thus, approximately equal proportions of eyes in the 2 arms had VA at the 24-month examination that had stabilized close to baseline VA or, in a few eyes in each arm, was better than at baseline.
Table 5.
Distribution of Patients by Change in Visual Acuity in the Study Eye from Baseline to Examinations at Specified Times after Enrollment, Submacular Surgery Trials Group N Trial
|
No. (%) of Patients, by Time After Enrollment |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
3 Months |
6 Months |
12 Months |
24 Months |
36 Months |
||||||
| Change in Visual Acuity (Lines) | Observation (n = 205) | Surgery (n = 217) | Observation (n = 203) | Surgery (n = 215) | Observation (n = 210) | Surgery (n = 214) | Observation (n = 194) | Surgery (n = 207) | Observation (n = 154) | Surgery (n = 150) |
| ≥4 lines better | 1 (<1) | 5 (2) | 4 (2) | 6 (3) | 8 (4) | 7 (3) | 7 (4) | 6 (3) | 4 (3) | 8 (5) |
| 2–3 lines better | 27 (13) | 25 (12) | 17 (8) | 20 (9) | 16 (8) | 18 (8) | 19 (10) | 19 (9) | 11 (7) | 12 (8) |
| <2-line change | 102 (50) | 74 (34) | 89 (44) | 74 (34) | 69 (33) | 72 (34) | 59 (30) | 60 (29) | 47 (31) | 37 (25) |
| 2–3 lines worse | 40 (20) | 49 (23) | 44 (22) | 48 (22) | 46 (22) | 48 (22) | 38 (20) | 55 (27) | 28 (18) | 36 (25) |
| 4–5 lines worse | 21 (10) | 35 (16) | 28 (14) | 39 (18) | 34 (16) | 37 (17) | 37 (19) | 27 (13) | 30 (19) | 27 (18) |
| 6–7 lines worse | 10 (5) | 15 (7) | 16 (8) | 17 (8) | 23 (11) | 18 (8) | 18 (9) | 25 (12) | 22 (14) | 16 (11) |
| ≥8 lines worse | 4 (2) | 14 (6) | 5 (2) | 11 (5) | 14 (7) | 14 (7) | 16 (8) | 15 (7) | 12 (8) | 14 (9) |
| Median change (no. of lines) | −0.6 | −1.8 | −1.2 | −1.6 | −2.0 | −1.8 | −2.1 | −2.0 | −2.6 | −2.5 |
Seventy eyes (31%) in the observation arm and 66 eyes (29%) in the surgery arm experienced improvements in VA from baseline levels of ≥2 lines based on measurements made at ≥1 examinations after enrollment. Of these eyes, 52 (74%) of 70 in the observation arm and 41 (62%) of 66 in the surgery arm later lost VA to the baseline level or worse. Figure 1 displays the percentages of study eyes with VA at each follow-up examination of 2 lines (8 letters) or more worse than at baseline (defined as failures by the SST Planning Committee). The percentages of eyes with successful outcomes at the 24-month examination (and 95% CIs) calculated using this approach, which takes account of both losses and recoveries of VA and all available data for each patient, are 43% (36%–50%) in the observation arm and 41% (35%–48%) in the surgery arm. The resulting 24-month success ratio was 0.96 (95% CI, 0.77–1.19), indicating no meaningful difference between treatment arms with respect to VA outcome. At no examination was there any evidence that eyes in the surgery arm had a better VA outcome than eyes in the observation arm.
Figure 1.
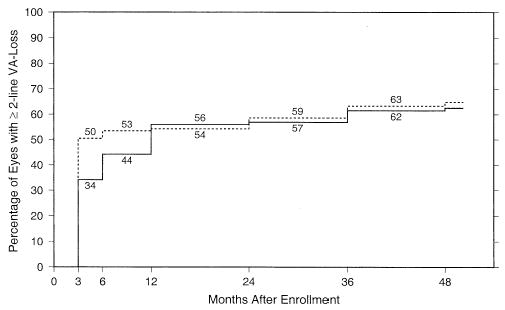
Percentage of patients in each treatment arm who had visual acuity (VA) of the study eye ≥2 lines worse than at baseline by each examination time after enrollment, based on a model that takes account of both losses and recoveries of VA and all examinations available for each patient. Solid line, observation arm (n = 228 patients); broken line, surgery arm (n = 226 patients).
The subgroups defined by baseline VA of the study eye were of particular interest because eyes with VA better than 20/200 had the potential of retaining moderately impaired vision. In addition, random assignment to treatment arm was stratified based on initial VA. Although eyes with VA better than 20/200 at baseline lost more lines of VA during follow-up than eyes with VA of 20/200 or worse initially, they retained better VA 24 months after enrollment than eyes with poorer VA initially: 20/320 versus 20/400, respectively, in both treatment arms. Mean 24-month losses of VA from baseline were 3.8 lines in the eyes with VA better than 20/200 initially and 1.1 lines in eyes with VA of 20/200 or worse initially, regardless of the treatment arm to which assigned. Thus, in neither subgroup was there clinically or statistically meaningful evidence of a beneficial effect of surgery.
Visual acuity outcomes also were compared between treatment arms within other subgroups of patients defined by baseline characteristics that were potential predictors of VA outcome to determine whether there was evidence to suggest that submacular surgery may benefit a subset of patients enrolled in the Group N Trial. Characteristics used to define subgroups included age (≤75 years, >75), gender, smoking history (ever, never), use of antihypertensive medication, baseline history of arthritis or rheumatism (yes, no), size of the subfoveal lesion (≤6 DAs, >6 DAs), percentage of the subfoveal lesion accounted for by classic choroidal neovascularization (≥50%, <50%), VA of the fellow eye (≥20/40, <20/40), and a choroidal neovascular lesion in the fellow eye (present, absent). In addition, outcomes by treatment arm were analyzed for subgroups of clinical centers that participated and did not participate in the pilot study. In none of these subgroups was there a suggestion that surgery may be beneficial for preventing or delaying further loss of VA in comparison with observation.
Reading Speed and Contrast Threshold
Changes during follow-up in study eye reading speed with enlarged text (letter size equivalent to approximately 20/1500 at the test distance) paralleled changes in VA shown in Table 5. The distributions of reading speed and of changes in reading speed favored observation eyes at the 3-month examination (P = 0.002 and P = 0.03, respectively; Wilcoxon rank-sum tests); reading speed findings in the 2 treatment arms were similar at all later examinations. Reading speed using only the study eye was slow for most patients. At the 24-month examination, only 58 (31%) of 188 observation eyes and 46 (24%) of 194 surgery eyes were reading at a rate of ≥40 words per minute; 90 (48%) observation eyes and 102 (53%) surgery eyes were either unable to read any of the enlarged text or able to read only at a rate of <20 words per minute.
Changes in contrast threshold of study eyes from baseline to each follow-up examination also were analyzed. The median change from baseline to each follow-up examination was no change in both treatment arms. Both the distributions of contrast threshold and the distributions of change in contrast threshold from baseline favored the surgery arm somewhat at the 6-month examination only (P = 0.03 and P = 0.08, respectively; Wilcoxon rank-sum tests).
Complications and Additional Treatments to Study Eyes
In accord with the recommendation of the Data and Safety Monitoring Committee in 1999 to modify the SST protocol to permit treatment of eligible eyes in the observation arm with photodynamic therapy with verteporfin, all untreated eyes (study eyes and fellow eyes) of all patients were evaluated early in 2000. The study eyes of 24 patients in the observation arm were judged eligible for photodynamic therapy and treated; 19 of these patients had treatment at the 12-month examination or earlier, 3 more by the 24-month examination, and 2 between the 24- and 48-month examinations. Two additional patients in the observation arm had laser photocoagulation in the study eye during follow-up, both by the 3-month examination.
Rhegmatogenous RDs were reported in the study eyes of 12 patients in the surgery arm, all by the 12-month examination, but in only 1 patient in the observation arm (36-month examination). Proliferative vitreoretinopathy developed in 4 of the 12 eyes with RDs in the surgery arm. In 11 of the surgery eyes, the RDs were repaired successfully with attachment of the macula; silicone oil was present in the remaining eye at the 36-month examination. This eye and 2 others that had RDs had VA worse than 20/1600 (<5/400) but at least as good as LP at the 24-month examination. Vitreous hemorrhage was observed in the study eyes of 4 patients in the surgery arm and 2 patients in the observation arm at ≥1 examinations; 2 of the surgery eyes had a vitrectomy during follow-up.
Leakage of fluorescein dye from choroidal neovascularization at the periphery of the area of retinal pigment disturbance was observed for 112 eyes after submacular surgery. Only 7 eyes had leakage observed solely within the disturbed area. Thus, a total of 119 eyes treated surgically had choroidal neovascularization (peripheral to the disturbed area, inside that area, or both) at some examination from 3 months through 24 months, inclusive, yielding a 24-month cumulative rate of 52% (95% CI, 46%–59%). However, at 24 months after enrollment, 119 (66%) of 181 eyes in the observation arm, versus only 41 (21%) of 195 eyes in the surgery arm, had classic or occult choroidal neovascularization (or both) visible on fluorescein angiography based on reviews at the Photograph Reading Center, as shown in Figure 2. Submacular surgery was repeated in 4 of the 119 surgery eyes with leakage observed during follow-up; 44 eyes had laser photocoagulation to recurrent choroidal neovascularization, and 7 had photodynamic therapy. Altogether, 53 (23%) surgically treated eyes had ≥1 additional treatments for choroidal neovascularization by the 24-month examination. Mean VA changes from baseline were compared for eyes that had peripheral leakage, central leakage, or no leakage at each examination time. Eyes without leakage had a mean change from baseline VA that was only 2 or 3 letters smaller than eyes with leakage. Eyes treated for leakage from choroidal neovascularization had VA changes 4 to 5 letters larger than those never treated.
Figure 2.
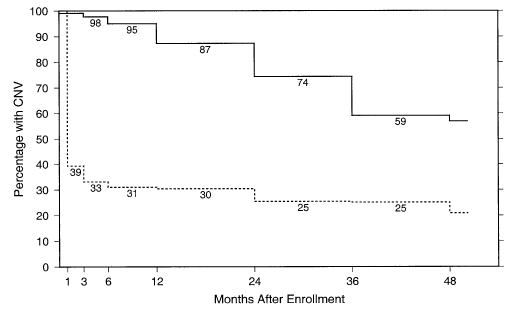
Percentage of study eyes at each follow-up examination that had leakage of dye from choroidal neovascularization (CNV) during fluorescein angiography identified during central review of photographs taken at follow-up examinations. Solid line, observation arm; broken line, surgery arm.
Visually significant cataract, defined as either cataract surgery or lens opacity reported by the SST ophthalmologist to be sufficient to reduce VA by ≥2 lines in a normal eye, developed in initially phakic study eyes of 50 of 133 patients in the observation arm and 123 of 142 patients in the surgery arm, for 2-year rates of 21% and 80%, respectively. The cumulative percentages of eyes that had cataract surgery are shown by treatment arm and follow-up time in Figure 3. Eighteen study eyes in the observation arm and 73 eyes in the surgery arm had cataract surgery. Eyes in the surgery arm that had cataract surgery during follow-up had a mean VA loss from baseline to the 24-month examination of 0.9 lines (4.5 letters), versus 2.6 lines (13 letters) lost in eyes that did not have cataract surgery. When VA changes were compared between treatment arms in eyes that were phakic at baseline and those that were aphakic or pseudophakic, there was no difference between eyes assigned to surgery and those assigned to observation in either subgroup of eyes.
Figure 3.
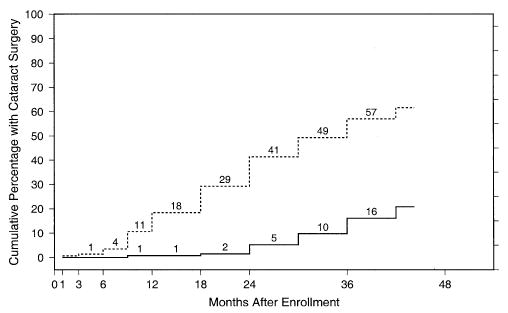
Percentage of patients in each treatment arm who had cataract surgery in the initially phakic study eye, by each examination and contact time after enrollment. Solid line, observation arm (n = 133 patients); broken line, surgery arm (n = 142 patients).
Changes in Size of Subfoveal Lesions
Three months after enrollment, the central macular lesions of 58 (28%) of 207 eyes in the surgery arm and 76 (38%) of 199 eyes in the observation arm were classified in the same size category as at baseline or in a smaller size category than at baseline (Table 6). Increases in the size of subfoveal lesions in surgery eyes were attributable to recurrent choroidal neovascularization and other factors.44 At each examination from 6 months through 36 months after enrollment, eyes in the observation arm showed more increases in size than eyes in the surgery arm (P<0.001, chi-square tests for trend). This trend for surgery eyes to have smaller lesions than observation eyes relative to baseline persisted through the 48-month examination, with 12 eyes (16%) of 76 in the surgery arm and 6 eyes (8%) of 76 in the observation arm having lesions of the same size or smaller compared with baseline. At the 24-month examination, 91 (54%) of 169 eyes in the observation arm but only 58 (34%) of 171 eyes in the surgery arm had subfoveal lesions that were ≥2 size categories larger than at baseline.
Table 6.
Changes in Size of Subfoveal Lesions from Baseline to Follow-up Examinations at Specified Times after Enrollment, Submacular Surgery Trials Group N Trial
|
No. (%) of Patients, by Time After Enrollment |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
3 Months |
6 Months |
12 Months |
24 Months |
36 Months |
||||||
| Change from Baseline (Categories) | Observation (n = 199) | Surgery (n = 207) | Observation (n = 194) | Surgery (n = 203) | Observation (n = 192) | Surgery (n = 193) | Observation (n = 169) | Surgery (n = 171) | Observation (n = 127) | Surgery (n = 123) |
| No change or smaller | 76 (38) | 58 (28) | 38 (20) | 63 (31) | 33 (17) | 58 (30) | 26 (15) | 41 (24) | 12 (9) | 24 (20) |
| 1 larger | 75 (38) | 74 (36) | 84 (43) | 81 (40) | 66 (34) | 77 (40) | 52 (31) | 72 (42) | 33 (26) | 49 (40) |
| 2 larger | 36 (18) | 52 (26) | 45 (23) | 45 (22) | 48 (25) | 39 (20) | 46 (27) | 37 (22) | 38 (30) | 32 (26) |
| ≥3 larger | 12 (6) | 23 (11) | 27 (14) | 14 (7) | 45 (23) | 19 (10) | 45 (27) | 21 (12) | 44 (35) | 18 (15) |
| Median change (no. of categories) | +1 | +1 | +1 | +1 | +1 | +1 | +2 | +1 | +2 | +1 |
Discussion
The SST Research Group was unable to demonstrate any benefit to submacular surgery compared with observation with respect to VA, reading speed with enlarged text, or contrast threshold in eyes with subfoveal choroidal neovascularization and age-related macular degeneration enrolled and followed in the Group N Trial. Both VA and reading speed of study eyes declined over time in both treatment arms. Despite high rates of recurrent choroidal neovascularization, eyes in the surgery arm maintained smaller sub-foveal lesions than eyes in the observation arm. Thus, the goal of a smaller subfoveal lesion after 2 years was achieved with surgery, but the smaller lesion did not result in better VA. The findings from health-related quality-of-life interviews conducted in parallel with clinical examinations and data collection suggest a small positive effect of surgery on self-reported visual function.26 However, a similar effect was not observed for any of the clinical outcomes analyzed for study eyes in the SST Group N Trial.
Although the target sample size was not enrolled in the SST Group N Trial, the study had >85% power to rule out differences in proportions of eyes with successful outcomes (improvement or stabilization of VA at 24 months after enrollment) in the 2 arms such as 35% versus 50%, 40% versus 55%, or 45% versus 60%. Thus, it is unlikely that a clinically significant difference in VA or other clinical outcomes favors submacular surgery in eyes eligible for the SST Group N Trial but was not detected due to the smaller achieved sample size. Furthermore, the percentage of eyes that had successful outcomes in each treatment arm, as defined a priori by the SST Planning Committee, was similar to that estimated from the observation arm in the earlier pilot study. When the SST pilot study ended, the 24-month success rates among patients with subfoveal neovascular lesions similar to those eligible for the SST Group N Trial were 56% in the surgery arm and 37% in the observation arm, for a success ratio of 1.5 (95% CI, 1.03–2.19). Although some differences in baseline characteristics have been noted between patients who enrolled in the pilot study and those who enrolled in the SST Group N Trial (e.g., age, presence of hypertension, VA of the study eye), no difference was large enough alone to account for the discrepancy in VA outcomes in the surgery arms. Furthermore, as discussed above, comparisons of VA outcomes by subgroups defined by age, health status, and characteristics of eyes and lesions identified none in which surgery resulted in a better outcome among the much larger number of patients who participated in the clinical trial. Thus, it is likely that poorer follow-up, less training of vision examiners, and usually unmasked VA measurements accounted for the apparent better outcome in the pilot study.
The SST Group N Trial was designed in 1995 after the results of the randomized trials of laser photocoagulation for subfoveal choroidal neovascularization conducted by the Macular Photocoagulation Study Group had been reported10–12 but before 1-year findings from the initial trial of photodynamic therapy with verteporfin13 were available. Patients selected for enrollment in the Group N Trial were those who would have been ineligible for the Macular Photocoagulation Study trial for new subfoveal choroidal neovascularization, because of large size or poorly demarcated boundaries of the subfoveal neovascular lesion or poor VA. After photodynamic therapy with verteporfin was reported to be beneficial for a subgroup of Group N–eligible eyes—namely, those with VA of 20/200 or better, particularly when classic choroidal neovascularization accounted for >50% of the total lesion—that treatment was offered to all patients in the Group N Trial who met the eligibility criteria established for the verteporfin trial. Because only 24 eyes (11%) in the observation arm and 7 eyes (3%) in the surgery arm ever were treated with photodynamic therapy, it is unlikely that a clinically meaningful beneficial effect of submacular surgery with respect to VA and other outcomes was masked by such treatment.
Unfortunately, treatments proven effective, as documented in the peer-reviewed literature, for delaying or preventing loss of VA after subfoveal choroidal neovascularization develops in eyes with age-related macular degeneration (i.e., laser photocoagulation and photodynamic therapy with verteporfin) are applicable only to subsets of patients eligible for the SST Group N Trial. Most patients who enrolled in this randomized trial had neovascular lesions that were too large or had lost too much vision in the study eye to be expected to benefit from those treatments. Submacular surgery, as performed in this clinical trial, has been shown to be ineffective for, and is not recommended by the SST Research Group for treating, eyes with subfoveal choroidal neovascularization due to age-related macular degeneration similar to those eligible for the SST Group N Trial. Nevertheless, the information emanating from this randomized trial in which all patients were observed for at least 2 years and more than half were observed for 3 years, summarized in this article and elsewhere,26 should be useful to researchers who design future trials of treatments for patients with subfoveal choroidal neovascularization in age-related macular degeneration. Pharmacologic agents currently under evaluation in randomized clinical trials may expand therapeutic options for these lesions in the future if proven to be effective.
Although the search for new effective treatments of choroidal neovascularization and other vision-threatening complications of age-related macular degeneration continues, research into methods to prevent development of these complications also must continue. To date, the only treatment demonstrated to be effective in preventing choroidal neovascularization in high-risk eyes is daily ingestion of a specific vitamin and mineral supplement.45 Long-term randomized trials of prophylactic laser treatment in high-risk eyes are underway; these include the Complications of Age-Related Macular Degeneration Prevention Trial.46 However, much more research, including a better understanding of the genetic, environmental, and other factors that contribute to the development of the high-risk characteristics that precede choroidal neovascularization and progression to the exudative stage, will be necessary before significant progress is achieved toward prevention of age-related macular degeneration and the vision-threatening complications.
Table 1.
Sociodemographic Characteristics and Health History of Patients at Time of Enrollment in the Submacular Surgery Trials Group N Trial
|
By VA and Treatment Arm |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
By Treatment Arm |
VA>20/200 |
VA<20/200 |
|||||||
| Total (n = 454) | Observation (n = 228) | Surgery (n = 226) | Total (n = 207) | Observation (n = 105) | Surgery (n = 102) | Total (n = 247) | Observation (n = 123) | Surgery (n = 124) | |
| Age (yrs) | |||||||||
| <60 | 5 (1) | 1 (<1) | 4 (2) | 2 (1) | 0 | 2 (2) | 3 (1) | 1 (1) | 2 (2) |
| 60–69 | 48 (11) | 22 (10) | 26 (12) | 19 (9) | 10 (10) | 9 (9) | 29 (12) | 12 (10) | 17 (14) |
| 70–79 | 241 (53) | 120 (53) | 121 (54) | 118 (57) | 61 (58) | 57 (56) | 123 (50) | 59 (48) | 64 (51) |
| 80–89 | 154 (34) | 81 (36) | 73 (32) | 67 (32) | 34 (32) | 33 (32) | 87 (35) | 47 (38) | 40 (32) |
| ≥90 | 6 (1) | 4 (2) | 2 (1) | 1 (<1) | 0 | 1 (1) | 5 (2) | 4 (3) | 1 (1) |
| Gender | |||||||||
| Woman | 241 (53) | 122 (54) | 119 (53) | 110 (53) | 56 (53) | 54 (53) | 131 (53) | 66 (54) | 65 (52) |
| Man | 213 (47) | 106 (46) | 107 (47) | 97 (47) | 49 (47) | 48 (47) | 116 (47) | 57 (46) | 59 (48) |
| Race/ethnicity* | |||||||||
| White, not Hispanic | 443 (98) | 223 (98) | 220 (97) | 202 (99) | 103 (98) | 99 (97) | 241 (98) | 120 (97) | 121 (97) |
| American Indian or Alaskan Native | 4 (1) | 2 (1) | 2 (1) | 2 (1) | 1 (1) | 1 (1) | 2 (1) | 1 (1) | 1 (1) |
| Asian or Pacific Islander | 4 (1) | 1 (<1) | 3 (1) | 2 (1) | 1 (1) | 1 (1) | 2 (1) | 0 | 2 (1) |
| Black, not Hispanic | 1 (<1) | 1 (<1) | 0 | 0 | 0 | 0 | 1 (<1) | 1 (1) | 0 |
| Unknown | 2 (<1) | 1 (<1) | 1 (<1) | 1 (<1) | 0 | 1 (1) | 1 (<1) | 1 (1) | 0 |
| Occupational status | |||||||||
| Retired | 401 (88) | 210 (92) | 191 (85) | 182 (89) | 96 (91) | 86 (84) | 219 (89) | 114 (93) | 105 (85) |
| Employed | 34 (7) | 10 (4) | 24 (11) | 15 (7) | 5 (5) | 10 (10) | 19 (8) | 5 (4) | 14 (11) |
| Housespouse | 15 (3) | 7 (3) | 8 (4) | 8 (4) | 4 (4) | 4 (4) | 7 (2) | 3 (2) | 4 (3) |
| Disabled due to vision | 3 (1) | 1 (<1) | 2 (1) | 1 (<1) | 0 | 1 (1) | 2 (1) | 1 (1) | 1 (1) |
| Unemployed | 1 (<1) | 0 | 1 (<1) | 1 (<1) | 0 | 1 (1) | 0 | 0 | 0 |
| Smoking history | |||||||||
| Never smoked | 166 (37) | 75 (33) | 91 (40) | 71 (34) | 32 (30) | 39 (38) | 95 (38) | 43 (35) | 52 (42) |
| Quit smoking | 232 (51) | 124 (54) | 108 (48) | 112 (54) | 63 (60) | 49 (48) | 120 (49) | 61 (50) | 59 (48) |
| Currently smoking | 56 (12) | 29 (13) | 27 (12) | 24 (12) | 10 (10) | 14 (14) | 32 (13) | 19 (15) | 13 (10) |
| Aspirin intake | |||||||||
| <7/wk | 283 (62) | 138 (61) | 145 (64) | 138 (67) | 69 (67) | 69 (67) | 145 (59) | 69 (57) | 76 (62) |
| ≥7/wk | 171 (38) | 90 (39) | 81 (36) | 69 (33) | 36 (34) | 33 (32) | 102 (41) | 54 (44) | 48 (39) |
| Warfarin use† | |||||||||
| ≤4 wks earlier | 11 (2) | 7 (3) | 4 (2) | 3 (1) | 1 (1) | 2 (2) | 8 (3) | 6 (5) | 2 (2) |
| >4 wks earlier | 44 (10) | 24 (11) | 20 (9) | 24 (12) | 11 (10) | 13 (13) | 20 (8) | 13 (11) | 7 (6) |
| Never | 398 (88) | 197 (86) | 201 (89) | 179 (86) | 93 (89) | 86 (85) | 219 (89) | 104 (85) | 115 (93) |
| Chronic conditions | |||||||||
| Hypertension‡ | 260 (57) | 133 (58) | 127 (56) | 118 (57) | 60 (57) | 58 (57) | 142 (57) | 73 (59) | 69 (56) |
| Diabetes mellitus | 48 (11) | 27 (12) | 21 (9) | 21 (10) | 14 (13) | 7 (7) | 27 (11) | 13 (11) | 14 (11) |
| Any other known§ | 315 (86) | 157 (87) | 158 (84) | 142 (88) | 67 (84) | 75 (93) | 173 (84) | 90 (90) | 83 (78) |
VA = visual acuity.
Data are nos. (%) of patients.
Based on self-reports.
Not reported for one patient in the surgery arm.
Based on history and/or current use of antihypertensive medication.
Based on responses from 180 patients in the observation arm and 188 in the surgery arm who were queried.
Acknowledgments
The Submacular Surgery Trials Research Group gratefully acknowledges the contributions of ophthalmologists who referred patients to the study and, in particular, of patients who enrolled, agreed to random assignment to treatment arm, and returned for scheduled follow-up examinations to benefit future patients.
Appendix 1: Members of the Submacular Surgery Trials Research Group, July 1998 through September 2003
Clinical Centers and Personnel Who Contributed Data for SST Patients with Age-Related Macular Degeneration: SST Groups N and B Trials
Centers are listed in alphabetic order by city. The number of patients enrolled in each trial is given in parentheses after the center location. Personnel listed are principal investigators and other personnel who performed ≥5 examinations or procedures for patients in these trials by the end of data collection on September 30, 2003.
Emory University Eye Center, Atlanta, Georgia (23 N, 14 B). Principal Investigator: G. Baker Hubbard III, MD, Paul Sternberg, Jr, MD (1999–2002), Antonio Capone, MD (1998–1999). Ophthalmologist: Thomas M. Aaberg, Jr, MD. SST coordinator: Jayne M. Brown. Vision examiners: Lindy G. Dubois, COMT, Judy Johnson, COMT, Natalie Schmitz. Photographers: James E. Gilman, CRA, Robert A. Myles, CRA, Ray Swords.
The Wilmer Ophthalmologic Institute, Baltimore, Maryland (42 N, 26 B). Principal Investigator: Julia A. Haller, MD. Ophthalmologists: Peter A. Campochiaro, MD, Mark Humayun, MD, Eugene de Juan, Jr, MD, Dante J. Pieramici, MD, Ingrid Zimmer-Galler, MD. SST coordinators and vision examiners: Michaele Hartnett, COT, Patricia L. Hawse, MS, COMT, Tracey L. Porter, COT, Ann Eager Youngblood, COA. Photographers: Judith E. Belt, Dennis Cain, CRA, David Emmert, Rachel E. Falk, Terry George, Mark Herring, Jacquelyn McDonald.
Massachusetts Eye and Ear Infirmary, Boston, Massachusetts (1 N, 1 B). Principal Investigator: Jorge G. Arroyo, MD.
Illinois Retina Associates, Chicago, Illinois (11 N, 19 B). Principal Investigator: Mathew W. MacCumber, MD, PhD. Ophthalmologists: Joseph Civantos, MD, Kirk H. Packo, MD. SST coordinators and vision examiners: Maggie De Alba, Michelle Franzyck, Bruce L. Gaynes, OD, Pharm D, Laurie Rago, Chris Morrison, Carrie Violetto. Photographers: Douglas A. Bryant, CRA, Donald Doherty, Frank Morini.
Northwestern University Medical School, Chicago, Illinois (4 N, 2 B). Principal Investigator: David V. Weinberg, MD. Ophthalmologist: Robert Schroeder, MD. SST coordinator: Jill Koecher. Vision examiner: Zuzanna Strugala. Photographers: Marsha Apushkin, Alexander Habib, Jim Yuhr.
Cole Eye Institute, Cleveland, Ohio (21 N, 17 B). Principal Investigator: Hilel Lewis, MD. Ophthalmologist: Peter K. Kaiser, MD. SST coordinators: Laura Holody, COA, Larissa S. Schaaf, RN. Vision examiners: Ginny Ambrose, Anthony Fattori, Helene Siegel. Photographers: Gloria M. Bartram, Stephanie L. Burke, Nicole Drozda, Tami Fecko, Deborah J. Ross, CRA.
Retina Associates of Cleveland, Cleveland, Ohio (20 N, 20 B). Principal Investigator: Lawrence J. Singerman, MD. Ophthalmologists: Michael A. Novak, MD, Scott D. Pendergast, MD. SST coordinators and vision examiners: Lori M. Campana, Kim Tilocco DuBois, COA, CCRP, Susan C. Rath, PA-C, Vivien Tanner, COA, CCRP. Photographers: John DuBois, CRA, Greg Greanoff, COA, CRA, David Lehnhardt, COA, Sheila Smith-Brewer, COMT, CRA, Kimberley Spagnoletta.
Ohio State University, Columbus, Ohio (7 N, 2 B). Principal Investigator: Frederick H. Davidorf, MD. Ophthalmologist: Robert Chambers, DO. SST coordinator: Cynthia Taylor. Vision examiners: Jill Milliron, COA, Jerilyn Perry, COT. Photographer: Scott Savage, EMT-A.
Texas Retina Associates, Dallas, Texas (8 N, 11 B). Principal Investigator: David G. Callanan, MD. Ophthalmologist: Gary Edd Fish, MD. SST coordinators: Jodi R. Creighton, COA, Jeff L. Harris, Nancy Resmini, Rubye Rollins. Vision examiner: Marilyn Andrews, COT. Photographers: Hank A. Aguado, CRA, Bob H. Boleman, Penny Ellenich.
Duke University Eye Center, Durham, North Carolina (10 N, 12 B). Principal Investigator: Cynthia A. Toth, MD. SST coordinators and vision examiners: Malcolm W. Anderson, PA-C, COT, Jennifer V. Caldwell. Photographers: Teresa Jackson Hawks, Gregory C. Hoffmeyer, Jeffrey M. Napoli.
Retina Associates of Hawaii, Honolulu, Hawaii (8 N, 1 B). Principal Investigator: Neal H. Atebara, MD. SST coordinator: Susan Pelke. Vision examiners and photographers: Deborah J. Nobler, Andrew Yuen.
Mid-America Retina Consultants, Kansas City, Missouri (7 N, 2 B). Principal Investigator: William N. Rosenthal, MD. Ophthalmologist: David S. Dyer, MD. SST coordinators and vision examiners: Denise Moore, RN, Barbara Petro, COT, Dalton J. Thibodeaux. Photographer: R. Scott Varner.
Southeastern Retina Associates, Knoxville, Tennessee (25 N, 24 B). Principal Investigator: John C. Hoskins, MD. Ophthalmologist: Joseph M. Googe, MD. SST coordinators: Katie E. Carter, COA, Stephanie M. Evans, Tina Thibodeaux Higdon, Jennifer L. Holton. Vision examiner: Bruce D. Gilliland, OD. Photographers: Paul Andrew Blais, Philip Michael Jacobus.
Retina and Vitreous Associates of Kentucky, Lexington, Kentucky (21 N, 8 B). Principal Investigator: William J. Wood, MD. Ophthalmologist: Rick D. Isernhagen, MD. SST coordinators: Michelle L. Buck, COA, J. Lynn Cruz, COT, Joni D. James, RN, Tammy L. Jordan, RN, Jenny L. Wolfe, RN. Vision examiners: Christine Brown, COT, Wanda Heath, COT, Catherine Millett, COA. Photographers: Marty Reid, COA, Edward Slade, CRA, COA.
Jules Stein Eye Institute, Los Angeles, California (35 N, 25 B). Principal Investigator: Steven D. Schwartz, MD. Ophthalmologists: Robert E. Engstrom, MD, Christine Gonzales, MD, Kent Small, MD. SST coordinators: Jo Eure, Jessica Hsu, Rosaleen Ostrick, MPH, MA, Dai Tran, Tina Wong. Vision examiners: Lisa Barnhart, Janine Chin, Melissa Chun, OD, Larisa Johnson, Jennie Y. Kageyama, OD. Photographers: Mirella Tetreault, Dennis Thayer, Bret Trump.
California Vitreoretinal Associates, Menlo Park, California (1 N). Principal Investigator: Mark S. Blumenkranz, MD. Clinic coordinator: Patricia Mattio. Vision examiner and photographer: Lora Lamborn.
Vitreoretinal Surgery, PA, Minneapolis, Minnesota (21 N, 14 B). Principal Investigator: David F. Williams, MD. Ophthalmologists: Sundeep Dev, MD, Robert A. Mittra, MD. Clinic coordinators and vision examiners: Julianne Enloe, Scott D. Marella, Neal Oestreich, COT. Photographer: Holly N. Cheshier.
McGee Eye Institute, Oklahoma City, Oklahoma (21 N, 10 B). Principal Investigator: Reagan H. Bradford, Jr, MD, Sumit K. Nanda, MD (1998–2002). SST coordinators and vision examiners: Angela Monlux, COT, Lisa M. Ogilbee. Photographer: Russ Burris, COT, CRA.
Retinal Consultants of Arizona, Phoenix, Arizona (44 N, 22 B). Principal Investigator: Jack O. Sipperley, MD. Ophthalmologist: Scott R. Sneed, MD. SST coordinators: Jaclin J. Jacobsen, CRA, COA, Eleonora Tysiac. Vision examiners: Denise Freistroffer, Nickie Perez, Pearl L. Rosas, Debra Tomaszewski. Photographers: John J. Bucci, Sharon H. Kosecki, John V. Martin.
Retina Vitreous Consultants, Pittsburgh, Pennsylvania (23 N, 17 B). Principal Investigator: Robert L. Bergren, MD. Ophthalmologist: Bernard Doft, MD. SST coordinators: Donna J. Metz, RN, Kathryn Sedory, RN, Christina Trombetta, CST. Vision examiners: Grace Rigoni, COA, Lynn Wellman, COA, Linda Wilcox, COA. Photographers: Alan Campbell, CRA, David Steinberg, CRA, Gary Vagstad, CRA.
Oregon Health Sciences University, Portland, Oregon (29 N, 22 B). Principal Investigator: David J. Wilson, MD. SST coordinator and vision examiner: Susan Pope. Photographers: Ellen Redenbo, Peter Steinkamp, Patrick Wallace.
Associated Retinal Consultants, Royal Oak, Michigan (22 N, 18 B). Principal Investigator: George A. Williams, MD. Ophthalmologists: Bruce R. Garretson, MD, Alan Ruby, MD. SST coordinators and vision examiners: Kristi L. Cumming, RN, MSN, Bobbie Lewis, RN, Patricia Manatrey, Mary Zajechewski. Photographers: Craig Bridges, Patricia Streasick, Lynette Szydlowski.
Barnes Retina Institute, St. Louis, Missouri (21 N, 18 B). Principal Investigator: Nancy M. Holekamp, MD. Ophthalmologists: Matthew A. Thomas, MD, Daniel P. Joseph, MD. SST coordinators and vision examiners: Julie Binning, COT, Lynda Boyd, COT, Janel Gualdoni, COT, Virginia S. Nobel, COT. Photographers: Rhonda Allen, Bryan D. Barts, Jon E. Dahl, Timothy S. Holle, Ella Ort, Matt Raeber, John Mark Rogers.
West Coast Retina Medical Group, Inc., San Francisco, California (13 N, 16 B). Principal Investigator: H. Richard McDonald, MD. Ophthalmologist: Robert N. Johnson, MD. SST coordinators: Margaret M. Stolarczuk, OD, Patricia Wood, LVN. Vision examiner: Kevan E. Curren, COA. Photographers: Kelly Ann DeBoer, Sarah M. Huggans, Jeremy R. Miller, John P. Uy.
St. Vincent Mercy Medical Center, Retina Vitreous Associates, Toledo, Ohio (16 N, 15 B). Principal Investigator: Samuel R. Pesin, MD. Ophthalmologists: Charles K. Dabbs, MD, Nicholas J. Leonardy, MD. SST coordinator and vision examiner: James M. Haener, COT. Photographers: Richard D. Hill, Dawn DeFalco, Lauren M. Cedoz, CRA.
Resource Centers: The Wilmer Ophthalmologic Institute, Baltimore, Maryland
Chairman’s Office, Retinal Vascular Center. Principal Investigator and SST Chair: Neil M. Bressler, MD. Traveling vision examiners: Peggy R. Orr, MPH, COMT (vision testing coordinator), Kristi L. Cumming, RN, MSN, Janel Gualdoni, COT, James M. Haener, COT, Michaele Hartnett, COT, Patricia L. Hawse, MS, COMT. Economic analyst: Eric B. Bass, MD, MPH. Other personnel: Dawn Childs, Connie Lawson, Irene L. Felicetti (1998–2002), Patricia Staflin (1998–2001).
Coordinating Center, Wilmer Clinical Trials and Biometry. Principal Investigator: Barbara S. Hawkins, PhD. Biostatisticians: Ashley L. Childs, MS, Li Ming Dong, PhD, Marta J. Marsh, MS. Epidemiologist: Päivi H. Miskala, PhD. Data coordination and telephone interviews: Rob G. Casper, MS, Alice D. Keith (1999–2003), Lee D. McCaffrey, MA (1998–2002), Dawn K. Smith (1998–2003). Systems management and programming: Kurt Dreger, Harris A. Jaffee, PhD, M. Marvin Newhouse, Stephen C. Grubb, MS (1998–1999). Other personnel: Patricia A. James, Lisa A. Lassiter, Kelly S. Manos, MAS, Gregory A. Surplus, Christine B. Alden (2000–2002), Takisha R. Kiah (1998–2002), Nancy A. Prusakowski, MS (1998–2000).
Photograph Reading Center, Wilmer Photograph Reading Center. Principal Investigator: Susan B. Bressler, MD. Ophthalmologists: Sharon D. Solomon, MD, Dante J. Pieramici, MD (1997–2001), Srinivas R. Sadda, MD (2000–2002). Consultant: Oliver D. Schein, MD. Operations Director: Rochelle E. Smith, Judith Alexander (1998), Kelly S. Manos, MAS (1998–2002). Coordinators: LaKaye Mbah, Reva W. Strozykowski (1998–2003), Isabel Mills (1999–2002). Photograph graders: Rita L. Denbow, MLA, Michael P. Minotti, Deborah A. Phillips, Yan Tian.
Sponsor: The National Eye Institute, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland. Director: Paul A. Sieving, MD, PhD, Carl Kupfer, MD (1998–2000). Deputy Director: Jack A. McLaughlin, PhD. Program Director: Maryann Redford, DDS, MPH, Mary Frances Cotch, PhD (1998–2001).
Committees and Members
Data and Safety Monitoring Committee. Voting members (appointed): Argye I. Hillis, PhD (Chair), Gary W. Abrams, MD, John E. Connett, PhD, Christine Grady, RN, PhD, Earl G. Harrison, LLD (deceased), Lee M. Jampol, MD. Non-voting members (ex officio): Neil M. Bressler, MD, Barbara S. Hawkins, PhD, Marta J. Marsh, MS, Maryann Redford, DDS, MPH, Mary Frances Cotch, PhD (1998–2000), Li Ming Dong, PhD (2000–2003).
Operations Committee. Neil M. Bressler, MD (SST Chair), Paul Sternberg, Jr, MD (SST Vice Chair), Matthew A. Thomas, MD (SST Vice Chair), Susan B. Bressler, MD, Barbara S. Hawkins, PhD, Maryann Redford, DDS, MPH.
Executive Committee (As of 2004). Neil M. Bressler, MD (Chair), Eric B. Bass, MD, MPH, Susan B. Bressler, MD, Jayne M. Brown, Hans E. Grossniklaus, MD, Julia A. Haller, MD, Barbara S. Hawkins, PhD, Nancy M. Holekamp, MD, Carol M. Mangione, MD, MSPH, H. Richard McDonald, MD, Peggy R. Orr, MPH, COMT, Maryann Redford, DDS, MPH, Paul Sternberg, Jr, MD, Matthew A. Thomas, MD, David J. Wilson, MD.
Adverse Event Review Committee. Voting members: Julia A. Haller, MD (Chair), Gary W. Abrams, MD, Lee M. Jampol, MD. Ex officio member: Barbara S. Hawkins, PhD.
Patient-Centered Outcomes Subcommittee. Carol M. Mangione, MD, MSPH (Chair), Eric B. Bass, MD, MPH (Vice Chair), Neil M. Bressler, MD, Ashley L. Childs, MS, Li Ming Dong, PhD, Barbara S. Hawkins, PhD, Harris A. Jaffee, PhD, Marta J. Marsh, MS, Päivi H. Miskala, PhD, Lee D. McCaffrey, MA (1998–2002). (Participation of Dr Mangione as chair of the Patient-Centered Outcomes Subcommittee is supported by a contract between the David Geffen School of Medicine, University of California, Los Angeles, California, and the Johns Hopkins University, Baltimore, Maryland.)
Quality Assurance and Monitoring Subcommittee. Barbara S. Hawkins, PhD (Chair), Susan B. Bressler, MD (Vice Chair), Judith E. Belt, Li Ming Dong, PhD, Julia A. Haller, MD, Michaele Hartnett, COT, Harris A. Jaffee, PhD, Carol M. Mangione, MD, MSPH, Marta J. Marsh, MS, Lee D. McCaffrey, MA, Päivi H. Miskala, PhD, Peggy R. Orr, MPH, COMT, Stephen C. Grubb, MS (1998–2000), Kelly S. Manos, MAS (1998–2002).
Surgery Subcommittee. Matthew A. Thomas, MD (Chair), Julia A. Haller, MD (Vice Chair), Eugene de Juan, Jr, MD, Paul Sternberg, Jr, MD.
Vision Testing Subcommittee. Peggy R. Orr, MPH, COMT (Chair), Michaele Hartnett, COT, Patricia L. Hawse, MS, COMT, Harris A. Jaffee, PhD, Marta J. Marsh, MS, Stephen C. Grubb, MS (1998–2000), Lee D. McCaffrey, MA (1998–2002), Gary S. Rubin, PhD (1998–1999).
Writing Committee for SST Report Number 11 on Behalf of the SST Research Group. Barbara S. Hawkins, PhD (Chair), Neil M. Bressler, MD, Päivi H. Miskala, PhD, Susan B. Bressler, MD, Nancy M. Holekamp, MD, Marta J. Marsh, MS, Maryann Redford, DDS, MPH, Steven D. Schwartz, MD, Paul Sternberg, Jr, MD, Matthew A. Thomas, MD, David J. Wilson, MD.
Appendix 2: Pertinent Financial Interests Reported by Members of the SST Research Group
Neil M. Bressler, MD: Dr Bressler’s employer, the Johns Hopkins University, but not Dr Bressler, receives funding from Novartis Pharma AG (Basel, Switzerland), QLT Inc. (Vancouver, Canada), and Genentech (South San Francisco, California) for consulting services and research efforts by Dr Bressler. The terms of these arrangements are managed by the Johns Hopkins University according to its policies regarding potential conflicts of interest.
Susan B. Bressler, MD: Dr Bressler’s employer, the Johns Hopkins University, but not Dr Bressler, receives funding from Novartis Pharma AG (Basel, Switzerland) and QLT Inc. (Vancouver, Canada) for research efforts by Dr Bressler. The terms of these arrangements are managed by the Johns Hopkins University according to its policies regarding potential conflicts of interest.
Steven D. Schwartz, MD: Scientific Advisory Board member and equity interest, Eyetech Pharmaceuticals, Inc. (New York, New York).
Footnotes
Pertinent financial interests reported by members of the SST Research Group are summarized in Appendix 2; individual statements are on file in the SST Chairman’s Office, Baltimore, Maryland. The Submacular Surgery Trials are sponsored by the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland (cooperative agreements U10 EY11547, EY11557, EY11558 with the Johns Hopkins University, Baltimore, Maryland). Participating clinical centers were supported by contracts with the Johns Hopkins University.
References
- 1.Macular Photocoagulation Study Group. Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Arch Ophthalmol. 1993;111:1189–99. doi: 10.1001/archopht.1993.01090090041018. [DOI] [PubMed] [Google Scholar]
- 2.Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol. 1997;115:741–7. doi: 10.1001/archopht.1997.01100150743009. [DOI] [PubMed] [Google Scholar]
- 3.Submacular Surgery Trials Research Group. Health- and vision-related quality of life among patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration at time of enrollment in randomized trials of submacular surgery. SST report no. 4. Am J Ophthalmol. 2004;138:91–108. doi: 10.1016/j.ajo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Macular Photocoagulation Study Group. Argon laser photocoagulation for senile macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1982;100:912–8. doi: 10.1001/archopht.1982.01030030920003. [DOI] [PubMed] [Google Scholar]
- 5.Macular Photocoagulation Study Group. Krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1990;108:816–24. doi: 10.1001/archopht.1990.01070080058036. [DOI] [PubMed] [Google Scholar]
- 6.Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Arch Ophthalmol. 1991;109:1109–14. [PubMed] [Google Scholar]
- 7.Macular Photocoagulation Study Group. Laser photocoagulation for juxtafoveal choroidal neovascularization. Five-year results from randomized clinical trials. Arch Ophthalmol. 1994;112:500–9. [PubMed] [Google Scholar]
- 8.Bermig J, Tylla H, Jochmann C, et al. Angiographic findings in patients with exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2002;240:169–75. doi: 10.1007/s00417-001-0378-2. [DOI] [PubMed] [Google Scholar]
- 9.Olsen TW, Feng X, Kasper TJ, et al. Fluorescein angiographic lesion type frequency in neovascular age-related macular degeneration. Ophthalmology. 2004;111:250–5. doi: 10.1016/j.ophtha.2003.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1220–31. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- 11.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal recurrent neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1232–41. doi: 10.1001/archopht.1991.01080090056026. [DOI] [PubMed] [Google Scholar]
- 12.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Arch Ophthalmol. 1993;111:1200–9. doi: 10.1001/archopht.1993.01090090052019. [DOI] [PubMed] [Google Scholar]
- 13.Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin. One-year results of 2 randomized clinical trials—TAP report 1. Arch Ophthalmol. 1999;117:1329–45. [PubMed] [Google Scholar]
- 14.Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials—TAP report 2. Arch Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- 15.Verteporfin in Photodynamic Therapy (VIP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—Verteporfin in Photodynamic Therapy report 2. Am J Ophthalmol. 2001;131:541–60. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 16.Macular Photocoagulation Study Group. Recurrent choroidal neovascularization after argon laser photocoagulation for neovascular maculopathy. Arch Ophthalmol. 1986;104:503–12. doi: 10.1001/archopht.1986.01050160059012. [DOI] [PubMed] [Google Scholar]
- 17.Macular Photocoagulation Study Group. Persistent and recurrent neovascularization after krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Arch Ophthalmol. 1990;108:825–36. doi: 10.1001/archopht.1990.01070080067037. [DOI] [PubMed] [Google Scholar]
- 18.Macular Photocoagulation Study Group. Persistent and recurrent neovascularization after laser photocoagulation for sub-foveal choroidal neovascularization of age-related macular degeneration. Arch Ophthalmol. 1994;112:489–99. doi: 10.1001/archopht.1994.01090160065024. [DOI] [PubMed] [Google Scholar]
- 19.Pharmacological Therapy for Macular Degeneration Study Group. Interferon alfa-2a is ineffective for patients with choroidal neovascularization secondary to age-related macular degeneration. Results of a prospective randomized placebo-controlled clinical trial. Arch Ophthalmol. 1997;115:865–72. doi: 10.1001/archopht.1997.01100160035005. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarthy U, Houston RF, Archer DB. Treatment of age-related subfoveal neovascular membranes by teletherapy: a pilot study. Br J Ophthalmol. 1993;77:265–73. doi: 10.1136/bjo.77.5.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radiation Therapy for Age-Related Macular Degeneration (RAD) Study Group. A prospective, randomized, double-masked trial of radiation therapy for neovascular age-related macular degeneration (RAD Study) Ophthalmology. 1999;106:2239–47. doi: 10.1016/s0161-6420(99)90522-5. [DOI] [PubMed] [Google Scholar]
- 22.Thomas MA, Grand MD, Williams DF, et al. Surgical management of subfoveal choroidal neovascularization. Ophthalmology. 1992;99:952–68. doi: 10.1016/s0161-6420(92)31888-3. discussion 975–6. [DOI] [PubMed] [Google Scholar]
- 23.Berger AS, Kaplan HJ. Clinical experience with the surgical removal of subfoveal neovascular membranes. Short-term postoperative results. Ophthalmology. 1992;99:969–75. doi: 10.1016/s0161-6420(92)31869-x. discussion 975–6. [DOI] [PubMed] [Google Scholar]
- 24.Lambert HM, Capone A, Jr, Aaberg TM, et al. Surgical excision of subfoveal neovascular membranes in age-related macular degeneration. Am J Ophthalmol. 1992;113:257–62. doi: 10.1016/s0002-9394(14)71576-4. [DOI] [PubMed] [Google Scholar]
- 25.Submacular Surgery Trials Pilot Study Investigators. Sub-macular Surgery Trials randomized pilot trial of laser photocoagulation versus surgery for recurrent choroidal neovascularization secondary to age-related macular degeneration. I. Ophthalmic outcomes. Submacular Surgery Trials Pilot Study report number 1. Am J Ophthalmol. 2000;130:387–407. doi: 10.1016/s0002-9394(00)00729-7. [DOI] [PubMed] [Google Scholar]
- 26.Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: quality-of-life findings. SST report no. 12. Ophthalmology. 2004;111:1981–92. doi: 10.1016/j.ophtha.2004.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Submacular Surgery Trials (SST) Research Group. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings. SST report no. 13. Ophthalmology. 2004;111:1993–2006. doi: 10.1016/j.ophtha.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Submacular Surgery Trials (SST) Research Group. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: quality-of-life findings. SST report no. 14. Ophthalmology. 2004;111:2007–14. doi: 10.1016/j.ophtha.2004.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Submacular Surgery Trials (SST) Manual of Procedures. Springfield, VA: National Technical Information Service; 1998. NTIS report no. PB98-166648
- 30.Submacular Surgery Trials (SST) Forms Book. Springfield, VA: National Technical Information Service; 1998. NTIS report no. PB98-159445.
- 31.Ferris FL, III, Kassoff A, Bresnick GH, et al. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–6. [PubMed] [Google Scholar]
- 32.Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci. 1988;2:187–99. [Google Scholar]
- 33.Rubin GS. Reliability and sensitivity of clinical contrast sensitivity tests. Clin Vis Sci. 1988;2:169–77. [Google Scholar]
- 34.Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the Macular Photocoagulation Study. Arch Ophthalmol. 1991;109:1242–57. [PubMed] [Google Scholar]
- 35.Macular Photocoagulation Study Group. Visual outcome after laser photocoagulation for subfoveal choroidal neovascularization secondary to age-related macular degeneration. The influence of intial lesion size and initial visual acuity. Arch Ophthalmol. 1994;112:480–8. doi: 10.1001/archopht.1994.01090160056023. [DOI] [PubMed] [Google Scholar]
- 36.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
- 37.Snedecor GW, Cochran WG. Statistical Methods. 7th ed. Ames, IA: Iowa State University Press; 1980:144–5
- 38.Armitage P, Berry G. Statistical Methods in Medical Research. 2nd ed. Boston: Blackwell Scientific; 1987:372–4
- 39.Katz D, Baptista J, Azen SP, Pike MC. Obtaining confidence intervals for the risk ratio in cohort studies. Biometrics. 1978;34:469–74. [Google Scholar]
- 40.Hillis AI, Maguire M, Hawkins BS, Newhouse MM. The Markov process as a general method for nonparametric analysis of right-censored medical data. J Chronic Dis. 1986;39:595–604. doi: 10.1016/0021-9681(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 42.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–7. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 43.Jampol LM, Tielsch J. Race, macular degeneration, and the Macular Photocoagulation Study. Arch Ophthalmol. 1992;110:1699–700. doi: 10.1001/archopht.1992.01080240039024. [DOI] [PubMed] [Google Scholar]
- 44.Sadda SR, Pieramici DJ, Marsh MJ, et al, Submacular Surgery Trials (SST) Pilot Study Investigators. Changes in lesion size after submacular surgery for subfoveal choroidal neovascularization in the Submacular Surgery Trials Pilot Study. Retina. In press. [DOI] [PubMed]
- 45.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Complications of Age-Related Macular Degeneration Prevention Trial Study Group. The Complications of Age-Related Macular Degeneration Prevention Trial (CAPT): rationale, design and methodology. Clin Trials. 2004;1:91–107. doi: 10.1191/1740774504cn007xx. [DOI] [PubMed] [Google Scholar]


