Abstract
The bacterial pathogen Yersinia pseudotuberculosis uses type III secretion machinery to translocate Yop effector proteins through host cell plasma membranes. A current model suggests that a type III translocation channel is inserted into the plasma membrane, and if Yops are not present to fill the channel, the channel will form a pore. We examined the possibility that Yops act within the host cell to prevent pore formation. Yop– mutants of Y.pseudotuberculosis were assayed for pore-forming activity in HeLa cells. A YopE– mutant exhibited high levels of pore-forming activity. The GTPase-downregulating function of YopE was required to prevent pore formation. YopE+ bacteria had increased pore-forming activity when HeLa cells expressed activated Rho GTPases. Pore formation by YopE– bacteria required actin polymerization. F-actin was concentrated at sites of contact between HeLa cells and YopE– bacteria. The data suggest that localized actin polymerization, triggered by the type III machinery, results in pore formation in cells infected with YopE– bacteria. Thus, translocated YopE inhibits actin polymerization to prevent membane damage to cells infected with wild-type bacteria.
Keywords: actin/pore/type III secretion/Yersinia/YopE
Introduction
A number of bacteria that are pathogenic for animals or plants use type III secretion systems to translocate virulence factors into host cells (Galán and Bliska, 1996; Hueck, 1998). Type III secretion systems produced by Salmonella typhimurium and Shigella flexnerii have been purified and visualized by electron microscopy (Blocker et al., 1999; Kubori et al., 2000; Tamano et al., 2000). These assemblages exhibit a basal body that spans the periplasm and a hollow projection that extends out from the bacterial surface like a needle. The basal body likely functions to allow protein transport from the bacterial cytoplasm across the inner and outer membranes. The ‘needle’ is thought to allow protein translocation into the host cell cytoplasm.
Members of the genus Yersinia that are pathogenic for humans (Yersinia pestis, Yersinia enterocolitica and Yersinia pseudotuberculosis) contain a plasmid-encoded type III secretion system that is required for virulence (Galán and Bliska, 1996; Cornelis et al., 1998). The type III system is activated when Yersinia binds to the surface of a host cell (Cornelis et al., 1998). In the enteropathogenic Yersinia spp. (Y.enterocolitica and Y.pseudotuberculosis) this tight contact can be achieved by a high affinity interaction between the adhesive protein invasin and β1 integrin receptors displayed on the eukaryotic host cell (Isberg and Leong, 1990; Isberg and Tran Van Nhieu, 1994). Upon activation of the secretion system, a set of at least six Yop effector proteins [YopE, H, J, M, O (YpkA) and T] are translocated into the cytoplasm of the cell. The Yops act to inhibit several key innate defense mechanisms, including phagocytosis, superoxide production and cytokine synthesis. By this method, Yersinia evade host defense mechanisms while replicating extracellularly in lymphoid tissues (Galán and Bliska, 1996; Cornelis et al., 1998).
YopE and YopT interfere with actin dynamics in host cells (Rosqvist et al., 1991; Iriarte and Cornelis, 1998). Following translocation of YopE or YopT, cultured cells lose actin filaments, round up, and eventually detach from the extracellular matrix (Bliska, 2000). In addition, YopE has been shown to have a potent antiphagocytic effect (Rosqvist et al., 1990, 1991). YopE is a GTPase-activating protein (GAP) for the Rho family of small GTP-binding proteins (RhoA, Rac1 and Cdc42) (Black and Bliska, 2000; Von Pawel-Rammingen et al., 2000). When bound to GTP, RhoA, Rac1 and Cdc42 stimulate different modes of actin polymerization (Van Aelst and D’Souza-Schorey, 1997; Hall, 1998). YopE switches RhoA, Rac1 and Cdc42 off by accelerating GTP hydrolysis. YopT appears to specifically inactivate RhoA, but by a mechanism distinct from that used by YopE. Evidence indicates that YopT covalently modifies RhoA, resulting in the displacement of RhoA from the host membrane to the cytoplasm (Zumbihl et al., 1999).
YopB, YopD and LcrV are thought to be central components of a translocation channel that is inserted in the host cell plasma membrane by the type III system (Rosqvist et al., 1994; Sory and Cornelis, 1994; Persson et al., 1995; Holmström et al., 2001). Several studies have demonstrated that YopB, YopD and LcrV are required for effector translocation into host cells (Persson et al., 1995; Håkansson et al., 1996; Sarker et al., 1998; Pettersson et al., 1999; Holmström et al., 2001). In addition, a pore-forming and lytic activity associated with expression of the type III system requires YopB, YopD and LcrV (Neyt and Cornelis, 1999; Tardy et al., 1999). Osmoprotection experiments have estimated the size of the pore generated by the Yersinia type III system to be 1.2–3.5 nm (Håkansson et al., 1996). Interestingly, mutants that are defective for multiple Yop effectors (multi-Yop– mutants) have higher pore-forming activity than wild-type Yersinia strains (Håkansson et al., 1996; Neyt and Cornelis, 1999). For example, Håkansson et al. (1996) found that HeLa cells exhibited membrane blebbing and an inability to exclude Trypan Blue after they were infected with a Y.pseudotuberculosis multi-Yop– mutant but not with the wild-type strain. In addition, lytic activity in red blood cells was reduced when YopE was overproduced in the multi-Yop– mutant (Håkansson et al., 1996). On the basis of these results it was proposed that Yop effectors prevent formation of a pore, and therefore cell lysis, by occupying the translocation channel (Håkansson et al., 1996). In this study, we investigated the mechanism by which Yop effectors prevent pore formation in host cells. We found that YopE plays a crucial role in preventing pore formation in HeLa cells by Y.pseudotuberculosis. A catalytically inactive form of YopE was unable to prevent pore formation, indicating that pore formation by YopE– mutant Yersinia involves activation of Rho GTPases and actin polymerization. Our results rule out the possibility that YopE prevents pore formation simply by filling translocation channels. A model is presented whereby translocated YopE downregulates Rho GTPases to prevent damage to the plasma membrane during Yersinia–host cell interactions.
Results
A multi-Yop– mutant of Y.pseudotuberculosis has a YopB-dependent pore-forming activity on HeLa cells
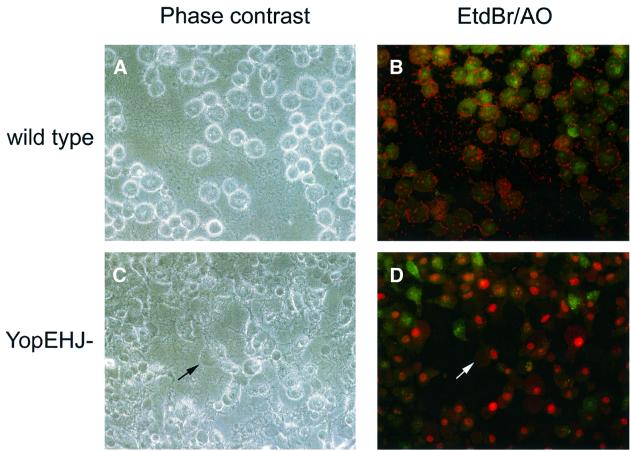
To investigate the role of Yop effectors in preventing pore formation, we infected HeLa cells at a multiplicity of infection of 100 with a wild-type strain of Y.pseudo tuberculosis (YP126) or an isogenic mutant (YP27) defective for expression of YopE, YopH and YopJ (YopEHJ– mutant). At different time points post infection we examined the morphology of the infected HeLa cells using phase contrast microscopy. HeLa cells infected with the YopEHJ– mutant exhibited dramatic membrane blebbing that could be detected within 1 h of infection (Figure 1C). In contrast, cells infected with the wild-type strain displayed the characteristic rounded morphology associated with YopE-mediated cytoskeletal disruption, but no membrane blebbing (Figure 1A). To assess membrane damage, infected cells were stained with ethidium bromide (EtdBr) and acridine orange (AO) and observed by fluorescence microscopy. AO penetrates cells with intact membranes and stains them green. EtdBr enters cells with perforated membranes and stains their nuclei red. The majority of cells infected with the YopEHJ– mutant displayed red nuclear staining, whereas cells infected with the wild-type strain stained only green (Figure 1D and B, respectively).
Fig. 1. Infection of HeLa cells with a multi-Yop– mutant of Y.pseudotuberculosis results in membrane blebbing and EtdBr uptake. HeLa cells grown on coverslips were infected with a wild-type strain (YP126) or a YopEHJ– mutant (YP27). After 3 h, coverslips were inverted onto a 5 µl drop of staining solution (25 µg/ml EtdBr and 5 µg/ml AO in PBS) placed on the surface of a glass slide. Infected cells were analyzed immediately by phase contrast (A and C) or epifluorescence (B and D) microscopy using a 40× objective. Images were recorded using a digital camera. The arrows in (C) and (D) point to a large bleb on a HeLa cell.
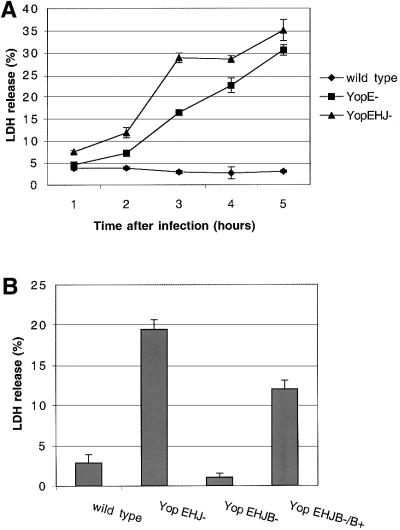
To quantify membrane damage, we assayed for release of the cytoplasmic enzyme lactate dehydrogenase (LDH) from HeLa cells infected with the wild-type strain or the YopEHJ– mutant. In a time course experiment, LDH release was detected within 1 h of infection with the YopEHJ– mutant (Figure 2A). LDH release increased linearly with time, reaching 30–40% of the total amount of LDH in uninfected cells after 5 h (Figure 2A). The amount of LDH released from HeLa cells infected with the YopEHJ– mutant after 3 h was ∼10-fold higher than that released from cells infected with the wild-type strain (Figure 2B). Similar amounts of LDH were released from HeLa cells infected with a YopEHJKOM– mutant (data not shown). As shown in Figure 2B, HeLa cells infected with a mutant (YP29) deficient in YopB (YopEHJB– mutant) did not release significant amounts of LDH. When a plasmid that encodes YopB was introduced into YP29 (YopEHJB–/YopB+), lytic activity was restored (Figure 2B).
Fig. 2. Release of LDH by HeLa cells infected with Y.pseudo tuberculosis. (A) Kinetics of LDH release after infection with a wild-type strain (YP126), a YopEHJ– mutant (YP27) or a YopE– mutant (YP6). Culture supernatants were collected from wells containing HeLa cells at indicated time points after infection. LDH in culture supernatants was measured using a CytoTox 96 assay kit (Promega). The percentage of LDH release was calculated by dividing the amount of LDH released from infected cells by the amount of LDH released from uninfected cells that were lysed by a freeze–thaw cycle. Error bars represent the standard deviation of the mean values obtained from triplicate samples. (B) YopB is required for LDH release. HeLa cells were infected with a wild-type strain (YP126), a YopEHJ– mutant (YP27), a YopEHJB– mutant (YP29) or a YopEHJB– mutant complemented with wild-type yopB+ (YP29/pYopB). Culture supernatants were collected 3 h post infection and assayed for LDH activity as described above.
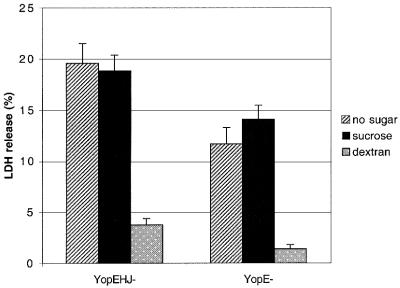
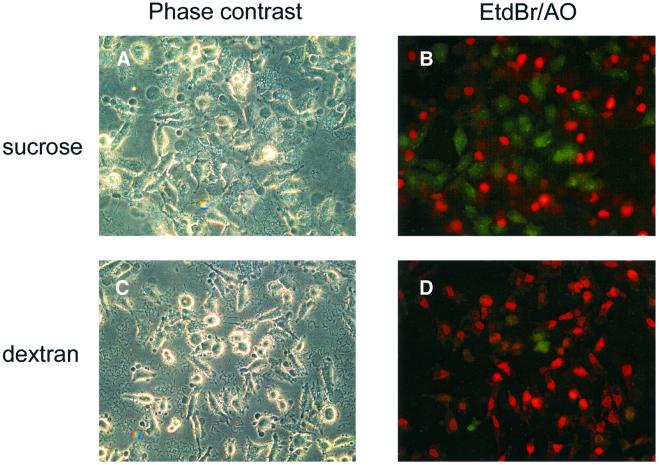
Osmoprotection experiments were performed to determine whether the membrane damage and cell lysis we observed was due to pore formation. Dextran 6000 (6000 Da), which is too large to diffuse efficiently through a small pore, should act as an osmoprotectant and prevent LDH release if membrane damage is due to pore formation (Kirby et al., 1998; Neyt and Cornelis, 1999; Holmström et al., 2001). However, Dextran 6000 should not interfere with the uptake of EtdBr, which is small enough (394.3 Da) to pass through pores. Release of LDH was diminished to background levels when HeLa cells were infected with the YopEHJ– mutant in the presence of 30 mM Dextran 6000 (Figure 3). In contrast, Dextran 6000 had no effect on EtdBr uptake (Figure 4D). Interestingly, Dextran 6000 did not prevent membrane blebbing, but did reduce the size of the blebs (Figure 4, compare A and C). As a control, we also carried out infections in the presence of 30 mM sucrose (342 Da). Addition of sucrose did not prevent LDH release (Figure 3) or EtdBr uptake (Figure 4B). These results confirm that multi-Yop– mutants of Y.pseudotuberculosis have a YopB-dependent pore-forming activity in HeLa cells, which results in osmotic lysis (Håkansson et al., 1996). LDH release was used in subsequent experiments as a quantitative assay of osmotic lysis resulting from pore formation.
Fig. 3. Effect of Dextran 6000 on release of LDH from HeLa cells infected with Y.pseudotuberculosis. HeLa cells were infected with a YopEHJ– mutant (YP27) or a YopE– mutant (YP6) in the presence or absence of 30 mM Dextran 6000 or 30 mM sucrose. LDH release 3 h post infection was determined as described in the legend to Figure 2.
Fig. 4. Effect of Dextran 6000 on membrane blebbing and uptake of EtdBr. HeLa cells were infected for 3 h with a YopEHJ– mutant (YP27) in the presence of 30 mM sucrose (A and B) or 30 mM Dextran 6000 (C and D). Images were obtained as described in the legend to Figure 1.
YopE plays a crucial role in preventing pore formation
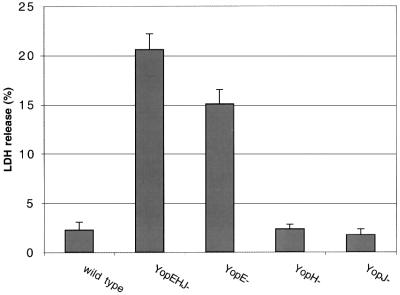
To determine whether pore-forming activity required inactivation of multiple effectors, we assayed for LDH release by HeLa cells infected with Y.pseudotuberculosis strains deficient for only one Yop (YopE–, YopH– or YopJ– mutants). As shown in Figure 5, HeLa cells infected for 3 h with a YopH– mutant or a YopJ– mutant did not release significant levels of LDH. However, high levels of LDH were detected in the culture supernatants of HeLa cells infected with the YopE– mutant (Figure 5). The kinetics of LDH release by HeLa cells infected with the YopE– mutant were comparable to those observed in cells infected with the YopEHJ– mutant (Figure 2A). Similarly, LDH released from HeLa cells infected with the YopE– mutant was prevented by addition of Dextran 6000 (Figure 3). Thus, YopE plays a critical role in preventing pore formation.
Fig. 5. YopE plays a critical role in preventing pore formation. HeLa cells were infected for 3 h with a wild-type strain (YP126), a YopEHJ– mutant (YP27), a YopE– mutant (YP6), a YopH– mutant (YP15), or a YopJ– mutant (YP26). LDH release was determined as described in the legend to Figure 2.
YopE GAP activity is required to inhibit pore formation
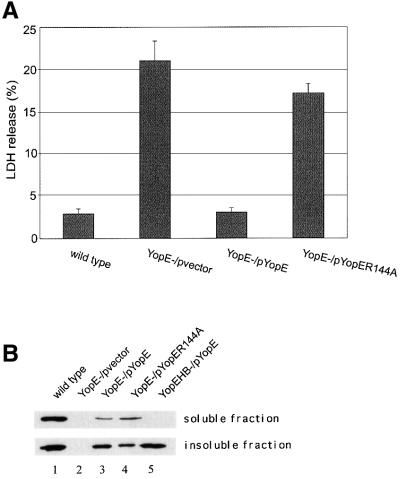
If YopE prevents pore formation by occupying translocation channels, it should not matter whether the protein is active or inactive. To examine this possibility, plasmids that encode either wild-type YopE (pYopE) or catalytically inactive YopE (pYopER144A) were introduced into the YopE– mutant (YP6). The resulting strains were tested for lytic activity in HeLa cells using the LDH release assay. YP6/pYopER144A and a control strain (YP6/pvector) stimulated similar levels of LDH release (Figure 6A). In contrast, YP6/pYopE had a low level of lytic activity that was comparable to the wild-type strain (Figure 6A).
Fig. 6. YopE GAP activity is required to inhibit pore formation. HeLa cells were infected with a wild-type strain (YP126), a YopE– mutant harboring an empty vector (YP6/vector), a YopE– mutant comple mented with yopE+ (YP6/pYopE), a YopE– mutant complemented with yopER144A (YP6/pYopER144A), or a YopEHB– mutant comple mented with yopE+ (YP19/pYopE). (A) LDH released from HeLa cells infected for 3 h was measured as described in the legend to Figure 2. (B) HeLa cells infected for 2 h were lysed in a buffer containing 1% Triton X-100. The lysates were separated by centrifugation into soluble and insoluble fractions. Samples of protein equivalent to 1 × 104 HeLa cells (soluble fraction) or 3 × 105 HeLa cells (insoluble fraction) were separated by SDS–PAGE and analyzed by immunoblotting with anti-YopE antibody.
To determine whether YopE and YopER144A were translocated into HeLa cells at equivalent levels, a Triton X-100 solubility assay was performed. HeLa cells infected as above for 2 h were lysed in 1% Triton X-100 and the lysates were centrifuged to separate translocated YopE (soluble fraction) from non-translocated YopE (insoluble fraction). Comparable amounts of YopE and YopER144A were detected in the soluble fractions by immunoblotting (Figure 6B, lanes 3 and 4). As a control, infections were also carried out with a strain deficient for YopB (YP19/pYopE). In this case, YopE was found only in the insoluble fraction (Figure 6B, lane 5). As YopE and YopER144A were translocated at similar levels, and only active YopE prevented pore formation, it is unlikely that YopE prevents pore formation by occupying translocation channels. Rather, it appears that YopE must enter the host cell and inactivate one or more Rho GTPases to inhibit pore formation.
YopT inhibits pore formation
As YopT has been shown to inhibit RhoA activity (Zumbihl et al., 1999), we examined whether YopT could prevent pore formation in HeLa cells infected with Y.pseudotuberculosis. YopT is not produced by our wild-type serogroup III strain YP126, but is produced by strains of other serogroups (our unpublished results). A segment of DNA encoding YopT and its chaperone SycT was isolated from a serogroup II strain and inserted into an expression vector (Materials and methods). The resulting plasmid (pYopT) was introduced into the YopE– mutant YP6. LDH released by HeLa cells infected with YP6/pYopE or YP6/pYopT was measured 3 h post infection. As shown in the first three columns of Figure 7A, similar amounts of LDH were released from cells infected with YP6/pYopE or YP6/pYopT, indicating that YopE and YopT have comparable anti-pore-forming activities.
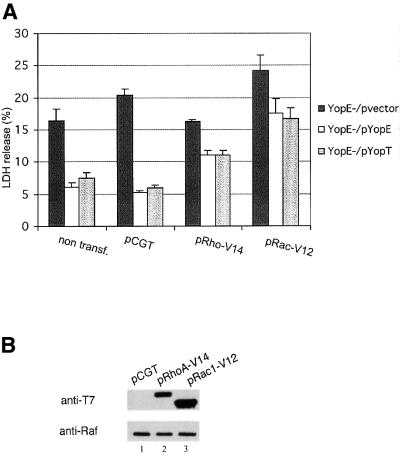
Fig. 7. Constitutively active forms of RhoA and Rac1 rescue pore-forming activity in the presence of YopE or YopT. HeLa cells were not transfected (non transf.) or were transfected with an empty vector (pCGT), pCGTRhoA-V14 (pRhoA-V14) or pCGTRac1-V12 (pRac1-V12). (A) LDH released from HeLa cells infected with YP6/vector, YP6/pYopE or YP6/pYopT for 3 h was determined as described in the legend to Figure 2. (B) Expression of RhoA-V14 and Rac1-V12 in HeLa cells was evaluated 24 h post transfection by immunoblotting. Transfected cells were lysed in detergent and samples of soluble protein equivalent to 1 × 104 HeLa cells were analyzed by immunoblotting with a monoclonal antibody that recognizes the T7 epitope tag appended to RhoA-V14 and Rac1-V12. Duplicate samples of protein were analyzed by immunoblotting with a monoclonal antibody specific for the Raf kinase, to control for loading.
Constitutively activated forms of RhoA or Rac1 rescue pore-forming activity in the presence of YopE or YopT
The above results encouraged us to ask whether Rho GTPases are directly involved in pore formation. Toward this end, constitutively activated forms of RhoA (RhoA-V14) or Rac1 (Rac1-V12) were produced in HeLa cells. HeLa cells were transfected with a control vector (pCGT) or vectors producing either RhoA-V14 or Rac1-V12. Twenty-four hours post transfection, HeLa cells were infected with YP6/pvector, YP6/pYopE or YP6/pYopT. LDH release was assayed 3 h post infection. In cells infected with YP6/pYopE or YP6/pYopT, release of LDH was increased 2-fold in the presence of RhoA-V14 and 3-fold in the presence Rac1-V12 (Figure 7A). In addition, the lytic activity of YP6/pvector increased slightly in cells containing Rac1-V12, but not in cells containing RhoA-V14 (Figure 7A). Non-infected HeLa cells producing RhoA-V14 or Rac1-V12 did not release more LDH than non-transfected cells (data not shown). Thus, production of either RhoA-V14 or Rac1-V12 in HeLa cells resulted in a partial rescue of pore-forming activity in the presence of YopE or YopT.
To determine the level of RhoA-V14 and Rac1-V12 expression in transfected cells, HeLa cells were lysed in detergent. Samples of the lysates were analyzed by immunoblotting using a monoclonal antibody that recognizes the T7 epitope fused to RhoA-V14 and Rac1-V12 (Figure 7B). The cell extracts were also analyzed by immunoblotting with a monoclonal antibody specific for the Raf kinase, to control for sample loading. As shown in Figure 7B both proteins were produced, although Rac1-V12 was produced at higher levels than RhoA-V14 (lanes 2 and 3). The higher expression level of Rac1-V12 may explain the increased rescue of pore-forming activity by Rac1-V12 as compared with RhoA-V14 (Figure 7A).
Inhibition of actin polymerization prevents pore formation and cell lysis
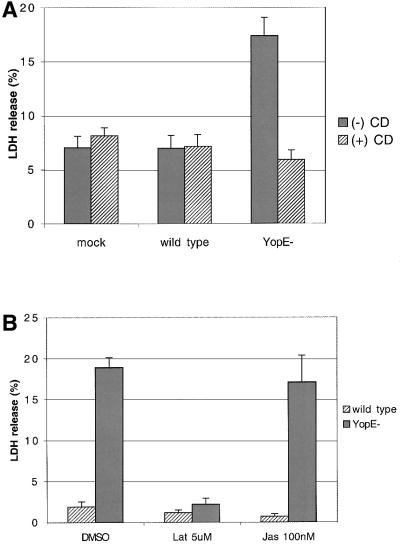
As RhoA and Rac1 are key regulators of actin polymerization, we investigated the role of actin polymerization in pore formation by Y.pseudotuberculosis. HeLa cells were exposed to the actin polymerization inhibitor cyto chalasin D (3.9 µM) or vehicle alone (DMSO), for 2 h prior to and during infection with the wild-type Y.pseudotuberculosis strain or the YopE– mutant. LDH release was assayed 3 h post infection. As shown in Figure 8A, release of LDH from cells infected with the YopE– mutant was significantly reduced in the presence of cytochalasin D. Cytocholasin D had no effect on LDH release in non-infected cells (mock) or in cells infected with the wild-type strain (Figure 8A). Analysis of infected cells by fluorescence microscopy after staining with EtdBr/AO showed that cytocholasin D prevented uptake of EtdBr by cells infected with the YopE– mutant (data not shown). From these results and from those described above we conclude that actin polymerization stimulated by Rho GTPases is required for pore formation by YopE– mutant Y.pseudotuberculosis. To examine further the role of actin polymerization in pore formation, we tested the effect of two other actin modifying agents, latrunculin B and jasplakinolide. The marine toxin latrunculin B forms a 1:1 complex with actin monomers and inhibits actin nucleation. In contrast, jasplakinolide enhances the rate of actin filament nucleation, inducing polymerization of monomeric actin in vivo. As predicted from the results with cytocholasin D, 5 µM latrunculin B had a strong inhibitory effect on LDH release by cells infected with the YopE– mutant (Figure 8B). Jasplakinolide (10 nM) had no significant effect on LDH release (Figure 8B). The lack of effect with jasplakinolide may indicate that actin polymerization must occur by a specific mechanism or in a specific location to trigger pore formation.
Fig. 8. Actin polymerization inhibitors prevent pore formation in HeLa cells infected with YopE– mutant Y.pseudotuberculosis. HeLa cells were exposed to the different actin modifying agents dissolved in DMSO or to DMSO only for 2 h prior to and during infection. Cells were either left uninfected (mock) or infected with a wild-type strain (YP126) or a YopE– mutant (YP6) for 3 h. Culture supernatants were assayed for LDH as described in the legend to Figure 2. (A) Cells were treated with 3.9 µM cytochalasin (+CD) or DMSO only (–CD). (B) Cells were treated with 5 µM latrunculin B (Lat), 100 nM Jasplakinolide (Jas) or DMSO only.
Bacterial internalization is not required for pore formation
We next considered the possibility that invasin-dependent bacterial entry into HeLa cells, which is dependent upon Rho GTPases and actin polymerization, was required for pore formation. Inhibitors of phosphatidylinositol (PI) 3-kinase antagonize uptake of Y.pseudotuberculosis by HeLa cells (Mecsas et al., 1998). PI 3-kinase inhibitors, such as wortmannin, impair closure of the phagocytic cup, but not actin polymerization per se (Araki et al., 1996; Cox et al., 1999). HeLa cells were treated with 100 nM wortmannin or vehicle alone (DMSO), for 2 h prior to and during infection with wild-type Y.pseudotuberculosis or the YopE– mutant. The effect of the inhibitor on bacterial uptake or LDH release was determined 1 or 3 h post infection, respectively. As determined by a gentamicin protection assay, wortmannin reduced entry of the YopE– mutant into HeLa cells by ∼2.5-fold (Figure 9A). However, wortmannin did not diminish LDH release from HeLa cells infected with the YopE– mutant (Figure 9B). Thus, bacterial uptake into HeLa cells did not appear to be required for pore formation.
Fig. 9. Wortmannin inhibits bacterial internalization but not LDH release. HeLa cells were exposed to 100 nM wortmannin in DMSO (+Wort.) or to DMSO alone (–Wort.) for 2 h prior to and during infections. Cells were infected with a wild-type strain (YP126) or a YopE– mutant (YP6). (A) Bacterial internalization (%) at 1 h post infection was measured by a gentamicin protection assay as described in Materials and methods. (B) LDH release at 3 h post infection was assayed as described in the legend to Figure 2.
Detection of F-actin at sites of contact between multi-Yop– mutants and HeLa cells
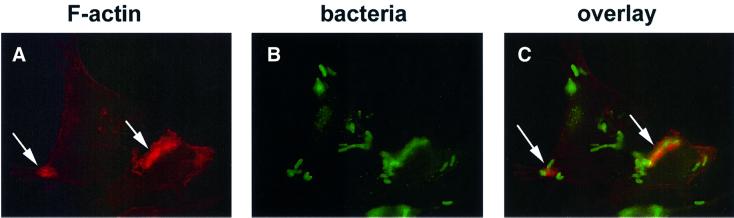
As actin polymerization appeared to stimulate pore formation, we looked for evidence of actin polymerization at sites of contact between HeLa cells and multi-Yop– mutants of Y.pseudotuberculosis. HeLa cells were infected with either the YopEHJ– mutant or the YopEHJB– mutant. At 30, 60, 90 and 120 min post infection, the cells were fixed and stained with rhodamine-phalloidin to label F-actin. The samples were then observed by epifluorescence microscopy. Intensely staining F-actin structures surrounding what appeared to be cell-associated bacteria were detected at all time points in cells infected with the YopEHJ– mutant, but not in cells infected with the YopEHJB– mutant (Figure 10, arrows, and data not shown). These ‘halos’ of F-actin were also observed in HeLa cells infected with the YopE– mutant, but not in cells infected with the wild-type strain (data not shown). Treatment with wortmannin did not prevent formation of the halos (Figure 11A, arrows). Therefore, Rho GTPase activity, but not PI 3-kinase activity, was required for assembly of F-actin halos. Treatment with cytocholasin D prevented formation of the F-actin halos in HeLa cells infected with YopE– mutant bacteria (data not shown). The infected HeLa cells were also stained with rabbit anti-Yersinia antibody and FITC-conjugated anti-rabbit IgG antibody to label bacteria (Figure 11B). As shown in Figure 11C, F-actin halos co-localized with a subset of cell-associated bacteria. These results indicate that YopE– mutant strains of Y.pseudotuberculosis stimulate actin polymerization at sites of bacterial–host cell contact.
Fig. 10. Formation of F-actin halos in HeLa cells infected with YopEHJ– mutant Y.pseudotuberculosis. Hela cells grown on coverslips were infected with a YopEHJ– mutant (YP27) (A and C) or a YopEHJB– mutant (YP29) (B and D). At 60 (A and B) or 90 (C and D) min post infection, the cells were fixed, permeabilized, and stained with rhodamine–phalloidin to label F-actin. F-actin was detected by epifluorescence microscopy using a 100× objective. Images were captured with a digital camera. Arrows in (A) and (C) point to F-actin halos. ‘Clumps’ of concentrated F-actin observed in HeLa cells infected with the YopEHJB– mutant are morphologically distinct from the F-actin halos observed in cells infected with the YopEHJ– mutant.
Fig. 11. Bacteria co-localized with F-actin halos. HeLa cells grown on coverslips were exposed to 100 nM wortmannin in DMSO for 2 h prior to and during infection. The HeLa cells were infected with a YopEHJ– mutant (YP27) for 60 min. The infected cells were fixed, permeabilized, and bacteria were labeled by sequential incubation with rabbit anti-Yersinia primary antibody and FITC-conjugated goat anti-rabbit IgG secondary antibody. F-actin was labeled with rhodamine–phalloidin. Cells were observed by epifluorescence microscopy using a 100× objective and filters to detect rhodamine (A) or FITC (B) fluorescence. Images were captured with a digital camera. A superimposition of (A) and (B) is presented in (C). Arrows point to F-actin halos in (A) and (C).
Discussion
Several lines of evidence suggest that Yop effectors are translocated into host cells through a channel introduced into the eukaryotic cell plasma membrane (Håkansson et al., 1996; Neyt and Cornelis, 1999; Tardy et al., 1999; Holmström et al., 2001). If such a channel is formed, it must be carefully gated because wild-type strains of Yersinia exhibit very low levels of pore-forming activity. Based on the observation that multi-Yop– mutants display high levels of pore-forming activity, it was suggested that Yop effectors prevent pore formation by filling translocation channels (Håkansson et al., 1996). In this work we examined the mechanism by which Yop effectors prevent pore formation.
Using osmoprotectants and assays that measure membrane damage (EtdBr/AO uptake) or cell lysis (LDH release) we confirmed that a multi-Yop– mutant of Y.pseudotuberculosis has a YopB-dependent pore-forming activity that leads to osmotic lysis of HeLa cells (Håkansson et al., 1996). Previously it was thought that pore-forming activity in Yersinia required the inactivation of multiple effectors (Håkansson et al., 1996; Neyt and Cornelis, 1999). However, Håkansson et al. (1996) showed that overproduction of YopE alone in a multi-Yop– mutant reduced lytic activity by 50%, suggesting that YopE plays a crucial role in preventing pore formation, possibly due to its ability to occupy translocation channels. We found that the lytic activity of a YopE– mutant was comparable to that of a YopEHJ– mutant or a YopEHJKMO– mutant, and that the GAP activity of YopE was required to prevent pore formation. In addition, the pore-forming activity of a YopE– mutant was reduced by ectopic expression of YopT. These data argue that the pore-forming activity of multi-Yop– mutants results from their inability to downregulate Rho-related GTPases.
Which Rho-related GTPases are involved in this pore-forming activity? YopE displays GAP activity towards RhoA, Rac1 and Cdc42 (Black and Bliska, 2000; Von Pawel-Rammingen et al., 2000), while YopT appears to specifically inactivate RhoA (Zumbihl et al., 1999). Therefore, it seems likely that RhoA is a key factor. However, we found that overproduction of Rac1-V12 in HeLa cells partially rescued pore-forming activity in the presence of YopT or YopE. These data suggest that activation of either Rac1 or RhoA can stimulate pore-forming activity. Determination of which GTPases are activated in HeLa cells infected with the YopE– mutant may help to clarify this issue.
As Rho family GTPases are responsible for stimulating actin polymerization, we examined the possibility that actin polymerization was involved in pore formation. Pore-formation was significantly reduced when HeLa cells were treated with the actin polymerization inhibitors cytocholasin D or latrunculin B. Actin polymerization inhibitors do not interfere with Yop translocation (Sory and Cornelis, 1994) and therefore should not reduce channel formation. However, inhibitors of actin polymerization do block invasin-mediated uptake of Yersinia by host cells, raising the possibility that pore formation requires bacterial entry. To address this issue, we used the PI 3-kinase inhibitor wortmannin, which antagonizes invasin-mediated entry of Y.pseudotuberculosis into HeLa cells, but does not block actin polymerization. Wortmannin decreased bacterial entry 2.5-fold, but had no effect on pore formation, indicating that bacterial entry is not required for pore formation. One problem with this interpretation is that wortmannin did not block entry completely. Thus, an extremely low level of bacterial internalization may be sufficient to trigger pore formation.
The most surprising finding in this work is that YopE– mutant strains stimulate formation of F-actin structures, which we term ‘halos’, at sites of contact with HeLa cells. Why the F-actin assembly is associated with only a subset of the cell-attached bacteria remains unclear. One possibility is that it is a transient process, and that at any given time only a subset of the cell-associated bacteria are stimulating actin polymerization.
Assembly of F-actin halos was dependent upon YopB, suggesting that channel formation is required for this process. YopE prevented formation of F-actin halos, indicating that Rho GTPases are required as well. We believe that the process leading to formation of F-actin at sites of bacterial attachment is somehow associated with, or responsible for, pore formation. One possibility is that local F-actin assembly drives uncontrolled membrane movement in the vicinity of the attached bacterium. If the bacterium assembles some type of needle that forms a stable conduit between the bacterial surface and the translocation channel, uncontrolled membrane movement could lead to breakage of the conduit. This could lead to pore formation if the translocation channel remains inserted in the membrane in the absence of an intact needle. In support of this concept, it has recently been shown that the type III sytem of Y.enterocolitica assembles needle structures during bacterial–host cell interaction (Hoiczyk and Blobel, 2001).
One important question raised by these findings is the nature of the stimulus responsible for triggering F-actin assembly at sites of host cell contact with YopE– mutant bacteria. As the process is dependent upon YopB, one possibility is that a bacterial protein that moves through the translocation channel is responsible for stimulating Rho GTPases and F-actin assembly. None of the known effectors, including YopO (YpkA) or YopM, appears to be involved in this process. An unidentified effector protein could function to activate Rho GTPases. Alternatively, one of the proteins involved in channel formation could perform this function. There is evidence that YopD and LcrV gain access to the host cell cytosol (Francis and Wolf-Watz, 1998; Fields and Straley, 1999). The Shigella IpaC protein, which is similar in overall structure to YopD, has been shown to induce actin polymerization through activation of Cdc42 and Rac1 (Tran Van Nhieu et al., 1999). If a translocated bacterial protein is responsible for activating this type of signaling, it is at present unclear what role this activity might play in the type III secretion/translocation mechanism. A second possibility is that introduction of the putative translocation channel into the plasma membrane stimulates a stress response at the membrane that leads to activation of Rho GTPases. This could be viewed as an unwanted side effect of the translocation channel.
In summary, our data suggest that the Yersinia type III secretion/translocation system triggers a signaling pathway in HeLa cells resulting in activation of Rho GTPases. Under normal conditions, GTPase activation is rapidly terminated following the delivery of YopE. In the absence of YopE, local F-actin assembly is triggered, leading to pore formation. It is interesting to draw a comparison between these series of events and the process of S.typhimurium invasion into epithelial cells. Salmonella typhimurium triggers local F-actin assembly through the actions of translocated type III effector proteins (Galán and Zhou, 2000). Dramatic membrane ruffling ensues, leading to bacterial internalization by macropinocytosis (Galán and Zhou, 2000). Following bacterial entry, F-actin assembly is downregulated by translocated SptP, a GAP sharing structural and functional similarity to YopE (Fú and Galán, 1999). Thus, in addition to its antiphagocytic function, YopE may be part of a mechanism that minimizes overt damage to the plasma membrane during the translocation of Yops into host cells.
Materials and methods
Bacterial strains and infection conditions
The wild-type serogroup III Y.pseudotuberculosis strain YP126 (Bölin et al., 1982) and the mutants derived thereof [YP15 (YopH–), YP6 (YopE–), YP26 (YopJ–), YP27 (YopEHJ–), YP29 (YopEHJB–) and YP19 (YopEHB–)] have been described (Black and Bliska, 1997; Palmer et al., 1998, 1999). Construction of YP37 (YopEHJKOM–; our unpublished data) will be described elsewhere. YP126 and its derivatives carry a naturally occurring deletion in virulence plasmid which inactivates the yopT gene and are thus devoid of YopT activity (our unpublished data). Prior to infection of HeLa cells the bacteria were grown in Luria–Bertani (LB) broth at 37°C under conditions that stimulate Yop expression (Palmer et al., 1998).
Plasmids
The plasmids pYopE and pYopER144A have been described previously (Black and Bliska, 2000). The plasmid pYopER144A encodes an inactive form of YopE due to conversion of the essential Arg at position 144 to Ala (R144A). Construction of pYopT (our unpublished data) will be described elsewhere. Plasmids were introduced into Y.pseudotubercu losis by conjugation as described previously (Bliska and Black, 1995). The eukaryotic expression plasmids pCGT, pCGT-Rac1-V12 and pCGT-RhoA-V14 were obtained from Dr D.Bar-Sagi (SUNY, Stony Brook, NY) and were purified by CsCl2/EtdBr gradient centrifugation.
Cell culture and transfections
HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco BRL) and 1 mM sodium pyruvate in a 5% CO2 humidified incubator at 37°C. For standard infection experiments 1 × 105 HeLa cells were seeded into wells of a 24-well tissue culture plate 24 h prior to assay. For experiments involving transfected cells 5 × 104 HeLa cells were seeded into wells of a 24-well tissue culture plate. Approximately 24 h later, the cells were transiently transfected with 0.5 µg of the indicated plasmid per well using FuGene 6 (Böehringer Mannheim) as specified by the manufacturer. Infections with Y.pseudotuberculosis were performed 24 h after transfection. For microscopic assays the HeLa cells were seeded onto glass coverslips in 24-well dishes and cultured as above.
Infection conditions
For experiments carried out in the presence of inhibitors, HeLa cells were preincubated for 2 h in the presence of 3.9 µM cytochalasin D (Sigma), 100 nM wortmannin (Sigma), 5 µM latrunculin B (Calbiochem) or 10 µM jasplakinolide (Molecular Probes). HeLa cells were also exposed to the appropriate concentration of vehicle alone (DMSO) as a control. Bacterial infections were performed in fresh media containing the same concentration of inhibitors or vehicle. Bacteria were used to infect HeLa cells at a multiplicity of infection of 100. The plates containing the infected cells were centrifuged for 5 min at 83 g and incubated at 37°C with 5% CO2 for different periods of time to allow bacterial–host cell interaction. In some cases, the medium was replaced 5 min post infection with fresh medium containing Dextran 6000 or sucrose at a final concentration of 30 mM.
EtdBr/AO uptake assay
After 3 h of infection, coverslips containing infected HeLa cells were inverted onto a 5 µl drop of 25 µg/ml EtdBr and 5 µg/ml AO in phosphate-buffered saline (PBS) placed on the surface of a glass slide. The cells were immediately analyzed by phase contrast or epifluorescence microscopy using a Zeiss AxioPlan 2 microscope equipped with a 40× objective (NA 0.75). Images were captured with a Diagnostic Instruments Spot digital camera and processed using Adobe Photoshop 5.5.
LDH assay
Samples of culture media from wells containing infected cells were collected at different time points post infection. Levels of LDH were assayed using the CytoTox 96 assay kit (Promega) according to the manufacturer’s instructions. After a 30 min incubation with the substrate, the reaction was stopped and absorbance at 490 nm was determined using an MRX microplate reader (Dynatech Laboratories). Total LDH was determined by assaying supernatants from uninfected cells that had been lysed by a freeze–thaw cycle. The percentage of LDH release was calculated by dividing the amount of LDH released from infected cells by the amount of total LDH.
Gentamicin protection assay
HeLa cells pretreated with wortmannin dissolved in DMSO, or with DMSO alone, were incubated with bacteria as described above. After 1 h of infection, the cells were exposed to 100 µg/ml gentamicin for 1 h. After washing with PBS, the cells are solubilized by treatment with 0.1% Triton X-100 for 5 min at room temperature. The number of viable bacteria was determined by plating serial 10-fold dilutions of the lysates on LB agar plates. Percentage bacterial internalization was calculated as the number of bacteria surviving gentamicin per well divided by the number of bacteria inoculated per well.
Immunoblotting
Infected HeLa cells were washed three times with ice-cold PBS containing 1 mM Na3VO4 and 10 mM NaF. To each well, 0.2 ml of cold 1% Triton X-100 buffer (10 mM Tris pH 7.6, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM Na3VO4, 10 mM NaF, 200 µM AEBSF, 20 µM leupeptin and 1 µM pepstatin) were added, and the plates were incubated for 15 min on ice with occasional rocking. The cells from three wells were scraped into a single microcentrifuge tube and the tube was centrifuged for 10 min at 12 000 g at 4°C. Supernatants (soluble fractions) were transferred to new tubes, and protein concentrations were determined using the Bio-Rad protein assay. Pellets (insoluble fractions) were carefully washed in lysis buffer, resuspended in 10 µl of Laemmli sample buffer and boiled. Samples of the fractions containing equivalent amounts of protein (or sample volume for insoluble fractions) were separated by SDS–PAGE under reducing conditions and transferred electrophoretically onto nitrocellulose. Immunoblotting was performed as described previously (Black and Bliska, 1997) using an affinity-purified polyclonal anti-YopE antibody (Black and Bliska, 2000) diluted 1:2000. Transfected cells in 24-well dishes were processed as above except that insoluble fractions were not analyzed, and immunoblotting was performed with either monoclonal anti-T7 antibody (Novagen) or monoclonal anti-Raf (Transduction Laboratories) antibody. Secondary anti-rabbit IgG and anti-mouse IgG antibodies conjugated to horseradish peroxidase (HRP) were purchased from Sigma.
Rhodamine–phalloidin staining and immunofluorescence assay
HeLa cells on coverslips were washed with PBS, fixed with 2% paraformaldehyde for 15 min and permeabilized with 0.2% Triton X-100 for 10 min. After washing, coverslips were incubated for 20 min with rhodamine–phalloidin (Molecular Probes) at a final concentration of 50 U/ml in PBS, to label F-actin. To label F-actin and bacteria, the fixed cells were first incubated with polyclonal anti-Yersinia antibody SB349 (Black and Bliska, 2000) diluted 1:1000 in PBS containing 3% BSA. After 40 min of incubation, the coverslips were washed three times with PBS containing 1% BSA. The fixed cells were then incubated for 40 min with FITC-conjugated goat anti-rabbit IgG (Jackson Laboratories) diluted 1:250 in PBS containing 3% BSA. After washing with PBS, the fixed cells were incubated with rhodamine–phalloidin at a final concentration of 0.2 U/ml in PBS for 20 min. Coverslips were washed with PBS before mounting in 10% Airvol (Air Products) dissolved in 100 mM Tris pH 8.5, 25% glycerol and 2.5% DABCO (Sigma). Samples were analyzed by epifluorescence microscopy using a Zeiss AxioPlan 2 microscope equipped with a 100× (NA 1.3) oil immersion objective. Images were captured with a Diagnostic Instruments Spot digital camera and processed using Adobe Photoshop 5.5.
Acknowledgments
Acknowledgements
We thank D.Bar-Sagi for providing mammalian expression plasmids, J.Zitzler for the construction of pYopT, and C.Pujol and D.Thanassi for reviewing the manuscript. This research was funded by a grant from the National Institute of Health (AI43389) to J.B.B.
References
- Araki N., Johnson,M.T. and Swanson,J.A. (1996) A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol., 135, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D.S. and Bliska,J.B. (1997) Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J., 16, 2730–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D.S. and Bliska,J.B. (2000) The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol., 37, 515–527. [DOI] [PubMed] [Google Scholar]
- Bliska J.B. (2000) Yop effectors of Yersinia spp. and actin rearrangements. Trends Microbiol., 8, 205–208. [DOI] [PubMed] [Google Scholar]
- Bliska J.B. and Black,D.S. (1995) Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun., 63, 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocker A., Gounon,P., Larquet,E., Niebuhr,K., Cabiaux,V., Parsot,C. and Sansonetti,P. (1999) The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol., 147, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Norlander,L. and Wolf-Watz,H. (1982) Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect. Immun., 37, 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G.R., Boland,A., Boyd,A.P., Geuijen,C., Iriarte,M., Neyt,C., Sory,M.P. and Stainier,I. (1998) The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev., 62, 1315–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D., Tseng,C.C., Bjekic,G. and Greenberg,S. (1999) A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem., 274, 1240–1247. [DOI] [PubMed] [Google Scholar]
- Fields K.A. and Straley,S.C. (1999) LcrV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism. Infect. Immun., 67, 4801–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M.S. and Wolf-Watz,H. (1998) YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol. Microbiol., 29, 799–813. [DOI] [PubMed] [Google Scholar]
- Fú Y. and Galán,J.E. (1999) A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature, 401, 293–297. [DOI] [PubMed] [Google Scholar]
- Galán J.E. and Bliska,J.B. (1996) Cross-talk between bacterial pathogens and their host cells. Annu. Rev. Cell. Dev. Biol., 12, 221–255. [DOI] [PubMed] [Google Scholar]
- Galán J.E. and Zhou,D. (2000) Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc. Natl Acad. Sci. USA, 97, 8754–8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson S., Schesser,K., Persson,C., Galyov,E.E., Rosqvist,R., Homblé,F. and Wolf-Watz,H. (1996) The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J., 15, 5812–5823. [PMC free article] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Hoiczyk E. and Blobel,G. (2001) Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl Acad. Sci. USA, 98, 8669–8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmström A., Olsson,J., Cherepanov,P., Maier,E., Nordfelth,R., Pettersson,J., Benz,R., Wolf-Watz,H. and Forsberg,Å. (2001) LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol., 39, 620–632. [DOI] [PubMed] [Google Scholar]
- Hueck C.J. (1998) Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev., 62, 379–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriarte M. and Cornelis,G.R. (1998) YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol., 29, 915–929. [DOI] [PubMed] [Google Scholar]
- Isberg R.R. and Leong,J.M. (1990) Multiple β 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell, 60, 861–871. [DOI] [PubMed] [Google Scholar]
- Isberg R.R. and Tran Van Nhieu,G. (1994) Binding and internalization of microorganisms by integrin receptors. Trends Microbiol., 2, 10–14. [DOI] [PubMed] [Google Scholar]
- Kirby J.E., Vogel,J.P., Andrews,H.L. and Isberg,R.R. (1998) Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol., 27, 323–336. [DOI] [PubMed] [Google Scholar]
- Kubori T., Sukhan,A., Aizawa,S.I. and Galán,J.E. (2000) Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc. Natl Acad. Sci. USA, 97, 10225–10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecsas J., Raupach,B. and Falkow,S. (1998) The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol. Microbiol., 28, 1269–1281. [DOI] [PubMed] [Google Scholar]
- Neyt C. and Cornelis,G.R. (1999) Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol., 33, 971–981. [DOI] [PubMed] [Google Scholar]
- Palmer L.E., Hobbie,S., Galán,J.E. and Bliska,J.B. (1998) YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol., 27, 953–965. [DOI] [PubMed] [Google Scholar]
- Palmer L.E., Pancetti,A.R., Greenberg,S. and Bliska,J.B. (1999) YopJ of Yersinia spp. is sufficient to cause downregulation of multiple mitogen-activated protein kinases in eukaryotic cells. Infect. Immun., 67, 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C., Nordfelth,R., Holmström,A., Håkansson,S., Rosqvist,R. and Wolf-Watz,H. (1995) Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol. Microbiol., 18, 135–150. [DOI] [PubMed] [Google Scholar]
- Pettersson J. et al. (1999) The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol., 32, 961–976. [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Forsberg,Å., Rimpiläinen,M., Bergman,T. and Wolf-Watz,H. (1990) The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol., 4, 657–667. [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Forsberg,Å. and Wolf-Watz,H. (1991) Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun., 59, 4562–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Magnusson,K.E. and Wolf-Watz,H. (1994) Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J., 13, 964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker M.R., Neyt,C., Stainier,I. and Cornelis,G.R. (1998) The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol., 180, 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sory M.P. and Cornelis,G.R. (1994) Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol., 14, 583–594. [DOI] [PubMed] [Google Scholar]
- Tamano K., Aizawa,S., Katayama,E., Nonaka,T., Imajoh-Ohmi,S., Kuwae,A., Nagai,S. and Sasakawa,C. (2000) Supramolecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J., 19, 3876–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardy F., Homblé,F., Neyt,C., Wattiez,R., Cornelis,G.R., Ruysschaert,J.M. and Cabiaux,V. (1999) Yersinia enterocolitica type III secretion-translocation system: channel formation by secreted Yops. EMBO J., 18, 6793–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Caron,E., Hall,A. and Sansonetti,P.J. (1999) IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J., 18, 3249–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L. and D’Souza-Schorey,C. (1997) Rho GTPases and signaling networks. Genes Dev., 11, 2295–2322. [DOI] [PubMed] [Google Scholar]
- Von Pawel-Rammingen U., Telepnev,M.V., Schmidt,G., Aktories,K., Wolf-Watz,H. and Rosqvist,R. (2000) GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol., 36, 737–748. [DOI] [PubMed] [Google Scholar]
- Zumbihl R., Aepfelbacher,M., Andor,A., Jacobi,C.A., Ruckdeschel,K., Rouot,B. and Heesemann,J. (1999) The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem., 274, 29289–29293. [DOI] [PubMed] [Google Scholar]