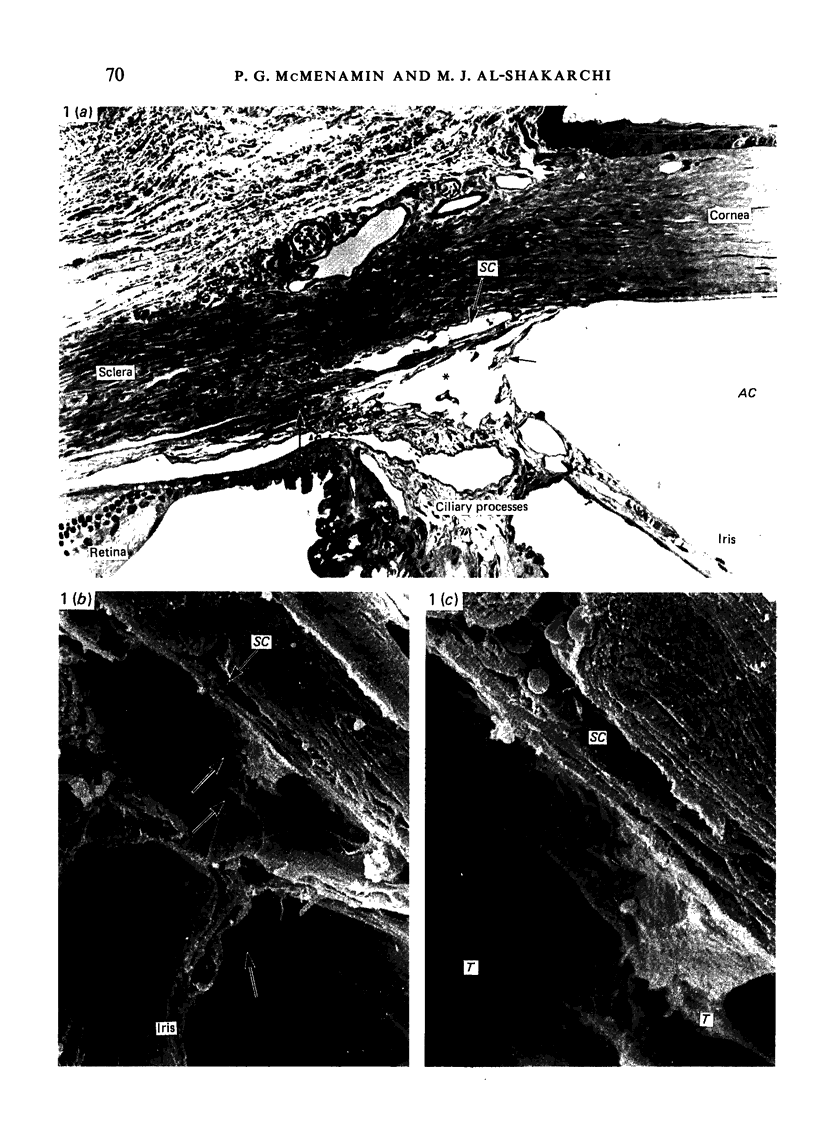
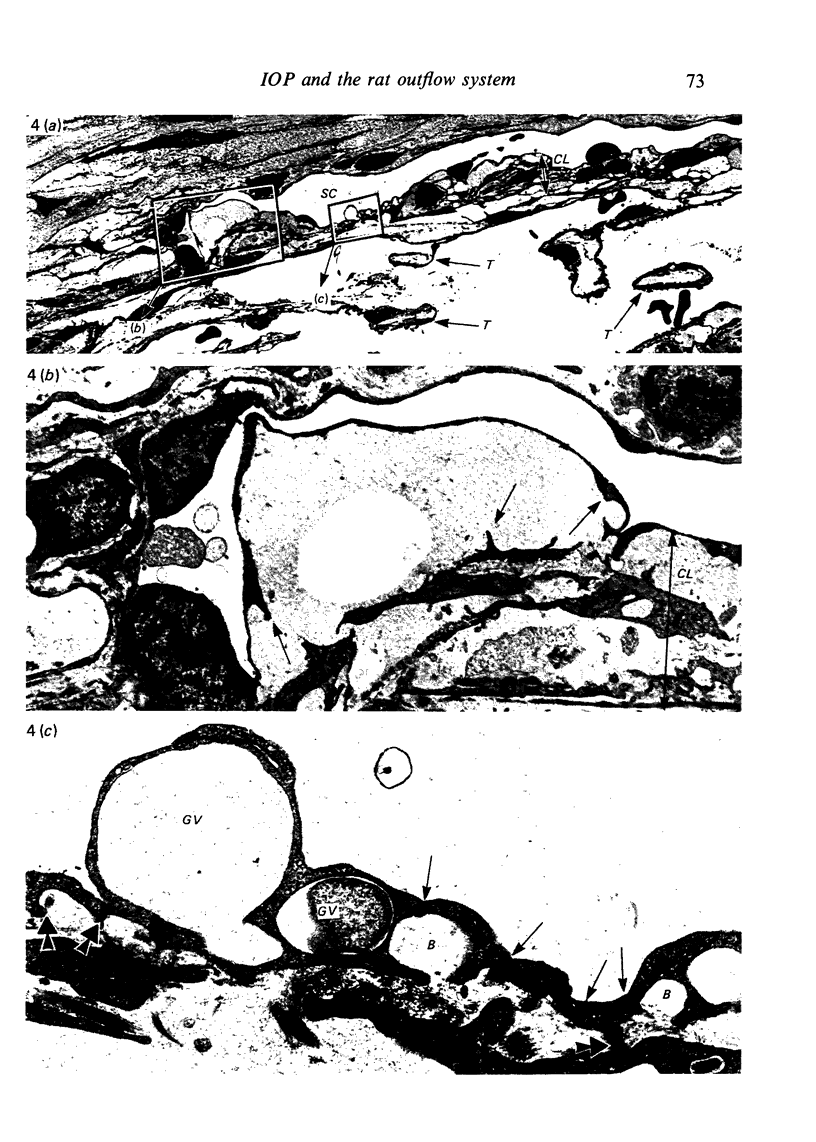
Abstract
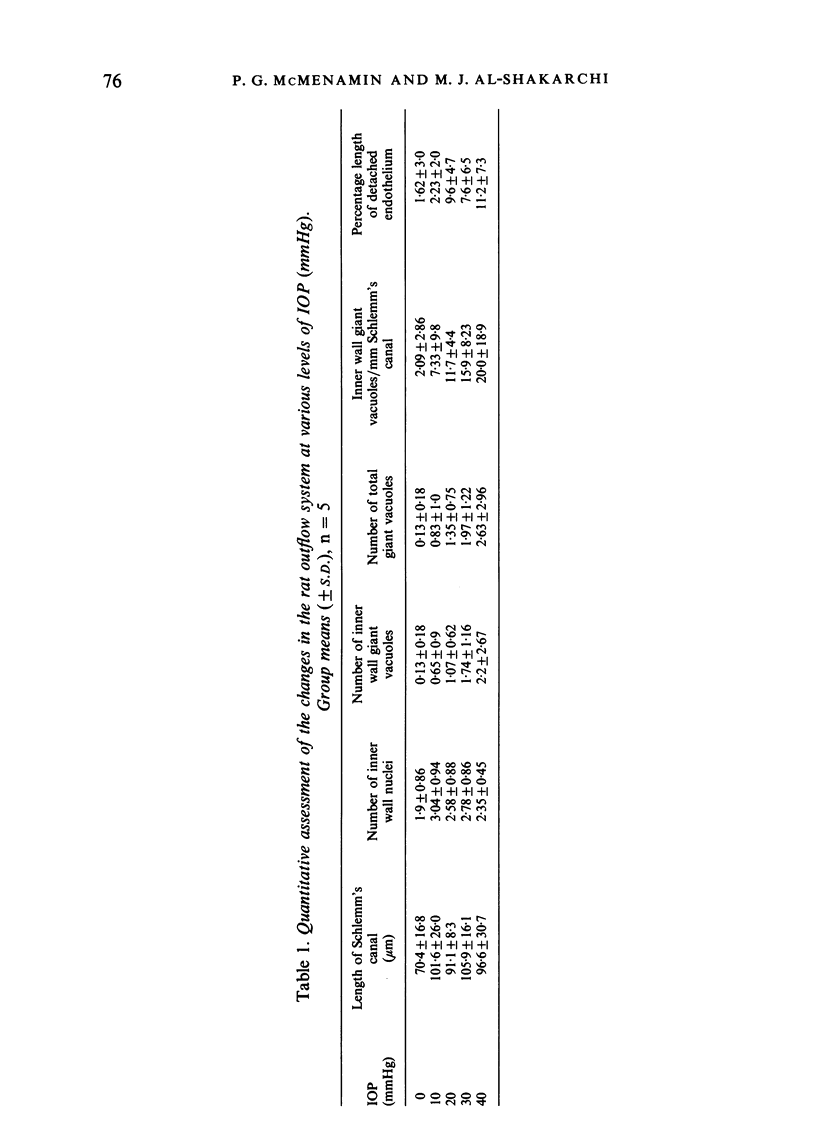
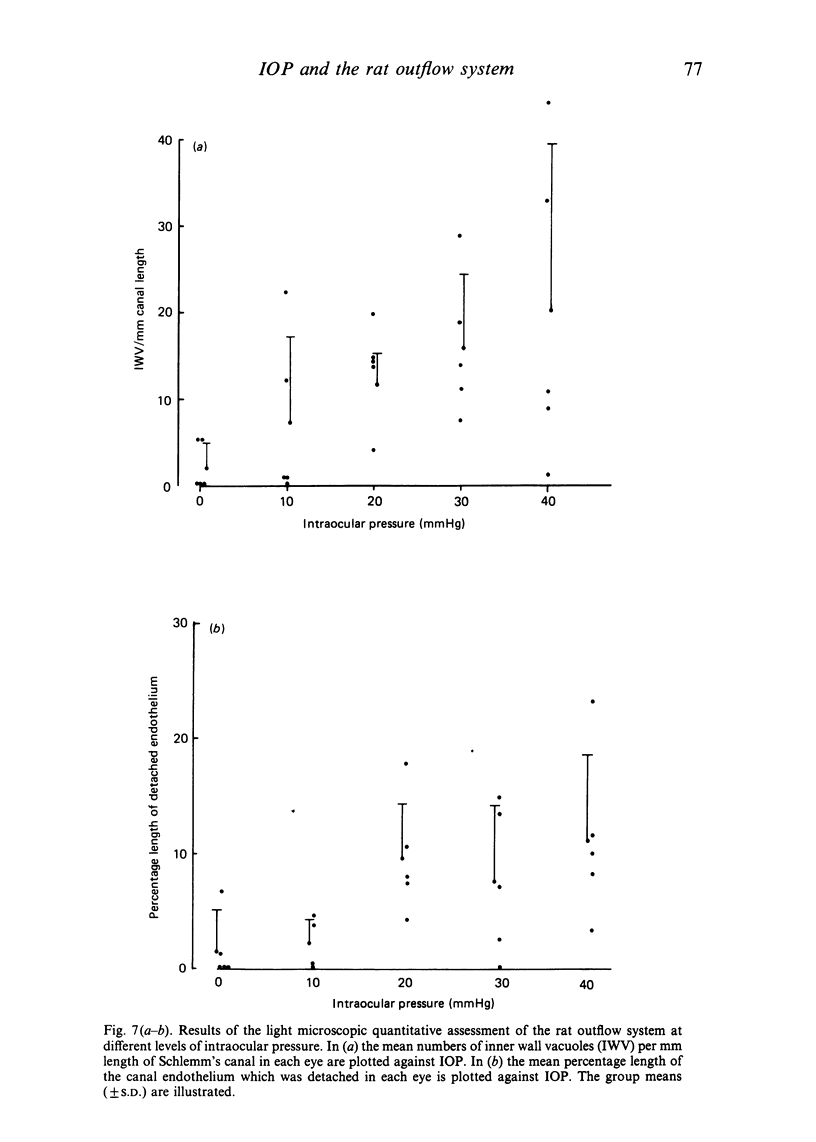
The eyes of 20 normal mature Swiss albino rats were fixed by intracameral perfusion with glutaraldehyde at various levels of intraocular pressure (10, 20, 30 and 40 mmHg). The anterior chamber was connected by a fine cannula to a reservoir of fixative for 10 minutes while the animal was maintained under anaesthesia for a further 20 minutes after death. Five animals were studied at each pressure. Fixation at 0 mmHg was achieved by rapid immersion of enucleated eyes from 5 animals whose eyes had been cannulated and open to atmospheric pressure for the first 10 minutes. The anterior segment tissues were studied by light microscopy and by scanning and transmission electron microscopy. The eyes from rats fixed by cardiac perfusion provided control tissue. Progressive increase in intraocular pressure produced varying degrees of structural alterations in the iridocorneal angle. These included widening of the ciliary cleft and enlargement of the spaces of Fontana; however, the pectinate ligaments remained intact even at the highest pressure. The trabecular tissues became more distended and there was a statistically significant relationship between the fixation pressure and the mean number of giant vacuoles in the inner wall of Schlemm's canal. The response between animals fixed at the same pressure was variable. This was most pronounced at 10 and 40 mmHg. The results indicate that the rat outflow system responds morphologically to various levels of experimentally induced intraocular pressure in a similar fashion to primates. These findings, together with the morphological similarities between the rat and primate aqueous humour outflow pathways, particularly the presence of a single canal of Schlemm, suggest that the rat may be a valuable model for future studies of the normal and abnormal mechanisms of aqueous drainage. The technical difficulties of experimental studies of the aqueous drainage mechanism in such a small eye are discussed.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J., Wang J., Epstein D. L. Metabolism of calf trabecular (reticular) meshwork. Invest Ophthalmol Vis Sci. 1980 Jan;19(1):13–20. [PubMed] [Google Scholar]
- Grierson I., Lee W. R. Changes in the monkey outflow apparatus at graded levels of intraocular pressure: a qualitative analysis by light microscopy and scanning electron microscopy. Exp Eye Res. 1974 Jul;19(1):21–33. doi: 10.1016/0014-4835(74)90068-2. [DOI] [PubMed] [Google Scholar]
- Grierson I., Lee W. R. Light microscopic quantitation of the endothelial vacuoles in Schlemm's canal. Am J Ophthalmol. 1977 Aug;84(2):234–246. doi: 10.1016/0002-9394(77)90857-1. [DOI] [PubMed] [Google Scholar]
- Grierson I., Lee W. R. Pressure effects on the distribution of extracellular materials in the rhesus monkey outflow apparatus. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1977 Sep 28;203(3-4):155–168. doi: 10.1007/BF00409822. [DOI] [PubMed] [Google Scholar]
- Grierson I., Lee W. R. Pressure-induced changes in the ultrastructure of the endothelium lining Schlemm's canal. Am J Ophthalmol. 1975 Nov;80(5):863–884. doi: 10.1016/0002-9394(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Grierson I., Lee W. R. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (1) Pressure effects within the near-physiological range (8-30 mmHg). Exp Eye Res. 1975 Jun;20(6):505–521. doi: 10.1016/0014-4835(75)90218-3. [DOI] [PubMed] [Google Scholar]
- Grierson I., Nagasubramanian S., Edwards J., Millar L. C., Ennis K. The effects of various levels of intraocular pressure on the rabbit's outflow system. Exp Eye Res. 1986 Apr;42(4):383–397. doi: 10.1016/0014-4835(86)90032-1. [DOI] [PubMed] [Google Scholar]
- Hernandez M. R., Weinstein B. I., Wenk E. J., Gordon G. G., Dunn M. W., Southren A. L. The effect of dexamethasone on the in vitro incorporation of precursors of extracellular matrix components in the outflow pathway region of the rabbit eye. Invest Ophthalmol Vis Sci. 1983 Jun;24(6):704–709. [PubMed] [Google Scholar]
- Johnstone M. A., Grant W. G. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973 Mar;75(3):365–383. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- Kayes J. Pressure gradient changes on the trabecular meshwork of monkeys. Am J Ophthalmol. 1975 Apr;79(4):549–556. doi: 10.1016/0002-9394(75)90791-6. [DOI] [PubMed] [Google Scholar]
- Levine J. E., Povlishock J. T., Becker D. P. The morphological correlates of primate cerebrospinal fluid absorption. Brain Res. 1982 Jun 3;241(1):31–41. doi: 10.1016/0006-8993(82)91225-2. [DOI] [PubMed] [Google Scholar]
- McMenamin P. G., Lee W. R. Effects of prolonged intracameral perfusion with mock aqueous humour on the morphology of the primate outflow apparatus. Exp Eye Res. 1986 Jul;43(1):129–141. doi: 10.1016/s0014-4835(86)80051-3. [DOI] [PubMed] [Google Scholar]
- Ohnishi Y., Taniguchi Y. Distributions of 35S-sulfate and 3H-glucosamine in the angular region of the hamster: light and electron microscopic autoradiography. Invest Ophthalmol Vis Sci. 1983 Jun;24(6):697–703. [PubMed] [Google Scholar]
- RUSKELL G. L. Aqueous drainage paths in the rabbit. A neoprene latex cast study. Arch Ophthalmol. 1961 Dec;66:861–870. doi: 10.1001/archopht.1961.00960010863013. [DOI] [PubMed] [Google Scholar]
- Remé C., Urner U., Aeberhard B. The development of the chamber angle in the rat eye. Morphological characteristics of developmental stages. Graefes Arch Clin Exp Ophthalmol. 1983;220(3):139–153. doi: 10.1007/BF02175946. [DOI] [PubMed] [Google Scholar]
- Remé C., Urner U., Aeberhard B. The occurrence of cell death during the remodelling of the chamber angle recess in the developing rat eye. Graefes Arch Clin Exp Ophthalmol. 1983;221(3):113–121. doi: 10.1007/BF02133849. [DOI] [PubMed] [Google Scholar]
- Remé C., d'Epinay S. L. Periods of development of the normal human chamber angle. Doc Ophthalmol. 1981 Jul 15;51(3):241–268. doi: 10.1007/BF00143888. [DOI] [PubMed] [Google Scholar]
- Richardson T. M., Marks M. S., Ausprunk D. H., Miller M. A morphologic and morphometric analysis of the aqueous outflow system of the developing cat eye. Exp Eye Res. 1985 Jul;41(1):31–51. doi: 10.1016/0014-4835(85)90092-2. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C. The functional morphology of the outflow systems of ocular and cerebrospinal fluids. Exp Eye Res. 1977;25 (Suppl):65–116. doi: 10.1016/s0014-4835(77)80010-9. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C., Tripathi B. J. The mechanism of aqueous outflow in lower mammals. Exp Eye Res. 1972 Jul;14(1):73–79. doi: 10.1016/0014-4835(72)90146-7. [DOI] [PubMed] [Google Scholar]
- Van Buskirk E. M. Anatomic correlates of changing aqueous outflow facility in excised human eyes. Invest Ophthalmol Vis Sci. 1982 May;22(5):625–632. [PubMed] [Google Scholar]
- Van Buskirk E. M. The canine eye: the vessels of aqueous drainage. Invest Ophthalmol Vis Sci. 1979 Mar;18(3):223–230. [PubMed] [Google Scholar]
- van der Zypen E. Experimental morphological study on structure and function of the filtration angel of the rat eye. Ophthalmologica. 1977;174(5):285–298. doi: 10.1159/000308617. [DOI] [PubMed] [Google Scholar]