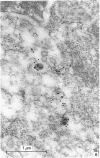
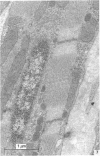
Abstract
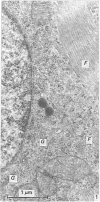
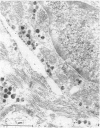
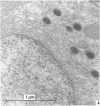
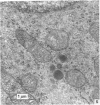
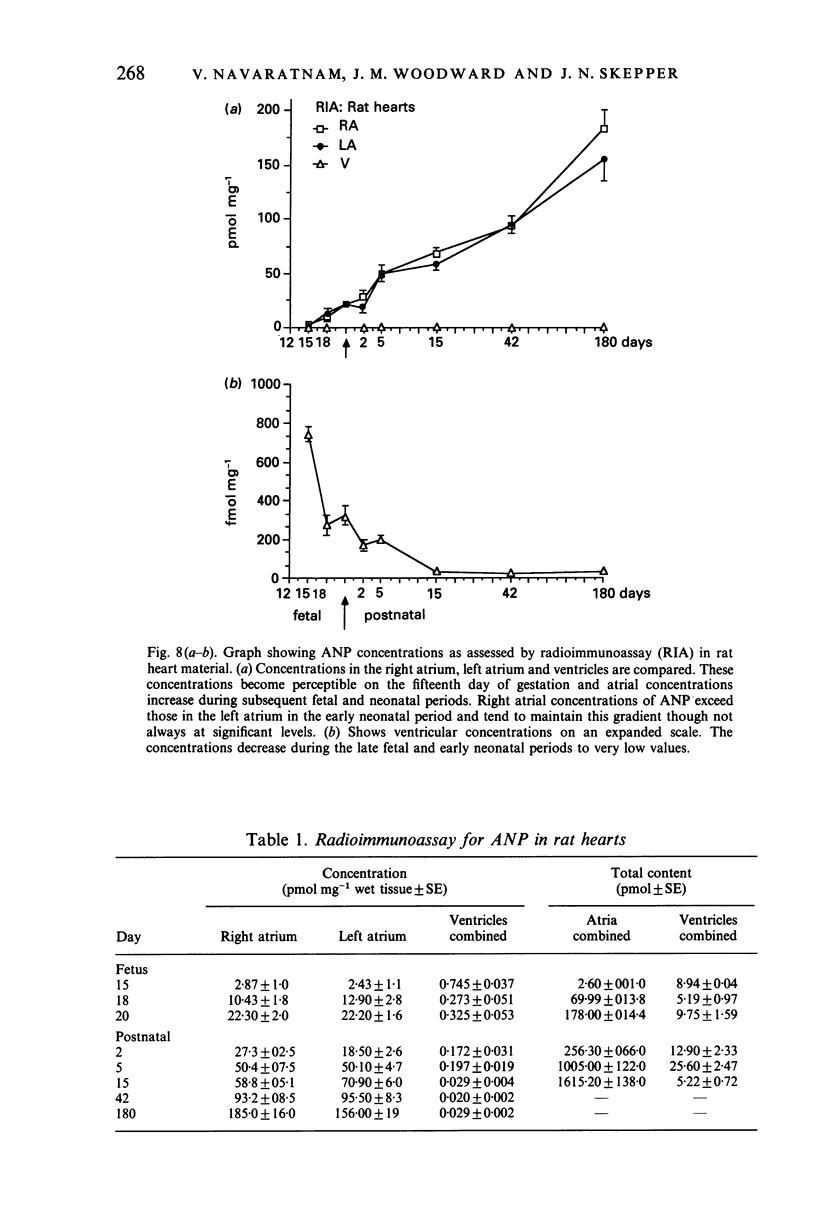
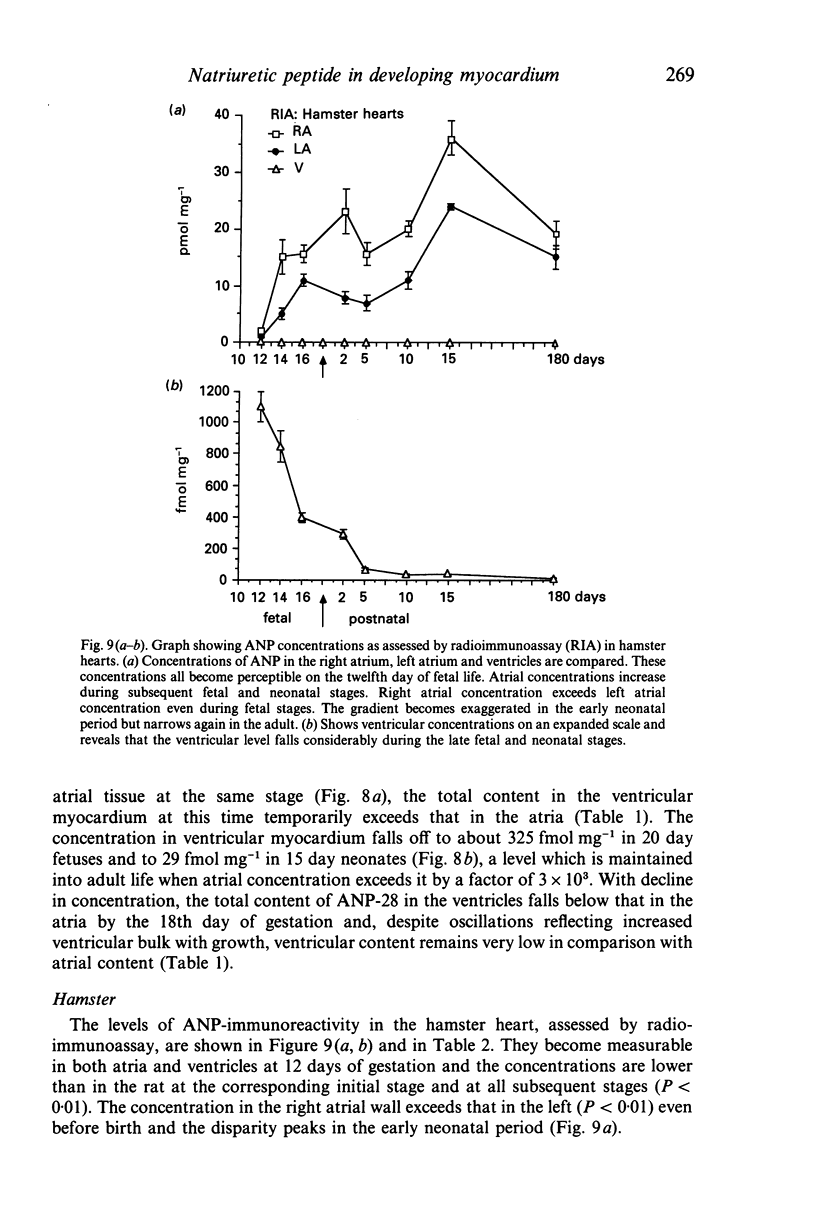
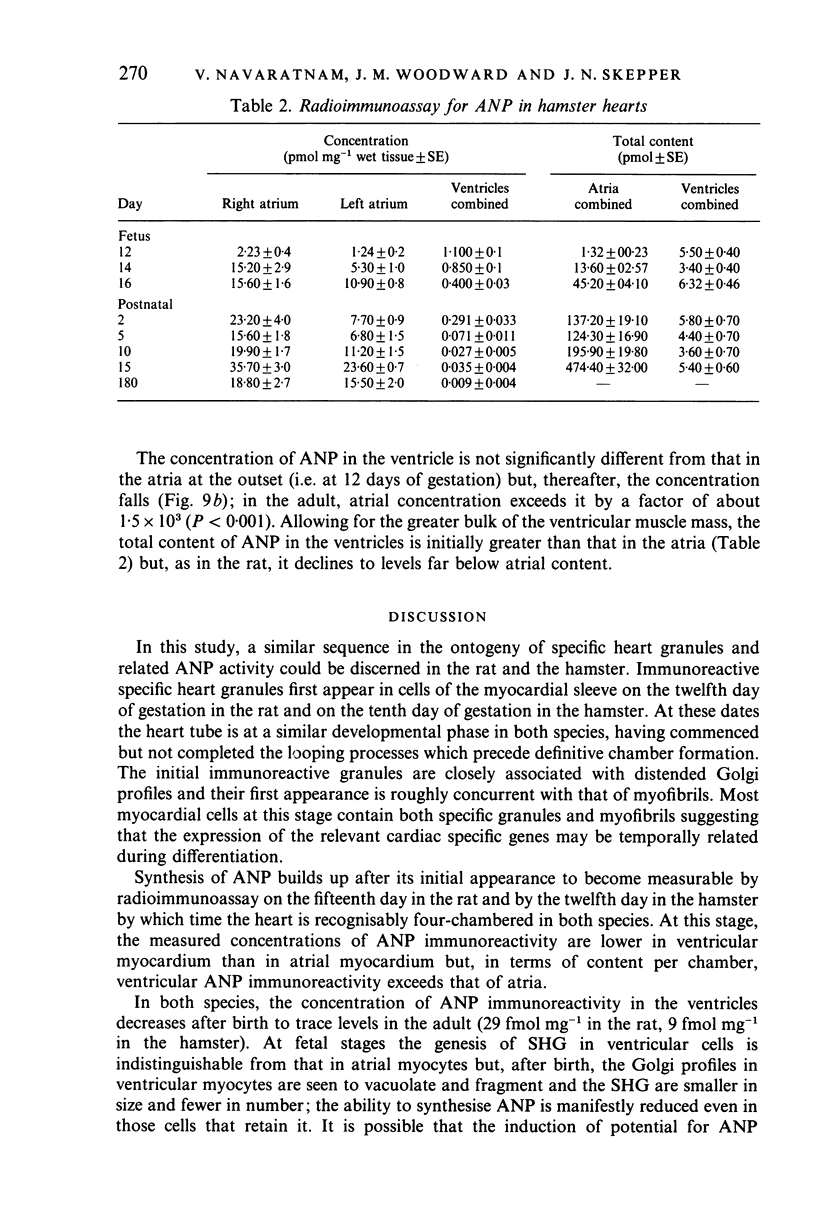
The ontogenesis of specific heart granules and of the related natriuretic peptide activity in heart muscle was studied in fetal and neonatal rats and golden hamsters by ultrastructural analysis including immunogold labelling for ANP-28 and by radioimmunoassay. In both species, immunoreactive granules first appear in the myocardial sleeve of the embryonic heart tube during the looping stages which precede chamber formation and the peptide becomes detectable by radioimmunoassay two or three days later by which time the chambers are identifiable. Granule density and ANP concentration in the rat are higher than in the hamster at all stages of development. Almost all atrial myocytes express ANP in fetal hearts whereas, in the ventricular wall, cells containing immunoreactive granules are scattered. The density of granules in atrial myocytes increases during further stages of fetal and neonatal development, while it decreases markedly even in those ventricular myocytes which are immunoreactive. Changes in the ultrastructural appearance of ventricular SHG suggest that the mode of production of ANP changes in ventricular myocytes after birth but does not change in atrial cells. There is no correlation between the distribution of immunoreactive ventricular myocytes and that of the conducting system. In both species, the concentration of ANP in the atrial well is higher than ventricular levels from the outset and the disparity becomes exaggerated with development till, in six months old adult animals, the atrial to ventricular concentration ratio is about 3 x 10(3):1 in the rat and 1.5 x 10(3): 1 in the hamster. In the hamster, a distinct gradient of ANP concentration between the right and left atria is already established in the early fetal period and it becomes enhanced in the neonatal period. In the rat, however, a slight difference becomes discernible only after birth.
Full text
PDF


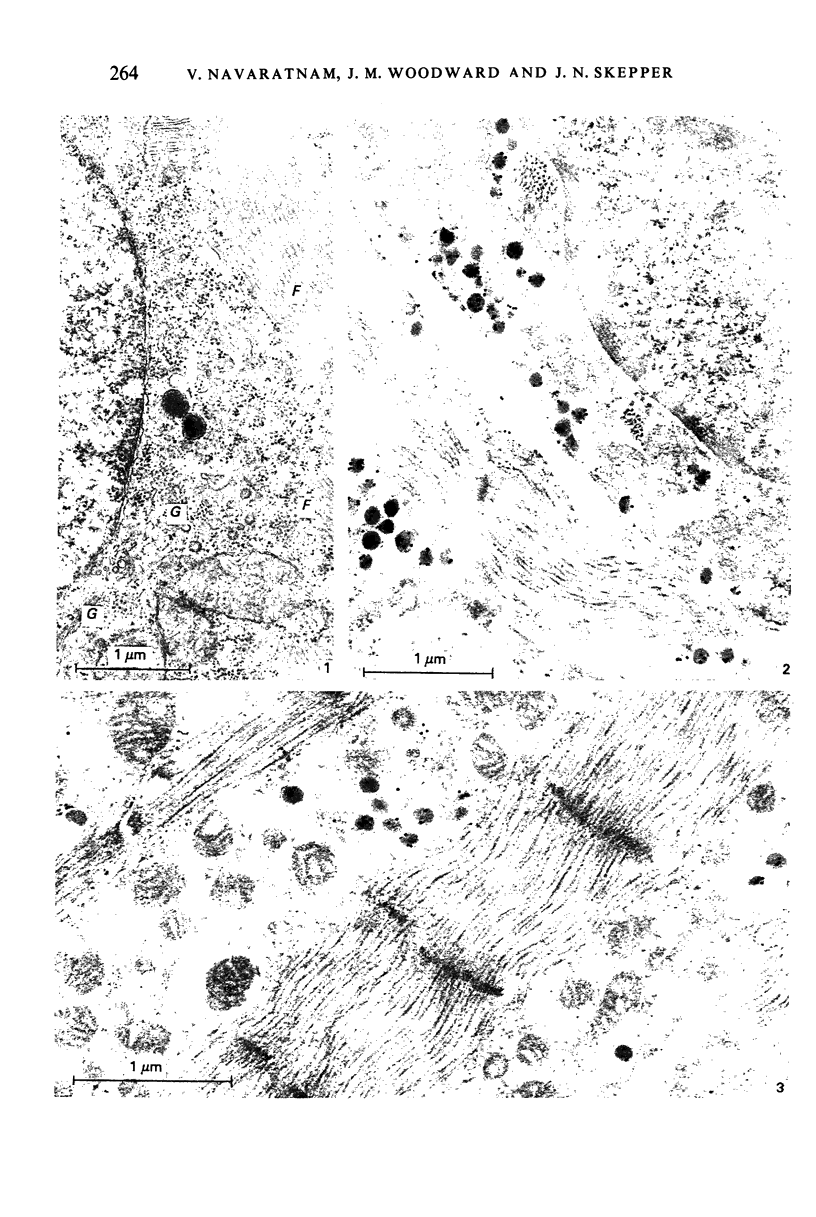

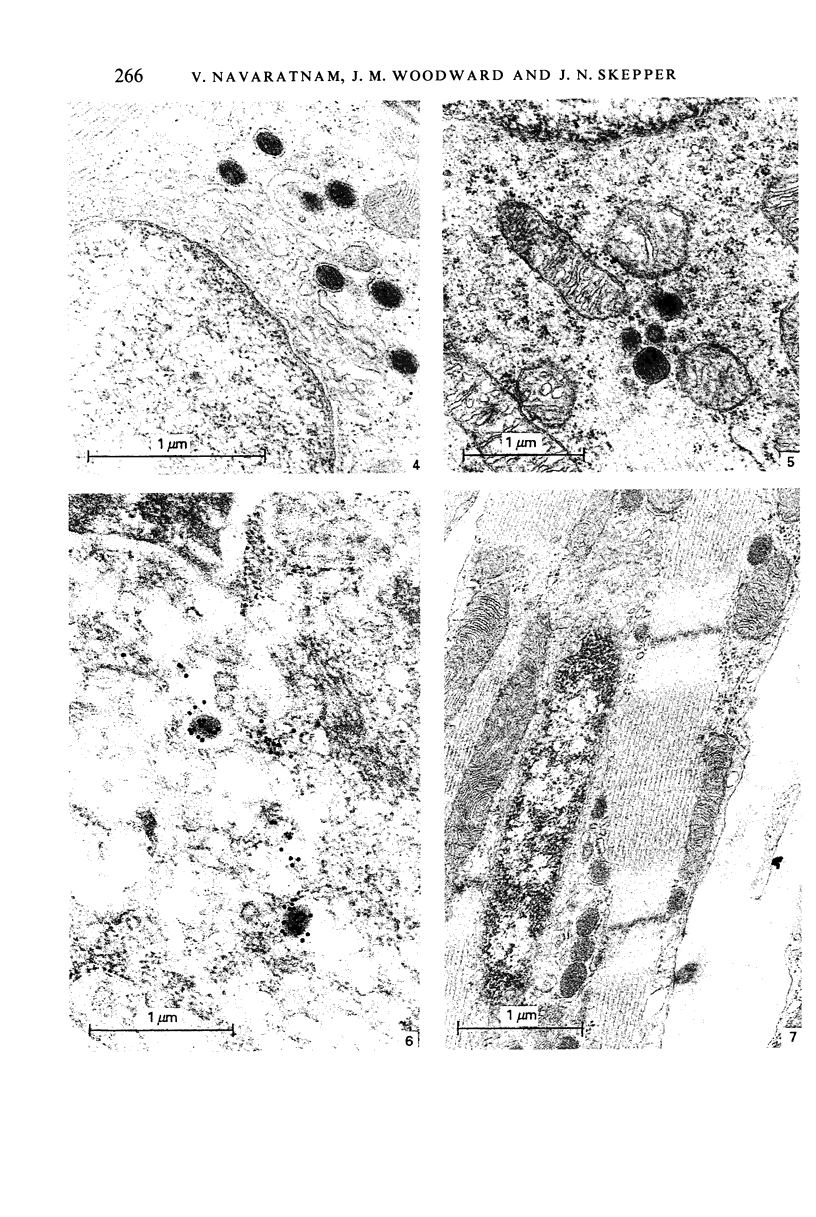




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bencosme S. A., Berger J. M. Specific granules in mammalian and non-mammalian vertebrate cardiocytes. Methods Achiev Exp Pathol. 1971;5:173–213. [PubMed] [Google Scholar]
- Cantin M., Genest J. The heart and the atrial natriuretic factor. Endocr Rev. 1985 Spring;6(2):107–127. doi: 10.1210/edrv-6-2-107. [DOI] [PubMed] [Google Scholar]
- Cantin M., Gutkowska J., Thibault G., Milne R. W., Ledoux S., MinLi S., Chapeau C., Garcia R., Hamet P., Genest J. Immunocytochemical localization of atrial natriuretic factor in the heart and salivary glands. Histochemistry. 1984;80(2):113–127. doi: 10.1007/BF00679984. [DOI] [PubMed] [Google Scholar]
- Currie M. G., Geller D. M., Cole B. R., Siegel N. R., Fok K. F., Adams S. P., Eubanks S. R., Galluppi G. R., Needleman P. Purification and sequence analysis of bioactive atrial peptides (atriopeptins). Science. 1984 Jan 6;223(4631):67–69. doi: 10.1126/science.6419347. [DOI] [PubMed] [Google Scholar]
- Danscher G., Rytter Nørgaard J. O. Ultrastructural autometallography: a method for silver amplification of catalytic metals. J Histochem Cytochem. 1985 Jul;33(7):706–710. doi: 10.1177/33.7.4008918. [DOI] [PubMed] [Google Scholar]
- Marie J. P., Guillemot H., Hatt P. Y. Le degré de granulation des cardiocytes auriculaires. Etude planimétrique au cours de différents apports d'eau et de sodium chez le rat. Pathol Biol (Paris) 1976 Oct;24(8):549–554. [PubMed] [Google Scholar]
- Scott J. N., Jennes L. Distribution of atrial natriuretic factor in fetal rat atria and ventricles. Cell Tissue Res. 1987 May;248(2):479–481. doi: 10.1007/BF00218216. [DOI] [PubMed] [Google Scholar]
- Skepper J. N., Navaratnam V. Analysis of the apparent heterogeneity of specific heart granules in rat atrial myocytes; an ultrastructural study including immunocytochemistry. Histochem J. 1988 Jan;20(1):1–10. doi: 10.1007/BF01745963. [DOI] [PubMed] [Google Scholar]
- Toshimori H., Toshimori K., Oura C., Matsuo H. Immunohistochemical study of atrial natriuretic polypeptides in the embryonic, fetal and neonatal rat heart. Cell Tissue Res. 1987 Jun;248(3):627–633. doi: 10.1007/BF00216493. [DOI] [PubMed] [Google Scholar]
- Toshimori H., Toshimori K., Oura C., Matsuo H. Immunohistochemistry and immunocytochemistry of atrial natriuretic polypeptide in porcine heart. Histochemistry. 1987;86(6):595–601. doi: 10.1007/BF00489553. [DOI] [PubMed] [Google Scholar]
- Yunge L., Ballak M., Beuzeron J., Lacasse J., Cantin M. Ultrastructural cytochemistry of atrial and ventricular cardiocytes of the bullfrog (Rana catesbeiana). Relationship of specific granules with reninlike activity of the myocardium. Can J Physiol Pharmacol. 1980 Dec;58(12):1463–1476. doi: 10.1139/y80-221. [DOI] [PubMed] [Google Scholar]