Abstract
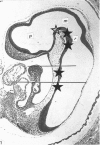
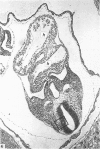
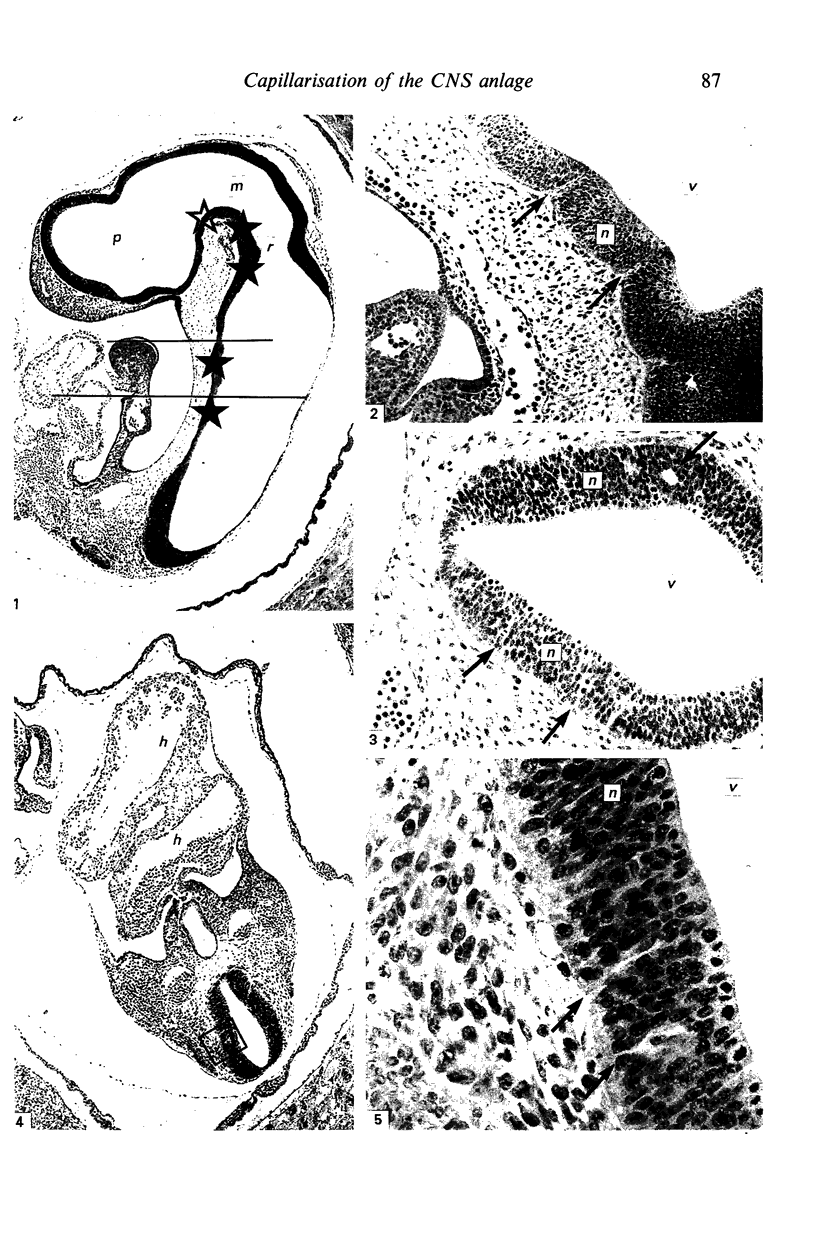
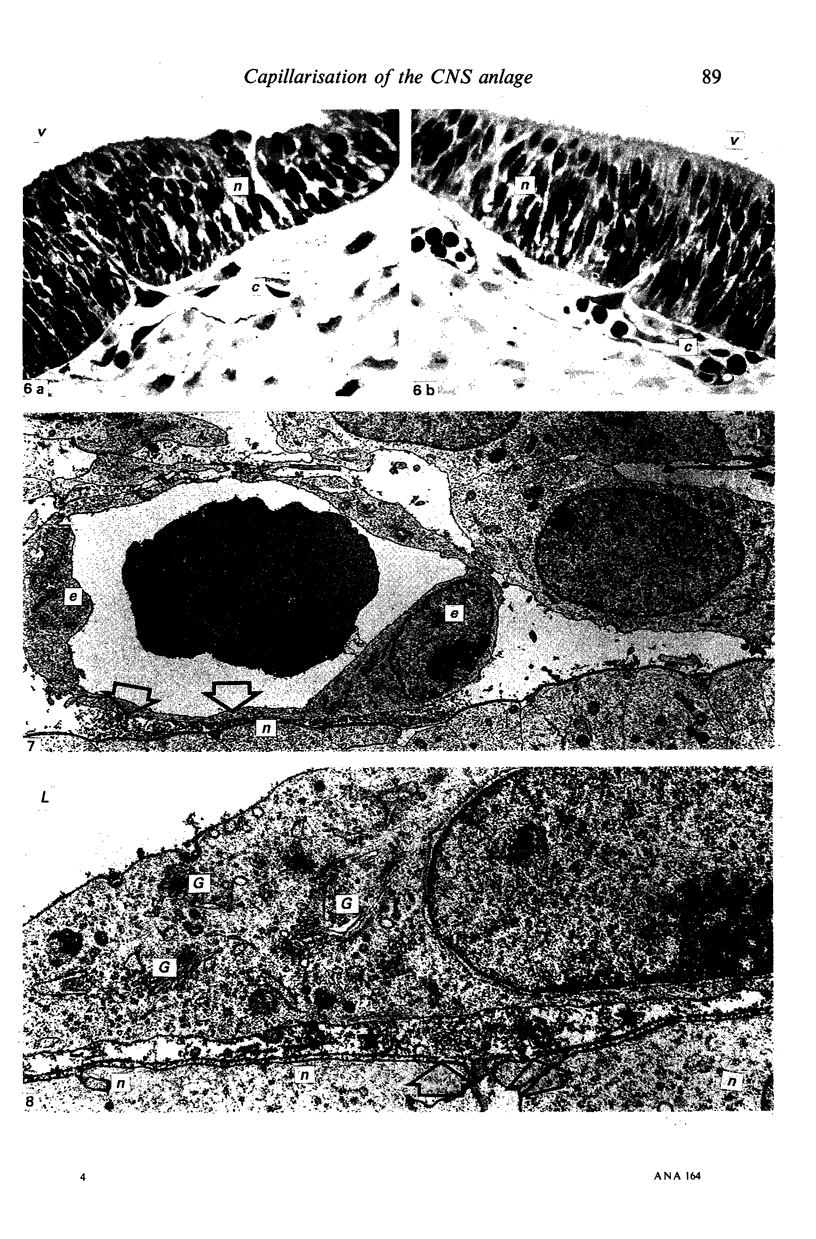
The developmental stage of the mouse embryo at which capillaries first occurred and their localisation in the neuroepithelium were investigated on serial sections of 9 and 10 days old embryos embedded in paraffin and Epon. In addition, areas of the neuroepithelium in which capillaries had been observed at the light microscopical level were investigated by electron microscopy carried out on embryos fixed with glutaraldehyde supplemented with tannic acid. In 5-7 microns serial sections of paraffin-embedded embryos, capillaries were initially seen in the CNS anlage at Theiler's Stage 14 (1972). At this stage, capillaries also occurred in the prosencephalon, the rhombencephalon and in the developing spinal cord. In 1 micron serial sections of resin-embedded embryos, capillaries could be identified in the neuroepithelium one stage earlier, i.e. at Stage 13. These very early capillaries were seen in the dorsal part of the lateral wall of the prosencephalon which later forms the diencephalon. At the ultrastructural level, those areas of the neuroepithelium in which leptomeningeal capillaries first started to spread into the neuroepithelium were characterised by the disintegration of the basement membrane of the neuroepithelium and the appearance of tannic acid-positive extracellular structures between the neuroepithelium and the adjacent leptomeningeal capillaries.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caley D. W., Maxwell D. S. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970 Jan;138(1):31–47. doi: 10.1002/cne.901380104. [DOI] [PubMed] [Google Scholar]
- Camosso M. E., Roncali L., Ambrosi G. Vascular patterns in the chick embryo spinal cord in normal and experimentally modified development. Acta Anat (Basel) 1976;95(3):349–367. doi: 10.1159/000144625. [DOI] [PubMed] [Google Scholar]
- Herken R., Barrach H. J. Ultrastructural localization of type IV collagen and laminin in the seven-day-old mouse embryo. Anat Embryol (Berl) 1985;171(3):365–371. doi: 10.1007/BF00347025. [DOI] [PubMed] [Google Scholar]
- Herken R., Merker H. J., Krowke R. Investigation of the effect of hydroxyurea on the cell cycle and the development of necrosis in the embryonic CNS of mice. Teratology. 1978 Aug;18(1):103–118. doi: 10.1002/tera.1420180113. [DOI] [PubMed] [Google Scholar]
- Herken R. Ultrastructural changes in the neural tube of 10-day-old mouse embryos exposed to colchicine and hydroxyurea. Teratology. 1985 Jun;31(3):345–352. doi: 10.1002/tera.1420310305. [DOI] [PubMed] [Google Scholar]
- LIERSE W. UBER DIE BEEINFLUSSUNG DER HIRNANGIOARCHITEKTUR DURCH DIE MORPHOGENESE. Acta Anat (Basel) 1963;53:1–54. [PubMed] [Google Scholar]
- STRONG L. H. THE EARLY EMBRYONIC PATTERN OF INTERNAL VASCULARIZATION OF THE MAMMALIAN CEREBRAL CORTEX. J Comp Neurol. 1964 Aug;123:121–138. doi: 10.1002/cne.901230111. [DOI] [PubMed] [Google Scholar]
- Sanders E. J. Development of the basal lamina and extracellular materials in the early chick embryo. Cell Tissue Res. 1979 May 25;198(3):527–537. doi: 10.1007/BF00234196. [DOI] [PubMed] [Google Scholar]
- Sannes P. L., Katsuyama T., Spicer S. S. Tannic acid-metal salt sequences for light and electron microscopic localization of complex carbohydrates. J Histochem Cytochem. 1978 Jan;26(1):55–61. doi: 10.1177/26.1.74385. [DOI] [PubMed] [Google Scholar]
- Simón-Marín R., Vilanova J. R., Aguinagalde A., Barberá-Guillem E. Vascular architecture of the developing spinal cord in the rat: a suggested model. J Embryol Exp Morphol. 1983 Aug;76:27–36. [PubMed] [Google Scholar]
- Singer I. I. The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell. 1979 Mar;16(3):675–685. doi: 10.1016/0092-8674(79)90040-0. [DOI] [PubMed] [Google Scholar]
- Singley C. T., Solursh M. The use of tannic acid for the ultrastructural visualization of hyaluronic acid. Histochemistry. 1980 Feb;65(2):93–102. doi: 10.1007/BF00493158. [DOI] [PubMed] [Google Scholar]
- Stewart P. A., Wiley M. J. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981 May;84(1):183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. A quantitative and morphological study of vascularisation of the developing mouse spinal cord. J Anat. 1981 Mar;132(Pt 2):203–221. [PMC free article] [PubMed] [Google Scholar]