Abstract
Purpose
To describe the guidelines followed by the Submacular Surgery Trials (SST) Research Group in the interpretation of color fundus photographs and fluorescein angiograms of subfoveal choroidal neovascular lesions evaluated in the SST and to assist ophthalmologists in applying the results of the SST.
Methods
Stereoscopic color fundus photographs and fluorescein angiograms of the study eye and nonstudy eye of 1,015 patients with subfoveal choroidal neovascular lesions secondary to age-related macular degeneration, ocular histoplasmosis syndrome, or idiopathic choroidal neovascularization (CNV) were obtained and graded by certified SST fundus photograph readers at the baseline examination in three randomized clinical trials comparing surgery with observation. Adherence to the inclusion and exclusion criteria and ocular features that might affect visual outcome were documented. Stereoscopic color fundus photography and fluorescein angiography were repeated 1 month after randomization for patients assigned to surgery to provide documentation that surgery was performed and to assess compliance with the surgery protocol. Photographs and fluorescein angiograms of both the study eye and the fellow eye in all patients then were obtained 3 months, 6 months, and 12 months after randomization and then annually up to 48 months. The κ statistic was used to evaluate interobserver reliability of photograph gradings.
Results
Lesion components at baseline included classic CNV, occult CNV, and features contiguous to CNV, including blood, fibrous tissue, hypofluorescence not corresponding to blood, serous detachment of the retinal pigment epithelium, and prior areas of laser photocoagulation. At follow-up, fluorescein leakage from CNV was assessed peripheral to or within the area of the retinal pigment epithelium abnormality after surgery. The lesion at follow-up could include any of the features identified at baseline as well as retinal pigment epithelium abnormalities, such as mottling of the retinal pigment epithelium with a subtle transition to normal retinal pigment epithelium or a very sharply demarcated, markedly hypopigmented area that was easily distinguished from the surrounding retinal pigment epithelium. κ statistics for interobserver reliability ranged from good (0.47) to excellent (1.00) for features graded at baseline and follow-up.
Conclusions
Although some of the definitions essential to the interpretation of the SST are similar to those used in the Macular Photocoagulation Study and randomized clinical trials of photodynamic therapy with verteporfin, this guideline provides new information regarding lesion components at baseline as well as standardized descriptions of lesions after submacular surgery. These descriptions from the SST assist in understanding what lesions were studied, when additional treatment was considered after surgery, and how anatomical results should be interpreted.
Age-related macular degeneration (AMD) is the most common cause of irreversible severe visual acuity loss in individuals 65 years of age or older in North America and Europe, affecting ≈5% of people in this age group.1,2 The neovascular form of AMD accounts for ≥80% of visual acuity loss,3 with many of these new choroidal neovascular lesions developing in the second eye of a patient who already has AMD with choroidal neovascularization (CNV) in the first eye.2
The Macular Photocoagulation Study (MPS) Group 4–6 and others have provided evidence from randomized clinical trials that treatment of CNV with focal laser photocoagulation of well-demarcated choroidal neovascular lesions reduces the risk of severe visual acuity loss for at least 5 years in patients with neovascular AMD, neovascular ocular histoplasmosis, and idiopathic CNV.4–6 Unfortunately, fewer than one half of CNV lesions secondary to AMD are well demarcated at presentation, making it more difficult to adequately cover the neovascular lesion in its entirety with intense laser treatment to reduce the chance of continued growth of the lesion from persistent CNV.7–9 More recently, photodynamic therapy with verteporfin has been shown to reduce the risk of moderate and severe vision loss in selected patients with subfoveal CNV secondary to AMD and other causes.10–12
Surgical removal of CNV has been under investigation as another potential treatment for CNV. Surgical extraction of a CNV lesion may not require identification of the exact boundaries of the neovascular lesion and potentially may be applicable to lesions in which most of the CNV is obscured by blood. The Submacular Surgery Trials (SST) have been designed to evaluate submacular surgery, in comparison with observation, within randomized clinical trials for a specific subset of subfoveal neovascular lesions.
The recognition of lesion components and the estimation of lesion proportions during fluorescein angiography are critical to understanding which lesions were eligible for surgery in the SST and in interpreting and applying the findings from each clinical trial. Furthermore, the alterations observed in the retina and choroid after surgical removal of choroidal neovascular lesions have not been described systematically. Use of common terminology to describe the retinal appearance after submacular surgery is helpful when describing morphologic outcomes after surgery and when comparing histopathologic descriptions of surgically removed lesions and the postoperative retinal appearance. To assist ophthalmologists in understanding terms adopted from the SST and their application, we describe the guidelines followed by the SST Research Group for interpreting and recording observations from the color fundus photographs and fluorescein angiograms of subfoveal choroidal neovascular lesions at study entry and during follow-up.
Patients and Methods
The SST are multicenter, randomized clinical trials designed to evaluate submacular surgery for removal of subfoveal choroidal neovascular lesions in selected patients with AMD, ocular histoplasmosis, or idiopathic CNV. Eligible patients, as assessed by the clinical center investigators, were enrolled and assigned randomly with equal probability to submacular surgery or to observation. The primary outcome to be assessed for all patients at specified times after enrollment was improvement or stabilization of visual acuity.
Stereoscopic color fundus photographs, red-free photographs, and fluorescein angiograms of the study eye were obtained at the baseline examination following the same protocol used in the MPS, including stereoscopic early-phase frames of the study eye during fluorescein angiography through 1 minute and then at 2, 3, 5, and 10 minutes; mid- and late-phase stereoscopic frames were taken of the fellow eye.13 These photographs were obtained to confirm adherence to the ophthalmoscopic and fluorescein angiographic inclusion and exclusion criteria and to describe baseline ocular features likely to affect visual outcome, such as lesion size or lesion composition. Stereoscopic color fundus photographs, red-free photographs, and limited fluorescein angiographic views of the nonstudy eye also were obtained to characterize fundus features that could confirm or refute the likely etiology of the neovascular lesion in the study eye as judged by the enrolling ophthalmologist. The baseline color photographs and angiograms were read independently by two trained readers from the SST Photograph Reading Center at The Johns Hopkins University School of Medicine (Baltimore, MD). Reading center staff had no knowledge when grading baseline photographs as to whether the management of the CNV had been assigned to surgery or to no surgery.
Definitions Essential to the Interpretation of Fluorescein Angiograms at the Initial Visit Before Randomization
A detailed description of terms used to describe fluorescein angiographic patterns of choroidal neovascular lesions in the retina of patients enrolled in the SST follows. Many of these definitions are similar to those used to currently describe features of lesions studied in randomized clinical trials evaluating laser photocoagulation in the MPS13 and photodynamic therapy with verteporfin in the management of CNV.14 Any differences in definitions from those used in the photodynamic therapy trials are emphasized below.
Classic and occult CNV
The SST investigators used definitions adopted from the MPS Group to identify areas of classic and occult CNV during fluorescein angiography.13 Several features observed at the boundaries of either classic or occult CNV were included as part of the overall neovascular lesion because these features could obscure the true boundaries of the CNV. These features included hypofluorescence that corresponded to blood seen on color fundus photographs (Fig. 1), hypofluorescence that did not correspond to blood on color fundus photographs and that may have been secondary to blood not visible on color fundus photographs, pigment (Fig. 2), or fibrous tissue (Fig. 3) and that often existed as a narrow rim of hypofluorescence surrounding CNV, hyperfluorescence from fibrous tissue that demonstrated fluorescein staining (Fig. 2), or hyperfluorescence from a serous pigment epithelial detachment (Fig. 4). Blood that was thick enough to obscure choroidal fluorescence but extended as a pseudopod from the remainder of the lesion so that, in the opinion of the grader, it was judged unlikely to obscure CNV (e.g., because of a linear shape of blood) was not considered part of the lesion (Fig. 5).
Fig. 1.
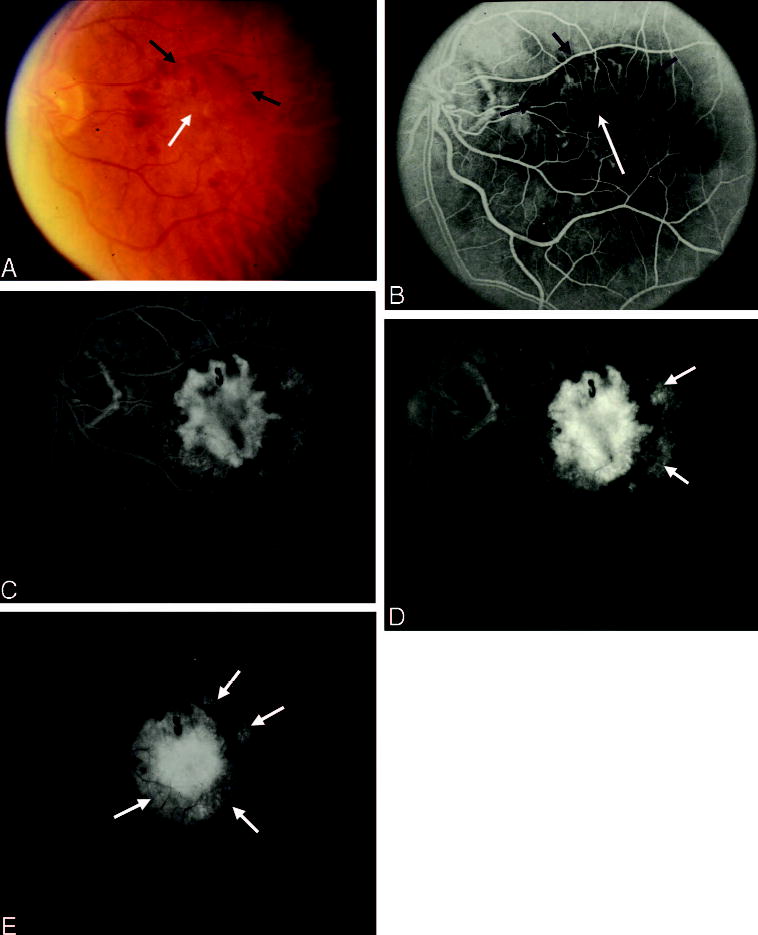
Blood obscuring choroidal neovascularization (CNV) boundaries. A, Color photograph shows central elevation of the retinal pigment epithelium (white arrow) associated with subretinal fluid surrounded by a thin rim of sub-retinal hemorrhage (black arrows). B, Early phase of the angiogram shows a well-defined area of hyperfluorescence consistent with classic CNV (white arrow) surrounded by a rim of blocked fluorescence (black arrows) that corresponds to the sub-retinal hemorrhage seen clinically. C, As the study progresses, a less intense stippled outer rim of hyperfluorescence (black arrows) is appreciated beyond the border of the blocked fluorescence inferiorly and temporally. D and E, This stippled hyperfluorescence progresses and now appears elevated, confirming the presence of occult CNV (white arrows) outside of central classic CNV.
Fig. 2.
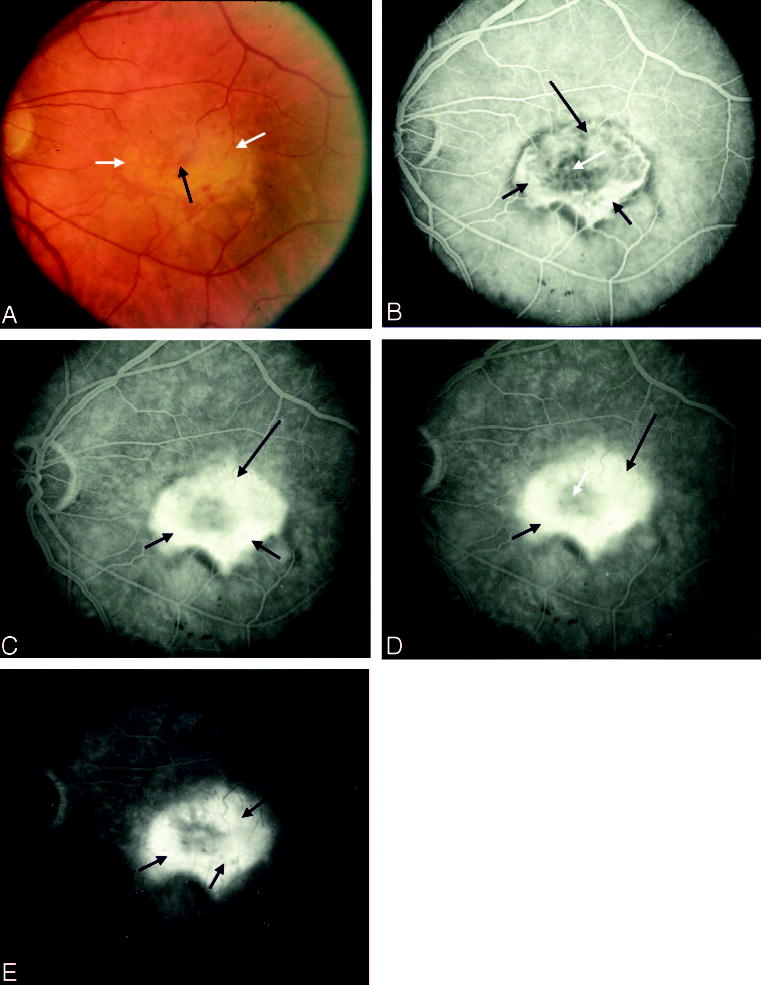
Pigment obscuring choroidal neovascularization (CNV) and staining of fibrous tissue. A, Color photograph shows a ring of fibrous tissue (white arrows) with central subretinal pigment (black arrow). B, Early phase of the angiogram shows blocked fluorescence from the central pigment (white arrow) with mild staining of the fibrous tissue (short black arrows) and early leakage from CNV superiorly (long black arrow). C and D, By mid phase, the angiogram shows increased hyperfluorescence and elevation in the region of CNV (long black arrow) with more intense staining of fibrous tissue (short black arrows). The central blocked fluorescence from pigment remains intact (white arrow). E, Late phase shows less fluorescence in the region of fibrous tissue (short black arrows).
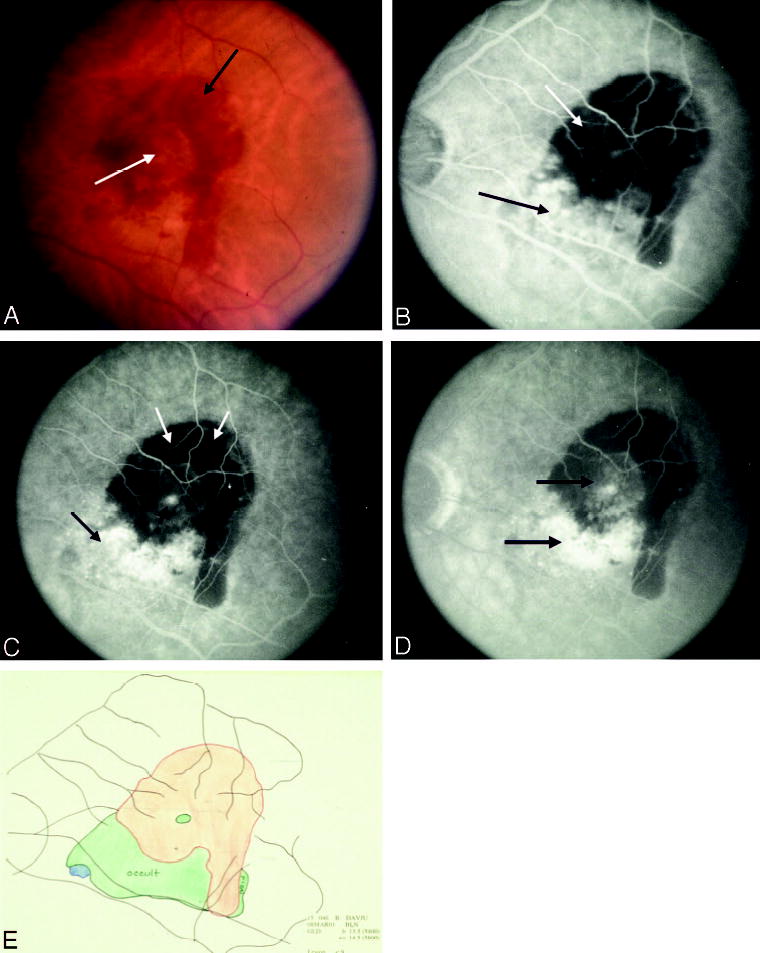
Fig. 3.
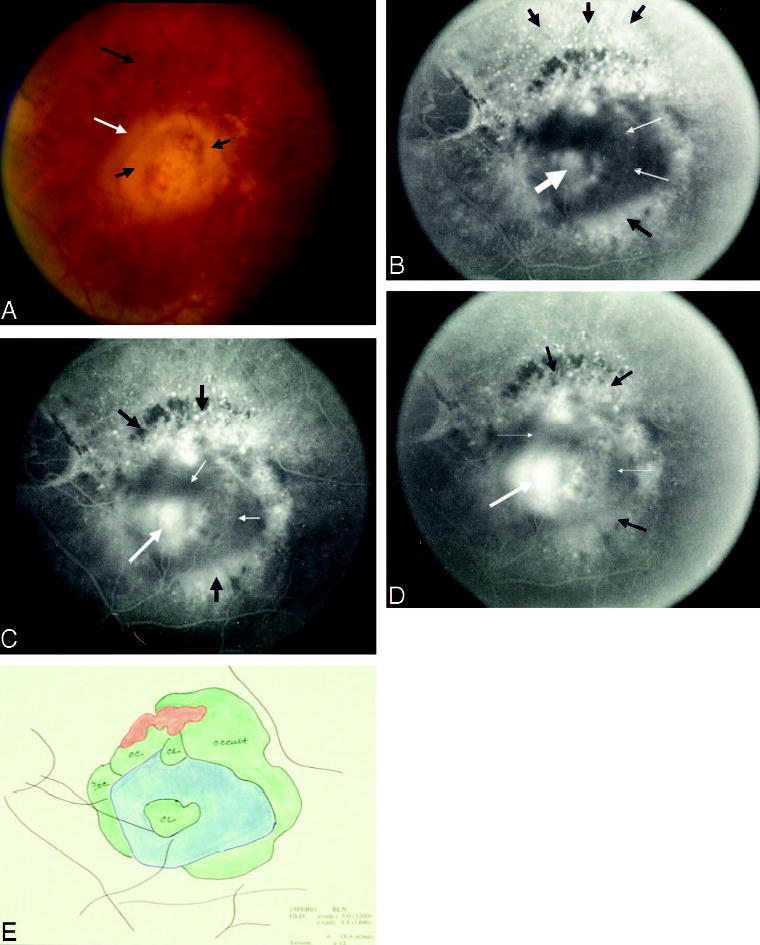
Fibrous tissue obscuring choroidal neovascularization (CNV). A, Color photograph shows a ring of fibrous tissue (short black arrows) surrounded by a rim of subretinal fluid (white arrow) with more peripheral subretinal hemorrhage (long black arrow). B, Early phase of the angiogram shows intense uniform hyperfluorescence (thick white arrow) surrounded by blocked fluorescence (thin white arrows) corresponding to fibrous tissue seen clinically with more peripheral speckled hyperfluorescence (black arrows). C and D, Mid to late phase of the angiogram shows progressive hyperfluorescence of central classic (thick white arrow) and peripheral occult (black arrows) CNV with preserved blocked fluorescence from fibrous tissue (thin white arrows). E, Composite drawing using multiple stereoscopic photographs of angiograms shows interpretation of the boundaries of the lesion components.
Fig. 4.
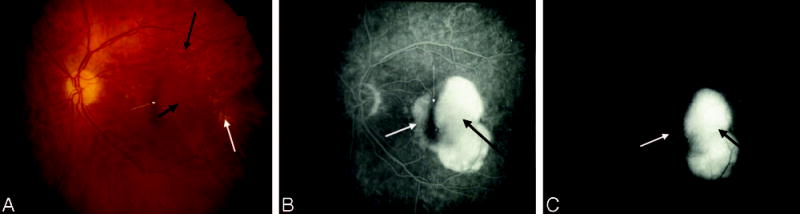
Serous pigment epithelial detachment obscuring choroidal neovascularization (CNV). A, Color photograph shows a large central blister of subretinal fluid (short black arrow) with temporal subretinal lipid (thick white arrow) and peripheral drusen (long black arrow) as well as subretinal pigment (thin white arrow). B, Early phase of the angiogram shows nasal hyperfluorescence that is poorly defined and elevated and suggestive of occult CNV (thick white arrow) with a large area temporally of early, uniform, intense hyperfluorescence (black arrow) that is adjacent to the thin subretinal pigment (thin white arrow). C, The temporal well-defined area of early, uniform hyperfluorescence is a serous pigment epithelial detachment (black arrow) that is appreciated to obscure the boundary of CNV (white arrow) by the late phase of the angiogram.
Fig. 5.
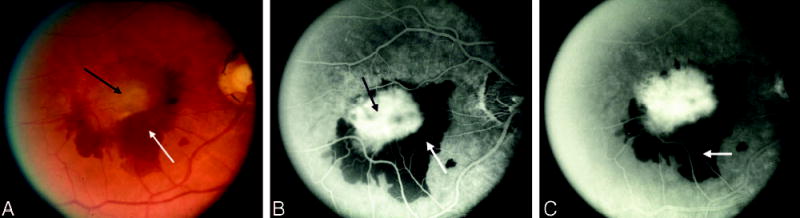
Blood as a pseudopod obscuring choroidal neovascularization (CNV). A, Color photograph shows central elevation of the retinal pigment epithelium (black arrow) with overlying subretinal fluid and a thick rim of subretinal hemorrhage (white arrow). B, Early phase of the angiogram shows well-defined intense hyperfluorescence (black arrow) consistent with classic CNV whose borders are obscured by blocked fluorescence from the subretinal hemorrhage (white arrow). C, Late phase of the angiogram shows the extent of leakage from CNV. The blocked fluorescence from subretinal hemorrhage is asymmetric with a pseudopod that extends inferonasally (white arrow). Although the borders of the CNV are obscured by subretinal hemorrhage, it is unlikely that the CNV leaks in an asymmetric pattern such that the pseudopod of subretinal hemorrhage is obscuring the boundaries of CNV.
Lesion component versus lesion at baseline
Two important terms also used to describe the angiographic characteristics of the CNV are lesion component and lesion. The term “lesion component” refers specifically to an area of the retina that exhibits angiographic characteristics that are CNV, including patterns of fluorescence corresponding to classic or occult CNV, as well as features that may obscure the boundaries of CNV, as explained previously. The term “lesion” at baseline is the complex of lesion components defined above. In the SST grading scheme, any areas of prior laser photocoagulation in the macula that were contiguous to the lesion were included as a lesion component at baseline (Fig. 6).
Fig. 6.
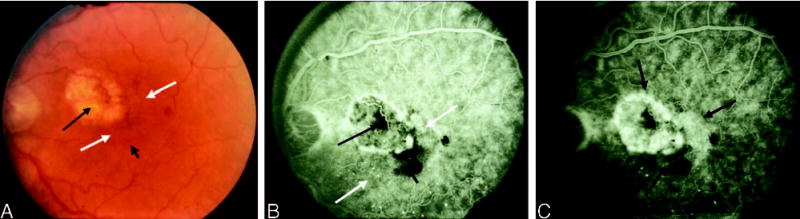
Lesion component versus lesion. A, Color photograph shows a laser photocoagulation scar (long black arrow) with adjacent subretinal fluid (white arrows) and inferior mild subretinal hemorrhage or pigment (short black arrow). B, Early phase of the angiogram shows staining of the laser-treated area (long black arrow) with blocked fluorescence from the subretinal pigment or hemorrhage (short black arrow) and punctate areas as well as speckled areas of hyperfluorescence (white arrows). Lesion component refers to an area of the retina that exhibits angiographic characteristics of choroidal neovascularization (CNV; classic and occult) or of features that may obscure the boundaries of CNV, such as subretinal hemorrhage or pigment. Each feature noted in this figure is a lesion component. C, The entire complex of lesion components constitutes the lesion (black arrows).
Other definitions useful for the interpretation of SST findings
The SST evaluated submacular surgery for removal of subfoveal choroidal neovascular lesions in selected patients with AMD, ocular histoplasmosis syndrome, or idiopathic CNV.
AMD is defined by the presence, in either the study eye or the fellow eye, of any of the following features within 3,000 μm of the geometric center of the foveal avascular zone (FAZ): at least one druse of at least medium size (i.e., at least 63 μm); abnormalities at the level of the retinal pigment epithelium (RPE) judged consistent with AMD; or a serous detachment at the level of the RPE. Abnormalities at the level of the RPE consistent with AMD include geographic atrophy (characterized by well-demarcated areas of hypopigmentation of the RPE with overlying thinning of the neurosensory retina), nongeographic atrophy (characterized by mottled hypopigmentation of the RPE with interspersed areas of hyperpigmentation), and focal areas of hyperpigmentation at the level of the outer retina judged not to conform to a pattern characteristic of other macular conditions with RPE abnormalities, such as a pattern dystrophy of the RPE, central serous chorioretinopathy, or angioid streaks in association with pseudoxanthoma elasticum.
Ocular histoplasmosis is a potential cause of CNV that is characterized by the ophthalmoscopic appearance of macular or peripheral “punched out” areas of focal atrophy or creamy yellow spots that may be round or slightly elongated and often accompanied by peripapillary atrophy or scarring (Fig. 7). These features are distinguished from multifocal choroiditis based on an absence of staining or leakage of the optic nerve in either eye as well as an absence of staining or leakage of choroidal lesions larger than focal spots.
Fig. 7.
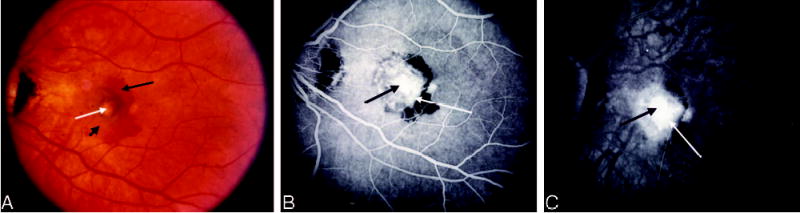
Group H lesion. A, Color photograph shows a central area of focal chorioretinal scarring (white arrow) with a rim of subretinal fluid (short black arrow) and subretinal hemorrhage (long black arrow). B, Early phase of the angiogram shows a transmission defect through the focal histoplasmosis spot with surrounding hyperfluorescence from choroidal neovascularization (white arrow). C, The hyperfluorescence progresses in the late frames of the angiogram.
Idiopathic CNV is defined by the occurrence of CNV in the absence of any appreciable underlying cause of CNV, as would be indicated by retinal abnormalities seen in AMD, ocular histoplasmosis syndrome, angioid streaks, or pathologic myopia.
Angiographic evidence of subfoveal neovascular lesions was a prerequisite for enrollment into the SST. For SST Groups H and N, CNV (classic or occult CNV) had to extend under the geometric center of the FAZ (Fig. 8). One other circumstance was considered to demonstrate CNV extending under the center of the FAZ, termed the “360° rule.” This rule specified that whenever blood, blocked fluorescence not due to visible blood, or a serous detachment of the RPE was completely surrounded by classic or occult CNV or both, the blood, blocked fluorescence, or serous detachment of the RPE was presumed to be obscuring CNV. In such a case, CNV was presumed to extend under the geometric center of the FAZ, meeting the criteria for subfoveal CNV. In the SST Group B Trial, either CNV (classic, occult, or based on the 360° rule) or blood could extend under the foveal center.
Fig. 8.
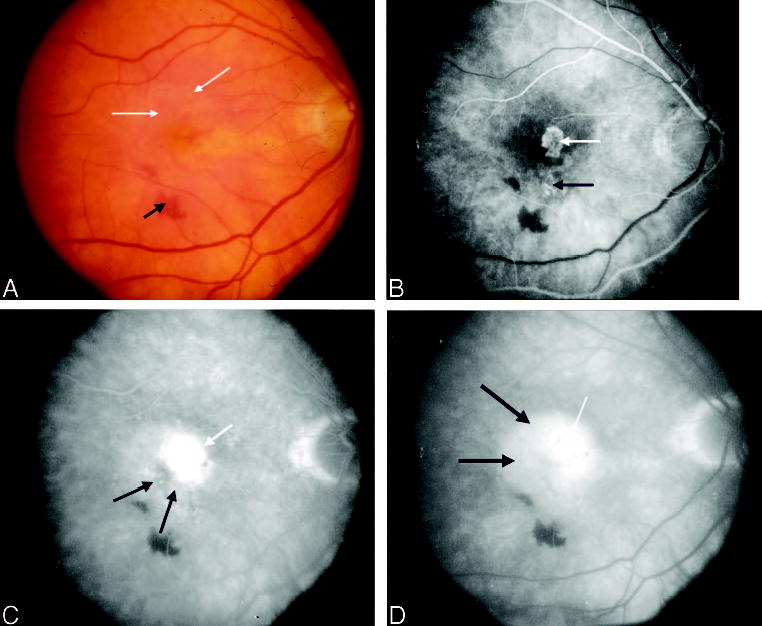
Group N lesion with subfoveal occult choroidal neovascularization (CNV), A. Color photograph shows sub-retinal fluid (white arrows) and subretinal hemorrhage (black arrow). B, Early phase of the angiogram shows a well-defined juxtafoveal area of hyperfluorescence suggestive of a small classic CNV lesion (white arrow) with more inferior subtle speckled fluorescence (black arrow). C, Mid phase of the angiogram shows progression of both the classic (white arrow) and the occult (black arrow) components of the lesion complex with significant expansion of the occult CNV temporally and subfoveally (black arrows) by the late phase, D.
Determining lesion size
Lesion size in the SST was measured in disk areas using a template developed originally for the MPS.13 The template contained 10 individual circles (Fig. 9) representing multiples of 1 standard disk area, defined as an area on the retina of 2.54 mm2, assuming a 2.5 magnification of the image on the film relative to the size of the lesion on the retina. The templates were used by the SST Photograph Reading Center readers on 35-mm-film angiograms.
Fig. 9.
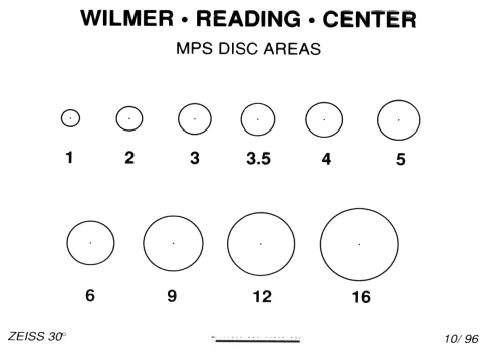
Disk area template. Template of clear acetate is placed on a 35-mm frame of the angiogram (made using a 30° fundus camera) to determine the lesion area. One Macular Photocoagulation Study (MPS) disk area is defined as an area of 2.54 mm2, assuming a 2.5 magnification of the image on the film relative to the size of the lesion on the retina. Thus, the 2 MPS disk area circle on the template has an area that covers 5.06 mm2 on the retina.
Judging lesion eligibility from baseline fundus photographs and fluorescein angiograms in the SST
After assessing whether photographs were gradable (readers were at least 90% certain that features needed to assess eligibility could be graded), SST Photograph Reading Center readers recorded evidence of the following from review of stereoscopic color fundus photographs and the original 35-mm-film-negative fluorescein angiogram: previous laser photocoagulation, CNV in the foveal center, the presence of classic CNV, the presence of occult CNV, blood that obscured the boundaries of CNV, blood occupying >50% of the lesion, size of the lesion, etiology of CNV, and the presence of fundus disease other than CNV. A summary of the principal eligibility criteria for each SST Group is given in Table 1.
Table 1.
Principal Ocular Eligibility Criteria for the Submacular Surgery Trials
| Criterion | Group B (Blood) | Group N (New CNV) | Group H (CNV Due to OHS or Idiopathic) |
|---|---|---|---|
| Age, y | ≥50 | ≥50 | ≥18 |
| CNV cause | AMD | AMD | OHS or unknown |
| Classic CNV | Optional | Required | Required |
| Occult CNV | Optional | Optional | Optional |
| Foveal center | Blood or CNV | CNV | CNV |
| Lesion size, MPS disk area | ≥3.5* | ≤9† | ≤9 |
| Blood (%) as a lesion component | ≥50 | <50 | Any |
| Prior laser photocoagulation for CNV‡ | Optional | Not allowed | Optional |
| Best visual acuity, approximate Snellen equivalent | 20/100 | 20/100 | 20/50 |
| Worst visual acuity, approximate Snellen equivalent | <20/1600 to light perception | 20/800 | 20/800 |
Area of CNV of ≤9 MPS disk areas; no limit to total lesion size including area of CNV, area of blood, and area of other components of lesion, except that ≥75% of blood must be posterior to the equator.
If total area of lesion is ≤3.5 MPS disk areas, then boundaries must be poorly demarcated.
Prior laser photocoagulation cannot extend under the center of the foveal avascular zone.
CNV, choroidal neovascularization; OHS, ocular histoplasmosis syndrome; AMD, age-related macular degeneration; MPS, Macular Photocoagulation Study.
Because recurrent subfoveal neovascular lesions were eligible for the SST for Groups H and B but not for Group N (new CNV), the readers determined whether there was evidence of previous laser treatment. Any area of prior laser photocoagulation, if present, was included in the total lesion size (Fig. 6). However, even for Group H and Group B, eyes were not eligible for the trials if prior laser treatment appeared to have involved the geometric center of the FAZ. A history of evidence of other treatments for CNV, such as photodynamic therapy with verteporfin, was an exclusion criterion for Groups H, B, and N Trials.
Summary of Eligibility Criteria for Groups B, N, and H in the SST
Patients enrolled in the SST met established color fundus photographic and fluorescein angiographic eligibility criteria particular to one of the groups, H, N, or B (Table 1). Enrollment into Group H required that the patient had either evidence of ocular histoplasmosis or at least no evidence of an underlying cause of CNV (Fig. 7). Fluorescein angiography had to reveal evidence of classic CNV with some CNV, either classic or occult, that extended through the geometric center of the FAZ. The entire size of the neovascular complex was not to exceed 9 MPS disk areas, including any previous area of nonfoveal laser treatment. Blood as a lesion component could occupy >50% of the lesion area provided that CNV was under the foveal center or the 360° rule was satisfied.
Patients enrolled in Group N had evidence of AMD, had not received prior photocoagulation, and had angiographic evidence of classic CNV with some CNV, either classic or occult, extending under the geometric center of the FAZ (Fig. 8). The CNV (classic plus occult) accounted for at least 50% of the entire lesion, which could not exceed 9 MPS disk areas; if the CNV was <3.5 MPS disk areas, the boundaries had to be poorly demarcated.
Group B patients had predominantly hemorrhagic lesions (blood as a lesion component accounted for at least 50% of the entire lesion) with either blood or CNV (classic or occult) extending under the geometric center of the FAZ (Fig. 10). The size of the entire lesion, including blood, had to exceed 3.5 MPS disk areas. Any visible CNV (in field 2) could not exceed 9 MPS disk areas, and the ophthalmologist had to confirm that at least 75% of the lesion was posterior to the equator.
Fig. 10.
Subfoveal blood with or without choroidal neovascularization (CNV), Group B. A, Color photograph shows extensive subretinal hemorrhage (black arrow) with a clear zone centrally (white arrow). B, Early phase of the angiogram shows blocked fluorescence from blood (white arrow) with mild speckled fluorescence at the inferior border (black arrow). C, By the mid phase of the angiogram, the occult CNV is evident both at the inferior border (long black arrow) and within the central area of blocked fluorescence from sub-retinal hemorrhage (short black arrow). D, Leakage progresses into the late phase. E, Composite drawing using multiple stereoscopic photographs of angiograms shows interpretation of the boundaries of the lesion components.
The stereoscopic color fundus photographs of fields 1 and 2 as well as the limited fluorescein angiographic views of the nonstudy (fellow) eye also were reviewed to identify the presence of retinal disease that would fulfill study inclusion criteria, such as features of AMD or ocular histoplasmosis. The most advanced manifestation of AMD noted in the nonstudy eye was categorized as having no features consistent with AMD, drusen with the largest maximal diameter of <63 μm, drusen in which at least 1 druse was at least 63 μm in size, geographic atrophy of the RPE, or CNV (with or without evidence of a visible scar or an area of laser photocoagulation on the color fundus photograph). In addition, the presence of any other retinal or optic nerve disease in the fellow eye was noted, including other conditions associated with CNV as well as conditions with the potential to affect vision during follow-up in the trials.
Evaluation of Photographs Obtained at Follow-up Examinations in the SST
Stereoscopic color fundus photographs and fluorescein angiograms were obtained again 1 month after randomization for all patients assigned to surgery in Groups H, N, and B, following the same protocol used at baseline, to provide documentation that surgery was performed, to assess compliance with the surgery protocol, and to monitor for complications associated with the procedure. Otherwise, fundus photographs and fluorescein angiograms of the study eye and fellow eye in all patients (whether assigned to surgery or to observation) were obtained at 3 months, 6 months, 12 months, and 24 months after randomization (as well as at 36 months and 48 months for those individuals who enrolled early enough to be eligible for examinations at those times). To permit correlation of anatomical outcomes with visual outcomes and to monitor for adverse events, study eye photographs were evaluated for the following features at follow-up: peripheral leakage from classic or occult CNV, non-peripheral leakage from classic or occult CNV, quantity of blood present compared with baseline finding, lesion size, and parafoveal involvement of lesion.
Evaluation of lesions at follow-up examinations
In characterizing the posttreatment lesion after surgery in all SST Groups (H, N, or B), one of several patterns of abnormality or alteration of the RPE was noted. At follow-up, these features occupied areas that were originally involved with or contiguous to the subfoveal neovascular lesion or blood.
On color fundus photographs of the study eye, a relatively subtle mottling of the RPE may have been present with an ambiguous border between surgically treated and uninvolved RPE (Fig. 11). For these eyes, fluorescein angiography demonstrated more definitive borders between the area of the RPE abnormality, which appeared as a transmission defect after surgery, and the contiguous normal appearing RPE. However, these borders were not sharply defined (Fig. 11). Another variation in the appearance of the RPE after surgery consisted of a clinically obvious, very well-demarcated lesion (i.e., a lesion with “cookie-cutter” boundaries), where the border of abnormality of the RPE and the normal appearing RPE could be detected easily on color photographs as well as on fluorescein angiograms (Fig. 12). Although more altered or degenerated than the postoperative RPE abnormalities exemplified in Figure 11, areas of the RPE disturbance after surgery such as seen in Figure 12 did not demonstrate features of fibrovascular tissue or evidence of scarring within the area of the RPE disturbance. A third posttreatment appearance was scarring at the level of the RPE after surgical excision of the original lesion. These eyes had fibrovascular tissue as seen by subretinal fibrosis clinically and intense staining of tissue shown by fluorescein angiography (Fig. 13). Any of these three patterns of the RPE disturbance was considered a lesion component at follow-up and was included in the assessment of lesion size at follow-up examinations. Eyes assigned to the observation arm usually developed areas of natural scar, both fibrovascular scar with staining of fibrosis and atrophic scar with staining of the RPE and choroid in the absence of visible fibrous tissue. These areas of fibrotic or atrophic scarring also were considered lesion components at follow-up examinations.
Fig. 11.
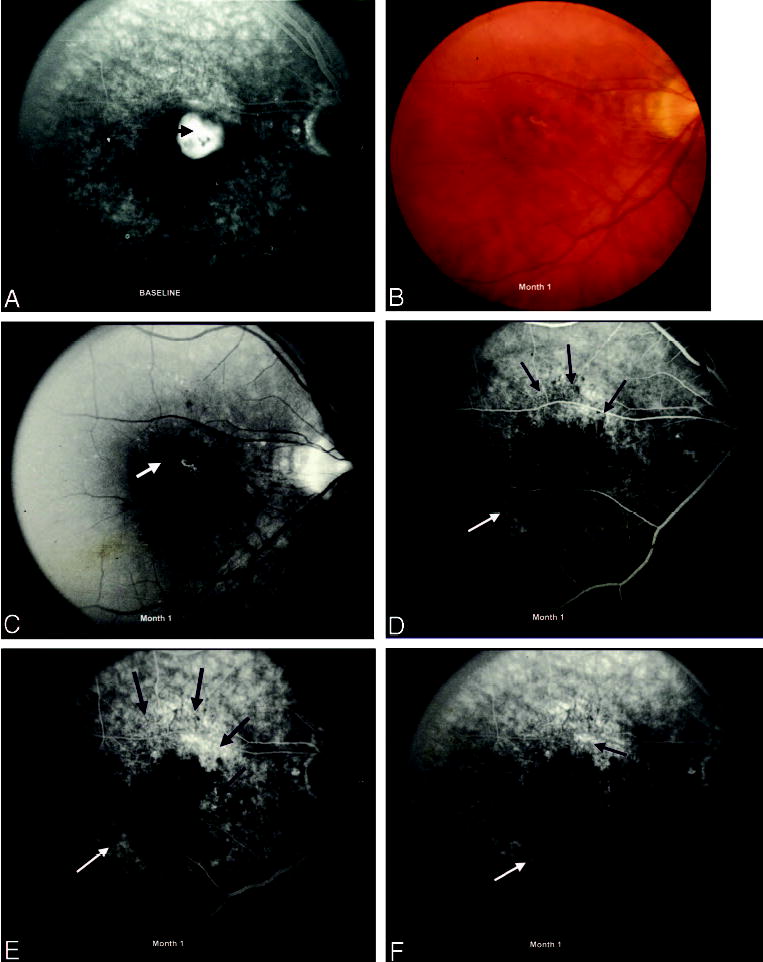
Disturbance of the retinal pigment epithelium (RPE) 1 month after surgical excision of choroidal neovascularization (CNV). A, Fluorescein angiogram demonstrates CNV at baseline (black arrow), before surgery. B, Color photograph shows residual subretinal hemorrhage and RPE alteration 1 month after surgical excision of CNV. C, Red-free photograph shows central subretinal hemorrhage (white arrow) and a focal area of scarring (black arrow) at the level of the RPE. The border between surgically treated and uninvolved RPE cannot be appreciated on the red-free photograph. D, Early phase of the fluorescein angiogram demonstrates a more definite border of the transmission defect from the area of surgically disturbed RPE (black arrows) compared with the uninvolved RPE (white arrow). E and F, Mid and late phases of the angiogram show staining of scar tissue (black arrows) that has formed at the level of the RPE after surgical excision of the original lesion.
Fig. 12.
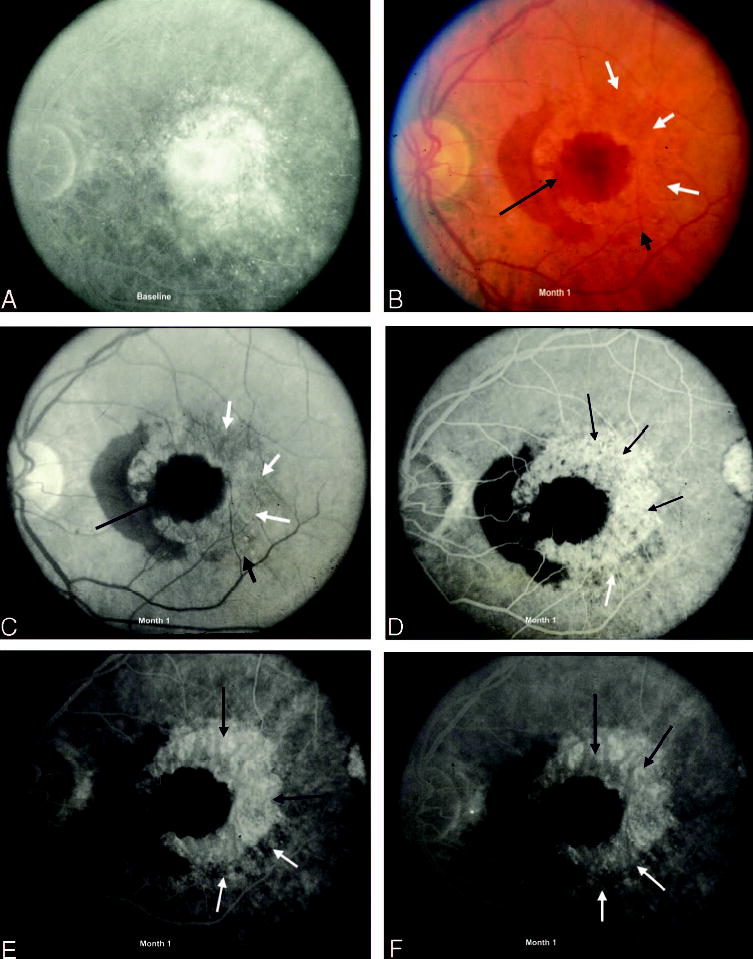
Disturbance of the retinal pigment epithelium (RPE) after surgical excision of choroidal neovascularization (CNV), 1-month follow-up. A, Fluorescein angiogram demonstrates CNV at baseline, before surgery. B, Color photograph shows extensive subretinal hemorrhage (black arrow) with a large temporal arcuate border of disturbed RPE (white arrows) 1 month after surgical excision of CNV. The borders of the surgically disturbed RPE (white arrows) are sharp in comparison with the mild alteration seen from the surgically unaltered RPE (short black arrow). C, The large, arcuate, cookie-cutter area of RPE alteration can be appreciated on the red-free photograph as well (white arrows) with the distinct, smaller, inferior area of RPE disturbance (short black arrow) adjacent. D, Early phase of the angiogram shows moderate hyperfluorescence from the transmission defect (black arrows) that corresponds to the region of surgically altered RPE and a less intense area of hyperfluorescence (white arrow) immediately inferior and adjacent to the surgically altered RPE that represents the transmission defect from the surgically unaltered RPE. E, By mid phase, there is staining of the arcuate area of the RPE disturbance (black arrows) with the transmission defect evident inferiorly (white arrow). F, Late phase of the angiogram shows decreased hyperfluorescence from the inferior region corresponding to the transmission defect from the surgically unaltered RPE (white arrows) and persistent staining of the surgically altered RPE temporally (black arrows).
Fig. 13.
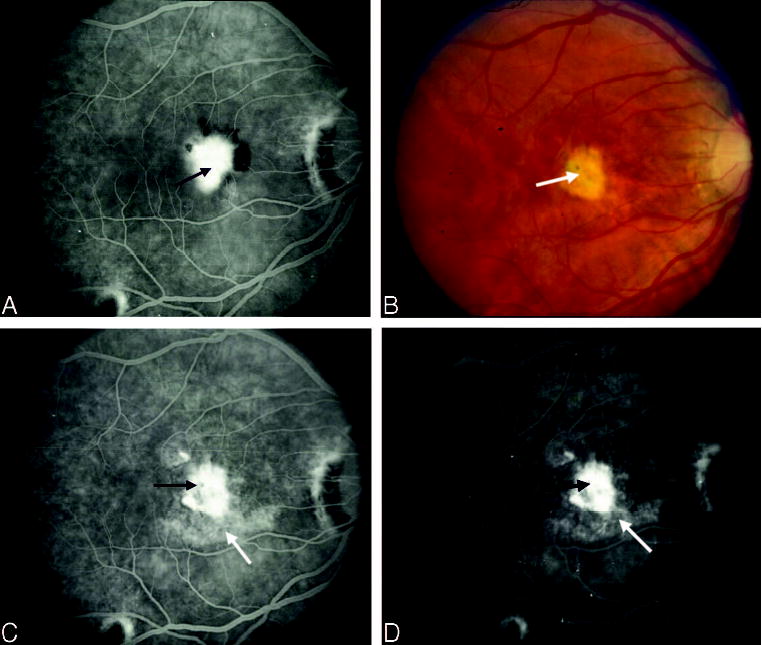
Disturbance of the retinal pigment epithelium (RPE) 1 month after surgical excision of choroidal neovascularization (CNV). A, Fluorescein angiogram demonstrates CNV at baseline, before surgery. B, Color photograph shows scarring at the level of the RPE (white arrow) after surgical excision of the original lesion. C and D, Mid and late phases show intense staining of subretinal fibrosis (black arrow) compared with the surgically uninvolved RPE (white arrow).
Peripheral leakage from classic or occult CNV at follow-up was judged to be present in a surgically managed eye if, during fluorescein angiography, fluorescein leakage extended beyond the periphery of the RPE abnormality noted after surgical excision of the lesion (Fig. 14). For patients enrolled in Group H only, assessment also was made as to whether fluorescein leakage from CNV occurred within the area of the RPE abnormality noted after surgical excision. Whenever observed, such leakage was classified as “nonperipheral” and further described as either “foveal” or “nonfoveal.” Whenever nonperipheral leakage extended under the foveal center (Fig. 15), it was recorded as foveal. Nonfoveal nonperipheral leakage was used to describe CNV leakage within the area of the RPE abnormality noted after surgical excision that did not extend under the foveal center and that also did not extend beyond the periphery of the lesion. Eyes assigned to observation were graded for CNV leakage (classic, occult, or both) only at the periphery of the lesion.
Fig. 14.
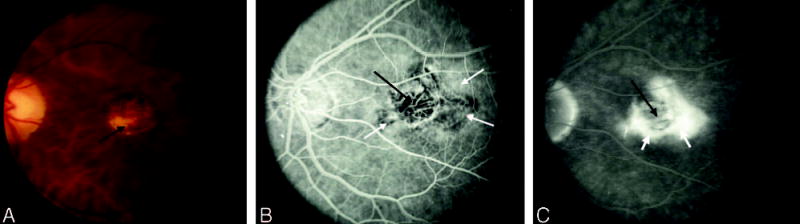
Peripheral leakage from choroidal neovascularization (CNV) after surgical excision. A, Color photograph shows a central retinal pigment epithelium (RPE) abnormality after surgical excision of CNV (black arrow). B, Early phase of the angiogram shows fluorescein leakage that has extended beyond the periphery of the RPE abnormality (white arrows). C, This peripheral leakage (white arrows) persists into the late phase of the study with only staining of the central RPE abnormality (black arrow).
Fig. 15.
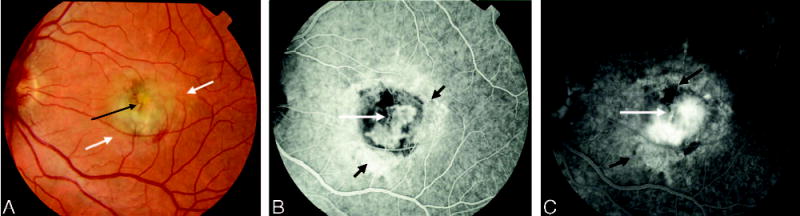
Nonperipheral leakage from choroidal neovascularization (CNV) after surgical excision of CNV. A, Color photograph shows a central area of fibrosis and pigment deposition (black arrow) in an area of retinal pigment epithelium (RPE) abnormality (white arrows) after surgical excision of CNV in a Group H patient. B, Early phase of the angiogram shows nonperipheral leakage within the center of the area of the RPE abnormality that appears to occur under the fovea, referred to as foveal nonperipheral leakage (long white arrow). The transmission defect is noted peripheral to the leakage (short black arrows). C, There is progressive hyperfluorescence in the late phase of the study from the foveal nonperipheral leakage (long white arrow) with fading from the more peripheral area of the transmission defect (short black arrows).
The presence and quantity of blood at follow-up examinations were noted and compared with findings of baseline photographs, characterized as less than, same as, or more than at baseline. When present, blood noted on follow-up photographs also was characterized as foveal or nonfoveal.
The lesion at follow-up was defined as the sum of the components that made up the lesion, such as any areas of disturbance of the RPE after surgical excision, fibrous tissue that stained during fluorescein angiography, scar that had a very atrophic appearance (i.e., appeared similar to geographic atrophy of the RPE except wisps of fibrous tissue, often with hyper-pigmentation, could be seen within or at the boundaries of the atrophic area), blocked fluorescence not corresponding to visible blood, classic CNV, occult CNV, leakage from CNV that could not be categorized as either classic or occult, serous pigment epithelial detachment, blood that may have obscured CNV, and areas of prior laser photocoagulation. Lesion size at follow-up was determined using the same methods and measurements in standard disk areas as described at baseline.
The extent of parafoveal involvement of the lesion was evaluated by centering the smallest possible disk area circle on the foveal center that left some area of uninvolved retina within the circle (Fig. 16). The size of the parafoveal involvement of the lesion also was classified within the same categories as described at baseline.
Fig. 16.
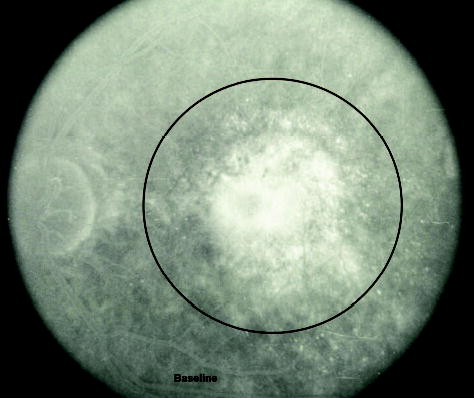
Extent of parafoveal involvement of the lesion. The fluorescein shows choroidal neovascularization with the smallest possible disk area circle, centered on the fovea, surrounding the lesion with some area of uninvolved retina within the circle.
The study eye photographs also were used to document any adverse events that may have occurred as a result of surgery to the study eye, such as retinal detachment, vitreous hemorrhage, or macular hole, as well as the presence of other retinal or optic nerve disease.
Reliability of Grading
As noted above, grading of all baseline and follow-up photographs was performed independently by two reading center readers, who were masked to treatment assignment at baseline. A comparison of their individual gradings of fluorescein angiographic lesion characteristics at baseline and follow-up was made, and discrepancies were discussed and resolved openly. Any discrepancies between the readers’ baseline and follow-up gradings that could not be resolved by mutual agreement were presented to a Photograph Reading Center ophthalmologist for resolution. A 5% sample of all photographs was selected randomly and regraded. κ statistics were calculated to compare the final original codes with the codes from the repeated assessment. The reliability of the classifications, assessed by the κ statistic for key baseline and follow-up lesion features, such as lesion composition and lesion size, was good to excellent, ranging from 0.47 to 1.0 for baseline characteristics and from 0.54 to 0.87 for follow-up features (Table 2).
Table 2.
κ Statistics for Reliability of Grading Selected Baseline Lesion Characteristics From Fluorescein Angiography as of June 30, 2002
| Characteristic | No. (Random Sample) | κ Statistic (95% Confidence Interval) |
|---|---|---|
| Baseline photograph | ||
| Lesion (%) that was classic CNV (<50%, ≥50%) | 25 (Group N only) | 0.90 (0.70–1.00) |
| Occult CNV (absent, present) | 50 | 0.47 (0.23–0.71) |
| Lesion size (MPS disk area category) | 50 | 0.78 (0.51–0.80)* |
| CNV subfoveal | 50 | 1.0 |
| Meets all photographic eligibility criteria (no, yes) | 50 | 0.86 (0.74–0.97) |
| Follow-up photograph | ||
| Classic CNV | 156 | 0.66 (0.57–0.76) |
| Occult CNV | 156 | 0.55 (0.45–0.66) |
| Nonperipheral leakage | 156 | 0.83 (0.77–0.90) |
| Lesion size | 156 | 0.74 (0.67–0.81)* |
| Lesion size centered on fovea | 156 | 0.58 (0.49–0.66)* |
| Most advanced AMD in fellow eye | 156 | 0.84 (0.77–0.90) |
Weighted κ; confidence interval for unweighted κ.
CNV, choroidal neovascularization; MPS, Macular Photocoagulation Study; AMD, age-related macular degeneration.
Discussion
Definitions essential to the interpretation of fluorescein angiograms of study eyes at baseline and follow-up examinations during the SST as well as examples of various lesions and lesion components have been described to assist ophthalmologists in interpreting and applying the findings from these clinical trials, conducted to evaluate surgical treatments of CNV. The terminology and definitions also may be useful for future trials involving surgical interventions for CNV. The definitions of classic CNV and occult CNV remain essentially unchanged since adoption for the MPS13; the same definitions were used in randomized clinical trials that evaluated photodynamic therapy with verteporfin,14 with only minimal editing to the wording of these definitions. One important addition to the lesion composition at baseline that was not included in previous angiographic guidelines13,14 for interpreting fluorescein angiograms is staining scar. This component was not noted routinely in the MPS when maximum eligible lesion sizes were relatively small,13 smaller than many lesions eligible for the SST or verteporfin trials.14 After observing lesions at baseline in study eyes with visual acuity as low as 20/800 (SST Groups H and N) or even light perception (Group B), the Photograph Reading Center ophthalmologists recognized that staining scar should be included among possible lesion components. Most patients enrolled in the SST did not have large areas of staining scar at baseline because at least 50% of the lesion had to be CNV for SST Groups H and N and at least 50% of the lesion had to be blood potentially obscuring CNV in Group B.
These guidelines also provide terminology for the uniform interpretation and description of lesions after submacular surgery. A variety of appearances were recognized that differed from those seen on fluorescein angiograms after laser photocoagulation or photodynamic therapy with verteporfin. Leakage of fluorescein dye after treatment of CNV deserves special mention. After laser photocoagulation, fluorescein leakage from CNV within the area of laser treatment that does not extend to the boundaries of the laser treatment is termed “central leakage.”13 Fluorescein leakage from CNV at the boundary of the laser-treated area within 6 weeks of laser photocoagulation is termed “persistent leakge,”13 while peripheral leakage after 6 weeks (usually with documentation of no leakage before this time) is termed “recurrent leakage.”13
In contrast, fluorescein leakage from CNV after photodynamic therapy with verteporfin beyond the area of the lesion defined at baseline is termed “progression,” while leakage at follow-up within the area of the lesion in the absence of progression is termed “moderate leakage” (when occupying ≥50% of the area of the lesion defined at baseline) or “minimal leakage” (when occupying <50% of the area of the lesion defined at baseline). No progression or no moderate or minimal leakage after photodynamic therapy was defined as absence of leakage.
With respect to submacular surgery, the complicated patterns observed on color fundus photographs and fluorescein angiograms required additional terms for adequate description. Additional features include the area of the RPE disturbance after surgical excision, fibrous tissue that stained during fluorescein angiography, and scar that had a very atrophic appearance. These terms and their definitions are required to interpret lesion size at follow-up and changes in lesion size from baseline as well as the parafoveal area of involvement of the lesion after surgery. In addition, terms for fluorescein leakage at the periphery of or within the area of the RPE disturbance after surgery were developed to apply protocols for management of these manifestations of leakage after surgery in a uniform manner.
The recognition of classic versus occult CNV, the subfoveal location of the lesion, and the size and composition of the neovascular lesion were important factors for confirming that a particular patient met eligibility criteria for the SST. As reports on outcomes of clinical trials become available, these guidelines should be helpful in the interpretation of trial results and should supplement previous guidelines13,14 to assist in patient management as well as in the design of future clinical trials of other treatments for CNV.
Footnotes
The Submacular Surgery Trials are supported by cooperative agreements U10 EY11547, EY11557, and EY11558 between the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland, and The Johns Hopkins University. Participating clinical centers are supported by contracts with The Johns Hopkins University.
A previous publication (Submacular Surgery Trials Research Group. Clinical trial performance of community-based compared with university-based practices: lessons from the Submacular Surgery Trials. Submacular Surgery Trials report no. 2. Arch Ophthalmol 2004;122:857–863) lists the investigators and other personnel who contributed data for this article, including the SST Clinical Center photographers and SST Photograph Reading Center personnel essential for this report. The members of the SST Writing Committee for this report, none of whom have any financial conflict of interest in the material presented, are Sharon D. Solomon, MD (Writing Committee Co-chair); Susan B. Bressler, MD (Writing Committee Co-chair); Barbara S. Hawkins, PhD; Marta J. Marsh, MS; and Neil M. Bressler, MD.
References
- 1.Klein R, Klein BE, Linton KC. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 2.The Age-Related Eye Disease Study Research Group. Potential public health impact of AREDS results: AREDS report no. 11. Arch Ophthalmol. 2003;121:1621–1624. doi: 10.1001/archopht.121.11.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferris FL. Senile macular degeneration: review of epidemiologic features. Am J Ophthalmol. 1983;118:132–151. doi: 10.1093/oxfordjournals.aje.a113624. [DOI] [PubMed] [Google Scholar]
- 4.Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy after five years. Results from randomized clinical trials. Arch Ophthalmol. 1991;109:1109–1114. [PubMed] [Google Scholar]
- 5.Macular Photocoagulation Study Group. Laser photocoagulation for juxtafoveal choroidal neovascularization: five-year results from randomized clinical trials. Arch Ophthalmol. 1994;112:500–509. [PubMed] [Google Scholar]
- 6.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration: updated findings from two clinical trials. Arch Ophthalmol. 1993;111:1200–1209. doi: 10.1001/archopht.1993.01090090052019. [DOI] [PubMed] [Google Scholar]
- 7.Bressler NM, Bressler SB, Gragoudas ES. Clinical characteristics of choroidal neovascular membranes. Arch Ophthalmol. 1987;105:209–213. doi: 10.1001/archopht.1987.01060020063030. [DOI] [PubMed] [Google Scholar]
- 8.Freund KB, Yannuzzi LA, Sorenson JA. Age-related macular degeneration and choroidal neovascularization. Am J Ophthalmol. 1993;115:786–791. doi: 10.1016/s0002-9394(14)73649-9. [DOI] [PubMed] [Google Scholar]
- 9.Moissiev J, Alhalel A, Masuri R, Treister G. The impact of the Macular Photocoagulation Study results on the treatment of exudative age-related macular degeneration. Arch Ophthalmol. 1995;113:185–189. doi: 10.1001/archopht.1995.01100020069031. [DOI] [PubMed] [Google Scholar]
- 10.Verteporfin Roundtable 2000 and 2001 Participants, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Principal Investigators, Verteporfin In Photodynamic Therapy (VIP) Study Group Principal Investigators. Guidelines for using verteporfin (Visudyne®) in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina. 2002;22:6–18. doi: 10.1097/00006982-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials—TAP report 2. Arch Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- 12.Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two year results of a randomized clinical trial including lesions with occult but no classic neovascularization—VIP report no. 2. Am J Ophthalmol. 2001;131:541–560. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 13.Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration: guidelines for evaluation and treatment in the Macular Photocoagulation Study. Arch Ophthalmol. 1991;109:1242–1257. [PubMed] [Google Scholar]
- 14.Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) and Verteporfin In Photodynamic Therapy Study Groups. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment—TAP and VIP report no. 2. Arch Ophthalmol. 2003;121:1253–1268. doi: 10.1001/archopht.121.9.1253. [DOI] [PubMed] [Google Scholar]


