Abstract
The hepatocyte growth factor-regulated tyrosine kinase substrate, Hrs, has been implicated in intracellular trafficking and signal transduction. Hrs contains a phosphatidylinositol 3-phosphate-binding FYVE domain that contributes to its endosomal targeting. Here we show that Hrs and EEA1, a FYVE domain protein involved in endocytic membrane fusion, are localized to different regions of early endosomes. We demonstrate that Hrs co-localizes with clathrin, and that the C-terminus of Hrs contains a functional clathrin box motif that interacts directly with the terminal β-propeller domain of clathrin heavy chain. A massive recruitment of clathrin to early endosomes was observed in cells transfected with Hrs, but not with Hrs lacking the C-terminus. Furthermore, the phosphatidylinositol 3-kinase inhibitor wortmannin caused the dissociation of both Hrs and clathrin from endosomes. While overexpression of Hrs did not affect endocytosis and recycling of transferrin, endocytosed epidermal growth factor and dextran were retained in early endosomes. These results provide a molecular mechanism for the recruitment of clathrin onto early endosomes and suggest a function for Hrs in trafficking from early to late endosomes.
Keywords: adaptor/clathrin/endocytosis/membrane traffic/phosphoinositide
Introduction
The uptake of macromolecules by way of endocytosis serves many important cellular functions, including the acquisition of extracellular nutrients, regulation of cell surface receptor expression, maintenance of cell polarity and antigen presentation (Gagescu et al., 2000; Mostov et al., 2000; Watts, 2001). A complex molecular machinery regulates vesicle-mediated transport of proteins and lipids between organelles in the endocytic pathway (Gu and Gruenberg, 1999; Marsh and McMahon, 1999; Sorkin, 2000). The phosphatidylinositol 3-kinase (PI 3-kinase) product, phosphatidylinositol 3-phosphate [PtdIns(3)P], is an example of a lipid that regulates endocytic trafficking in yeast and higher eukaryotes (Wurmser et al., 1999; Gillooly et al., 2001). Potential effectors of PtdIns(3)P have emerged through the observation that a conserved domain, the FYVE zinc finger, binds specifically to this lipid (Burd and Emr, 1998; Gaullier et al., 1998; Patki et al., 1998). Several FYVE domain proteins have functions in endocytic membrane trafficking and receptor signalling (Stenmark and Aasland, 1999; Gillooly et al., 2001).
We previously have studied the subcellular localization of two FYVE domain proteins. Membrane targeting of the early endosomal autoantigen EEA1 is thought to be mediated via a cooperative binding of its FYVE and Rab5-binding domains to PtdIns(3)P and Rab5, respectively (Simonsen et al., 1998b; Gaullier et al., 2000). Recently, we found that FYVE and coiled-coil domains of the hepatocyte growth factor-regulated tyrosine kinase substrate Hrs target this protein to early endosomes (Raiborg et al., 2001). Thus, like EEA1, the endosomal targeting of Hrs is mediated by a PtdIns(3)P-binding FYVE domain in cooperation with an additional domain. This raises the questions as to whether EEA1 and Hrs share the same localization on the early endosomal membrane, and whether both proteins function in the same biochemical pathways.
While the function of EEA1 as a tethering molecule involved in the homo- and heterotypic fusion of early endosomes is well characterized (Mills et al., 1998; Simonsen et al., 1998b; McBride et al., 1999; Rubino et al., 2000), little is known about the role of Hrs in endocytic trafficking. Hrs was identified originally as a tyrosine-phosphorylated protein in melanoma cells stimulated with hepatocyte growth factor (Komada and Kitamura, 1995). The protein is ubiquitously expressed and is also tyrosine phosphorylated upon stimulation of cells with epidermal growth factor (EGF), platelet-derived growth factor, interleukin-2 and granulocyte–macrophage colony-stimulating factor (Komada and Kitamura, 1995; Asao et al., 1997). The phosphorylation of Hrs is prevented when endocytosis is inhibited, suggesting that this post-translational modification takes place at the endosome (Urbé et al., 2000). In addition to the FYVE domain, Hrs contains two coiled-coil domains, a VHS domain and proline/glutamine-rich regions. Through its various domains, Hrs has been shown to bind the signal transducing adaptor molecules STAM (Asao et al., 1997) and STAM2/Hbp (Endo et al., 2000; Takata et al., 2000), the SNARE protein SNAP-25 (Kwong et al., 2000) and Smad2, which is known to transmit signals by activated transforming growth factor-β/activin receptors (Miura et al., 2000). Hrs is required for ventral folding morphogenesis, and Hrs–/– mouse embryos accumulate enlarged endosomes and die at the 11-day stage (Komada and Soriano, 1999). A putative yeast homologue of Hrs, Vps27p, is involved in vacuolar protein sorting (Piper et al., 1995; Odorizzi et al., 1998). Given the essentiality of Hrs and its implication in both signalling and membrane trafficking, it will be of great importance to study the function of Hrs on early endosomes. Here we show that the C-terminal domain of Hrs recruits the membrane coat protein clathrin to early endosomes. Moreover, we provide evidence for a function of Hrs in trafficking from early to late endosomes.
Results
The two PtdIns(3)P-binding proteins EEA1 and Hrs localize to distinct regions of Rab5Q79L-induced giant early endosomes
In order to study the relative distribution of the two PtdIns(3)P-binding proteins EEA1 and Hrs on the early endosomal membrane, we transfected BHK cells with a GTPase-deficient mutant form of Rab5 (Rab5Q79L) that leads to the formation of enlarged early endosomes due to increased homo- and heterotypic fusion (Stenmark et al., 1994). The enlarged perimeter of the Rab5Q79L-positive endosomes provides the opportunity to study the relative localization of proteins in the vesicle membrane. As expected, EEA1 was non-uniformly located in regions of the limiting membrane of giant early endosomes (Figure 1A, inset). These ‘hot-spots’ have been demonstrated previously by video microscopy to be involved in membrane fusion (McBride et al., 1999; Roberts et al., 1999). In contrast, while Hrs was also detected on the surface of the enlarged endosomes (Figure 1B, inset), this protein localized to regions different from EEA1. These results indicate that EEA1 and Hrs are associated with distinct molecular complexes in the endosomal membrane.
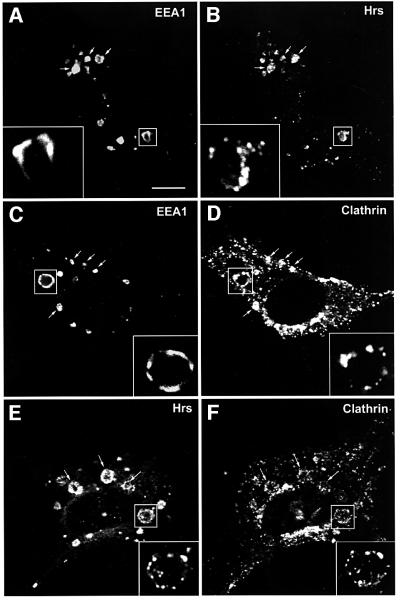
Fig. 1. Localization of Hrs, EEA1 and clathrin in Rab5Q79L-overexpressing BHK cells. BHK cells were transfected with Rab5Q79L and permeabilized with 0.05% saponin prior to fixation. They were then stained with anti-EEA1 (A and C), anti-Hrs (B and E) or anti-clathrin (D and F) and studied by confocal immunofluorescence microscopy. Arrows indicate examples of co-localization. Bar, 5 µm.
Clathrin localizes to Hrs-containing regions on endosomes
The lack of precise co-localization between EEA1 and Hrs suggests that these two proteins are involved in distinct biochemical pathways. Since EEA1 regulates membrane fusion, we reasoned that Hrs might regulate some other endosomal trafficking events, such as receptor sorting or vesicle budding. We therefore investigated the possible co-localization between Hrs and molecules implicated in the latter events. Previous studies have identified clathrin on early endosomes (Stoorvogel et al., 1996; Sorkina et al., 1999; Raposo et al., 2001). In order to detect endosomal clathrin by confocal immunofluorescence microscopy, we transfected BHK cells with Rab5Q79L and stained the cells with an anti-clathrin antibody. As expected, the anti-clathrin antibody stained numerous punctae that presumably correspond to coated pits in the plasma membrane and coated vesicles throughout the cytoplasm of the cell (Figure 1D). In addition, a subset of the clathrin was found on the enlarged Rab5Q79L-positive endosomes (Figure 1D, arrows), confirming the existence of an endosomal pool of clathrin. We did not detect the clathrin adaptors AP1 and AP2 on the enlarged endosomes, and endosomal AP3 was not found to co-localize with clathrin (not shown). Like EEA1 and Hrs, clathrin was detected on regions of the enlarged endosomes (Figure 1D, inset). Significantly, whereas clathrin localized to regions different from EEA1 (Figure 1C and D, inset), it was found to co-localize strongly with Hrs-containing regions on the endosomal membrane (Figure 1E and F, inset).
Having established that Hrs co-localizes with clathrin on Rab5Q79L-expanded early endosomes, we set out to determine whether the same is the case in non-transfected cells. Hrs was identified originally in melanoma cells (Komada and Kitamura, 1995), and melanoma cells recently have been shown by electron microscopy to contain clathrin-coated endosomes involved in melanosome formation (Raposo et al., 2001). To investigate the co-localization of Hrs and clathrin further, we therefore triple stained non-transfected melanoma cells with anti-EEA1, anti-Hrs and anti-clathrin antibodies (Figure 2). In agreement with the data obtained with Rab5Q79L-transfected BHK cells, we observed an extensive co-localization between Hrs and clathrin on EEA1-positive endosomes of melanoma cells (Figure 2A–D). Moreover, when magnifying these early endosomes (Figure 2E–I), we could observe that EEA1 and Hrs had a differential localization, and that clathrin co-localized with the Hrs-containing regions. The same observation was also made in non-transfected HEp-2 cells (not shown), although these cells had less clathrin on endosomes. The striking co-localization between Hrs and clathrin suggests that these proteins may be found in the same molecular complexes on early endosomes.
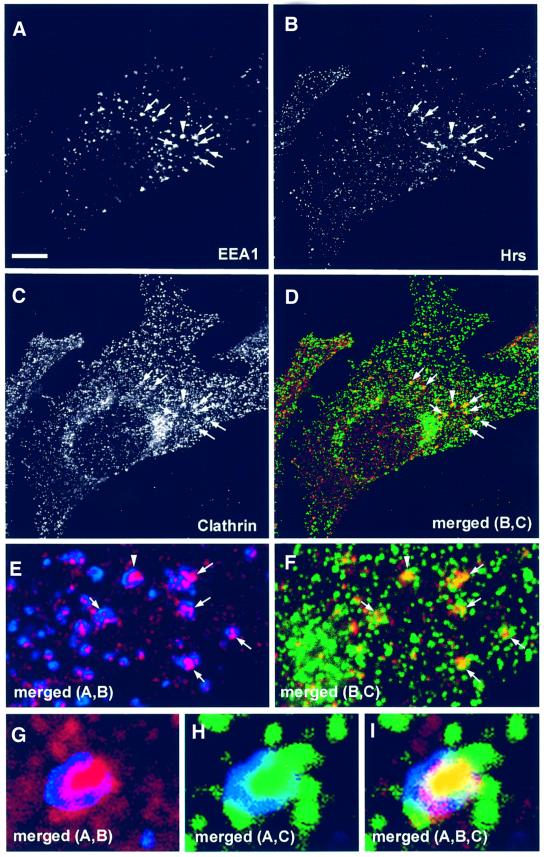
Fig. 2. Localization of Hrs, EEA1 and clathrin in melanoma cells. A melanoma cell, permeabilized with 0.05% saponin prior to fixation, was stained with anti-EEA1 (A), anti-Hrs (B) and anti-clathrin (C). Arrows indicate co-localization between EEA1, Hrs and clathrin. Yellow indicates co-localization between Hrs and clathrin (D). The arrowhead points to an endosome that is shown magnified in (G–I). Bar, 5 µm. (E and F) Magnification of the endosomes highlighted by arrows in (A–D). The arrowhead points to an endosome that is shown magnified in (G–I). (G–I) Merged images of a triple-stained magnified endosome. Note the differential localization of EEA1 in blue and Hrs in red, and the co-localization (yellow) between clathrin in green and Hrs.
Hrs binds directly to the terminal domain of clathrin heavy chain
When we compared the amino acid sequence of Hrs with those of different clathrin-binding proteins such as amphiphysins, β-arrestins and adaptor proteins, we detected a cluster of five conserved residues in the very C-terminus of Hrs. In the other clathrin-binding proteins, this sequence, which consists of one polar amino acid flanked by hydrophobic and acidic amino acids [LLpL(–)], has been described as the ‘clathrin box’ and interacts specifically with the terminal domain (TD) of clathrin heavy chain (HC) (Goodman et al., 1997; Dell’Angelica et al., 1998; ter Haar et al., 2000). An alignment of the clathrin box motif from different clathrin-binding proteins and that of Hrs is shown in Figure 3A.
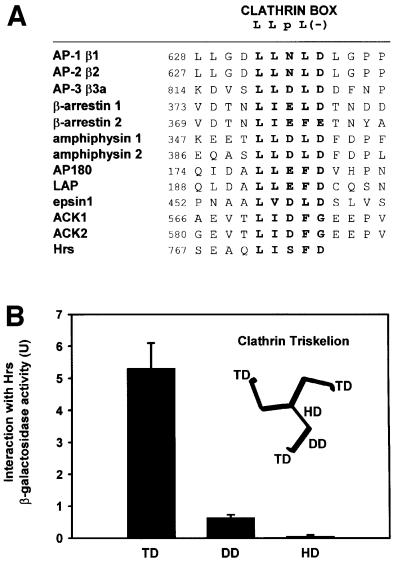
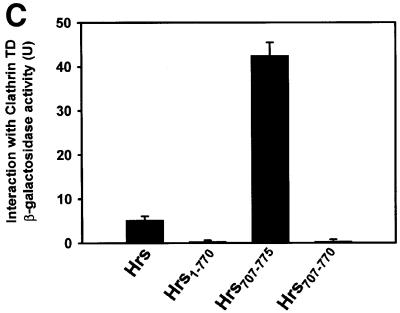
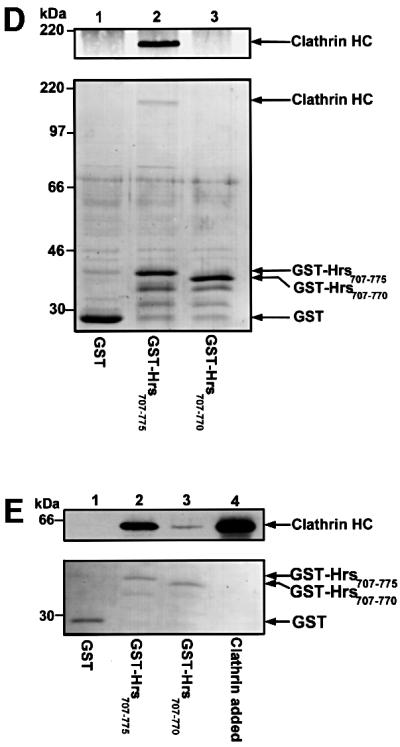
Fig. 3. The C-terminus of Hrs binds clathrin TD. (A) Alignment of sequences found in proteins that bind clathrin (ter Haar et al., 2000; Teo et al., 2001; Yang et al., 2001). This illustrates the existence of a potential clathrin-binding motif within residues 770–775 of Hrs. Hrs is the only protein that has the clathrin box motif at the very C-terminus. (B–E) Interaction of Hrs with clathrin. (B and C) L40 reporter yeast cells were transformed with bait constructs in pLexA and prey constructs in pGAD. Reporter β-galactosidase activities (in arbitrary units) indicate binding and are represented as mean values of two independent experiments performed in duplicate. Error bars denote ± SEM. In (B), a clathrin triskelion (consisting of three heavy chains) is illustrated, with the terminal domain (TD), distal domain (DD) and hub domain (HD) indicated. (D) Recombinant GST (lane 1), GST–Hrs707–775 (lane 2) or GST–Hrs707–770 (lane 3) were immobilized on glutathione–Sepharose beads and incubated with pig brain cytosol. The beads were recovered by centrifugation and washed. Pellet fractions were resolved by SDS–PAGE and transferred to nitrocellulose. The blot was stained with Ponceau S (lower panel) prior to detection of clathrin with anti-clathrin heavy chain antibodies (upper panel). (E) Recombinant GST (lane 1), GST–Hrs707–775 (lane 2) or GST–Hrs707–770 (lane 3), were immobilized on glutathione–Sepharose beads and incubated with purified recombinant clathrin terminal domain (TD1–579). The beads were recovered by centrifugation and washed. Pellet fractions were resolved by SDS–PAGE and transferred to nitrocellulose. The blot was stained with Ponceau S (lower panel) prior to detection of clathrin-TD1–579 with anti-clathrin heavy chain (upper panel). Lane 4 represents the total amount of recombinant clathrin-TD1–579 added to the beads.
Based on these findings, we wanted to investigate whether Hrs could interact with clathrin. Using the yeast two-hybrid system, we first tested whether there was an interaction between Hrs and three different domains of the clathrin HC (Figure 3B). While we found no significant interaction between full-length Hrs and the distal domain (residues 493–1072) or the hub domain (residues 1066–1675) of clathrin HC, we observed a strong interaction between Hrs and the terminal domain (TD) of clathrin HC (residues 1–579) (Figure 3B). An interaction was also found with a shorter fragment of clathrin HC (residues 1–330) (not shown), constituting the β-propeller domain of the clathrin TD (ter Haar et al., 2000). These results suggest that Hrs binds specifically to clathrin TD. While a deletion mutant lacking the 102 most C-terminal amino acids of Hrs (Hrs1–673) did not bind (not shown), a small construct consisting of the 69 most C-terminal amino acids (Hrs707–775) showed a strong interaction with clathrin TD (Figure 3C), indicating that the C-terminal part of Hrs is an autonomous clathrin-binding domain. Further, deletion of the clathrin box motif of full-length Hrs (Hrs1–770) or the clathrin-binding domain (Hrs707–770) largely abolished the interaction with clathrin TD (Figure 3C), demonstrating the importance of these five amino acids for clathrin binding.
To confirm the two-hybrid data biochemically, we studied the interaction between Hrs and clathrin-TD in a GST pull-down assay. When GST–Hrs707–775 coupled to glutathione–Sepharose was incubated with pig brain cytosol, clathrin HC bound to the fusion protein and was pelleted with the beads (Figure 3D, lane 2). No detectable clathrin was pulled down with GST alone or with GST–Hrs707–770, confirming the importance of the clathrin box motif (Figure 3D). To investigate whether Hrs binds directly to the clathrin TD, we examined the interaction between these proteins by using purified recombinant clathrin TD (clathrin-TD1–579). When incubated with GST–Hrs707–775 immobilized on glutathione–Sepharose, clathrin-TD1–579 was retained in the bead fraction (Figure 3E, lane 2), whereas no binding was observed with GST alone. It is worth noting that a small fraction of clathrin-TD1–579 bound to GST–Hrs707–770 (Figure 3E, lane 3), indicating that the deletion of the clathrin box reduces, but does not completely abolish, the affinity of Hrs for clathrin.
Hrs recruits clathrin to early endosomes
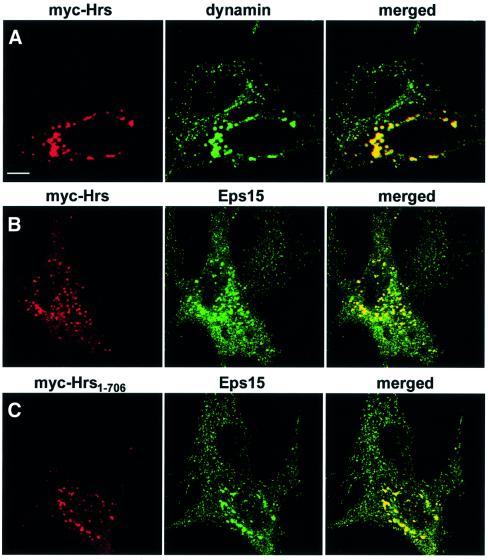
In order to study whether Hrs is able to recruit clathrin to early endosomes in vivo, we expressed myc epitope-tagged Hrs constructs in BHK cells and examined their co-localization with clathrin. As previously described (Urbé et al., 2000; Raiborg et al., 2001), overexpressed Hrs co-localized with EEA1 on clustered early endosomes (Figure 4A). Significantly, a massive recruitment of both clathrin HC (Figure 4B) and clathrin light chain (LC) (not shown) was observed on these structures, as compared with non-transfected cells (Figure 4B). Co-localization with clathrin was also observed with a deletion mutant of Hrs lacking the N-terminal VHS domain (not shown). Hrs still showed some co-localization with clathrin after deletion of the five amino acids constituting the clathrin box (not shown), indicating that this motif is not absolutely essential for clathrin recruitment, and that additional residues in the C-terminus of Hrs may participate in the interaction. This is consistent with results with β-arrestin (ter Haar et al., 2000) and epsin 1 (Drake et al., 2000) and in agreement with our results obtained with the GST pull-down assay (Figure 3E). However, a deletion mutant lacking the 69 most C-terminal amino acids (Hrs1–706) did not show any recruitment of clathrin to the Hrs-positive endosomes (Figure 4C). These findings indicate that the C-terminal part of Hrs recruits clathrin to early endosomes.
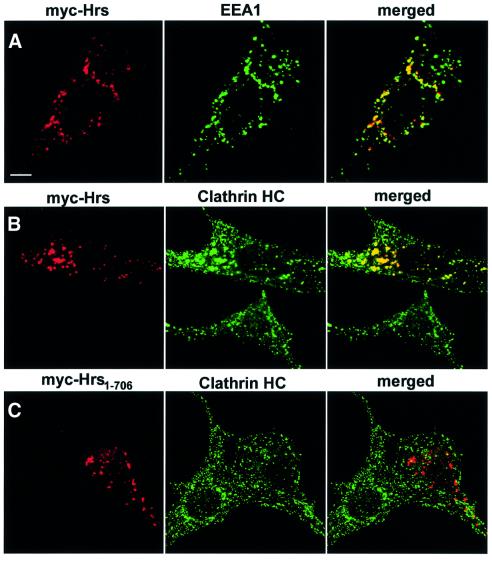
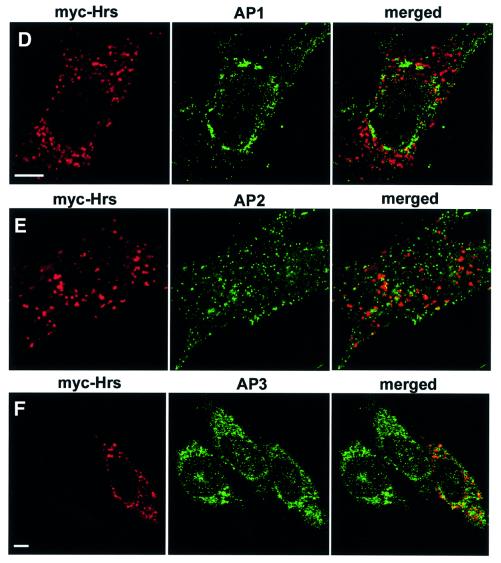
Fig. 4. (A–C) Overexpressed Hrs recruits clathrin to early endosomes. BHK cells were transfected with myc-tagged Hrs (A and B) or Hrs1–706 (C) and permeabilized with 0.05% saponin prior to fixation. They were stained with anti-myc (left panels), anti-EEA1 (A, middle panel) or anti-clathrin (B and C, middle panels) and studied by confocal immunofluorescence microscopy. (D–F) Overexpressed Hrs does not recruit AP1, AP2 or AP3. BHK cells or HEp-2 cells (in the case of AP3) were transfected with myc-tagged Hrs and permeabilized with 0.05% saponin prior to fixation. The cells were stained with anti-myc (left panels), anti-AP1 (D, middle panel), anti-AP2 (E, middle panel) or anti-AP3 (F, middle panel) and studied by confocal immunofluorescence microscopy. The right panels show the merged images, with yellow indicating co-localization. Bar, 5 µm.
The clathrin adaptor proteins AP1 and AP2 are found in the trans-Golgi network (TGN) and in the plasma membrane, respectively, whereas AP3 is found in the TGN and on endosomes (Dell’Angelica et al., 1997; Hirst and Robinson, 1998; Kirchhausen, 1999). Because these adaptor complexes interact directly with clathrin through a clathrin box sequence (Shih et al., 1995; Dell’Angelica et al., 1998), we investigated their possible recruitment to Hrs-overexpressing endosomes. As expected, AP1 was found in the trans-Golgi region (Figure 4D), while AP2 was detected on the plasma membrane and in coated vesicles (Figure 4E), and AP3 had mainly a perinuclear distribution (Figure 4F). In contrast to clathrin, no recruitment of AP1, AP2 or AP3 onto Hrs-overexpressing endosomes was observed (Figure 4D–F). AP1 and AP2 have been previously reported to co-localize with EGF receptors and clathrin on endosomes in A431 cells stimulated with EGF (Sorkina et al., 1999), whereas very little AP2 was found on endosomes in Cos-1 cells (Sorkina et al., 1999). Since we also did not detect the presence of AP1 or AP2 on endosomes, the endosomal localization of these clathrin adaptors may be cell type dependent. Unlike the adaptor proteins, the coated vesicle-associated GTPase, dynamin, was found to be recruited by Hrs onto early endosomes (Figure 5A), together with Eps15 (Figure 5B), which has recently been shown to bind directly to the N-terminal half of Hrs (Bean et al., 2000). Similarly to clathrin, dynamin did not co-localize with Hrs lacking the C-terminus (Hrs1–706), suggesting that it may be recruited via clathrin (not shown). In contrast, Eps15 was recruited to endosomes by Hrs1–706 (Figure 5C), consistent with Eps15 binding to a region of Hrs different from clathrin.
Fig. 5. Overexpressed Hrs recruits dynamin and Eps15 to endosomes. BHK cells were transfected with myc-tagged Hrs (A and B) or Hrs1–706 (C) and permeabilized with 0.05% saponin prior to fixation. They were stained with anti-myc (left panels), anti-dynamin (A, middle panel) or anti-Eps15 (B and C, middle panels) and studied by confocal immunofluorescence microscopy. The right panels show the merged images, with yellow indicating co-localization. Bar, 5 µm.
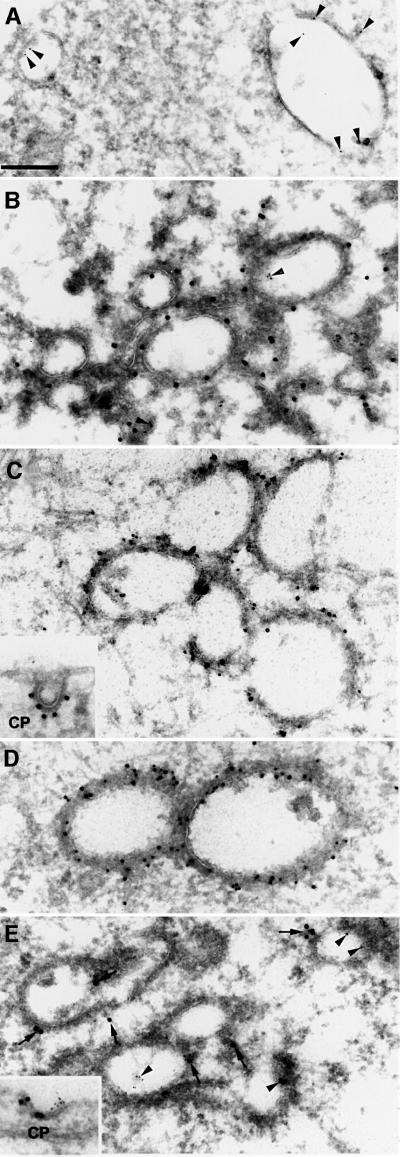
Early endosomes in BHK cells have been well characterized by electron microscopy (Griffiths et al., 1989) and typically consist of isolated cisternae as exemplified in Figure 6A. When cells overexpressing Hrs were analysed, a clustering of bovine serum albumin (BSA)–gold-containing Hrs-positive endosomes was observed (Figure 6B), in agreement with the effect seen by confocal microscopy. The Hrs-positive vesicles contained an electron-dense flat coat, which gives these structures a characteristic morphology (Figure 6B). Consistent with the immunofluorescence data, the coat contained clathrin (Figure 6C), Eps15 (Figure 6D) and dynamin (Figure 6E). The Hrs-induced coat was slightly thicker than a typical clathrin coat observed on clathrin-coated pits (Figure 6C, inset). Moreover, a coat could also be observed in cells transfected with a mutant deficient in clathrin binding, Hrs1–706 (not shown), indicating that it consists of additional proteins.
Fig. 6. Electron microscopy of Hrs-overexpressing endosomes. (A) Early endosomes from a non-transfected BHK cell that prior to fixation had endocytosed 5 nm BSA–gold (arrowheads) for 10 min. (B) Localization of Hrs in a myc-Hrs-transfected BHK cell that prior to fixation had endocytosed 5 nm BSA–gold (arrowheads) for 1 h. Thawed cryosections were labelled with anti-myc antibodies followed by rabbit anti-mouse antibodies and 15 nm protein A–gold. Note the coated appearance and clustering of the Hrs-positive compartments. (C) Immunoelectron microscopic localization of clathrin in myc-Hrs-transfected cells. Thawed cryosections were double labelled using rabbit anti-clathrin LC (10 nm gold) followed by mouse anti-myc and rabbit anti-mouse antibodies (15 nm gold). The labelling showed that clathrin localized to the characteristic myc-Hrs-induced endosomal coat. (C, inset) A clathrin-coated pit labelled with goat anti-clathrin followed by rabbit anti-goat and 15 nm protein A–gold. (D) Immunoelectron microscopic localization of Eps15 in myc-Hrs-transfected cells. Thawed cryosections were double labelled with anti-Eps15 (10 nm protein A–gold) followed by labelling for the myc epitope (15 nm protein A–gold). There is a strong Eps15 labelling on the characteristic Hrs-positive endosomal coat. (E) Immunoelectron microscopic localization of dynamin in myc-Hrs-transfected cells. Thawed cryosections of cells that had endocytosed 5 nm BSA–gold for 1 h (arrowheads) were labelled with mouse anti-dynamin 1 antibody (Hudy 1) followed by rabbit anti-mouse antibodies and 15 nm protein A–gold (arrows). In cells showing the characteristic clustering of coated endosomes due to Hrs overexpression, labelling of dynamin is localized to the endosomal coat as well as to typical clathrin-coated pits at the plasma membrane (CP, inset). Bar, 200 nm.
Overexpressed Hrs does not interfere with transferrin endocytosis and recycling
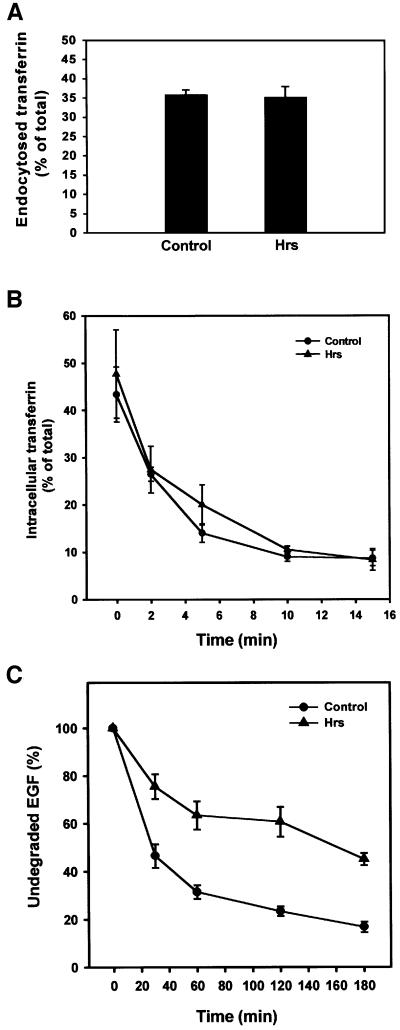
Due to the strong recruitment of clathrin to Hrs-positive early endosomes, we wanted to assess the role of Hrs in clathrin-mediated endocytosis. Under our transfection conditions, BHK cells co-transfected with transferrin (Tf) receptor and Hrs or various fragments of Hrs (Hrs707–775, Hrs707–770 and Hrs1–706, not shown) showed no reduction in the endocytosis of Tf when compared with cells transfected with Tf receptor alone (Figure 7A). Moreover, the cytosolic clathrin-binding fragment of Hrs (Hrs707–775) did not behave as a dominant-negative mutant for clathrin recruitment on endosomes (not shown). Overexpression of Hrs also did not affect the recycling of Tf (Figure 7B). These results indicate that Hrs does not play a role in the endocytic internalization or recycling pathways.
Fig. 7. Overexpressed Hrs does not interfere with transferrin endocytosis or recycling, but inhibits EGF degradation. (A) Effect of Hrs on transferrin endocytosis. BHK cells were transfected with transferrin receptor alone (control) or co-transfected with transferrin receptor and Hrs for 6 h. The cells were incubated with Ru-tag-labelled transferrin for 15 min at 37°C and surface-bound transferrin was removed by pronase. The amount of cell-associated transferrin was measured using an ORIGEN analyser, and endocytosed transferrin is presented as a percentage of total cell-associated transferrin. Error bars represent the SEM of three independent experiments performed in duplicate. (B) Effect of Hrs on transferrin recycling. BHK cells were transfected with transferrin receptor alone (control) or co-transfected with transferrin receptor and Hrs for 6 h. The cells were incubated with Ru-tag-labelled transferrin for 30 min at 37°C and surface-bound transferrin was removed in a portion of the wells by MESNA. The MESNA-treated cells were incubated further for the times indicated. The amount of cell-associated transferrin was measured using an ORIGEN analyser, and intracellular transferrin is presented as a percentage of total cell-associated transferrin. Error bars represent the SEM of two independent experiments performed in duplicate. (C) Effect of Hrs on EGF degradation. HEp-2 cells were transfected with myc-tagged Hrs for 6 h, and rhodamine-EGF was internalized for 1 h at 37°C. The cells were washed and incubated further for different times up to 3 h. The cells were permeabilized with 0.05% saponin prior to fixation. They were stained with anti-myc and studied by confocal immunofluorescence microscopy. The mean intensity of the rhodamine-EGF signal from 10 transfected and 10 non-transfected cells from each time point was quantified and is presented as the percentage of undegraded EGF compared with the rhodamine-EGF signal before chase. Error bars denote± SEM.
Overexpressed Hrs inhibits trafficking from early to late endosomes
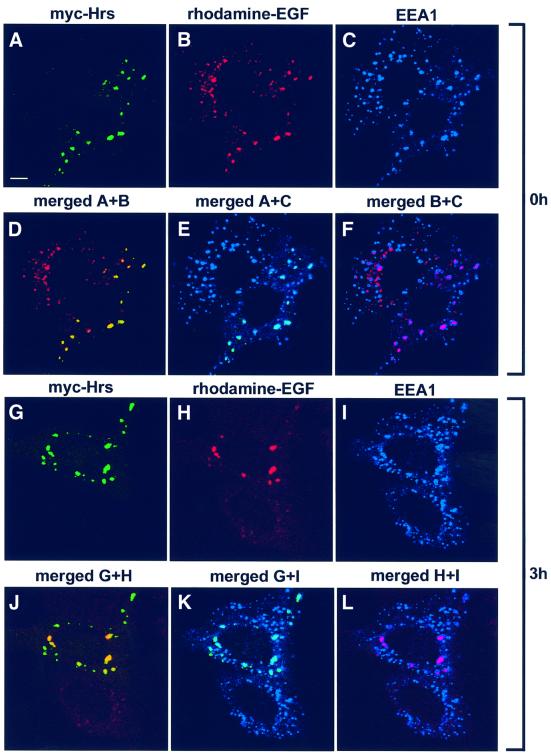
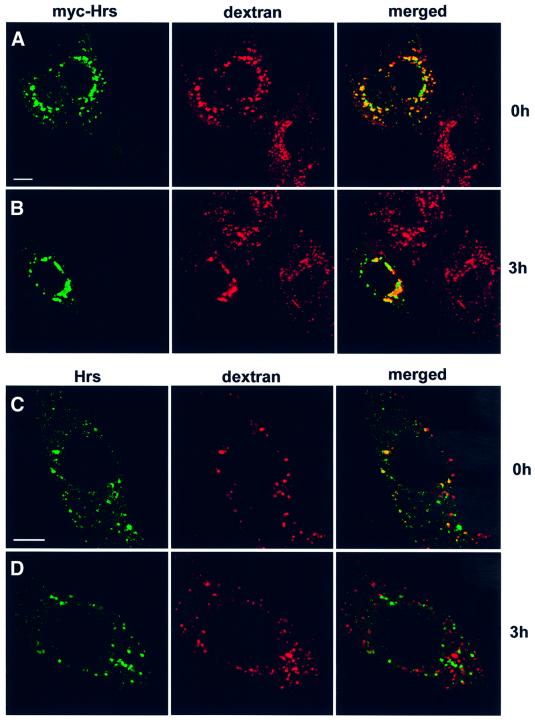
Recently, overexpressed Hrs was shown to inhibit degradation of the EGF receptor (Chin et al., 2001), and it was suggested that Hrs may regulate the sorting of this receptor for transport to late endocytic compartments. We investigated whether this inhibition is specific to EGF or whether overexpression of Hrs could also affect transport of molecules taken up by fluid-phase endocytosis. By internalizing rhodamine-labelled EGF for 1 h, followed by chasing EGF for different time periods, we indeed observed a strong reduction of EGF degradation in cells transfected with Hrs compared with non-transfected cells (Figure 7C). Rhodamine-labelled EGF co-localized strongly with Hrs after both 0 (Figure 8A, B and D) and 3 h of chase (Figure 8G, H and J). However, similar results were obtained with internalized biotin–dextran (Figure 9), demonstrating that overexpression of Hrs leads to a general inhibition of transport. The inhibitory effect was observed in cells expressing both high and low levels of Hrs. Somewhat surprisingly, the non-clathrin-binding deletion mutant of Hrs (Hrs1–706) also caused a general inhibition of transport (not shown). While there was a strong co-localization between overexpressed Hrs and dextran after both 0 and 3 h of chase (Figure 9A and B), endogenous Hrs in non-transfected cells only co-localized with internalized dextran after 0 h and not after the chase period (Figure 9C and D). This is consistent with the localization of Hrs to early endosomes.
Fig. 8. Overexpressed Hrs inhibits EGF trafficking from early to late endosomes. HEp-2 cells were transfected with myc-tagged Hrs for 6 h and incubated with rhodamine-EGF for 1 h at 37°C (A–F). A portion of the cells was washed and incubated further for 3 h (G–L). The cells were permeabilized with 0.05% saponin prior to fixation. They were stained with anti-myc (A and G) or anti-EEA1 antibodies (C and I) and studied by confocal immunofluorescence microscopy. Turquoise indicates co-localization between Hrs and EEA1 (E and K). Purple indicates co-localization between EGF and EEA1 (F and L). Yellow indicates co-localization between Hrs and EGF (D and J). Bar, 5 µm.
Fig. 9. The transport of endocytosed dextran from early to late endosomes is inhibited in Hrs-overexpressing cells. HEp-2 cells were transfected with myc-tagged Hrs for 6 h and incubated with biotin–dextran for 1 h at 37°C (A). The cells were washed and incubated further for 3 h (B). (C) A non-transfected cell incubated with biotin–dextran for 1 h at 37°C. (D) A non-transfected cell after 3 h of chase. The cells were stained with anti-myc (left panels A and B), anti-Hrs (left panels C and D) or streptavidin–Cy3 (middle panels) and studied by confocal fluorescence microscopy. The right panels show the merged images, with yellow indicating co-localization. Bar, 5 µm.
Remarkably, in Hrs-overexpressing cells, the EGF-containing endosomes (Figure 8) or dextran-containing endosomes (not shown) were still positive for EEA1 after 3 h of chase, whereas they were negative for the late endosome/lysosome marker LAMP-1 (not shown), indicating that internalized EGF and dextran are retained in early endosomes in cells transfected with Hrs. Thus, under conditions in which Tf uptake and recycling are unaffected, Hrs overexpression inhibits the trafficking of both a receptor-bound and a fluid-phase marker from early to late endosomes. We next studied this effect at the ultrastructural level. In contrast to non-transfected cells, in which the majority of the internalized BSA–gold was found within multivesicular bodies after 1 h of internalization, BSA–gold accumulated in myc-Hrs-positive structures (not shown), whereas labelling of multivesicular bodies and lysosomes was never observed. Taken together, our data suggest that Hrs is a specific regulator of membrane trafficking from early to late endosomes.
Wortmannin removes Hrs and clathrin from transferrin-positive endosomes
The association of Hrs with early endosomes requires PI 3-kinase activity (Komada and Soriano, 1999; Urbé et al., 2000). To test our conclusion that Hrs recruits clathrin to endosomes, we therefore investigated whether the PI 3-kinase inhibitor wortmannin could disrupt the endosomal localization of clathrin. In order to study the presence of Hrs and clathrin on endosomes, internalized Tf was used as an endosomal marker. In control cells, we observed a co-localization between Hrs and clathrin on Tf-positive endosomes (Figure 10A, arrows). As expected, a 30 min wortmannin treatment led to the dissociation of Hrs from endosome membranes (Figure 10B) and caused a tubulation of endosomes (Shpetner et al., 1996). Signific antly, wortmannin also disrupted the association of clathrin with Tf-positive endosomes (Figure 10B), while clathrin could still be detected in the presumed TGN region (arrowhead) and on the plasma membrane. These results confirm the role of Hrs in the recruitment of clathrin to endosomes.
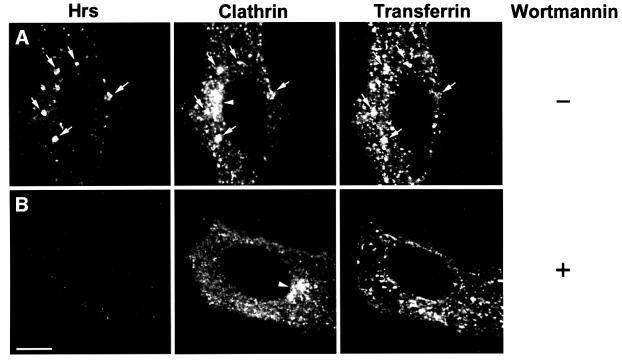
Fig. 10. Wortmannin disrupts the association of Hrs and clathrin with transferrin-positive endosomes. HEp-2 cells were incubated with Alexa488–transferrin for 30 min at 37°C in the absence (A) or presence (B) of 100 nM wortmannin. The cells were permeabilized with 0.05% saponin prior to fixation in order to remove cytosolic proteins. They were stained with anti-Hrs (left panels) and anti-clathrin (middle panels) and studied by confocal fluorescence microscopy. Arrows indicate examples of co-localization between Hrs, clathrin and internalized transferrin. Arrowheads point at the presumed TGN region stained with anti-clathrin. Bar, 5 µm.
Discussion
In this report, we have shown that the two PtdIns(3)P-binding proteins, EEA1 and Hrs, localize to distinct regions of early endosomes, and that the C-terminal 69 residues of Hrs constitute a novel clathrin-binding domain. This provides a mechanism for the specific recruitment of clathrin onto early endosomes and suggests that Hrs may function as an endosomal clathrin adaptor. By recruiting clathrin, Eps15 and other proteins into restricted domains of early endosomes, Hrs appears to regulate trafficking (or maturation) from early to late endosomes.
Differential localization of Hrs and EEA1 on early endosomes
The mechanisms that define distinct membrane domains of early endosomes are not known, but it has been suggested that the differential localization of endosomal Rab GTPases may contribute to define membrane identity (Sönnichsen et al., 2000). Even though most FYVE domain proteins studied so far have been localized to early endosomes, their relative distribution on these organelles has not been investigated. Our finding that EEA1 and Hrs localize to distinct regions of early endosomes now shows that the precise localization of FYVE domain proteins is not only determined by the FYVE-PtdIns(3)P interaction. This is consistent with the fact that a Rab5-binding domain of EEA1 and a coiled-coil domain of Hrs are also involved in the subcellular targeting of these proteins (Simonsen et al., 1998b; Raiborg et al., 2001). While the EEA1-containing regions are clearly involved in membrane tethering and fusion, our present results suggest that the Hrs-containing regions may play a role in protein sorting.
Endosomal clathrin
The molecular machinery responsible for active sorting of integral membrane proteins in early sorting endosomes has so far not been identified. Such sorting can be expected to employ cytoplasmic coats analogous to the different types of coats identified for Golgi cisternae, the endoplasmic reticulum, the TGN and the plasma membrane (Rothman and Wieland, 1996; Hirst and Robinson, 1998; Gu and Gruenberg, 1999; Scales et al., 2000). There is indeed evidence for coats on endosomes (Aniento et al., 1996; Stoorvogel et al., 1996; Futter et al., 1998; Sorkina et al., 1999), and our detection of clathrin on the enlarged Rab5Q79L-positive endosomes, and on endosomes in non-transfected melanoma and HEp-2 cells, confirms the presence of clathrin on restricted regions of endosomes (Stoorvogel et al., 1996; Raposo et al., 2001). In melanoma cells, the endosomal clathrin coat consists of planar or invaginated lattices without budding profiles (Raposo et al., 2001) while, in A431 cells, clathrin-coated buds have been detected, suggesting the formation of endosome-derived clathrin-coated vesicles (Stoorvogel et al., 1996). Thus, there are probably at least two different clathrin coats on endosomes, and our present results are most consistent with a function of Hrs in the formation of the flat clathrin coat previously identified on endosomes in melanoma cells. Our finding that wortmannin-induced dissociation of Hrs from early endosome membranes in HEp-2 cells is accompanied by the disappearance of endosomal clathrin further suggests that Hrs plays an important role in clathrin recruitment to endosomes in these cells. Whether the dissociation of clathrin from endosomes may explain some of the effects of wortmannin on endosome morphology and function (Shpetner et al., 1996) remains to be investigated.
Hrs as a regulator of endocytic trafficking
Our finding that overexpression of Hrs did not affect Tf endocytosis contradicts a previous report in which Hrs was found to inhibit Tf endocytosis when expressed at high level (Bean et al., 2000). The reason for this discrepancy is not clear, but one possibility is that the reported inhibition was caused by a higher level of overexpression, which might cause a redistribution of clathrin from the plasma membrane to endosomes. Importantly, under our conditions in which Tf uptake and recycling were unaffected, Hrs overexpression was found to inhibit the trafficking of both a receptor-bound and a fluid-phase marker from early to late endosomes. This indicates that Hrs functions in the trafficking from early to late endosomes rather than between the plasma membrane and early endosomes.
Somewhat surprisingly, the inhibitory effect of Hrs overexpression did not seem to be due to the massive recruitment of clathrin to early endosomes, as a non-clathrin-binding deletion mutant of Hrs (Hrs1–706) also caused a general inhibition of transport. The overexpression of endosome-targeted Hrs constructs leads to a clustering of early endosomes (see Figure 6B), irrespective of the presence of the clathrin-binding C-terminus (Raiborg et al., 2001). We therefore speculate that the overexpression of these constructs may lead to a hyper-recruitment or sequestering of specific proteins that regulate endosome docking or motility.
Hrs as a possible clathrin adaptor
Clathrin adaptors are recruited from the cytosol to specific membranes through a cooperative binding to receptor proteins and phosphoinositides (Gaidarov and Keen, 1999; Kirchhausen, 1999). Here we demonstrate that Hrs, which binds PtdIns(3)P and is localized to early endosomes, contains a C-terminal clathrin-binding domain, and that overexpression of Hrs recruits clathrin to early endosomes. Hrs therefore is a possible candidate for an endosomal clathrin adaptor. A well-established function of adaptors is to recognize the sorting signals present in the cytosolic tails of those transmembrane proteins that are recruited to coated pits and vesicles. So far, we have been unable to detect any complex between Hrs and receptors, but it is interesting to note that EAST, the chicken homologue of the tightly Hrs-associated protein STAM, has been found to interact with the EGF receptor in a ligand-dependent fashion (Lohi et al., 1998). Moreover, Hrs has been found to interact with sorting nexin-1 (Snx1), which recognizes the lysosomal sorting motif of the EGF receptor (Chin et al., 2001). Even more intriguing are the recent findings that GGA proteins, which contain VHS domains, appear to function as monomeric clathrin adaptors in the TGN, and that the VHS domain interacts with receptor tails (Nielsen et al., 2001; Puertollano et al., 2001). Since both Hrs and STAM contain VHS domains, we speculate that the Hrs–STAM complex may function as a dimeric clathrin adaptor on early endosomes. Our findings that Hrs co-localizes with clathrin but not with the adaptor proteins AP1, AP2 and AP3 on endosomes, and that wortmannin causes the dissociation of clathrin from endosomes, support the idea that Hrs may itself act as an endosomal clathrin adaptor.
A recruitment of receptors into flat clathrin lattices may be a mechanism to sort cargo that is destined for late endosomes. The endosomal clathrin coat would then have to dissociate before the formation of endosomal invaginations, and a possible mechanism is suggested by the finding that phosphorylated Hrs dissociates from endosomes (Urbé et al., 2000). In order to test these hypotheses it will be necessary to identify receptors that are recognized by Hrs–STAM.
Materials and methods
Antibodies and labelled proteins
Anti-myc antibodies were from the 9E10 hybridoma (Evan et al., 1985). Human anti-EEA1 serum was a gift from Ban-Hock Toh. For immunoblotting, an antiserum against Hrs was prepared by injecting two rabbits with a synthetic peptide corresponding to residues 242–256 of mouse Hrs, coupled to keyhole limpet haemocyanin. The antiserum was affinity purified on recombinant Hrs1–289 (Gaullier et al., 1998) immobilized on Affi-Gel (Bio-Rad, Hercules, CA). For immunocytochemistry, an antiserum against recombinant maltose-binding protein (MBP)–Hrs was raised in two rabbits. The antiserum was first adsorbed against immobilized MBP and then affinity purified on Affi-Gel beads containing immobilized MBP–Hrs. Mouse anti-clathrin heavy chain (HC) was from Research Diagnostics, Inc. (Flanders, NL) and goat anti-clathrin HC (C-20) was from Santa Cruz Biotechnology, Inc. Rabbit anti-clathrin LC (Liu et al., 2001) was a gift from Frances Brodsky. Anti-AP1 (γ-adaptin) was purchased from Sigma, while anti-AP2 (α-adaptin) was from Affinity Bioreagents, Inc. Anti-AP3 (δ-adaptin) was provided by Stefan Höning. Rabbit anti-Eps15 (C-terminus) was from Berkeley Antibody Company (Berkeley, CA). Anti-dynamin (Hudy 1) was from Upstate Biotechnology. Rhodamine-, fluorescein isothiocyanate-, Cy3-, Cy5- and horseradish peroxidase-labelled secondary antibodies were from Jackson Immunoresearch (West Grove, PE). Rhodamine-labelled EGF and biotinylated dextran were from Molecular Probes, Inc. Rabbit-anti mouse IgG and rabbit-anti goat IgG antibodies used for electron microscopy were from Cappel.
Plasmid constructs
The Hrs constructs indicated were generated by PCR with mouse Hrs (Komada and Kitamura, 1995) as the template. Synthetic oligonucleotides were from MedProbe (Oslo, Norway). For expression in mammalian cells with the T7 RNA polymerase vaccinia virus system, constructs were cloned behind the myc epitope of pGEM-myc4 (Simonsen et al., 1998a). For use in the two-hybrid system, constructs were cloned into pLexA/pBTM116 (Vojtek et al., 1993) as bait and pGAD GH (Clontech) as prey. For expression of GST fusion proteins in Escherichia coli, pGEX-Hrs or the deletion constructs indicated were obtained by subcloning the respective cDNAs into the polylinker sites of pGEX-6P (Amersham Pharmacia). pGEX-2T-clathrin1–579 was a gift from Dr Jim Keen (Goodman et al., 1997). For expression of a fusion protein between MBP and Hrs (MBP–Hrs), Hrs was cloned into the polylinker of pMAL-c2 (New England Biolabs).
Transient expression in BHK or HEp-2 cells
pGEM and pcDNA3 constructs were expressed in cells using modified Ankara T7 RNA polymerase recombinant vaccinia virus and lipofection as decribed (Stenmark et al., 1995). Cells were analysed 6 h after transfection.
Confocal fluorescence microscopy
BHK, HEp-2 or FEMX-III melanoma cells (Fodstad et al., 1988) grown on coverslips were fixed with 3% (w/v) paraformaldehyde (PFA) and stained for fluorescence microscopy as described (Simonsen et al., 1998a). In some experiments, cells were permeabilized with 0.05% saponin prior to fixation. When indicated, cells were incubated with biotinylated dextran, rhodamine-labelled EGF, Alexa488–Tf (25 µg/ml) or wortmannin (100 nM) prior to fixation. Coverslips were examined using a Leica TCS NT confocal microscope equipped with a Kr/Ar laser and a PL Fluotar 100×/1.30 oil immersion objective. Appropriate emission filter settings and controls were included to exclude bleed-through effects.
Yeast two-hybrid methods
The yeast reporter strain L40 (Vojtek et al., 1993) was co-transformed (Schiestl and Gietz, 1989) with the indicated pLexA and pGAD plasmids, and β-galactosidase activities of transformants were determined as previously described (Guarente, 1983).
Protein expression and purification
GST and various GST fusion proteins were produced in E.coli BL21 (DE3) cells as described previously (Callaghan et al., 1999). The recombinant proteins were purified on glutathione–Sepharose 4B (Amersham Pharmacia Biotech AB, Uppsala, Sweeden) after lysis of the bacteria in B-PER™ reagent (Pierce). GST was cleaved from clathrin-TD1–579 by digestion with thrombin protease (Amersham Pharmacia Biotech), using the conditions recommended by the manufacturer. MBP–Hrs was expressed in E.coli BL21 (DE3) cells as described previously (Callaghan et al., 1999) and purified on amylose resin (New England Biolabs) according to the manufacturer’s instructions. Pig brain cytosol was prepared as previously described (Garred et al., 2001).
GST pull-down assay
Aliquots (50 or 25 µl) of glutathione–Sepharose 4B beads (Amersham Pharmacia Biotech) were washed three times with assay buffer (25 mM HEPES–KOH pH 7.2, 125 mM potassium acetate, 2.5 mM magnesium acetate, 5 mM EGTA and 1 mM dithiothreitol) before incubation with 0.4 or 0.1 nmol of GST, GST–Hrs707–775 or GST–Hrs707–770 in 200 µl of assay buffer for 30 min at room temperature. The beads were then washed twice in assay buffer before incubation with either 200 µl of pig brain cytosol or with 25 pmol recombinant clathrin-TD1–579 [in assay buffer containing 10% fetal calf serum (FCS)] for 1 h at 4°C. Finally, the beads were washed four times with assay buffer and resuspended in SDS–PAGE sample buffer. Cytosolic clathrin HC associated with the beads was detected by SDS–PAGE, followed by immunoblotting with the goat anti-clathrin HC polyclonal antibody from Santa Cruz Biotechnology, Inc. and a SuperSignal chemiluminescence kit from Pierce. Recombinant clathrin-TD1–579 associated with the beads was detected with the mouse anti-clathrin HC monoclonal antibody from Research Diagnostics, Inc.
Electron microscopy
Cells were either fixed immediately or incubated with 5 nm BSA-coated colloidal gold (Slot and Geuze, 1985) in the medium at 37°C for 1 h or 10 min to identify endosomal compartments. At the end of the incubation with BSA–gold, the cells were washed with phosphate-buffered saline (PBS) and immediately fixed with 0.1% glutaraldehyde/4% PFA in Soerensen phosphate buffer. Following fixation, the cells were scraped from the culture dish, pelleted, infused with 2.3 M sucrose, mounted, frozen and stored in liquid nitrogen. Immunocytochemical labelling was performed on thawed cryosections as described (Griffiths et al., 1984), using different primary antibodies followed by 10 or 15 nm protein A–gold (purchased from G.Posthuma and J.Slot, Utrecht, The Netherlands) either directly or after incubation with secondary antibodies. The labelled cryosections were viewed in a Philips CM120 electron microscope.
Transferrin endocytosis and recycling
In order to study the effect of overexpressed Hrs on Tf endocytosis and recycling, BHK cells, which have low endogenous levels of Tf receptors, were co-transfected with human Tf receptor and the Hrs constructs indicated. With the vaccinia system, the level of co-transfection was found to be >95%, and non-transfected cells only contributed to a minor extent to the measured endocytosis. Endocytosis and recycling of Tf were measured using the ORIGEN analyser (IGEN Inc., Rockville, MD), which is based on electrochemiluminescence detection. Human holo-Tf (Sigma, St Louis, MO) was labelled with N-hydroxysuccinimide ester-activated tris (bipyridine)-chelated ruthenium(II) (Ru-tag) (IGEN Inc.) according to the manufacturer’s instructions, and simultaneously labelled with the reducable NHS-SS-Biotin (Pierce) for recycling measurements or with Biotin-LC-Sulpho NHS Ester (IGEN Inc.) for the study of endocytosis. For the measurement of Tf recycling, transfected BHK cells were washed twice with HEPES medium and incubated with Ru-tag-labelled Tf (50 ng/ml) in the presence of BSA (2 mg/ml) for 30 min at 37°C. A portion of the cells were then treated with 0.1 M 2-mercaptoethanesulfonic acid (MESNA) for 1 h to reduce the SS-linked biotin in the cell surface-bound Tf. Only Tf that is Ru-tag-labelled and still biotinylated is detected in the cell lysate using streptavidin beads (Dynal, Oslo, Norway) and the ORIGEN analyser. Cells treated with MESNA correspond to the amount of endocytosed Tf, whilst untreated cells correspond to the total amount of Tf associated with the cells. A portion of the MESNA-treated cells were incubated for a further 2–15 min at 37°C for recycling measurements, and re-treated with MESNA to remove signal from any surface-bound Tf. For endocytosis experiments, cells were incubated with Ru-tag-labelled Tf for 15 min at 37°C for the measurement of total cell-associated Tf. Endocytosed Tf was measured after removal of surface-bound Tf by 2 mg/ml pronase (Sigma).
Degradation and transport of EGF and dextran
Transfected HEp-2 cells were washed twice in HEPES medium and incubated with rhodamine-labelled EGF (200 ng/ml) for 1 h at 37°C in the presence of BSA (2 mg/ml). In the case of dextran, cells were pre-incubated with 10% FCS on ice for 30 min followed by internalization of biotinylated dextran (5 mg/ml) for 1 h at 37°C. The cells were then washed three times in HEPES medium and incubated for various times up to 3 h in HEPES medium containing cycloheximide (10 µg/ml). The cells were permeabilized with 0.05% saponin prior to fixation in 3% PFA, and stained for confocal microscopy. For the quantitation of EGF degradation, images from 10 transfected and 10 non-transfected cells from each time point were taken, each without changing intensity settings for the laser and photomultiplier. The mean intensity of the rhodamine-EGF signal from each cell was quantified with the software provided with the Leica TCS NT confocal microscope.
Acknowledgments
Acknowledgements
We thank Eva Rønning for technical assistance, Rob Parton and Kirsten Sandvig for helpful suggestions, Frances Brodsky for anti-clathrin LC antibodies, Stefan Höning for anti-AP3 antibodies, Jim Keen for pGEX-2T-clathrin1–579, Gunhild Mælandsmo for FEMX-III melanoma cells, Silje Ugland for pig brain cytosol, and David Gillooly, Kirsten Sandvig and Anne Simonsen for critical reading of the manuscript. This work was supported by the Top Research Programme, the Research Council of Norway, the Norwegian Cancer Society, the Novo-Nordisk Foundation and the Anders Jahre Foundation.
References
- Aniento F., Gu,F., Parton,R.G. and Gruenberg,J. (1996) An endosomal βCOP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol., 133, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asao H. et al. (1997) Hrs is associated with STAM, a signal-transducing adaptor molecule. J. Biol. Chem., 272, 32785–32791. [DOI] [PubMed] [Google Scholar]
- Bean A.J., Davanger,S., Chou,M.F., Gerhardt,B., Tsujimoto,S. and Chang,Y. (2000) Hrs-2 regulates receptor-mediated endocytosis via interactions with Eps15. J. Biol. Chem., 275, 15271–15278. [DOI] [PubMed] [Google Scholar]
- Burd C.G. and Emr,S.D. (1998) Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell, 2, 157–162. [DOI] [PubMed] [Google Scholar]
- Callaghan J., Simonsen,A., Gaullier,J.-M., Toh,B.-H. and Stenmark,H. (1999) The endosome fusion regulator EEA1 is a dimer. Biochem. J., 338, 539–543. [PMC free article] [PubMed] [Google Scholar]
- Chin L.-S., Raynor,M.C., Wei,X., Chen,H.-Q. and Li,L. (2001) Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J. Biol. Chem., 276, 7069–7078. [DOI] [PubMed] [Google Scholar]
- Dell’Angelica E.C., Ohno,H., Ooi,C.E., Rabinovich,E., Roche,K.W. and Bonifacino,J.S. (1997) AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J., 16, 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Angelica E.C., Klumperman,J., Stoorvogel,W. and Bonifacino,J.S. (1998) Association of the AP-3 adaptor complex with clathrin. Science, 280, 431–434. [DOI] [PubMed] [Google Scholar]
- Drake M.T., Downs,M.A. and Traub,L.M. (2000) Epsin binds to clathrin by associating directly with the clathrin-terminal domain. Evidence for cooperative binding through two discrete sites. J. Biol. Chem., 275, 6479–6489. [DOI] [PubMed] [Google Scholar]
- Endo K. et al. (2000) STAM2, a new member of the STAM family, binding to the Janus kinases. FEBS Lett., 477, 55–61. [DOI] [PubMed] [Google Scholar]
- Evan G.I., Lewis,G.K., Ramsay,G. and Bishop,J.M. (1985) Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol., 5, 3610–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodstad Ø., Kjønniksen,I., Aamdal,S., Nesland,J.M., Boyd,M.R. and Pihl,A. (1988) Extrapulmonary, tissue-specific metastasis formation in nude mice injected with FEMX-I human melanoma cells. Cancer Res., 48, 4382–4388. [PubMed] [Google Scholar]
- Futter C.E., Gibson,A., Allchin,E.H., Maxwell,S., Ruddock,L.J., Odorizzi,G., Domingo,D., Trowbridge,I.S. and Hopkins,C.R. (1998) In polarized MDCK cells basolateral vesicles arise from clathrin–γ-adaptin-coated domains on endosomal tubules. J. Cell Biol., 141, 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagescu R., Gruenberg,J. and Smythe,E. (2000) Membrane dynamics in endocytosis: structure–function relationship. Traffic, 1, 84–88. [DOI] [PubMed] [Google Scholar]
- Gaidarov I. and Keen,J.H. (1999) Phosphoinositide–AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J. Cell Biol., 146, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garred Ø., Rodal,S.K., van Deurs,B. and Sandvig,K. (2001) Reconstitution of clathrin-independent endocytosis at the apical domain of permeabilized MDCK II cells: requirement for a Rho-family GTPase. Traffic, 2, 26–36. [DOI] [PubMed] [Google Scholar]
- Gaullier J.-M., Simonsen,A., D’Arrigo,A., Bremnes,B., Aasland,R. and Stenmark,H. (1998) FYVE fingers bind PtdIns(3)P. Nature, 394, 432–433. [DOI] [PubMed] [Google Scholar]
- Gaullier J.-M., Rønning,E., Gillooly,D.J. and Stenmark,H. (2000) Interaction of the EEA1 FYVE finger with phosphatidylinositol 3-phosphate and early endosomes. Role of conserved residues. J. Biol. Chem., 275, 24595–24600. [DOI] [PubMed] [Google Scholar]
- Gillooly D.J., Simonsen,A. and Stenmark,H. (2001) Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem. J., 355, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman O.B., Krupnick,J.G., Gurevich,V.V., Benovic,J.L. and Keen,J.H. (1997) Arrestin/clathrin interaction. Localization of the arrestin binding locus to the clathrin terminal domain. J. Biol. Chem., 272, 15017–15022. [DOI] [PubMed] [Google Scholar]
- Griffiths G., McDowall,A., Back,R. and Dubochet,J. (1984) On the preparation of cryosections for immunocytochemistry. J. Ultrastruct. Res., 89, 65–78. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Back,R. and Marsh,M. (1989) A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J. Cell Biol., 109, 2703–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F. and Gruenberg,J. (1999) Biogenesis of transport intermediates in the endocytic pathway. FEBS Lett., 452, 61–66. [DOI] [PubMed] [Google Scholar]
- Guarente L. (1983) Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol., 101, 181–189. [DOI] [PubMed] [Google Scholar]
- Hirst J. and Robinson,M.S. (1998) Clathrin and adaptors. Biochim. Biophys. Acta, 1404, 173–193. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. (1999) Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol., 15, 705–732. [DOI] [PubMed] [Google Scholar]
- Komada M. and Kitamura,N. (1995) Growth factor-induced tyrosine phosphorylation of Hrs, a novel 115-kilodalton protein with a structurally conserved putative zinc finger domain. Mol. Cell. Biol., 15, 6213–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M. and Soriano,P. (1999) Hrs, a FYVE finger protein localized to early endosomes, is implicated in vesicular traffic and required for ventral folding morphogenesis. Genes Dev., 13, 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong J., Roudabush,F.L., Moore,P.H., Montague,M., Oldham,W., Li,Y., Chin,L.-S. and Li,L. (2000) Hrs interacts with SNAP-25 and regulates Ca2+-dependent exocytosis. J. Cell Sci., 113, 2273–2284. [DOI] [PubMed] [Google Scholar]
- Liu S.-H., Towler,M.C., Chen,E., Chen,C.-Y., Song,W., Apodaca,G. and Brodsky,F.M. (2001) A novel clathrin homolog that co-distributes with cytoskeletal components functions in the trans-Golgi network. EMBO J., 20, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi O., Poussu,A., Meriläinen,J., Kellokumpu,S., Wasenius,V.-M. and Lehto,V.-P. (1998) EAST, an epidermal growth factor receptor- and Eps15-associated protein with Src homology 3 and tyrosine-based activation motif domains. J. Biol. Chem., 273, 21408–21415. [DOI] [PubMed] [Google Scholar]
- Marsh M. and McMahon,H.T. (1999) Cell biology—the structural era of endocytosis. Science, 285, 215–220. [DOI] [PubMed] [Google Scholar]
- McBride H.M., Rybin,V., Murphy,C., Giner,A., Teasdale,R. and Zerial,M. (1999) Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell, 98, 377–386. [DOI] [PubMed] [Google Scholar]
- Mills I.G., Jones,A.T. and Clague,M.J. (1998) Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr. Biol., 8, 881–884. [DOI] [PubMed] [Google Scholar]
- Miura S. et al. (2000) Hgs (Hrs), a FYVE domain protein, is involved in Smad signalling through cooperation with SARA. Mol. Cell. Biol., 20, 9346–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K.E., Verges,M. and Altschuler,Y. (2000) Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol., 12, 483–490. [DOI] [PubMed] [Google Scholar]
- Nielsen M.S., Madsen,P., Christensen,E.I., Nykjaer,A., Glieman,J., Kasper,D., Pohlmann,R. and Petersen,C.M. (2001) The sortilin cytoplasmic tail conveys Golgi–endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J., 20, 2180–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G., Babst,M. and Emr,S.D. (1998) Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell, 95, 847–858. [DOI] [PubMed] [Google Scholar]
- Patki V., Lawe,D.C., Corvera,S., Virbasius,J.V. and Chawla,A. (1998) A functional PtdIns(3)P-binding motif. Nature, 394, 433–434. [DOI] [PubMed] [Google Scholar]
- Piper R.C., Cooper,A.A., Yang,H. and Stevens,T.H. (1995) VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol., 131, 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R., Randazzo,P.A., Presley,J.F., Hartnell,L.M. and Bonifacino,J.S. (2001) The ggas promote arf-dependent recruitment of clathrin to the tgn. Cell, 105, 93–102. [DOI] [PubMed] [Google Scholar]
- Raiborg C., Bremnes,B., Mehlum,A., Gillooly,D.J., Stang,E. and Stenmark,H. (2001) FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J. Cell Sci., 114, 2255–2263. [DOI] [PubMed] [Google Scholar]
- Raposo G., Tenza,D., Murphy,D.M., Berson,J.F. and Marks,M.S. (2001) Distinct protein sorting and localization to premelanosomes, melanosomes and lysosomes in pigmented melanocytic cells. J. Cell Biol., 152, 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R.L., Barbieri,M.A., Pryse,K.M., Chua,M. and Stahl,P.D. (1999) Endosome fusion in living cells overexpressing GFP–rab5. J. Cell Sci., 112, 3667–3675. [DOI] [PubMed] [Google Scholar]
- Rothman J.E. and Wieland,F.T. (1996) Protein sorting by transport vesicles. Science, 272, 227–234. [DOI] [PubMed] [Google Scholar]
- Rubino M., Miaczynska,M., Lippé,R. and Zerial,M. (2000) Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J. Biol. Chem., 275, 3745–3748. [DOI] [PubMed] [Google Scholar]
- Scales S.J., Gomez,M. and Kreis,T.E. (2000) Coat proteins regulating membrane traffic. Int. Rev. Cytol., 195, 67–144. [DOI] [PubMed] [Google Scholar]
- Schiestl R.H. and Gietz,R.D. (1989) High efficiency transformation of intact cells using single stranded nucleic acid as a carrier. Curr. Genet., 16, 339–346. [DOI] [PubMed] [Google Scholar]
- Shih W., Gallusser,A. and Kirchhausen,T. (1995) A clathrin-binding site in the hinge of the β2 chain of mammalian AP-2 complexes. J. Biol. Chem., 270, 31083–31090. [DOI] [PubMed] [Google Scholar]
- Shpetner H., Joly,M., Hartley,D. and Corvera,S. (1996) Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J. Cell Biol., 132, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Bremnes,B., Rønning,E., Aasland,R. and Stenmark,H. (1998a) Syntaxin-16, a putative Golgi t-SNARE. Eur. J. Cell Biol., 75, 223–231. [DOI] [PubMed] [Google Scholar]
- Simonsen A. et al. (1998b) EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature, 394, 494–498. [DOI] [PubMed] [Google Scholar]
- Slot J.W. and Geuze,H.J. (1985) A new method for preparing gold probes for immunoelectron microscopy. Eur. J. Cell Biol., 38, 87–93. [PubMed] [Google Scholar]
- Sönnichsen B., De Renzis,S., Nielsen,E., Rietdorf,J. and Zerial,M. (2000) Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5 and Rab11. J. Cell Biol., 149, 901–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A. (2000) The endocytosis machinery. J. Cell Sci., 113, 4375–4376. [DOI] [PubMed] [Google Scholar]
- Sorkina T., Bild,A., Tebar,F. and Sorkin,A. (1999) Clathrin, adaptors and eps15 in endosomes containing activated epidermal growth factor receptors. J. Cell Sci., 112, 317–327. [DOI] [PubMed] [Google Scholar]
- Stenmark H. and Aasland,R. (1999) FYVE-finger proteins—effectors of an inositol lipid. J. Cell Sci., 112, 4175–4183. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Parton,R.G., Steele-Mortimer,O., Lütcke,A., Gruenberg,J. and Zerial,M. (1994) Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J., 13, 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Bucci,C. and Zerial,M. (1995) Expression of Rab GTPases using recombinant vaccinia viruses. Methods Enzymol., 257, 155–164. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W., Oorschot,V. and Geuze,H.J. (1996) A novel class of clathrin-coated vesicles budding from endosomes. J. Cell Biol., 132, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata H., Kato,M., Denda,K. and Kitamura,N. (2000) A Hrs binding protein having a Src homology 3 domain is involved in intracellular degradation of growth factors and their receptors. Genes Cells, 5, 57–69. [DOI] [PubMed] [Google Scholar]
- Teo M., Tan,L., Lim,L. and Manser,E. (2001) The tyrosine kinase ACK1 associates with clathrin coated vesicles through a binding motif shared by arrestin and other adaptors. J. Biol. Chem., 276, 18392–18398. [DOI] [PubMed] [Google Scholar]
- ter Haar E., Harrison,S.C. and Kirchhausen,T. (2000) Peptide-in-groove interactions link target proteins to the β-propeller of clathrin. Proc. Natl Acad. Sci. USA, 97, 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbé S., Mills,I.G., Stenmark,H., Kitamura,N. and Clague,M.J. (2000) Endosomal localisation and receptor dynamics determine tyrosine phosphorylation of hepatocyte growth factor-regulated tyrosine kinase substrate. Mol. Cell. Biol., 20, 7685–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A.B., Hollenberg,S.M. and Cooper,J.A. (1993) Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell, 74, 205–214. [DOI] [PubMed] [Google Scholar]
- Watts C. (2001) Antigen processing in the endocytic compartment. Curr. Opin. Immunol., 13, 26–31. [DOI] [PubMed] [Google Scholar]
- Wurmser A.E., Gary,J.D. and Emr,S.D. (1999) Phosphoinositide 3-kinases and their FYVE domain-containing effectors as regulators of vacuolar/lysosomal membrane trafficking pathways. J. Biol. Chem., 274, 9129–9132. [DOI] [PubMed] [Google Scholar]
- Yang W., Lo,C.G., Dispenza,T. and Cerione,R.A. (2001) The Cdc42-target ACK2 directly interacts with clathrin and influences clathrin assembly. J. Biol. Chem., 276, 17468–17473. [DOI] [PubMed] [Google Scholar]