Abstract
Type XIII collagen is a type II transmembrane protein found at sites of cell adhesion. Transgenic mouse lines were generated by microinjection of a DNA construct directing the synthesis of truncated α1(XIII) chains. Shortened α1(XIII) chains were synthesized by fibroblasts from mutant mice, and the lack of intracellular accumulation in immunofluorescent staining of tissues suggested that the mutant molecules were expressed on the cell surface. Transgene expression led to fetal lethality in offspring from heterozygous mating with two distinct phenotypes. The early phenotype fetuses were aborted by day 10.5 of development due to a lack of fusion of the chorionic and allantoic membranes. The late phenotype fetuses were aborted by day 13.5 of development and displayed a weak heartbeat, defects of the adherence junctions in the heart with detachment of myofilaments and abnormal staining for the adherence junction component cadherin. Decreased microvessel formation was observed in certain regions of the fetus and the placenta. These results indicate that type XIII collagen has an important role in certain adhesive interactions that are necessary for normal development.
Keywords: collagen/fetal lethality/placentation/transgenic mice/vascularization
Introduction
The collagen superfamily of proteins consists of >19 types of collagen and several other proteins with collagen-like domains (Vuorio and de Crombrugghe, 1990; Prockop and Kivirikko, 1995). Type XIII collagen and type XVII collagen form a subfamily of membrane-bound collagens (Pihlajaniemi and Rehn, 1995). Type XIII collagen consists of three collagenous domains (COL1–3) separated and flanked by four non-collagenous domains (NC1–4) (Pihlajaniemi and Tamminen, 1990). It is predicted that the structures of the COL1, NC2 and COL3 domains of the human and mouse type XIII collagen chains are affected by alternative splicing (Pihlajaniemi and Tamminen, 1990; Tikka et al., 1991; Juvonen and Pihlajaniemi, 1992; Juvonen et al., 1992, 1993; Peltonen et al., 1997). Type XIII collagen produced in insect cells forms α1(XIII) homotrimers, and the three collagenous domains fold into a stable triple helical conformation (Snellman et al., 2000a). The N-terminal NC1 domain contains a highly hydrophobic transmembrane domain, and the type XIII collagen molecules reside on the plasma membrane of cells in a ‘type II’ orientation with a short N-terminal cytosolic portion, a transmembrane domain and an extensive collagenous ectodomain (Snellman et al., 2000b). Sequences that are important for the association of the three α1(XIII) chains reside in the N-terminal region, and triple helix formation is thought to proceed from the N-terminus to the C-terminus, in the opposite orientation to that known to occur in the fibrillar collagens (Snellman et al., 2000b). The ligands of type XIII collagen are not known, but recent studies with recombinant protein demonstrate that it interacts strongly with the I-domain of α1 integrin (Nykvist et al., 2000).
Immunohistochemical studies of mature human and mouse tissues have shown that type XIII collagen is located at many cell matrix adhesion sites, and at cell–cell adhesion sites such as the intercalated discs in cardiac muscle cell (Peltonen et al., 1999; Hägg et al., 2001). Type XIII collagen is expressed throughout fetal development in the mouse, with strongest expression initially in the developing nervous system and the heart, but later in development also in several other tissues, with clear developmental shifts in expression rate and pattern (Sund et al., 2001).
In order to understand the biological function of type XIII collagen, we have used microinjection of oocytes to generate transgenic mice synthesizing α1(XIII) chains with a 90 amino acid in-frame deletion mutation of their COL2 sequences. A fragment of the endogenous mouse type XIII collagen gene promoter was used to drive the expression of the transgene construct. The large in-frame deletion was designed such that mutant α1(XIII) chains would associate with normal ones and result in synthesis of abnormal type XIII collagen. Several transgenic lines were generated, and expression of the mutant α1(XIII) chains resulted in embryonic lethality due either to a lack of placental formation or to cardiovascular defects in offspring from heterozygous mating.
Results
Generation and identification of type XIII collagen COL2del mice
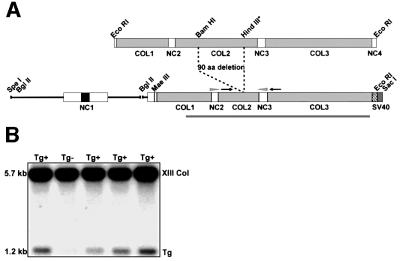
The construct used for oocyte microinjections was generated utilizing both mouse genomic and cDNA clones (Figure 1A), and consisted of 1 kb promoter and 5′ flanking sequences, the first exon, and 0.8 and 0.2 kb of the 5′ and 3′ sequences of the 18 kb first intron, respectively, the rest of the coded sequences and SV40-derived transcription termination and polyadenylation signals, but with a 270 bp in-frame deletion of sequences affecting the conserved COL2 domain. Subsequently, a 3′ base insertion was noted near the end of the cDNA, causing the shortening of the COL3 domain consisting of 78 collagenous Gly-X-Y repeats by six triplets and a lack of the short NC4 domain. As transcription termination signals were added in all three reading frames, the base insertion did not cause defects in the termination and polyadenylation of the construct. Since nucleation of the type XIII collagen occurs at the N-terminal region and triple helix formation proceeds from the N- to the C-terminus (Snellman et al., 2000b), the large deletion of the COL2 domain is predicted to cause the C-terminal half of the molecule to be out of register. Thus, the lack of the extreme C-terminal residues in the mutant molecules was not considered to be of functional significance.
Fig. 1. Structure of the type XIII collagen COL2del transgene construct and identification of transgenic mice by Southern blotting analysis. (A) Polypeptide structure of the mouse cDNA and the COL2del transgene construct resulting in a 90 residue deletion of the COL2 sequences. Collagenous domains are shown in gray and non-collagenous domains in white. The transmembrane domain is indicated by a black box, the SV40-derived polyadenylation sequence by a dark gray box and a sequence 3′ to a single base insertion by a hatched box. The insertion leads to premature termination and lack of the extreme 36 residues, indicated by the hatched box. Restriction sites used when generating the transgenic construct are shown. The probe used for genotyping is shown as a gray bar beneath the transgene construct. The PCR primers used when genotyping are indicated by the arrows and the RT–PCR primers by the arrowheads. (B) The 1.2 kb transgene and the 5.7 kb endogenous gene bands in Southern blots of KpnI- and XbaI-digested genomic DNA.
Ten founder mice were obtained that were positive for the transgene in both PCR and Southern blot hybridization (Figure 1B), nine of which showed germ line integration of the transgene and thus gave rise to separate transgenic lines. In order to evaluate the numbers of transgene copies in the genomes of the different lines, a genomic DNA fragment recognizing the first exon of the type XIII collagen gene was used to probe Southern blots containing SphI-digested genomic DNA from these lines. The probe recognized a 6 kb endogenous type XIII collagen gene fragment and a 3.5 kb transgene fragment (data not shown). The estimated number of transgene copies ranged from 1 to 20 between the lines (Table I).
Table I. Comparison of transgene and endogenous type XIII collagen expression rates by RT–PCR analysis and numbers of transgene copies.
| Transgenic line | Intestine | Cartilage | Skin | Brain | Lung | Liver | Spleen | Kidney | Heart | Skeletal muscle | Transgene copy no. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | L | E | L | H | L | E | E | E | L | L | 1 |
| 14 | E | E | E | H | L | L | E | H | L | L | 20 |
| 17 | E | E | H | E | E | L | E | E | H | E | 2 |
| 18 | E | H | E | H | H | H | E | E | E | H | 15 |
| 23 | E | E | E | E | L | L | E | E | H | E | 3 |
| 26 | E | H | H | E | L | L | E | E | E | E | 2 |
| 32 | L | L | L | L | L | L | L | L | L | L | 8 |
| 35 | L | L | L | L | L | L | L | L | L | L | 2 |
| 41 | E | L | L | L | L | L | E | L | L | L | 15 |
H = higher transgene than endogenous type XIII collagen gene expression; E = equal transgene and endogenous type XIII collagen gene expression level; L = lower transgene than endogenous type XIII collagen gene expression.
Expression of mutant type XIII collagen mRNA and protein
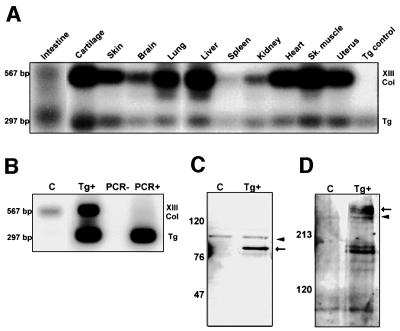
To study the expression of the transgene, RT–PCR was carried out on total RNA isolated from several tissues of mice heterozygous for the transgene (Table I). The specific oligonucleotide primers used for the PCR amplification were chosen by bracketing the COL2 deletion (see Figure 1A), and thus transgene and endogenous gene transcriptions could be distinguished in the same reactions. The transgene was expressed in all the tissues studied in all nine lines, with a pattern closely resembling that of the endogenous type XIII collagen gene, but at a lower rate in most lines and tissues studied, which indicates that most of the control sequences necessary for correct transcription are included in the promoter sequences used in the construct (Table I; Figure 2A). The number of transgene copies in the genome did not correlate with the rate of transgene expression.
Fig. 2. Expression of COL2del RNA and protein. (A) RT–PCR analysis of gene expression in transgenic line 32. The pattern of transgene expression (Tg) closely resembles that of the endogenous type XIII collagen (Col XIII) gene, but the level of expression is lower in most tissues. (B) RT–PCR analysis of transgene expression in primary cultured fibroblasts (passage 3) derived from control (Con) and mutant (Tg+) fetuses. The relative expression rate of the mutant mRNA is clearly higher in the fibroblasts than in any of the tissues of heterozygote mice (compare A and B). (C) SDS–PAGE under reducing conditions and western blotting analysis of protein expression using an anti-type XIII collagen NC3 domain antibody (Hägg et al., 1998) of the same fibroblasts derived from control (Con) and mutant (Tg+) fetuses. The mutant fibroblasts express both endogenous type XIII collagen (arrowhead in C) and truncated type XIII collagen polypeptides (arrow in C). (D) SDS–PAGE under non-reducing conditions and western blotting analysis indicate that the mutant polypeptides (Tg+) form several disulfide-bonded trimers with a slower mobility (arrow in D) than the endogenous type XIII collagen (Con; arrowhead in D).
In order to verify that the transgene was translated to shortened polypeptides with an internal in-frame deletion, cell extracts derived from mutant and wild-type fetal cultured fibroblasts were fractionated by SDS–PAGE and analyzed by western blotting using a type XIII collagen antibody recognizing the NC3 domain. In reduced samples, a band with an identical mobility was observed in both mutant and control fibroblasts, representing the endogenous type XIII collagen α-chains (Figure 2C). In addition, a smaller 80 kDa band was observed in the mutant fibroblasts (Figure 2C). The difference in size between the two bands was comparable to that observed when full-length α1(XIII) chains and α1(XIII) chains lacking the first 83 residues were expressed in insect cells (Snellman et al., 2000b), suggesting that the COL2del RNA is translated to shortened polypeptides of the expected size (Figure 2C). Since the antibody used detects the NC3 domain situated downstream from the 90 residue COL2 deletion, the result also indicates that the correct reading frame is maintained in the mutant polypeptides. Under non-reduced conditions, the mutant polypeptides were shown to associate into disulfide-bonded trimers (Figure 2D). In the mutant fibroblasts, the trimers appeared to have a slower mobility than the endogenous trimers, which may be due to conformational changes affecting their mobility on the gel (Figure 2D). However, the mutant α1(XIII) chains were more strongly expressed by the cultured fibroblasts, and similarly, RT–PCR analysis indicated that the mutant mRNA was also more strongly expressed than the endogenous one (Figure 2B). This is in contrast to RT–PCR analyses of tissues indicating lower transgene expression in almost all tissues (Figure 2A; Table I). Interestingly, when establishing the cultured fibroblasts, it was noticed that from the third passage onwards, coinciding with the high expression rate of the transgene, the cells were found to undergo morphological changes, suggesting that the mutant fibroblasts are susceptible to undergoing transformational change (A.Tuomisto, unpublished data). All in all, the COL2del transgene-derived RNA was found to be translated to truncated α1(XIII) chains that are able to form disulfide-bonded trimeric molecules.
Subsequently, the localization of the type XIII collagen protein was studied in heterozygous mice from lines 17, 23 and 26 with the type XIII collagen-specific antibody recognizing the NC3 domain, thus detecting both the mutant and the endogenous protein. The staining pattern of the mutant and control tissues (data not shown) did not differ from that previously described for type XIII collagen (Hägg et al., 2001; Sund et al., 2001). Since no signs of intracellular accumulation were seen, the mutant protein was presumed to be correctly transported to the plasma membrane in the cells of tissues positive for the transgene RNA. Owing to the insensitivity of the immunosignal, expression of the mutant product was not expected to result in a readily identifiable increase in the immunosignal in most tissues. However, increases in type XIII collagen staining were observed in the proliferating chondrocytes of bones undergoing endochondral ossification, in the osteoblasts of the developing bones and in the capsule of the liver, suggesting that the promoter sequences used to drive expression of the transgene were particularly active in the above cells (data not shown).
Lethality of COL2del mice during fetal development
As the heterozygous mice expressed no overt phenotypes, they were bred further in order to increase the load of mutant protein. When analyzing the genotype distribution of the offspring born from heterozygous mating of the nine lines, it became evident that there were four lines (lines 17, 23, 26 and 35) in which the expected Mendelian ratio of 75% transgene-positive and 25% transgene-negative mice could not be observed (Table II). The distribution of genotypes indicated that this distortion of the birth rates was caused by a lack of transgene-positive offspring. In subsequent pregnancy terminations, spontaneous abortion of a portion of the offspring was observed between days 10.5 and 13.5 of development (Table II). The abortions were observed to take place at two distinct points in time, allowing two separate phenotypes to be distinguished among the fetuses.
Table II. Genotypes of offspring from heterozygous mating of type XIII collagen transgenic mice.
| Day of development | No. of litters | Total fetuses | Normal | Aborted | pb | ||
|---|---|---|---|---|---|---|---|
| |
|
|
Tg– (%) |
Tg+ (%) |
Tg+ (%) |
Resorbed (%)a |
|
| 9.5 | 5 | 46 | 14 (30.4) | 32 (69.6) | 0 | 0 | 0.3 |
| 10.5 | 10 | 79 | 22 (27.8) | 36 (45.6) | 18 (22.8) | 3 (3.8) | 0.002 |
| 11.5 | 14 | 96 | 25 (26.0) | 38 (39.6) | 13 (13.6) | 20 (20.8) | 0.00006 |
| 12.5 | 10 | 77 | 19 (24.6) | 29 (37.7) | 12 (15.6) | 17 (22.1) | 0.0001 |
| 13.5 | 10 | 80 | 27 (33.7) | 30 (37.5) | 9 (11.3) | 14 (17.5) | 0.00003 |
| 14.5–15.5 | 10 | 64 | 13 (20.3) | 26 (40.6) | 2 (3.1) | 23 (36.0) | 0.0001 |
| p >21 | 58 | 273 | 127 (46.5) | 146 (53.5) | 0 | 0 | 1.4 × 10–13 |
The genotype distributions of the offspring from heterozygous mating in transgenic lines 17, 23, 26 and 35 are pooled together in the table.
aA portion of the aborted fetuses could not be genotyped due to extensive resorption.
bThe p value was evaluated by the χ2 test for actual genotype segregation compared with the expected genotype segregation.
The early phenotype
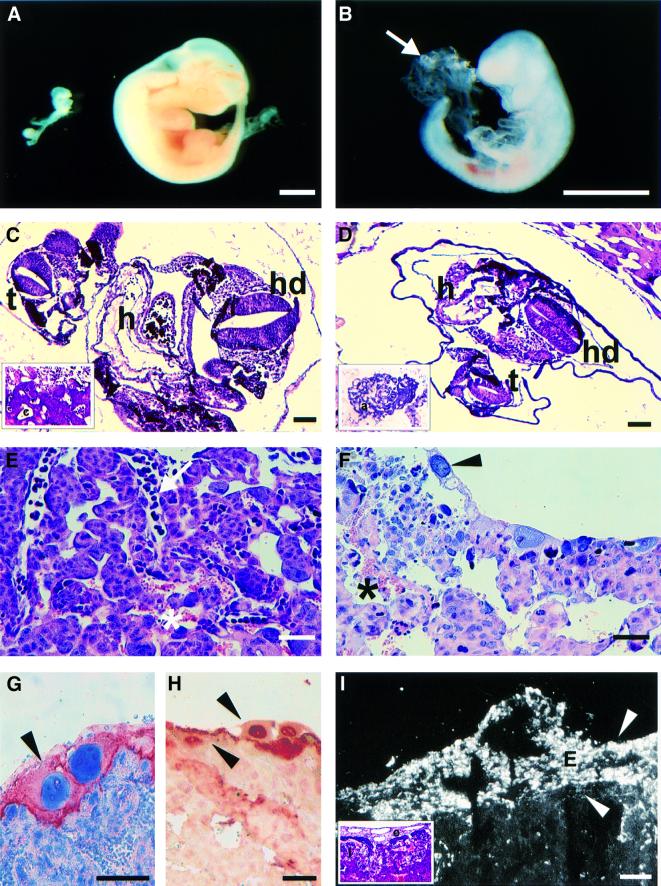
The fetuses of the early phenotype, aborted by day 10.5 of development, were small and severely retarded (Figure 3A and B). During dissection from the uterus, a balloon-shaped sac could be observed next to the fetus (Figure 3B), indicating a failure in the fusion of the chorionic and amniotic membranes, leading to a defect in the formation of a functioning placenta. Approximately half of the aborted fetuses represented this early phenotype. We also analyzed fetal day 7.5–9.5 litters from heterozygote mating. No abnormalities of the fetuses could be observed at day 7.5 (data not shown). However, at fetal day 8.5 in some of the mutant fetuses the chorion and allantois did not fuse and placental formation was perturbed, whereas in the controls the formation of the placenta was well under way (Figure 3C and D). The same was also observed at fetal day 9.5, and at this stage the mutant fetus was clearly smaller when compared with controls (data not shown). At day 10.5, histological analysis of control fetuses indicated that the labyrinth layer of the placenta had formed, and both nucleated fetal blood cells and anucleated maternal blood cells were observed (Figure 3E). In the mutant, there was a complete lack of formation of the fetal portion of the placenta, and thus no fetal blood cells could be distinguished in it (Figure 3F), although a single layer of trophoblast giant cells lining the maternal portion of the mutant placenta (Figure 3G) indicated that implantation of the fetus had initially occurred successfully. These giant trophoblast cells were observed by TUNEL assay to be undergoing apoptosis (Figure 3H). Interestingly, the ectoplacental cone, which is formed after the fusion of the allantois and chorion (Cross et al., 1994; Kaufman, 1994), is a site of strong type XIII collagen expression in both control and mutant fetuses (Figure 3I and data not shown), and thus the expression of abnormal type XIII collagen at this site might well disturb the delicate adhesive processes and cause the observed defect in placental formation.
Fig. 3. The early phenotype fetuses. (A) The mutant fetus is severely retarded (left) compared with the control littermate (right). (B) The unfused allantois (arrow) can be seen as a balloon-shaped sac next to a mutant fetus. (C and D) At fetal day 8.5, the control fetus (C) has completed the turning process and shows a successful fusion of the chorionic and allantoic membranes with penetration of embryonic vessels into the chorionic plate (c in insert of C). The mutant fetus (D) is also well into the turning process, but still has an unfused allantois (a in insert of D), despite being beyond the 7–10 somite stage when fusion normally takes place. (E and F) No fetal blood cells were distinguished in the mutant placenta (F), whereas the labyrinth layer of the control placenta (E) contained both nucleated fetal blood cells (arrow) and anucleated maternal blood cells (asterisk). (G) A single layer of cytokeratin-positive trophoblast giant cells lined the mutant placenta (arrowheads in G and F). (H) These giant trophoblast cells were observed by TUNEL assay to be undergoing apoptosis (arrowheads). (I) Staining of the placenta of a control mouse with a type XIII collagen antibody. Strong type XIII collagen staining is seen in the ectoplacental cone (arrowheads and e in insert), which is formed after fusion of the chorion and allantois. No difference in the staining pattern of the placenta of mutant mice surviving the initial chorioallantoic fusion could be observed (data not shown). The insert shows the same area stained by hematoxylin–eosin where the distinct structures of the ectoplacental cone (e) and the labyrinth layer of the placenta (l) can be appreciated. hd, head; h, heart; t, tail; c, chorionic plate; a, allantois; e, ectoplacental cone; l, labyrinth layer. Bars: 1.5 mm (A and B), 50 µm (C and D) and 20 µm (E–I).
Defective adhesive junctions in the hearts of late phenotype fetuses
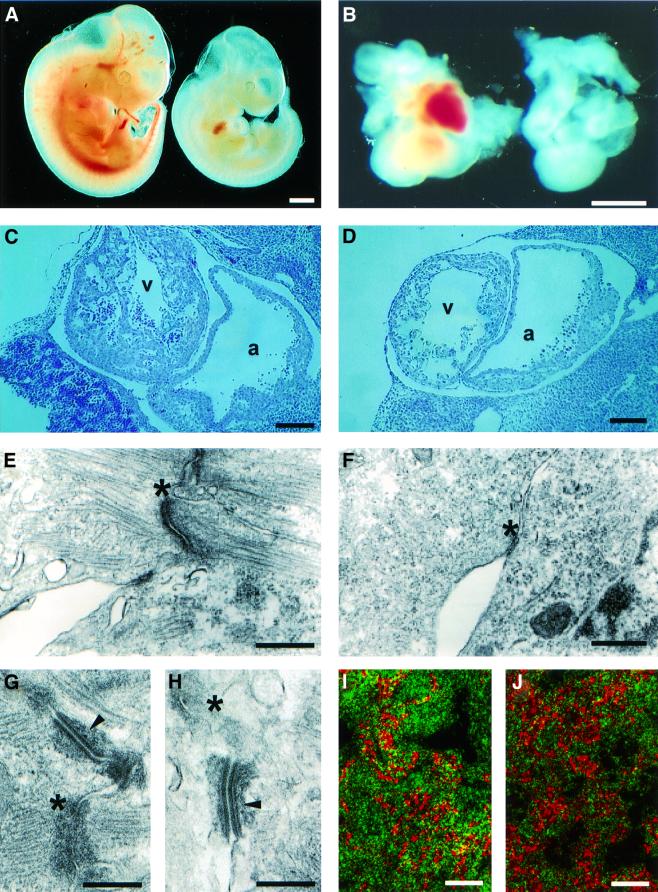
The late phenotype fetuses, aborted by day 13.5 of development, were small and pale by comparison with their transgene-negative littermates (Figure 4A). It was observed during dissection from the uterus that the hearts of the aborting fetuses were beating irregularly and weakly. This cardiac dysfunction and the marked paleness of the fetuses were suggestive of a cardiovascular defect.
Fig. 4. Macroscopic features of the late phenotype fetuses and details of the heart defect. (A) The mutant fetus (right) is small and pale relative to its control littermate (left). (B) The four-chambered structure and the inflow and outflow tracts of the heart are intact in the mutant (right). (C and D) The myocardial layer is thin and the trabeculation of the ventricles (v) reduced in the mutant (D) relative to the control (C). (E and F) Electron microscopy of the mutant (F) and control (E) heart indicated less dense adherence junctions (asterisks in E and F) and detachment of myofilaments in the mutant. (G and H) Abnormal adherence junctions (asterisks in H) but normal desmosomes (arrowhead in H) in the heart of a mutant fetus, while the junctions in the control are normal (asterisk and arrowhead in G). (I and J) Immunostaining with desmoplakin (red) and cadherin (green) of mutant (J) and control (I) hearts analyzed by confocal microscopy. In the mutant, the cadherin signal is reduced and appears disorganized when compared with the control. The desmoplakin signals do not differ between the mutant and the control. Bars: 1.5 mm (A and B), 20 µm (C and D), 0.5 µm (E–H) and 2 µm (I and J).
The four-chambered structure of the heart, and its inflow and outflow tracts, appeared to be intact and properly developed in the mutants upon macroscopic and histological analysis (Figure 4B). The endocardium, myocardium and epicardium had developed, but the myocardial layer appeared thinner than in the control fetuses and the trabeculation of the ventricles was reduced (Figure 4C and D). Ultrastructural analysis pointed to clear differences in the structure of the myocardium, the structure of the adherence junctions being less electron dense with detachment of myofilaments from these structures. The number of myofilaments also appeared to be reduced and the cardiomyocytes showed degenerative signs (Figure 4E and F). On the other hand, the desmosomes had developed normally in the mutant heart and were indistinguishable from those of the control (Figure 4G and H). In the samples analyzed, also no differences of the developing gap junctions in the mutant and control hearts could be observed (data not shown).
In antibody stainings of fetal hearts with markers for the different junctions we could not observe any differences in the staining pattern for the desmosomal component desmoplakin between the mutant and the control, but staining for the adherence junction marker cadherin revealed a disorganized pattern and reduced signal intensities in the mutant (Figure 4I and J). This finding is well in line with the ultrastructural data, where the adherence junctions are markedly abnormal in the transgenic fetuses but the desmosomes are indistinguishable from the controls (Figure 4G and H).
Defective angiogenesis in the late phenotype fetuses
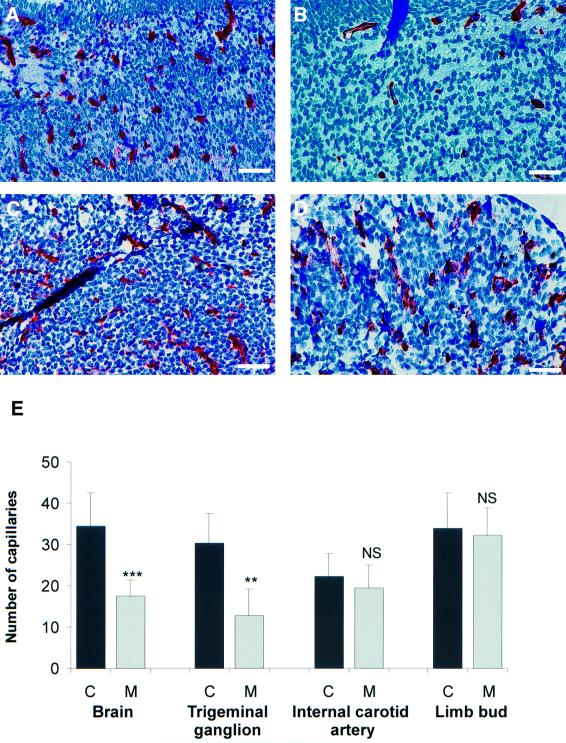
The development of the vasculature of the mutant fetuses was studied by immunohistochemical staining of the tissues with the endothelial markers CD31 (PECAM) and CD34. Vasculogenesis appeared to proceed normally in the mutants, but a defect in angiogenesis was evident, leading to a perturbation of microvascularization in some regions of the fetuses. The number of small vessels in the developing central nervous system and the ganglia was reduced in the cranial region (Figure 5A and B and data not shown), whereas the development of the microvessels in other parts, such as the cephalic mesenchyme and the upper limb of the same fetuses, was normal (Figure 5C and D and data not shown). These findings were verified by morphometric analysis of the number of capillaries (Figure 5E). No statistically significant difference in the number of capillaries could be observed in the limb or in the area surrounding the internal carotid artery, but such a difference was evident in both the developing central nervous system and the trigeminal ganglia. As no staining of capillary endothelia is normally detected with type XIII collagen antibodies (Sund et al., 2001), the possibility of abnormal expression of the mutant protein in the endothelia of the transgenic mice was analyzed by double staining with a type XIII collagen antibody recognizing both the mutant and the endogenous protein and the endothelial marker CD34 in sections of fetuses. However, no expression of type XIII collagen was observed in the endothelia at these sites either in mutant or control, whereas the surrounding tissue stained strongly for type XIII collagen (data not shown). Expression of the mutant mRNA in these tissues was confirmed by RT–PCR analysis (Table I).
Fig. 5. Vascular defects in the late phenotype fetuses. (A and B) The mutant brain (B) contains less small vessels than the control brain (A). (C and D) No differences in the microvascularization in the limbs of the same mutant (D) and control mice (C) can be observed. Bars: 20 µm (A–D). (E) Morphometric analysis reveals statistically significant differences in the number of capillaries in the developing central nervous system and the trigeminal ganglia, whereas no differences are seen in the limb or the area surrounding the internal carotid artery. Statistical significance was evaluated by the Mann–Whitney U-test.
Impaired placental development in the late phenotype
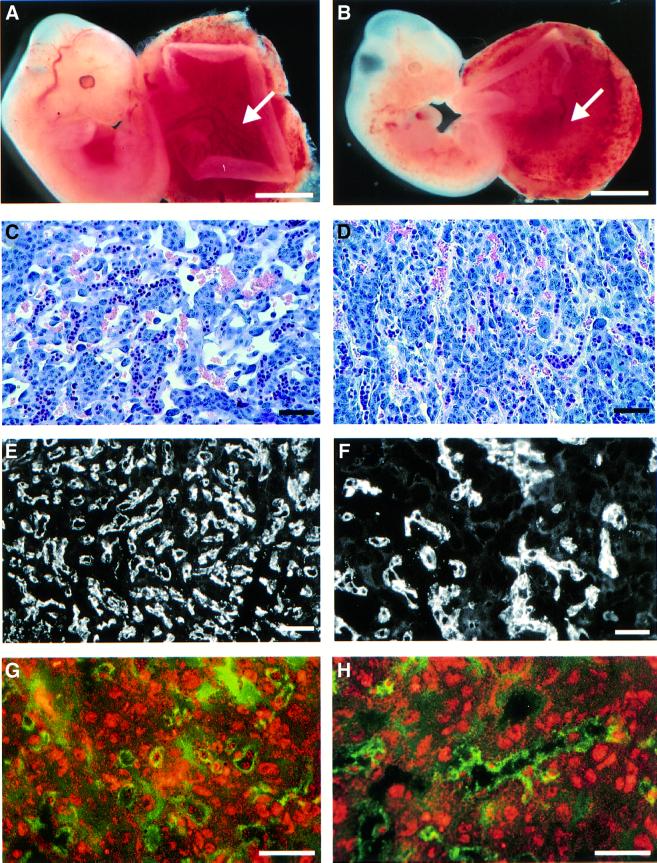
During dissection it was observed that the placental blood vessels were not as well developed in the mutants as in the controls (Figure 6A and B), and that in histological sections the labyrinth layer of the placenta was thick and less vascularized in appearance (Figure 6C and D). The cell lineages of the placenta were studied with the endothelial marker CD34, the trophoblast marker cytokeratin and the decidual markers vimentin and desmin. Apoptosis was studied with the TUNEL assay and cell proliferation with proliferating cell nuclear antigen (PCNA) antibody.
Fig. 6. Placental defects in the late phenotype fetuses. (A and B) The placental blood vessels are well developed in the control (arrow in A), but not as clearly defined in the mutant (arrow in B). (C and D) In the mutant (D) the labyrinth layer is thick and less well vascularized when compared with the control (C). (E and F) Endothelial marker CD34 reveals a reduced number of vessels in the mutant placenta (F) relative to the control placenta (E). (G and H) Double staining of the labyrinth layer of mutant (H) and control (G) placenta with type XIII collagen (red) and CD34 antibodies (green) does not indicate any differences in the staining pattern of type XIII collagen in the placenta. Expression of either transgenic or endogenous type XIII collagen cannot be detected in the endothelia of the mutant (H) or control (G) placenta, as the signals of type XIII collagen and CD34 do not overlap. Bars: 0.3 mm (A and B) and 20 µm (C–H).
Similar defects were observed in the development of the vascularization of the placenta to those in the fetus (see above). Expression of both mutant and endogenous type XIII collagen in the placentas of transgenic mice was verified by RT–PCR analysis (data not shown). The fetal vessels showed less spreading in the labyrinth layer of the mutant placentas than in the control placentas (Figure 6E and F). Morphometric analysis of placentas from mutant and control fetuses indicated that the defect in the development of fetal vascularization had led to a statistically significant increase in the average distance between the fetal vessels and the maternal blood lacunae (average distance 24.5 µm, SD 5.75 µm for the mutant; and average distance 6 µm, SD 1.5 µm for the control, p <0.001).
In double staining of the labyrinth layer with antibodies recognizing type XIII collagen and the endothelial marker CD34, no overlapping immunosignals could be detected in the mutant and control (Figure 6G and H). This confirms the lack of type XIII collagen in endothelial structures (Sund et al., 2001), and rules out the possibility of abnormal expression of type XIII collagen in the endothelia of the mutant placenta. Nevertheless, strong staining for type XIII collagen could be detected in the placental stroma, and these staining patterns and intensities were essentially identical when comparing the labyrinth layers of the mutant and the control (Figure 6G and H and data not shown). In order to study the expression of type XIII collagen mRNAs in placenta, in situ hybridizations were performed using a cDNA probe recognizing both the mutant and the endogenous type XIII collagen mRNAs. Strong in situ signals were detected in the endodermal cells of the visceral yolk sac and moderate signals were seen in the trophoblastic cells, while clear signals were not detected in endothelial cells (data not shown). All in all, both at the protein and mRNA levels, the expression pattern of type XIII collagen did not differ between the mutant and the control placenta.
The staining with cytokeratin, vimentin and desmin revealed no defects in the development of the other compartments of the mutant placenta (data not shown). The TUNEL assay pointed to somewhat more numerous apoptotic cells in the mutant placentas, most likely secondary to the poorly developed vascularization. Staining of the placentas with PCNA did not indicate any changes in cell proliferation between the mutant and control placentas.
Discussion
The function of type XIII collagen is not known, although its occurrence at many sites of cell–matrix interaction and of cell–cell adhesion is suggestive of a role in cell adhesion (Peltonen et al., 1999; Hägg et al., 2001). It has been shown in human disorders as well as in mouse models, with respect to the fibrillar collagens and some of the non-fibrillar types, that in cases where a mutation does not involve the chain association sequences, mutant and normal chains associate stoichiometrically, leading to the formation of abnormal collagen and a dominant-negative phenotype (Jacenko et al., 1993; Kivirikko, 1993; Prockop and Kivirikko, 1995). Thus, generation of transgenic mice expressing mutant α1(XIII) collagen chains in addition to the endogenous α-chains was used to gain information on the role of type XIII collagen. The 90 amino acid in-frame deletion in the central portion of the molecule was designed not to affect sequences known to be involved in the association of α1(XIII) chains (Snellman et al., 2000b). Furthermore, the deletion mutation was chosen to affect sequences not subject to the alternative splicing known to affect other portions of the mouse α1(XIII) chains (Peltonen et al., 1997); thus, the deletion was expected to impair a functionally important domain. RT–PCR analysis of a range of tissues of the mutant mouse lines produced indicated that the transgene was transcribed in a similar manner to the endogenous gene.
Analysis of cultured fibroblasts derived from the mutant mice showed that the COL2del transgene led to synthesis of shortened α1(XIII) chains, which were able to associate into disulfide-bonded trimeric molecules. Interestingly, trimers of different sizes were observed under non-reducing electrophoretic conditions. Thus, it is likely that the cultured fibroblasts as well as cells in the mutant mice that expressed type XIII collagen contained a mixture of molecules, including heterotrimers of COL2del and normal α1(XIII) chains, homotrimers of normal chains and homotrimers of COL2del chains. Furthermore, immunostaining of mutant fetal tissues did not reveal any signs of intracellular accumulation of type XIII collagen. In separate experiments we have also produced transgenic mice that express type XIII collagen α-chains with a hemagglutinin tag at their C-terminus (R.Ylönen, unpublished data), and the same promoter was used to direct the synthesis of the tagged α1(XIII) chains as in the case of the COL2del mice reported here. Staining of the transgenic fetuses with an anti-tag antibody revealed identical patterns when compared with anti-type XIII collagen NC3 antibody staining. Taken together, the mutant α1(XIII) chains with a 90 residue COL2 deletion are likely to associate stoichiometrically with both mutant and endogenous normal α1(XIII) chains and be transported to the plasma membrane of cells. Owing to a defect in making the mutant cDNA construct, the molecules also lack the extreme 36 C-terminal residues. Since this defect is downstream of the sequences responsible for nucleation and triple helix formation, and the central deletion placing all subsequent sequences out of register, it is not predicted to be of functional importance.
The expression of truncated type XIII collagen α-chains in transgenic mice was found to result in arrest of fetal development between days 10.5 and 13.5 of gestation. Homozygous offspring were not obtained, indicating recessive lethality as the amount of mutant protein increased. Some of the heterozygous offspring were also lost, however, suggesting individual differences in the actual transcription level of the mutant protein during development. The fetuses were aborted at two stages, with ∼50% representing each of the phenotypes.
The early phenotype fetuses were aborted by day 10.5 of development when the chorionic and allantoic membranes failed to fuse and the formation of a functioning placenta was subsequently hindered. The latter is a critical step for development (Rossant, 1996), and once this connection failed it led to severe retardation and abortion of the fetuses.
The fetuses that were able to form a functioning placenta and overcome this initial critical step developed further and represent the late phenotype. These were aborted by day 13.5 of development due to cardiovascular defects. During this period of gestation, the fetus becomes dependent on its own blood circulation and on a well functioning cardiovascular system for the subsequent developmental processes to proceed (Rossant, 1996). Previous studies indicate that type XIII collagen is strongly expressed in the heart during early development, being localized to cell–cell contacts and becoming accentuated in the intercalated discs (Sund et al., 2001). The hearts of the fetuses expressing mutant type XIII collagen beat poorly during dissection from the uterus, the myocardium of the ventricles in the mutant hearts was thin, with reduced trabeculae, and ultrastructural analyses demonstrated poorly developed adherence junctions, with detachment of myofilaments. Cardiomyocytes also displayed overall signs of degeneration and appeared to contain fewer myofilaments, a secondary effect most likely caused by the lack of organization when the contacts with the adherence junctions failed. Interestingly, the desmosomes were intact and did not differ between the mutant and the control. Antibody staining of mutant and control hearts with markers for the different junctions indicated disorganized and reduced signal for the adherence junction component cadherin, while the staining pattern for the desmosomal component desmoplakin did not differ between the mutant and the control. Previous analyses of skin indicate that type XIII collagen is located to epidermal adherence junctions and appears to be absent from desmosomes (Peltonen et al., 1999). Here the expression of COL2del α1(XIII) chains greatly impaired the development of adherence junctions in the heart. Taken together, these results suggest that type XIII collagen is a component of adherence junctions, and that expression of mutant type XIII collagen causes a cascade of reactions affecting the various proteins, such as cadherin and myofilaments, found in the adherence junctions of the heart, and leading to deterioration of these junctions. The preservation of the normal appearance of desmosomes may explain why the heart functions until day 13.5 in the mutant fetuses.
Previous studies indicate that notable structures lacking in type XIII collagen protein are the endothelia of capillaries and large blood vessels (Sund et al., 2001). In agreement with this, no ectopic expression of type XIII collagen in the endothelia of the COL2del transgenic mice was observed in double staining of tissues with type XIII collagen- and endothelial-specific antibodies. We were thus surprised to find reduced microvascularization in the central nervous system and the trigeminal ganglion in the COL2del mice, especially since the number of capillaries in the region surrounding the internal carotid artery and the limbs in the same fetuses did not differ. The ability of the endothelial cells to adopt a migratory phenotype during angiogenesis is known to be influenced by alterations in the expression levels of adhesive glycoproteins and integrins, and of other proteins produced by the endothelial cells and the extracellular matrix surrounding them (Luscinskas and Lawler, 1994). On the other hand, the central nervous system and the ganglia are known to be sites of strong type XIII collagen expression during development, whereas the expression rate is lower in the mesenchyme of the head and the developing limb (Sund et al., 2001). Thus, decreased angiogenesis is observed in the COL2del mice in tissues normally expressing high levels of type XIII collagen in non-endothelial cells, and therefore also likely to express high levels of the mutant molecules in such cells.
Interestingly, α1β1 integrin, which is known to be among the collagen receptors expressed in both large vessel and microvessel endothelial cells (Enenstein et al., 1992; Klein et al., 1993), has recently been shown to interact strongly with recombinant type XIII collagen (Nykvist et al., 2000). Thus, we suggest that the impaired angiogenesis in the COL2del mice is not due to abnormal endothelial cells, but rather to expression of altered type XIII collagen in the surrounding tissue where the newly forming vessels are invading. The mutant type XIII collagen could affect binding or signal transduction between the endothelial cells and the matrix, causing the observed defect of angiogenesis.
Defects were also observed in the placentas of the late phenotype embryos. The labyrinth layer is usually well vascularized, with the fetal vessels and maternal blood lacunae in close contact for effective exchange of nutrients and other substances (Cross et al., 1994). In the type XIII collagen mutants, the labyrinth layer was dense and less vascularized, and the fetal vessels in the placenta showed defects similar to those observed in the fetus proper. Type XIII collagen RNA is expressed in the human placenta by fibroblastic stromal cells in the villi, developing endothelia, cytotrophoblastic cells and decidual cells (Juvonen et al., 1993). Immunostaining and in situ hybridization of the murine placenta revealed type XIII collagen in the trophoblasts of the labyrinth and the spongiotrophoblastic layer; it was not associated with endothelia in either the control or mutant fetuses. It therefore appears that the expression of mutant type XIII collagen in the placenta has the same effect on the developing fetal vessels as was observed in the fetus itself, in that regions normally expressing this collagen strongly are subject to impaired angiogenesis.
The phenotype of the transgenic mice expressing mutant type XIII collagen closely resembles that of a number of mouse mutants where adhesion molecules have either been subjected to mutation or knocked out, e.g. the phenotypes of the targeted disruptions of the genes for plakoglobin (γ-catenin) and vinculin (Bierkamp et al., 1996; Ruiz et al., 1996; Xu et al., 1998). Many of these disruptions have led to lethality during fetal development due to cardiovascular and placental defects. The plakoglobin mice displayed a poorly functioning heart caused by the formation of fused desmosome and adherence junctions (Ruiz et al., 1996). The mice lacking in vinculin also represented a fetal lethal phenotype characterized by a dysfunctional heart (Xu et al., 1998). The type XIII collagen mutants also resemble to some extent the α4 integrin (Yang et al., 1995), VCAM-1 (Gurtner et al., 1995; Kwee et al., 1995), N-cadherin (Radice et al., 1997), αv integrin (Bader et al., 1998) and β3 integrin (Hodivala-Dilke et al., 1999) mutants. Correct sequential formation of the cellular junctions is of importance with respect to the formation of the intercalated discs, which are essential for generating the mechanical strength needed in cell–cell adhesions in the myocardium (Angst et al., 1997). Data obtained from different transgenic mice, including the type XIII collagen mutant mice, indicate that alterations in the structure of the junctions can have deleterious effects on this process, leading to a dysfunctional heart.
The importance of adhesion molecules and the extracellular matrix during development, for guiding the migrating cells, regulating morphogenesis and providing mechanical strength, has been well established (Lin and Bissell, 1993; Gumbiner, 1996). Type XIII collagen is expressed at the early stages of development of the mouse fetus (Sund et al., 2001) and the present results show that expression of mutant type XIII collagen results in fetal mortality from cardiovascular and placental defects very similar to those displayed by mice with mutations for several classic cell adhesion molecules. Furthermore, direct evidence was obtained for the role of type XIII collagen in the formation of adherence junctions in the heart.
We thus conclude that type XIII collagen is a molecule involved in cell adhesion with functions that are essential for normal development to proceed. All in all, the role of adhesion molecules in placentation and the development of the cardiovascular system is well documented in work with transgenic mice (Hynes, 1996), and the observed defects in mice expressing mutated type XIII collagen can most likely be correlated to the events leading to the spontaneous abortions of human first trimester pregnancies or congenital heart defects.
Materials and methods
Type XIII collagen COL2 deletion construct
A mouse cDNA clone P40 (Peltonen et al., 1997), corresponding to exons 2–41, was used to generate the transgenic construct. A HindIII site (underlined), created by introducing a point mutation (asterisk) into exon 24 with the U.S.E. kit (Pharmacia) and the oligonucelotides Hmut1 (5′-CCAAAGGGAGAAGCTT*GTGTTGATGGCC-3′) and (Hmutrev1 5′-GGCCATCAACACAAGCTT*CTCCCTTTGG-3′), and a BamHI site in exon 18 were used to delete 270 bp of the COL2 domain (Figure 1A), and the ends were ligated with BHlink (5′-GATCAGGGCTATGGAGA-3′) and BHlinkrev (5′-AGCTTCTCCATAGCCCTG-3′). A genomic DNA fragment containing the 159 bp extreme 3′ sequences of the first intron and the first 91 bp of the second exon was generated by PCR of the plasmid 3HA (M.Sund, unpublished results) with the primers PCRmbeg (5′-GAAGATCTGTATGAACTGCCATGCTTTC-3′) and PCRmend (5′-CAGTTACATCCTGGAGACATCTTCGGGGC-3′). The fragment generated was digested with BglII and MaeIII, and ligated 5′ of the P40 cDNA clone. A 2.5 kb BglII-digested genomic DNA fragment containing 1 kb of promoter and 5′ flanking sequences, the complete first exon and 0.8 kb of the first intron was further cloned 5′ of the cDNA. Transcription termination and polyadenylation signals were derived from the SV40 poly(A) DNA and cloned 3′ of the cDNA. The transgene construct (COL2del) was released from the vector by SpeI and SacI digestion, purified, and used for microinjection. A base insertion was later detected at the 3′ end of the cDNA causing the shortening of the COL3 domain by six collagenous repeats and the lack of the NC4 domain.
Generation and identification of transgenic mice
The transgene construct was microinjected into the pronuclei of the one-cell-stage embryos from B6D2F1 hybrid mice, cultured overnight at 37°C and implanted into the oviducts of NMRI pseudopregnant mice. DNA was extracted from tail or yolk sac samples by overnight incubation at 55°C in STE buffer (50 nM Tris, 10 mM EDTA, 0.1% SDS) with 400 µg of proteinase K (Boehringer Mannheim) followed by isopropanol precipitation. Genotyping was performed by PCR with primers MutScreen2 (5′-GGTTTACCGGGGCCTCCTGGACCAAAGGG-3′) and MutScreen2rev (5′-GGCCTGCTTGTCCTGTCTCCCCTTTCTCC-3′) (arrows in Figure 1A), or Southern hybridization of genomic DNA digested with KpnI and XbaI. A 1.2 kb cDNA fragment generated by KpnI and XbaI digestion of clone 3VPL6 (M.Sund, unpublished data) (Figure 1A) was used to probe the Southern blots (Figure 1B). Densitometric analysis of signals generated when probing Southern blots of SphI-digested genomic DNA with a 0.85 kb SphI fragment of the clone P7 (M.Sund, unpublished results) was used to evaluate the number of transgene copies in the different transgenic lines.
Analysis of mutant type XIII collagen mRNA and protein
Total RNA was isolated from tissues and cells as described previously (Chomczynski and Sacchi, 1987). The RT reaction with 2.5 µg of total RNA and 150 ng of random oligohexamers (Gibco-BRL) was carried out at 42°C for 50 min with subsequent treatment of the products with two units of RNaseH (Gibco-BRL) at 37°C for 20 min. The PCR primers were RTpcr1 (5′-GATGCTGCCATTATAATCCACCATCTC-3′) and RTpcr2 (5′-CCTAAAGGGGAACAAAGTCAGACTGGC-3′) (arrowheads in Figure 1A).
Western blotting was performed on cell extracts isolated from fibroblasts derived from 12.5 day mutant and control mouse fetuses, and grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal calf serum and non-essential amino acids. Cells were extracted with a Triton X-100 lysis buffer and prepared for SDS–PAGE as described previously (Hägg et al., 1998). Western blotting was performed under reducing and non-reducing conditions with a type XIII collagen-specific antibody recognizing the NC3 domain of the molecule (Hägg et al., 1998).
Pregnancy terminations and dissection of fetuses
Heterozygous mice were mated overnight and the appearance of a vaginal plug in the morning was designated day 0.5 of gestation. Terminations were performed between days 9.5 and 15.5 of gestation with dissection of fetuses from the uterus. The fetuses and placentas were either embedded in Tissue Tec compound (Sakura Finetek) and frozen in liquid nitrogen or fixed in 10% buffered formalin and embedded in paraffin for subsequent histological and immunohistochemical analysis.
Histological, immunohistochemical, morphometric analysis and in situ hybridization
Specimens were stained with hematoxylin–eosin according to standard procedures for general histology. Antibodies used were anti-CD31 (PECAM) and CD34 (PharMingen), anti-cytokeratin, anti-vimentin (Zymed), anti-desmin (Sigma), anti-type IV collagen (Chemicon), anti-PCNA (Santa Cruz Biotechnology), anti-desmoplakin I+II (Boehringer Mannheim), anti-pancadherin (Santa Cruz Biotechnology) and anti-type XIII collagen (Hägg et al., 2001). Apoptosis was studied by performing the TUNEL assay (Boehringer Mannheim) according to the manufacturer’s protocol. Samples were analyzed by conventional light microscopy or confocal microscopy (Zeiss).
Areas of interest for morphometric analysis were photographed in both mutant and control specimens. The number of capillaries or distance between the capillaries and maternal blood lacunae was measured in 15 squares of a grid containing 25 squares of 6.25 cm2 imposed on the photographs.
Four-micrometer paraffin sections of a control and a mutant placenta were used for in situ hybridization. The probes for the in situ hybridization were generated from plasmid JA-2 containing a 720 bp fragment, corresponding to nucleotides 1419–2139 of the mouse type XIII collagen cDNA (Hägg et al., 1998). The same conditions as described previously were used for the in situ hybridizations (Sund et al., 2001).
Ultrastructural studies
Hearts were dissected from day 10.5 fetuses, fixed in 2.5% glutaraldehyde, 0.1 M phosphate buffer, postfixed in 1% osmium tetroxide, dehydrated in acetone and embedded in Epon LX112. Thin sections were cut with a Reihert Ultracut E-ultramicrotome (Reichert-Jung). Electron microscopy was performed with a Philips CM100 transmission electron microscope (Philips Export B.V.) using an accelerating voltage of 80 kV.
Acknowledgments
Acknowledgements
We thank Ritva Savilaakso, Maija Seppänen, Sirpa Kellokumpu and Heli Auno for their expert technical assistance. Grants from the Health Sciences Council of the Academy of Finland, the Finnish Centre of Excellence Programme (2000–2005) of the Academy of Finland (44843), the Sigrid Juselius Foundation and Finska Läkaresällskapet supported this work.
References
- Angst B.D., Khan,L.U., Severs,N.J., Whitely,K., Rothery,S., Thompson,R.P., Magee,A.I. and Gourdie,R.G. (1997) Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ. Res., 80, 88–94. [DOI] [PubMed] [Google Scholar]
- Bader B.L., Rayburn,H., Crowley,D. and Hynes,R.O. (1998) Extensive vasculogenesis, angiogenesis and organogenesis precede lethality in mice lacking all α v integrins. Cell, 95, 507–519. [DOI] [PubMed] [Google Scholar]
- Bierkamp C., Mclaughlin,K.J., Schwarz,H., Huber,O. and Kemler,R. (1996) Embryonic heart and skin defects in mice lacking plakoglobin. Dev. Biol., 180, 780–785. [DOI] [PubMed] [Google Scholar]
- Chomczynski P. and Sacchi,N. (1987) Single-step method of RNA isolation by acid guanidium thiocyanate–phenol–chloroform extraction. Anal. Biochem., 162, 156–159. [DOI] [PubMed] [Google Scholar]
- Cross J.C., Werb,Z. and Fisher,S.J. (1994) Implantation and the placenta: key pieces of the development puzzle. Science, 266, 1508–1518. [DOI] [PubMed] [Google Scholar]
- Enenstein J., Waleh,N.S. and Kramer,R.H. (1992) Basic FGF and TGF-β differentially modulate integrin expression of human microvascular endothelial cells. Exp. Cell Res., 203, 499–503. [DOI] [PubMed] [Google Scholar]
- Gumbiner B.M. (1996) Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell, 84, 345–357. [DOI] [PubMed] [Google Scholar]
- Gurtner G.C., Davis,V., Li,H., McCoy,M.J., Sharpe,A. and Cybulsky,M.I. (1995) Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev., 9, 1–14. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke K.M. et al. (1999) β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest., 103, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. (1996) Targeted mutations in cell adhesion genes: what have we learned from them? Dev. Biol., 180, 402–412. [DOI] [PubMed] [Google Scholar]
- Hägg P., Rehn,M., Huhtala,P., Väisänen,T., Tamminen,M. and Pihlajaniemi,T. (1998) Type XIII collagen is identified as a plasma membrane protein. J. Biol. Chem., 273, 15590–15597. [DOI] [PubMed] [Google Scholar]
- Hägg P., Väisänen,T., Tuomisto,A., Rehn,M., Tu,H., Huhtala,P., Eskelinen,S. and Pihlajaniemi,T. (2001) Type XIII collagen: a novel cell adhesion component present in a range of cell–matrix adhesions and in the intercalated discs between cardiac muscle cells. Matrix Biol., 19, 727–742. [DOI] [PubMed] [Google Scholar]
- Jacenko O., LuValle,P.A. and Olsen,B.R. (1993) Spondylometaphyseal dysplasia in mice carrying a dominant negative mutation in a matrix protein specific for cartilage-to-bone transition. Nature, 365, 56–61. [DOI] [PubMed] [Google Scholar]
- Juvonen M. and Pihlajaniemi,T. (1992) Characterization of the spectrum of alternative splicing of α1(XIII) collagen transcripts in HT-1080 cells and calvarial tissue resulted in identification of two previously unidentified alternatively spliced sequences, one previously unidentified exon and nine new mRNA variants. J. Biol. Chem., 267, 24693–24699. [PubMed] [Google Scholar]
- Juvonen M., Sandberg,M. and Pihlajaniemi,T. (1992) Patterns of expression of the six alternatively spliced exons affecting the structures of the COL1 and NC2 domains of the α1(XIII) collagen chain in human tissues and cell lines. J. Biol. Chem., 267, 24700–24707. [PubMed] [Google Scholar]
- Juvonen M., Pihlajaniemi,T. and Autio-Harmainen,H. (1993) Location and alternative splicing of type XIII collagen RNA in the early human placenta. Lab. Invest., 69, 541–551. [PubMed] [Google Scholar]
- Kaufman M.H. (1994) Atlas of Mouse Development. Academic Press, London, UK, pp. 472–477.
- Kivirikko K.I. (1993) Collagens and their abnormalities in a wide spectrum of diseases. Ann. Med., 25, 113–126. [DOI] [PubMed] [Google Scholar]
- Klein S., Giancotti,F.G., Presta,M., Albelda,S.M., Buck,C.A. and Rifkin,D.B. (1993) Basic fibroblast growth factor modulates integrin expression in microvascular endothelial cells. Mol. Biol. Cell, 4, 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee L., Baldwin,H.S., Shen,H.M., Stewart,C.L., Buck,C., Buck,C.A. and Labow,M.A. (1995) Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development, 121, 489–503. [DOI] [PubMed] [Google Scholar]
- Lin C.Q. and Bissell,M.J. (1993) Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J., 7, 737–743. [DOI] [PubMed] [Google Scholar]
- Luscinskas F.W. and Lawler,J. (1994) Integrins as dynamic regulators of vascular function. FASEB J., 8, 929–938. [DOI] [PubMed] [Google Scholar]
- Nykvist P., Tu,H., Ivaska,J., Käpylä,J., Pihlajaniemi,T. and Heino,J. (2000) Distinct recognition of collagen subtypes by α1β1 and α2β1 integrins. α1β1 mediates cell adhesion to type XIII collagen. J. Biol. Chem., 275, 8255–8261. [DOI] [PubMed] [Google Scholar]
- Peltonen S., Rehn,M. and Pihlajaniemi,T. (1997) Alternative splicing of mouse α1(XIII) collagen RNAs results in at least 17 different transcripts, predicting α1(XIII) collagen chains with length varying between 651 and 710 amino acid residues. DNA Cell Biol., 16, 227–234. [DOI] [PubMed] [Google Scholar]
- Peltonen S., Hentula,M., Hagg,P., Yla-Outinen,H., Tuukkanen,J., Lakkakorpi,J., Rehn,M., Pihlajaniemi,T. and Peltonen,J. (1999) A novel component of epidermal cell–matrix and cell–cell contacts: transmembrane protein type XIII collagen. J. Invest. Dermatol., 113, 635–642. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T. and Rehn,M. (1995) Two new collagen subgroups: membrane associated collagens and types XV and XVIII. Prog. Nucleic Acid Res. Mol. Biol., 50, 225–262. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T. and Tamminen,M. (1990) The α1 chain of type XIII collagen consists of three collagenous and four noncollagenous domains and its primary transcript undergoes complex alternative splicing. J. Biol. Chem., 265, 16922–16928. [PubMed] [Google Scholar]
- Prockop D.J. and Kivirikko,K.I. (1995) Collagens: molecular biology, diseases and potentials for therapy. Annu. Rev. Biochem., 64, 403–434. [DOI] [PubMed] [Google Scholar]
- Radice G.L., Rayburn,H., Matsunami,H., Knudsen,K.A., Takeichi,M. and Hynes,R.O. (1997) Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol., 181, 64–78. [DOI] [PubMed] [Google Scholar]
- Rossant J. (1996) Mouse mutants and cardiac development: new molecular insights into cardiogenesis. Circ. Res., 78, 349–353. [DOI] [PubMed] [Google Scholar]
- Ruiz P. et al. (1996) Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J. Cell Biol., 135, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman A., Keränen,M.R., Hägg,P.O., Lamberg,A., Hiltunen,J.K., Kivirikko,K.I. and Pihlajaniemi,T. (2000a) Type XIII collagen forms homotrimers with three triple helical collagenous domains and its association into disulfide-bonded trimers is enhanced by prolyl 4-hydroxylase. J. Biol. Chem., 275, 8936–8944. [DOI] [PubMed] [Google Scholar]
- Snellman A., Tu,H., Väisänen,T., Kvist,A.P., Huhtala,P. and Pihlajaniemi,T. (2000b) A short sequence in the N-terminal region is required for the trimerization of type XIII collagen and is conserved in other collagenous transmembrane proteins. EMBO J., 19, 5051–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund M., Väisänen,T., Kaukinen,S., Ilves,M., Tu,H., Autio-Harmainen,H., Rauvala,H. and Pihlajaniemi,T. (2001) Distinct expression of type XIII collagen in neuronal structures and other tissues during mouse development. Matrix Biol., 20, 215–231. [DOI] [PubMed] [Google Scholar]
- Tikka L., Elomaa,O., Pihlajaniemi,T. and Tryggvason,K. (1991) Human α1(XIII) collagen gene. Multiple forms of the gene transcripts are generated through complex alternative splicing of several short exons. J. Biol. Chem., 266, 17713–17719. [PubMed] [Google Scholar]
- Vuorio E. and de Crombrugghe,B. (1990) The family of collagen genes. Annu. Rev. Biochem., 59, 837–872. [DOI] [PubMed] [Google Scholar]
- Xu W., Baribault,H. and Adamson,E.D. (1998) Vinculin knockout results in heart and brain defects during embryonic development. Development, 125, 327–337. [DOI] [PubMed] [Google Scholar]
- Yang J.T., Rayburn,H. and Hynes,R.O. (1995) Cell adhesion events mediated by α 4 integrins are essential in placental and cardiac development. Development, 121, 549–560. [DOI] [PubMed] [Google Scholar]