Abstract
During the switch from human γ- (fetal) to β- (adult) globin gene expression, the γ and β genes are expressed competitively by an alternating transcription mechanism. The –50 region of the γ gene promoter has been proposed to be responsible for the early competitive advantage of the γ genes and to act as a stage selector element (SSE) in hemoglobin switching. We analyzed the effect of mutating the –50 region of the γ gene in the presence of a competing β gene in transgenic mice. This shows that the –50 region does not affect silencing of the β gene in early development and does not act as a stage selector. However, it affects the ratio of γ versus β gene expression in the early, but not later, stages of fetal development. Interestingly, both the wild-type and mutant minilocus constructs show a higher frequency of alternate transcription than observed in the complete locus, suggesting that sequences normally present between the γ and β genes facilitate the interaction of the locus control region (LCR) and β-globin gene in the complete locus.
Keywords: globin switching/in situ hybridizations/SSE
Introduction
The human β-globin locus, present on chromosome 11, comprises five expressed genes (ε, Gγ, Aγ, δ and β), which are arranged in the order in which they are expressed during development in erythroid cells. The ε gene is expressed in the embryonic yolk sac, the major site of erythropoiesis from conception until the fifth week of gestation. Between 6 and 10 weeks of gestation, the first switch in globin subtypes results in the expression of the fetal globin genes (Gγ, Aγ), accompanied by a change in the site of erythropoiesis to fetal liver. Starting in the late fetal liver and continuing through the neonatal stages of development, there is a second switch from γ- to adult β-globin gene expression coincident with the bone marrow becoming the major erythropoietic organ (reviewed by Stamatoyannopoulos and Grosveld, 2001). A similar expression profile is observed when the complete human β-globin locus is incorporated in transgenic mice, although the γ genes are expressed earlier during embryonic development and are switched off at day 16 in the late fetal liver (Strouboulis et al., 1992). A similar expression pattern is observed when the γβ minilocus construct, in which the locus control region (LCR) is directly linked to the γ and β genes in the correct configuration, is introduced into mice (Berry et al., 1992; Ronchi et al., 1996).
In the absence of the LCR, the locus shows a low level of expression, which is sensitive to the site of integration. However, the individual globin genes themselves display correct temporal and tissue-specific expression (Chada et al., 1985; Magram et al., 1985; Kollias et al., 1986; Starck et al., 1994). Studies in transgenic mice with constructs where the individual ε-, γ- and β-globin genes were linked to the LCR demonstrate that the ε and γ genes are silenced autonomously by sequences immediately flanking the genes (Raich et al., 1990; Dillon and Grosveld, 1991). However, the developmental specificity of the β gene is disrupted in these experiments, resulting in expression in the yolk sac (Enver et al., 1989; Behringer et al., 1990; Lindenbaum et al., 1990). Correct temporal regulation of the β gene is obtained when a copy of the γ-globin gene is introduced in a position between the LCR and the β gene, indicating that the silencing of the latter during early development is non-autonomous and is due to the competitive advantage of the more proximal γ gene interacting preferentially with the LCR (Hanscombe et al., 1991). Choi and Engel (1988) proposed that the genes in the chicken β-globin locus competed for interaction with the same enhancer. They found that a stage selector element (SSE) in the promoter of the chicken β-globin gene was essential for preferential interaction of that promoter with the enhancer in the adult stage. Studies using in situ hybridization to visualize transcription in the nucleus in transgenic mouse liver or human erythroid cells suggest that the LCR acts as a single unit to activate a single globin gene at any given time. Activation of the genes is thought to take place through a looping mechanism (Wijgerde et al., 1995; Gribnau et al., 1998; Bulger and Groudine, 1999), rather than some other mechanism (Herendeen et al., 1992; Martin et al., 1996), where the LCR alternates between the γ- and β-globin genes at the time of the fetal to adult globin switch. As the nuclear environment changes during development, the stability of LCR–globin gene interactions changes in favor of the β-globin gene. The resulting suppression of the γ genes may be further enhanced by changes in chromatin structure (Gribnau et al., 2000).
Jane et al. (1992) originally suggested that the presence of an SSE in the human γ-globin gene promoter at position –50 would be important for the silencing of the β-globin gene in cis. They showed that the SSE confers a competitive advantage to the γ gene over the β gene in its interaction with HS2 in transient transfection assays. The –50 element binds a heteromeric complex between CP2, a ubiquitous transcription factor, and a protein present in erythroid cells called NF-E4 or the stage selector protein (SSP) (Jane et al., 1995; Zhou et al., 2000). We have looked at the role of this region of the human γ-globin promoter in vivo in transgenic mice and the results show that this region is not a ‘stage selector element’ involved in keeping the β-globin gene in cis silent in the early stages of development. However, the mutation of the NF-E4/SSP region causes an alteration in the relative levels of the linked γ- and β-globin genes in the fetal stage when both genes are active. We therefore propose that NF-E4/SSP plays a role in the γ-globin promoter similar to that of the transcription factor erythroid Kruppel-like factor (EKLF), which provides a competitive advantage to the β- over the γ-globin gene promoter in the fetal stages of development.
Results
The effect of mutating the –50 region of the human γ-globin promoter
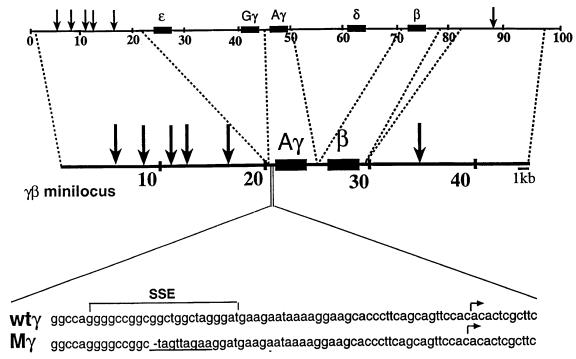
Developmental expression analysis of the wild-type and mutated human γ-globin genes was performed using an γβ minilocus construct that contained the LCR, the human Aγ-globin gene and the human β-globin gene cloned in the correct orientation with respect to the LCR (Berry et al., 1992; Ronchi et al., 1996). Using the minilocus construct rather than a complete whole locus allows us to measure the effect of the –50 region on the γ-globin gene promoter relative to that of the β-globin gene promoter without interference by competition from the ε gene in the early embryo (Strouboulis et al., 1992). Oligonucleotide mutagenesis was used to mutate the –50 region (Jane et al., 1992) of the Aγ promoter. The nucleotide sequences of the wild-type and mutated region are shown in Figure 1. This mutation inhibits the binding of SSP (Jane et al., 1992). Transgenic mice containing the mutated or wild-type γβ minilocus were generated and these were mated with wild-type mice to collect embryonic blood, early and late fetal liver, and adult blood. A total of six founder animals were generated from the wild-type γβ minilocus cosmid construct (wtγβmini) and four founders for the mutated γ construct (–50γ) (Figure 1, wtγ and Mγ). The wtγβmini lines behaved like those published previously (Berry et al., 1992) and therefore only one was used in the quantitative analysis. This founder, together with three of the –50γ founders, was mated to produce the transgenic lines wtγβmini and –50γln2, ln4 and ln7 (the fourth –50 founder did not transmit). Transgene copy number determination of these lines revealed that –50γln4 and –50γln7 each have two copies of the minilocus, while –50γln2 and wtγβmini are both single-copy transgenic lines.
Fig. 1. Schematic representation of the γβ minilocus. The top line shows the normal human β-globin locus. The second line shows the map of the γβ minilocus. Dotted lines indicate the fragments of the normal locus that have been used to construct the minilocus (for details see Grosveld et al., 1987; Berry et al., 1992). The bottom two lines show the sequence of the proximal part of the γ-globin gene promoter. The SSE region of the γ-globin gene promoter that has been mutagenized is underlined.
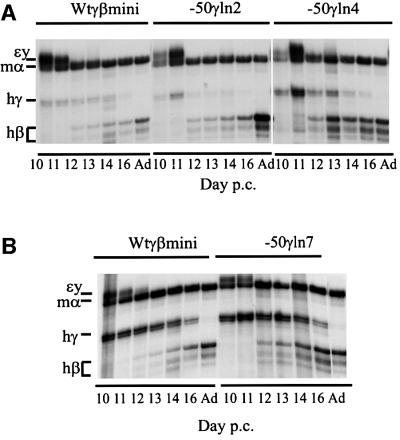
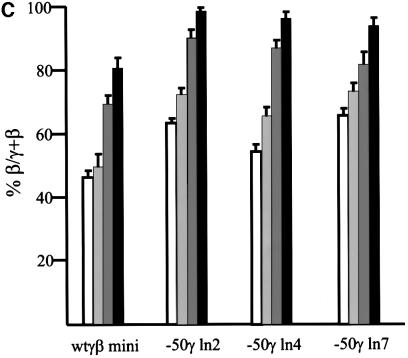
The levels of RNA for the γ and β transgenes were determined by S1 nuclease analysis at different stages of development. Results were quantitated as human β-globin RNA as a percentage of total human γ + β RNA. This showed that in the three mutant lines –50γln2, ln4 and ln7, as in the control wtγβmini, the β-globin gene is silent at day 10 (Figure 2A and B). Three independent experiments at the fetal stage, when both γ and β are expressed, show no significant difference in the output of the γ + β genes (γ +β mRNA per copy versus endogenous mouse α genes) between the single-copy –50γln2 and the control wtγβmini (Table I). On the other hand, all three mutant lines have a higher level of β gene RNA in fetal liver when compared with the β levels at the same stages in the control line, wtγβmini (Figure 2). At day 12, the mutant lines express the human β-globin gene on average at a level 60% of the total γ + β level compared with 45% in the control line wtγβmini (Figure 2C). This effect is still observable at day 13, but diminishes at day 14 to a very small difference. These data suggest that the human β-globin gene has gained a competitive advantage in its interaction with the LCR as a result of a ‘weakened’ γ-globin gene caused by the –50 promoter mutation.
Fig. 2. Expression levels of β- and γ-globin genes by S1 nuclease protection assays. Probes for the human γ- and β-globin and mouse εy and α-globin were used as described before (e.g. by Berry et al., 1992). Protected fragments are indicated to the left of each panel. All mice analyzed were hemizygous for the transgene. (A) Total RNA from days 10, 11, 12, 13, 14, 16 and adult for wtγβmini, –50γln2 and –50γln4. (B) Total RNA from days 10, 11, 12, 13, 14, 16 and adult for wtγβmini and –50γln7. (C) Bar diagram of the results described in (A) and (B). The level of β expression is given as β RNA as a percentage of the total γ + β RNA. The different colors indicate the different days of development: 12 (white), 13 (light gray), 14 (dark gray) and 16 (black) days. The mean of at least three independent experiments is plotted.
Table I. Ratio of the human (γ + β) mRNA to mouse α mRNA in the fetal liver per copy of the human locus as determined by S1 nuclease protection analysis.
| Day of development |
|||
|---|---|---|---|
| 12 | 14 | 16 | |
| wtγβ | 0.22 | 0.30 | 0.42 |
| –50γln2 | 0.24 | 0.35 | 0.45 |
| –50γln2+/– | 0.21 | 0.31 | 0.42 |
| –50γln4 | 0.20 | 0.32 | 0.33 |
| –50γln7 | 0.28 | 0.32 | 0.35 |
The left hand column shows mouse lines (ln2+/– is ln2 in the EKLF+/– background). The other columns are the ratios at days 12, 14 and 16 of development.
The effect of EKLF dosage on the –50 γ promoter mutation
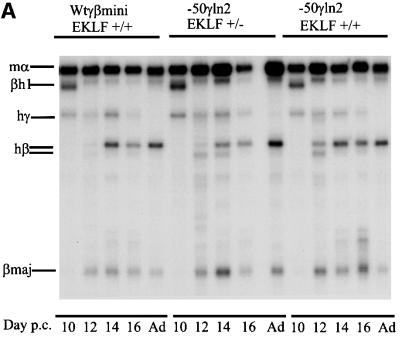
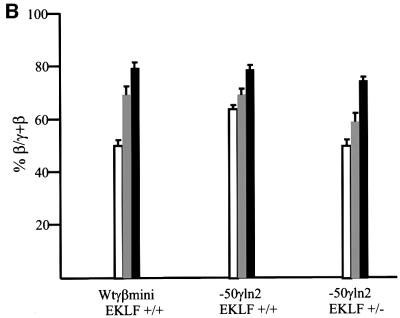
The erythroid transcription factor EKLF (Miller and Bieker, 1993) has been shown to be a crucial determinant in the transcriptional activation of the β-globin gene (Nuez et al., 1995; Perkins et al., 1995). EKLF plays a major role in the competition between the γ- and β-globin genes in the fetal liver. EKLF hemizygous fetal liver cells display a decrease in transcription of the β-globin gene and a reciprocal increase in the γ gene (Wijgerde et al., 1996). Our results show that a loss of NF-E4/SSP binding to the –50γ region of the γ-globin gene results in a loss of γ transcription relative to that of the β gene, suggesting that it plays a role in the γ versus β competition. If this is the case, it would be predicted that a decrease in the level of EKLF should compensate for the loss of γ transcription due to the –50 NFE4/SSP mutation. EKLF binds to the CACC box in the β-globin promoter and we have shown previously that a lower level of EKLF results in a competitive disadvantage to β compared with γ (Wijgerde et al., 1996). The competing γ gene, though weakened by the –50 promoter mutation, would then be able to interact more efficiently with the LCR. This would alter the relative ratios of the transcripts of the two genes in favor of the γ gene. To verify whether these proteins (SSP and EKLF) play an antagonistic role during the switching process, we crossed the wtγβmini and –50γln2 mice with EKLF+/– mice (Wijgerde et al., 1996), and compared the levels of γ and β mRNAs with those obtained in an EKLF+/+ background (Figure 3A). Quantitative RNA analysis showed that the β gene loses its increase in competitive advantage over the mutated γ gene in EKLF+/– mice (Figure 3B). The different β/γ + β ratios observed in the EKLF+/– and +/+ mice in both the wtγβmini and –50γln2 lines have changed, to the advantage of γ. However, the total output of the minilocus constructs (calculated as a percentage of the endogenous mouse α-globin expression) is not significantly different in an EKLF+/– or +/+ background, as assayed in three independent experiments (Table I, ln2+/–).
Fig. 3. Expression levels in different EKLF backgrounds. (A) S1 nuclease protection assay. Probes for the human γ- and β-globin and mouse εy and α-globin were as above. Protected fragments are indicated to the left of each panel. All mice analyzed were hemizygous for the wtγβmini and –50γln2 in either the EKLF+/+ or EKLF+/– backgrounds as indicated above each line (p.c., post-conception). (B) Bar diagrams of the expression level (% β/γ + β) of the wtγβmini compared with the –50γln2 in the EKLF+/+ and EKLF+/– backgrounds at 12 (white), 14 (light gray) and 16 (black) days of development.
Analysis of primary transcripts by fluorescent in situ hybridization (FISH)
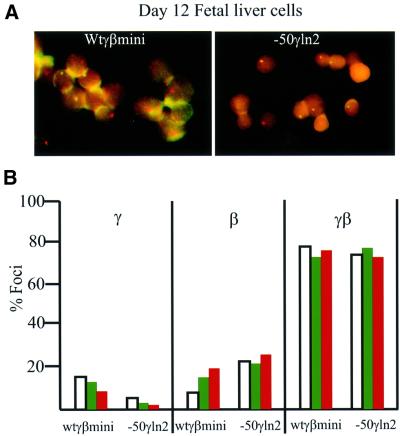
We have described previously how the number of primary transcription signals in the nucleus correlates with the mRNA levels detected in the cytoplasm, providing an independent method to measure the level of transcription (Wijgerde et al., 1995). To confirm whether the –50 mutation indeed results in a decrease in the number of transcriptional events of the γ gene versus the β gene, we carried out FISH detection of primary transcripts (Figure 4) after fixation of the fetal liver cells on poly-l-lysine slides and processing as described earlier (Wijgerde et al., 1995). The results of in situ hybridizations of primary transcripts of fetal liver cells from days 12, 13 and 14 of either the control line (wtγβmini) or the mutant line –50γln2, each of which carries a single copy of the transgene, are shown in Figure 4. Analysis of single primary transcript signals from cells of day 12 fetal liver of –50γln2 shows that 5% of the cells show a γ only signal compared with 15% of the cells in the control line (Figure 4). Later time points show that the number of γ-globin primary transcript signals in the wtγβmini line remains higher than in the –50γln2 line (Figure 4), confirming the results obtained by S1 analysis (Figures 2 and 3). Interestingly, the in situ results obtained with the γβ miniloci showed a considerable difference in terms of the number of single γ or β primary transcript signals versus double (γ + β) signals per nucleus when compared with those obtained with a complete β-globin locus (Wijgerde et al., 1995). The number of double signals (γ + β) is considerably higher in the γβ miniloci than in a complete β-globin locus [71–76% versus 18% at day 12 (Wijgerde et al., 1995)]. There are two major differences between the γβ miniloci when compared with a normal wild-type locus. In the former, the γ and β genes are situated much closer to the LCR, and the DNA normally present between the genes is absent (Figure 6). The increase in double signals suggests that alternate transcription (and looping) may be taking place at a higher frequency, or may not be taking place at all, and that the genes are regulated by a different mechanism.
Fig. 4. (A) In situ hybridization of day 12 fetal liver cells containing a single copy of the wild-type minilocus (wtγβmini, left) or the mutated –50γ promoter line2 (–50γln2, right). Red signals (Texas red) are γ-globin primary gene transcripts, green signals (fluorescein isothio cyanate) are β-globin gene primary transcripts; yellow signals are the combination of both. (B) Bar diagrams of the percentages of transcriptional cell types observed at day 12 (white), 13 (green) and 14 (red) of development in transgenic fetal liver cells of the wtγβmini and –50γln2 lines. Left panel: γ signal only; middle panel: β signal only; right panel: overlapping γ and β signal.
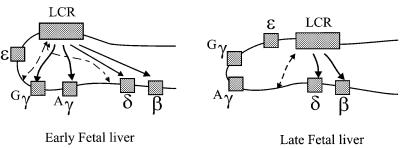
Fig. 6. A model to show the possible role of sequence(s) upstream of the δ-globin gene which may be involved in tethering the LCR to the 3′ end of the human β-globin locus in late fetal liver (shown by the broken line in late fetal liver) and which may further facilitate the switching process. The role of the –50 region is similarly indicated in the early fetal liver stage.
Kinetic analysis of transcription by the reversible inhibitor DRB
We used the reversible transcription inhibitor DRB (Fraser et al., 1978; Marshall et al., 1996; Gribnau et al., 1998) to determine whether the γ and β genes in the γβ mini locus are transcribed as single genes but alternating at a higher rate than normal, or whether they are co-transcribed.
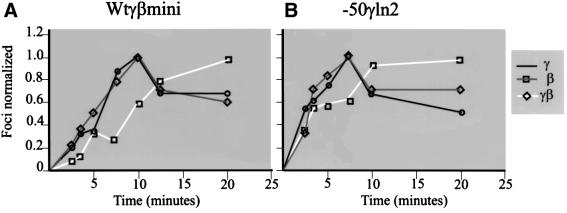
Day 12 fetal liver cells of the control line (wtγβmini) and the single-copy mutant line –50γln2 were treated with DRB for 15 min as described by Gribnau et al. (1998). Cells were then washed to remove the DRB and incubated at 25°C in fresh medium for different times and fixed to measure the reappearance of transcription signals. A slower reappearance of double signals in comparison to single signals would strongly suggest that the two globin genes are transcribed alternately, while a similar reappearance would indicate co-transcription (or a very rapid alternation) (Gribnau et al., 1998). As can be seen from Figure 5, the reappearance of double signals lags behind single signals for both the control line as well as the mutant line; we conclude that, even in this configuration, the transcription of γ and β is alternating with only a single gene being activated at any given time. However, as the number of double signals, which are caused by an overlap in the detection of the RNA (half-life ∼3 min; Wijgerde et al., 1995) from the gene that has just finished being transcribed and the RNA produced from the newly transcribed gene, is increased relative to that observed in a complete locus (Wijgerde et al., 1995), we conclude that the genes are being transcribed alternately, but at a higher rate than in the normal locus.
Fig. 5. Kinetics of reappearance of the single (γ or β) versus double (γβ) primary transcription signals after release of the DRB block to transcription elongation (see Gribnau et al., 1998). Transcription foci were counted in 12 day fetal liver nuclei after in situ hybridization with γ- and β-globin intron probes in nuclei fixed at different times after the release of the DRB block. The number of black (γ), gray (β) or white (γ + β) signals was plotted against time of fixation after the release of the block. (A) Signals observed in mice containing a single copy of the wtγβmini locus. (B) Signals from the line –50γln2. Symbols corresponding to the γ, β or γβ foci are shown on the right.
Discussion
Stage selection
The SSE was first described in the chicken β-globin locus, which lacks the γ gene, in which it is required for definitive cell stage-specific expression of the adult β-globin (Choi and Engel, 1988). A second such element was mapped at position –53 to –5 of the γ promoter in the human globin cluster (the –50γ region). In contrast to the chicken locus, the proposed function of the human SSE was to silence the human β-globin gene by a competitive mechanism for interaction with the LCR during the early stages of erythroid development (Jane et al., 1992). This proposal was based on a study of the –50 region of the γ-globin gene in transient transfection assays (Jane et al., 1992). Later studies focusing on the proteins binding to this sequence have identified both CP2 and NF-E4 as forming a heteromeric complex (SSP) (Jane et al., 1995; Zhou et al., 2000). Overexpression of NF-E4 in cord blood progenitors induces the human γ gene with a concomitant reduction in the expression of the β gene (Zhou et al., 2000). This suggests that the levels of the NF-E4 complex can alter the balance between the transcription of the two promoters to favor the fetal gene over the adult one (Zhou et al., 2000). However, the latter experiments do not address the function of the –50 region of the γ-globin gene in ‘stage selection’ or in the silencing of the β-globin gene early during development. In the present study, we have examined the function of this region in vivo by mutating this sequence as part of a construct that carries a linked copy of the β-globin gene and the complete LCR. Our results shows that the –50 region of the γ promoter is not responsible for the silencing of the β-globin gene in cis (Figure 2A and B) because the human β-globin gene is silenced properly at the embryonic stage in all the lines we analyzed.
The –50 region also does not appear to be a typical promoter element such as the CCAAT box (Ronchi et al., 1996) because the level of γ-globin RNA at the embryonic stage is very similar with or without an intact –50 region. This suggests that the –50γ region binding NF-E4/SSP may be a promoter element that plays a role in the early embryo by contributing to the competitive ability and resulting activity of the γ-globin genes in the context of the whole locus when the ε gene is also present. However, such an effect can only be tested in the context of the whole locus.
The role of the –50 region in the fetal liver
The effect of the –50 mutation becomes apparent in the early fetal liver. When it is mutated, a higher level of steady-state β-globin RNA is observed (an increased ratio of human β/γ + β). The in situ hybridization assays, which detect primary RNA transcripts, confirm these results. Because the overall output of the locus measured against the mouse α-globin gene has remained the same, it shows that the level of transcription of the γ-globin gene relative to the β-globin gene has been decreased by the mutation of the –50 region. Thus the –50 region contributes to the competitive ability of the γ-globin gene promoter relative to that of the β-globin gene in the early fetal liver in mice. This contribution appears to be of a similar nature to the contributions of the transcription factor EKLF and the CACC box to the competitive ability of the β-globin promoter (Perkins et al., 1996; Wijgerde et al., 1996) because a decrease in the level of EKLF compensates for the loss of competitive ability of the –50γ-globin gene (Figures 4 and 5). This experiment shows the interesting phenomenon that the output of the γ-globin gene has been restored to normal levels even though the –50 region is not functional, implying that the –50 region contributes to the activity of the promoter, but does not play a critical or rate-limiting role. It also suggests that the CACC box (Asano et al., 1999, 2000; Ryan et al., 2000) and the CAAT box (Ronchi et al., 1996) play a more important role in switching.
Alternating transcription
We observed a high percentage of γ–β double signals in the miniloci when compared with the full locus, suggesting that the genes were transcribed concurrently rather than alternately in the fetal liver. However, DRB transcription inhibition followed by removal of the inhibitor shows that the single signals reappear before double signals. Thus, the genes are transcribed in an alternate fashion, albeit at a higher frequency than that observed in the normal β-globin locus. On the basis of data obtained in the complete globin locus, we originally suggested that the transcriptional periods of the β-globin gene increase as development and the switch to β progress. However, we were unable to distinguish whether a transcriptional period consisted of one stable interaction or a number of short interactions. The results described here suggest that the latter is the case. There are two possibilities to explain such an increase in the frequency of alternating transcription: changes in distance caused by the absence of all other sequences but the γ and β genes, or a destabilization of the interaction between the LCR and the genes (or a combination of both).
The change in distance from the normal locus to the minilocus per se does not provide a good explanation for a shorter interaction between the LCR and the β gene. The β gene has been moved closer to the LCR in the minilocus when compared with a normal locus, and in relative terms has been moved nearer to the LCR than the γ gene. As a result, it would be expected to have gained a bigger advantage over γ, which it has not. It is possible that moving the genes closer to the LCR could cause some constraint in the chromatin, resulting in less stable interactions. However, this should also lead to a lower output of the locus, which is not the case. The alternative explanation for the change in the stability of the interactions would be the absence of sequences that lie between the genes and potentially stabilize such interactions. Both the γ and β genes have a similar amount of flanking sequence (at least 2 kb either side), which makes it unlikely that the direct gene–LCR interactions are disturbed. However, other possible interactions within the full locus, which may indirectly affect LCR–gene interactions, would not take place in the minilocus due to the absence of the relevant sequences (Figure 6). This possibility is supported by a number of recent observations. First, our data on the deletion of a region 5′ to the δ-globin gene suggest that this region may facilitate the interaction between the LCR and the β-globin genes (Calzolari et al., 1999). Deletion of this region results in a defect in β-globin activation in a number of the cells due to position effect varigation (PEV) and, importantly, also in a reduced level of initiation of transcription in the cells in which the β-globin gene is transcribed. Additional supporting evidence for such a mechanism is derived from the expression pattern of the normal locus. Early in development, the 5′ Gγ-globin gene, which is located closer to the LCR (Figure 6), is expressed at a higher level than the 3′ Aγ-globin gene. However, when expression of the γ-globin genes is reduced and switched to the β gene, the level of Aγ expression is higher than that of Gγ. An obvious explanation for this switch in Gγ/Aγ expression is that there is a difference in the regulatory elements surrounding these genes; however, no such developmentally specific elements have been described. The alternative is that in fact the Aγ gene is now closer to the LCR than the Gγ gene, a situation that would be created if, for example, the element 5′ to the δ gene tethers or facilitates an interaction with the LCR (Figure 6).
In conclusion, this study indicates that the –50 region is not a stage selector sequence, as originally proposed (Jane et al., 1992), but that it plays a role in early fetal development by enhancing transcription from the γ-globin gene promoter in a γβ minilocus context. Interestingly, the use of this minilocus and a primary transcript analysis indicate that regions well outside the γ and β genes play a more important role in the γ/β balance than the –50 region during the switching process.
Materials and methods
Cosmid constructs and production of transgenic mice
The γβ minilocus cosmid was as described earlier (Dillon and Grosveld, 1991; Berry et al., 1992) and is represented schematically in Figure 1. The –50 region of the γ promoter was mutagenized using PCR in the following way. The forward primer 5′-GAGGCCAGGGGCCGGCTAGTTAGAAGGGAT-3′ (comprising the mutation) was used together with the reverse primer 5′-ACCTTGCCCCACAGGCTTGTG-3′ to generate a 174 bp fragment spanning the region from –65 to +109 of the Aγ gene. This mutation has been shown to hamper the binding of the SSP to the SSE (Jane et al., 1992). Following digestion of the amplified fragment with NaeI and BglI, it was cloned in the 5.6 kb ClaI–KpnI fragment of the γ-globin gene present in a plasmid. The presence of the mutation was confirmed by sequencing. The mutagenized γ gene was then used to replace the original fragment cloned as a ClaI–KpnI fragment in the γβ minilocus (Figure 1) construct by in vitro packaging. Cosmids carrying the 40 kb inserts of the wild-type (γβmini) and mutated γ promoters (–50γ) were excised by digestion with SalI and purified by centrifugation on NaCl gradients. DNA fragments were prepared for microinjection as described previously (Ellis et al., 1996). A solution containing 0.25 ng of DNA/µl was injected into the male pronucleus of (FVB × FVB) fertilized eggs and transferred to pseudopregnant females as described by Ellis et al. (1996). Transgenic mice were identified as described earlier (Ellis et al., 1996) and founder mice were bred with non-transgenic BCBA mates to establish transgenic lines. These were used to obtain fetal liver and embryonic blood samples by timed matings of adult transgenic males with non-transgenic females. The wt γβmini and –50γln2 lines were also bred with mice heterozygous for EKLF knock-out (Nuez et al., 1995) and embryo samples obtained after timed mating as earlier.
RNA and S1 protection analysis
RNA extracted from embryonic blood, fetal livers and adult blood was analyzed by S1 nuclease protection assay as described by Berry et al. (1992) and Strouboulis et al. (1992). Quantitation of signals obtained from S1 analyses was carried out using a phosphoimager.
Primary transcript in situ hybridization
Fetal liver samples were disrupted in phosphate-buffered saline (PBS) at room temperature. Cells were spotted and immobilized on poly-l-lysine-coated slides, and subsequently fixed in 4% formaldehyde/5% acetic acid in saline for 20 min at room temperature. Slides were processed and used for in situ hybridization and antibody detection as described by Wijgerde et al. (1995). Probes were labeled with either biotin or digoxigenin. Transcription signals were quantitated by counting cells using epifluorescence microscopy and recorded with a CCD camera. Reversible inhibition of transcriptional elongation with DRB was as described by Gribnau et al. (1998). Briefly, adult mice transgenic for either a single integrated copy of the wild-type γ promoter minilocus (wtγmini) or the mutated γ promoter minilocus (–50γsln2) were bred to give timed matings. Fetal livers were processed and cells treated with DRB (Sigma), and samples for in situ hybridizations were processed as described by Gribnau et al. (1998).
DNA analysis
Tail DNA was extracted, digested with restriction enzymes and 10 µg of each sample run on 0.7% agarose gels. Southern blotting and genotyping was performed as described by Berry et al. (1992). Transgene copy numbers were determined using a phosphoimager.
Acknowledgments
Acknowledgements
We are grateful to Ton de Wit for support with the animal work, and to Sjaak Philipsen and Dave Whyatt for critically reading the manuscript. This work is supported by the CBG, EUR, EC, NWO (NL) and Regione Autonoma della Sardegna. L.R. n.11 of 30-04-90, year 2001.
References
- Asano H., Li,X.S. and Stamatoyannopoulos,G. (1999) FKLF, a novel Kruppel-like factor that activates human embryonic and fetal β-like globin genes. Mol. Cell. Biol., 19, 3571–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano H., Li,X.S. and Stamatoyannopoulos,G. (2000) FKLF-2, a novel Kruppel-like factor that activates globin and other erythroid lineage genes. Blood, 95, 3578–3584. [PubMed] [Google Scholar]
- Behringer R.R., Ryan,T.M., Palmiter,R.D., Brinster,R.L. and Townes,T.M. (1990) Human γ- to β-globin gene switching in transgenic mice. Genes Dev., 4, 380–389. [DOI] [PubMed] [Google Scholar]
- Berry M., Dillon,N. and Grosveld,F. (1992) A single point mutation is the cause of the Greek form of hereditary persistence of fetal hemoglobin. Nature, 358, 499–452. [DOI] [PubMed] [Google Scholar]
- Bulger M. and Groudine,M. (1999) Looping versus linking: toward a model for long distance gene activation. Genes Dev., 13, 2465–2477. [DOI] [PubMed] [Google Scholar]
- Calzolari R., McMorrow,T., Yannoutsos,N., Langeveld,A. and Grosveld, F. (1999) Deletion of a region that is a candidate for the difference between the deletion forms of hereditary persistence of fetal hemoglobin and δβ-thalassemia affects β- but not γ-globin gene expression. EMBO J., 18, 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada, K., Magram,J., Raphael,K., Radice,G., Lacy,E. and Costantini,F. (1985) Specific expression of a foreign β-globin gene in erythroid cells of transgenic mice. Nature, 314, 377–380. [DOI] [PubMed] [Google Scholar]
- Choi O.R. and Engel,J.D. (1988) Developmental regulation of the β-globin gene switching. Cell, 55, 17–26. [DOI] [PubMed] [Google Scholar]
- Dillon N. and Grosveld,F. (1991) Human γ-globin genes silenced independently of other genes in the β-globin locus. Nature, 350, 252–254. [DOI] [PubMed] [Google Scholar]
- Ellis J., Tan-Un,K.C., Harper,A., Michalovich,D., Yannoutsos,N., Philipsen,S. and Grosveld,F. (1996) A dominant chromatin-opening activity in 5′-hypersensitive site 3 of the human β-globin locus control region. EMBO J., 15, 562–568. [PMC free article] [PubMed] [Google Scholar]
- Enver T., Ebens,A.J., Forrester,W.C. and Stamatoyannopoulos,G. (1989) The human β-globin locus activation region alters the developmental fate of a human fetal globin gene in transgenic mice. Proc. Natl Acad. Sci. USA, 86, 7033–7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N.W., Sehgal,P.B. and Darnell,J.E. (1978) DRB-induced premature termination of late adenovirus transcription. Nature, 272, 590–593. [DOI] [PubMed] [Google Scholar]
- Gribnau J., de Boer,E., Trimborn,T., Wijgerde,M., Milot,E., Grosveld,F. and Fraser,P. (1998) Chromatin interaction mechanism of tran scriptional control in vivo. EMBO J., 17, 6020–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribnau J., Diderich,K., Pruzina,S., Calzolari,R. and Fraser,P. (2000) Intergenic transcription and developmental remodeling of chromatin sub domains in the human β-globin locus. Mol. Cell, 5, 377–386. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft,G.B., Greaves,D.R. and Kollias,G. (1987) Position independent, high-level expression of the human β-globin gene in transgenic mice. Cell, 51, 975–985. [DOI] [PubMed] [Google Scholar]
- Hanscombe O., Whyatt,D., Fraser,P., Yannoutsous,N., Greaves,D., Dillon,N. and Grosveld,F. (1991) Importance of globin gene order for correct developmental expression. Genes Dev., 5, 1387–1394. [DOI] [PubMed] [Google Scholar]
- Herendeen D.R., Kassavetis,G.A. and Geiduschek,E.P. (1992) A transcriptional enhancer whose function imposes a requirement that proteins track along DNA. Science, 256, 1298–1303. [DOI] [PubMed] [Google Scholar]
- Jane S.M., Ney,P.A., Vanin,E.F., Gumucio,D.L. and Nienhuis,A.W. (1992) Identification of a stage selector element in the human γ-globin gene promoter that fosters preferential interaction with the 5′-HS2 enhancer when in competition with the β-promoter. EMBO J., 11, 2961–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane S.M., Nienhuis,A.W. and Cunningham,J.M. (1995) Hemoglobin switching in man and chicken is mediated by a heteromeric complex between the ubiquitous transcription factor CP2 and a developmental specific protein. EMBO J., 14, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G., Wrighton,N., Hurst,J. and Grosveld,F. (1986) Regulated expression of human A γ-, β- and hybrid γ–β globin genes in transgenic mice: manipulation of the developmental expression pattern. Cell, 46, 89–94. [DOI] [PubMed] [Google Scholar]
- Lindenbaum M.H. and Grosveld,F. (1990) An in vitro globin gene switching model based on differentiated embryonic stem cells. Genes Dev., 4, 2075–2085. [DOI] [PubMed] [Google Scholar]
- Magram J., Chada,K. and Costantini,F. (1985) Developmental regulation of a cloned adult β-globin gene in transgenic mice. Nature, 315, 338–340. [DOI] [PubMed] [Google Scholar]
- Marshall N.F., Peng,J., Xie,Z. and Price,D.H. (1996) Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J. Biol. Chem., 271, 27176–27183. [DOI] [PubMed] [Google Scholar]
- Martin D.I., Fiering,S. and Groudine,M. (1996) Regulation of β-globin gene expression: straightening out the locus. Curr. Opin. Genet. Dev., 6, 488–495. [DOI] [PubMed] [Google Scholar]
- Miller I.J. and Bieker,J.J. (1993) A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol. Cell. Biol., 13, 2776–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuez B., Michailovich,D., Bygrave,A., Ploemacher,R. and Grosveld,F. (1995) Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature, 375, 316–318. [DOI] [PubMed] [Google Scholar]
- Perkins A.C., Gaensler,K.M. and Orkin,S.H. (1996) Silencing of the human fetal globin expression is impaired in the absence of the adult β-globin gene activator protein EKLF. Proc. Natl Acad. Sci. USA, 93, 12267–12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S.C., Sharp,A.H. and Orkin,S.H. (1995) Lethal β-thalassemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature, 375, 318–322. [DOI] [PubMed] [Google Scholar]
- Raich N., Enver,T., Nakamoto,B., Josephson,B., Papayannopoulou,T. and Stamatoyannopoulos,G. (1990) Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science, 250, 1147–1149. [DOI] [PubMed] [Google Scholar]
- Ronchi A., Berry,M., Raguz,S., Imam,A., Yannoutsos,N., Ottolenghi,S., Grosveld,F. and Dillon,N. (1996) Role of the duplicated CCAAT box region in γ-globin gene regulation and hereditary persistence of fetal hemoglobin. EMBO J., 15, 143–149. [PMC free article] [PubMed] [Google Scholar]
- Ryan T.M., Sun,C.-W., Ren,J. and Townes,T.M. (2000) Human γ-globin gene promoter element regulates human β-globin gene developmental specificity. Nucleic Acids Res., 28, 2736–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G. and Grosveld,F. (2001) Hemoglobin switching. In Stamatoyannopoulos,G., Majerus,P.W., Perlmutter,A.M. and Varmus,H. (eds), The Molecular Basis of Blood Diseases. W.B.Saunders, Philadelphia, PA, pp. 135–182.
- Starck J., Sarkar,R., Romana,M., Bhargava,A., Scarpa,A., Tanaka,M., Chamberlain,J., Weissman,S. and Forget,B. (1994) Developmental regulation of human γ and β globin genes in the absence of the LCR. Blood, 84, 1656–1665. [PubMed] [Google Scholar]
- Strouboulis J., Dillon,N. and Grosveld,F. (1992) Developmental regulation of a complete 70 kb human β globin locus in transgenic mice. Genes Dev., 6, 1857–1864. [DOI] [PubMed] [Google Scholar]
- Wijgerde M., Grosveld,F. and Fraser,P. (1995) Transcription complex stability and chromatin dynamics in vivo. Nature, 377, 209–213. [DOI] [PubMed] [Google Scholar]
- Wijgerde M., Gribnau,J., Trimborn,T., Nuez,B., Philipsen,S., Grosveld, F. and Fraser,P. (1996) The role of EKLF in human β-globin gene competition. Genes Dev., 10, 2894–2902. [DOI] [PubMed] [Google Scholar]
- Zhou W., Clouston,D.R., Wang,X., Cerruti,L., Cunningham,J.M. and Jane,S.M. (2000) Induction of human fetal globin gene expression by a novel erythroid factor, NF-E4. Mol. Cell. Biol., 20, 7662–7672. [DOI] [PMC free article] [PubMed] [Google Scholar]