Abstract
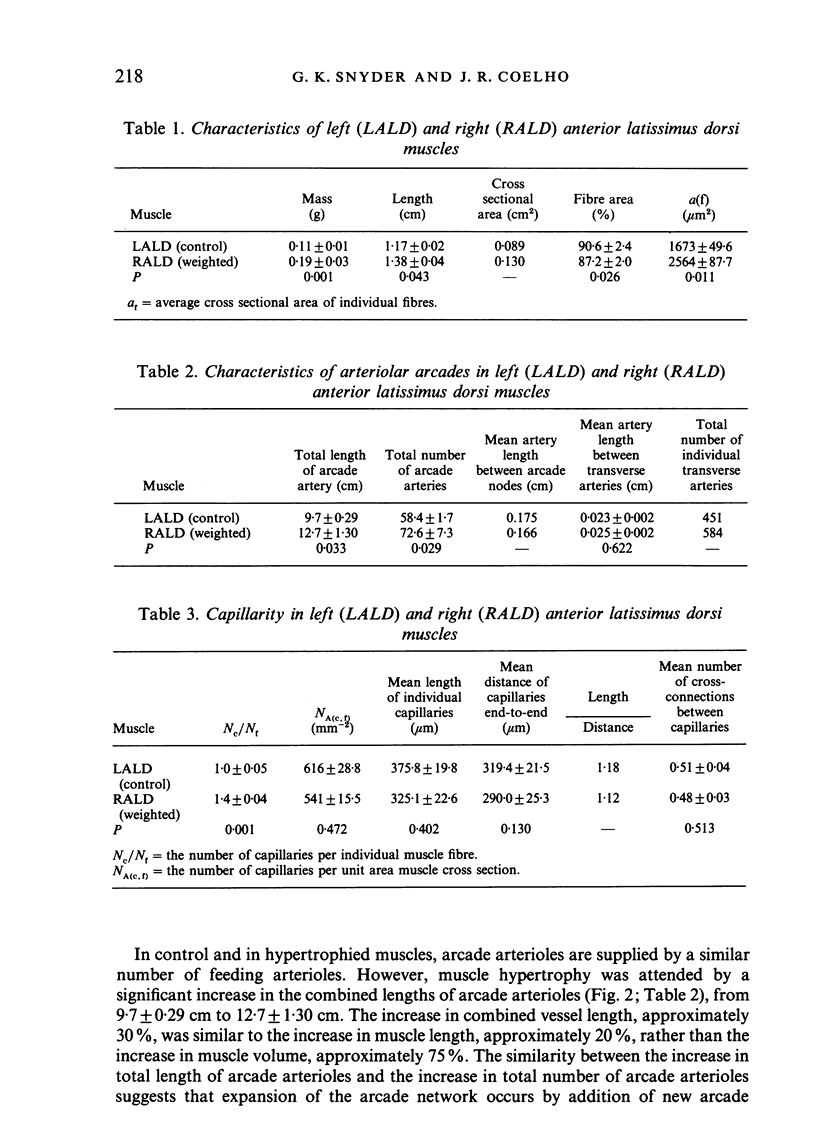
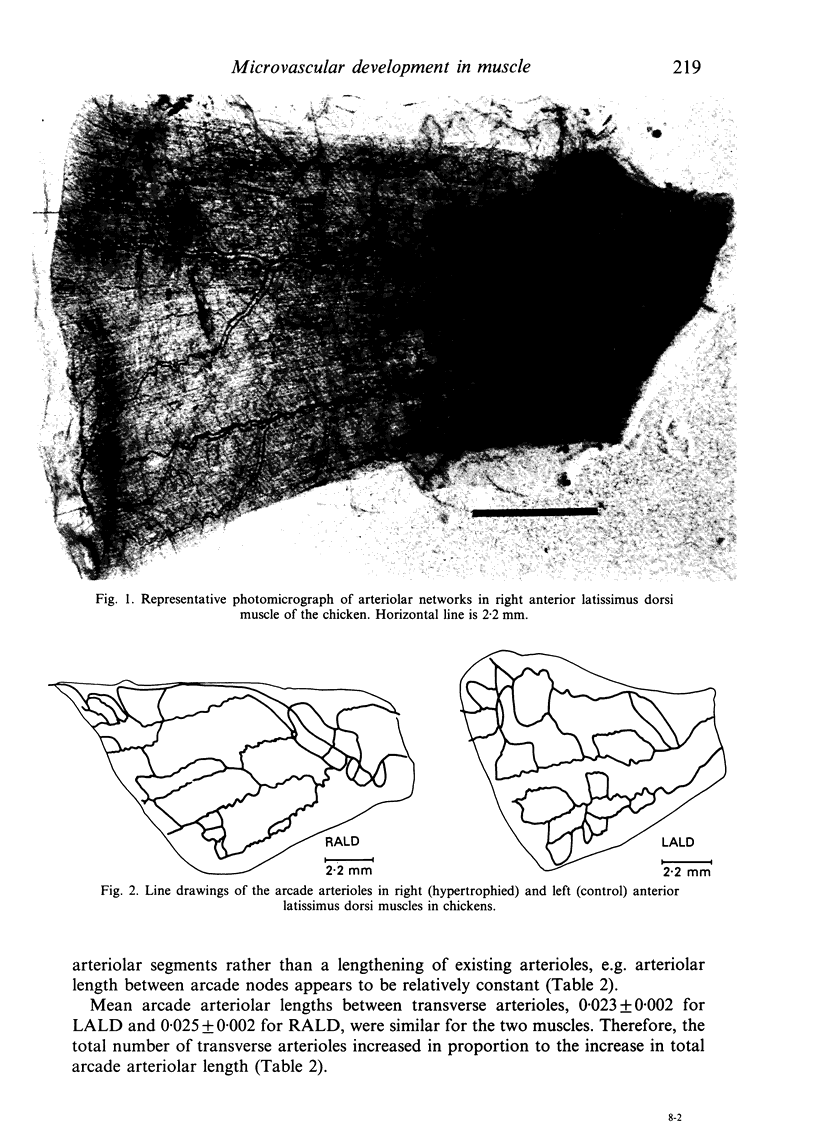
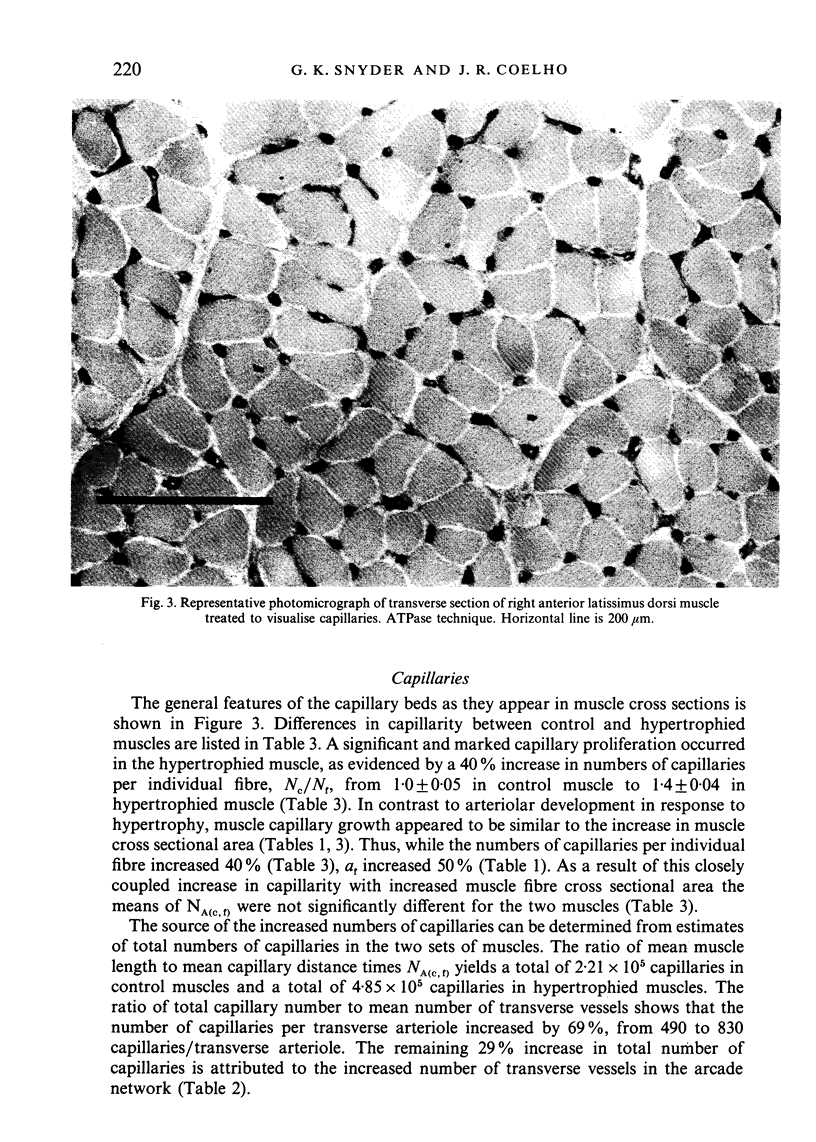
Right (hypertrophied) and left (control) anterior latissimus dorsi muscles (ALD) were injected with India ink to visualise microvascular geometry in these muscles. The ALD muscles have multiple feeding arterioles that branch into meshworks of arcade arterioles. Transverse arterioles arise from the arcade system and give rise to capillaries. Hypertrophy is attended by an increase in the combined length of arterioles in the arcade system due to an increase in the number of arcade segments. Morphometric characteristics of each segment remained constant. Hypertrophy produced a significant increase in the numbers of capillaries per individual muscle fibre due to an increase in number of transverse arterioles and an increase in number of capillaries per transverse arteriole. However, the increased numbers of capillaries per fibre just matched fibre hypertrophy, so that the number of exchange vessels per cross sectional area of muscle remained constant. These results are consistent with the argument that muscle growth is the primary determinant of capillarity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977 Sep;270(3):677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Kroese A. J. Capillary supply in soleus and gastrocnemius muscles of man. Pflugers Arch. 1978 Aug;375(3):245–249. doi: 10.1007/BF00582437. [DOI] [PubMed] [Google Scholar]
- Asmussen G., Kiessling A., Wohlrab F. Histochemische differenzierbare Sorten von Muskelfasern im M. latissimus dorsi des Huhnes. Experientia. 1969 Sep 15;25(9):959–961. doi: 10.1007/BF01898089. [DOI] [PubMed] [Google Scholar]
- Chen I. I., Prewitt R. L. A mathematical representation for vessel network. J Theor Biol. 1982 Sep 21;98(2):211–219. doi: 10.1016/0022-5193(82)90259-4. [DOI] [PubMed] [Google Scholar]
- Engelson E. T., Schmid-Schönbein G. W., Zweifach B. W. The microvasculature in skeletal muscle. II. Arteriolar network anatomy in normotensive and spontaneously hypertensive rats. Microvasc Res. 1986 May;31(3):356–374. doi: 10.1016/0026-2862(86)90024-5. [DOI] [PubMed] [Google Scholar]
- Engelson E. T., Skalak T. C., Schmid-Schönbein G. W. The microvasculature in skeletal muscle. I. Arteriolar network in rat spinotrapezius muscle. Microvasc Res. 1985 Jul;30(1):29–44. doi: 10.1016/0026-2862(85)90035-4. [DOI] [PubMed] [Google Scholar]
- Hermansen L., Wachtlova M. Capillary density of skeletal muscle in well-trained and untrained men. J Appl Physiol. 1971 Jun;30(6):860–863. doi: 10.1152/jappl.1971.30.6.860. [DOI] [PubMed] [Google Scholar]
- Holly R. G., Barnett J. G., Ashmore C. R., Taylor R. G., Molé P. A. Stretch-induced growth in chicken wing muscles: a new model of stretch hypertrophy. Am J Physiol. 1980 Jan;238(1):C62–C71. doi: 10.1152/ajpcell.1980.238.1.C62. [DOI] [PubMed] [Google Scholar]
- Hoppeler H., Mathieu O., Weibel E. R., Krauer R., Lindstedt S. L., Taylor C. R. Design of the mammalian respiratory system. VIII Capillaries in skeletal muscles. Respir Physiol. 1981 Apr;44(1):129–150. doi: 10.1016/0034-5687(81)90080-3. [DOI] [PubMed] [Google Scholar]
- Lipowsky H. H., Zweifach B. W. Network analysis of microcirculation of cat mesentery. Microvasc Res. 1974 Jan;7(1):73–83. doi: 10.1016/0026-2862(74)90038-7. [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O. Capillary tortuosity and degree of contraction or extension of skeletal muscles. Microvasc Res. 1987 Jan;33(1):98–117. doi: 10.1016/0026-2862(87)90010-0. [DOI] [PubMed] [Google Scholar]
- Mathieu O., Cruz-Orive L. M., Hoppeler H., Weibel E. R. Estimating length density and quantifying anisotropy in skeletal muscle capillaries. J Microsc. 1983 Aug;131(Pt 2):131–146. doi: 10.1111/j.1365-2818.1983.tb04240.x. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Plyley M. J., Groom A. C. Geometrical distribution of capillaries in mammalian striated muscle. Am J Physiol. 1975 May;228(5):1376–1383. doi: 10.1152/ajplegacy.1975.228.5.1376. [DOI] [PubMed] [Google Scholar]
- ROMANUL F. C. CAPILLARY SUPPLY AND METABOLISM OF MUSCLE FIBERS. Arch Neurol. 1965 May;12:497–509. doi: 10.1001/archneur.1965.00460290053007. [DOI] [PubMed] [Google Scholar]
- Schantz P., Henriksson J., Jansson E. Adaptation of human skeletal muscle to endurance training of long duration. Clin Physiol. 1983 Apr;3(2):141–151. doi: 10.1111/j.1475-097x.1983.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Sillau A. H., Banchero N. Effect of maturation on capillary density, fiber size and composition in rat skeletal muscle. Proc Soc Exp Biol Med. 1977 Mar;154(3):461–466. doi: 10.3181/00379727-154-39694. [DOI] [PubMed] [Google Scholar]
- Snyder G. K. Anatomical organisation of the microvasculature in the anterior and posterior latissimus dorsi muscles of the chicken. J Anat. 1988 Feb;156:97–106. [PMC free article] [PubMed] [Google Scholar]
- Snyder G. K., Wilcox E. E., Burnham E. W. Effects of hypoxia on muscle capillarity in rats. Respir Physiol. 1985 Oct;62(1):135–140. doi: 10.1016/0034-5687(85)90057-x. [DOI] [PubMed] [Google Scholar]
- ZWEIFACH B. W., METZ D. B. Selective distribution of blood through the terminal vascular bed of mesenteric structures and skeletal muscle. Angiology. 1955 Aug;6(4):282–290. doi: 10.1177/000331975500600402. [DOI] [PubMed] [Google Scholar]




