Structural analysis of adenylate cyclases from Trypanosoma brucei in their monomeric state
Boris Bieger and Lars-Oliver Essen
The EMBO Journal, 20, 433–445, 2001
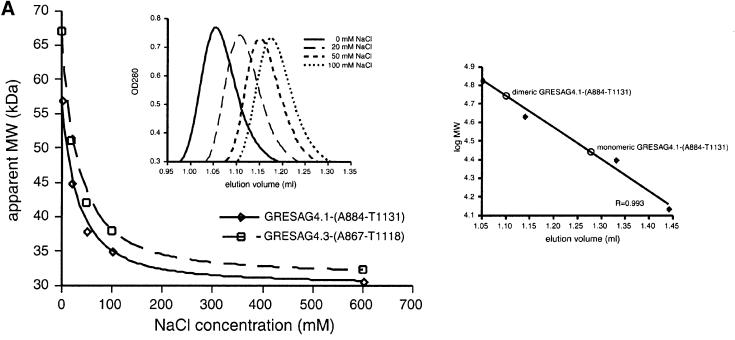
In Figure 2A, the proteolytic fragment GRESAG4.1-(A884-T1131) was used for the gel filtrations instead of GRESAG4.1-(A884-Y1241) and the labelling of the monomeric and dimeric forms was erraneously interchanged in the right inlay. The corrected Figure 2A is shown below.
Unlike indicated in the legend of Figure 2B and part of the Discussion, the titrations were performed in the presence of 0.33 µM (0.67 µM) GRESAG4.1-(A884- Y1241) instead of 2 nM (4 nM) when being titrated with the mutant D949A (D949A/R1053A). These titrations demonstrated that the mutant D949A (D949A/R1053A) caused a decrease in AC activity by GRESAG4.1-(A884-Y1241) to <40% (10%). We later found that the activity of GRESAG4.1-(A884-Y1241) can be similarly titrated down to ∼0% by the single-site mutant D949A if fresh preparations of 0.17 µM GRESAG4.1-(A884- Y1241) were co-incubated with 0–11 µM D949A mutant. According to our discussed model of a GRESAG4.1 homodimer, a functionally relevant pairing of the D949A mutant with the wild-type AC domain should leave the concentration of intact active sites constant, which is obviously not consistent with the observed loss of activity upon titration with the D949A mutant.
Further experiments also revealed that the increased gain of catalytic activity by complementary mixtures of GRESAG4.1 mutants, which was reported in Figure 2C, proved to be irreproducible. Overall, from this the authors conclude that a formation of catalytically active GRESAG4.1 homodimers in solution is not corroborated by the available biochemical data. Interestingly, homodimerization was recently found to be a prerequisite for the formation of catalytically active species for the mycobacterial AC Rv1625c (EMBO J., 20, 3667–3675, 2001).
Energetic contribution of non-essential 5′ sequence to catalysis in a hepatitis delta virus ribozyme
I.-hung Shih and Michael D.Been
The EMBO Journal, 20, 4884–4891, 2001
On page 4888, second column, third line from the bottom, the sentence should read: ‘In the case of the hammerhead ribozyme, which catalyzes a similar chemical reaction, the cleavage reaction is endothermic due to the penalty paid in product formation of the 2′,3′-cyclic phosphate, but the reaction is spontaneous due to a large gain in entropy upon formation of two cleavage products (Hertel et al., 1994).’