Abstract
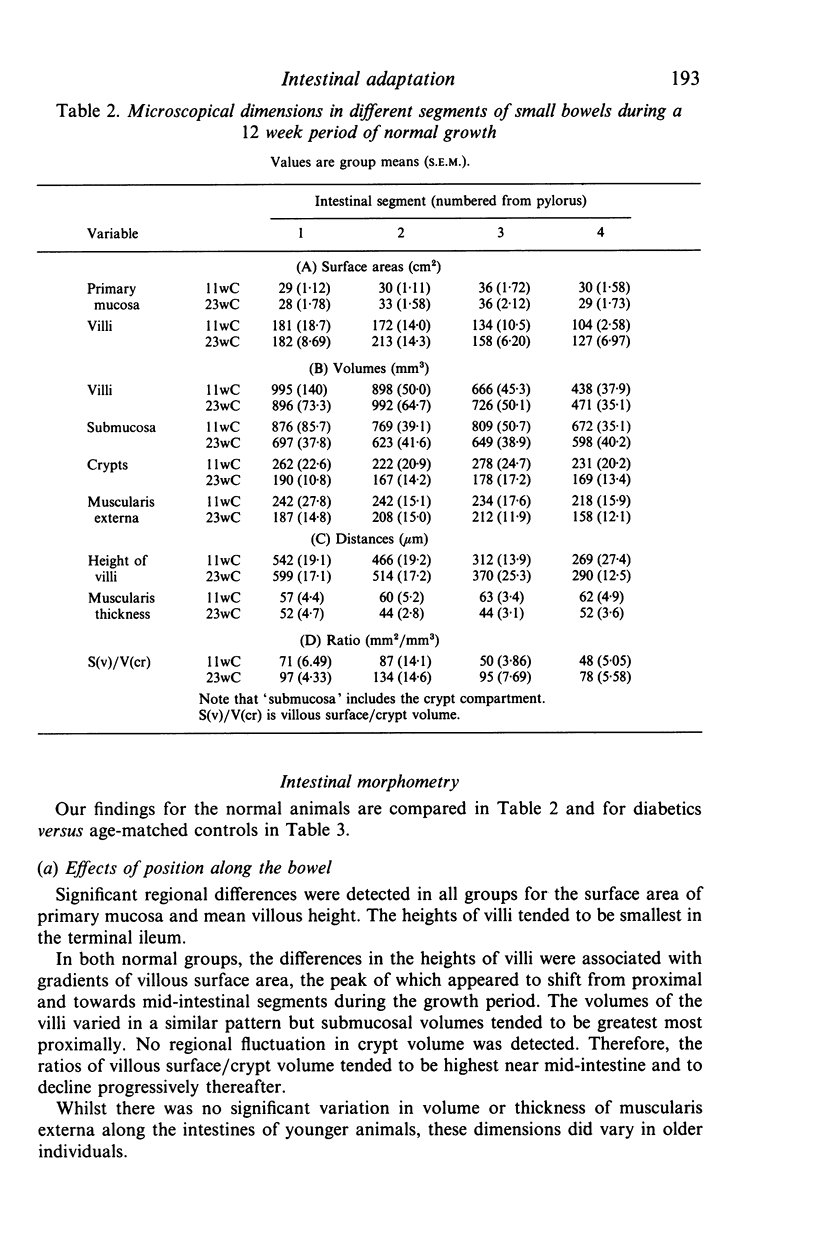
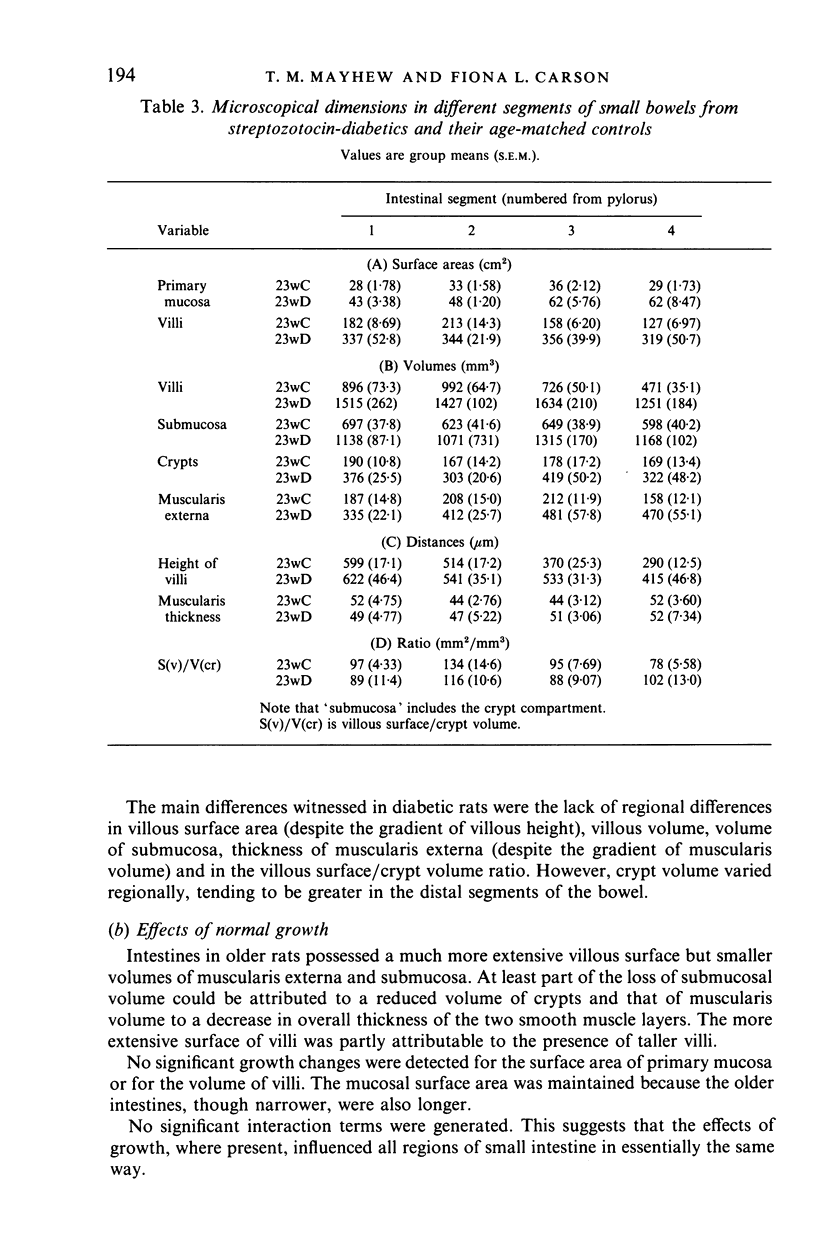
The gross and microscopical dimensions of small intestines from three groups of rats were investigated by morphometric (mainly stereological) methods. The groups were chosen to represent relatively 'steady state' situations: normal growth (over a 12 week period) and intestinal hyperplasia due to streptozotocin-diabetes of 12 weeks duration. Four intestinal segments were sampled along each intestine. For normal groups, no interaction effects were found, suggesting that growth affected all regions of the small intestine in the same way. Older rats were heavier and their intestines were longer and narrower. In addition, villous surface area was more extensive and the villi differed in shape. Volumes of crypts, submucosa and muscularis externa were all reduced. Diabetic animals weighed less than age-matched controls and their intestines were wider but not significantly longer. All surface areas and volumes were increased substantially. However, hypertrophy of the muscularis externa was not detected by measuring muscularis thickness. Villi altered their shape. At least for villous height, the effects of diabetes were greater in terminal segments. These findings are discussed in the context of the reported effects of age and experimental hyperplasia (including diabetes) on intestinal architecture and behaviour.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldewachi H. S., Wright N. A., Appleton D. R., Watson A. J. The effect of starvation and refeeding on cell population kinetics in the rat small bowel mucosa. J Anat. 1975 Feb;119(Pt 1):105–121. [PMC free article] [PubMed] [Google Scholar]
- Altmann G. G., Enesco M. Cell number as a measure of distribution and renewal of epithelial cells in the small intestine of growing and adult rats. Am J Anat. 1967 Sep;121(2):319–336. doi: 10.1002/aja.1001210210. [DOI] [PubMed] [Google Scholar]
- Altmann G. G. Influence of starvation and refeeding on mucosal size and epithelial renewal in the rat small intestine. Am J Anat. 1972 Apr;133(4):391–400. doi: 10.1002/aja.1001330403. [DOI] [PubMed] [Google Scholar]
- BAKER S. J., MATHAN V. I., CHERIAN V. The nature of the villi in the small intestine of the rat. Lancet. 1963 Apr 20;1(7286):860–860. doi: 10.1016/s0140-6736(63)91630-1. [DOI] [PubMed] [Google Scholar]
- BROWN A. L., Jr Microvilli of the human jejunal epithelial cell. J Cell Biol. 1962 Mar;12:623–627. doi: 10.1083/jcb.12.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastie M. J., Balas D., Laval J., Senegas-Balas F., Bertrand C., Frexinos J., Ribet A. Histological variations of jejunal and ileal mucosa on days 8 and 15 after hypophysectomy in rat: morphometrical analysis on light and electron microscopy. Acta Anat (Basel) 1982;112(4):321–337. doi: 10.1159/000145525. [DOI] [PubMed] [Google Scholar]
- Bhoyrul S., Sharma A. K., Stribling D., Mirrlees D. D., Peterson R. G., Farber M. O., Thomas P. K. Ultrastructural observations on myelinated fibres in experimental diabetes: effect of the aldose reductase inhibitor ponalrestat given alone or in conjunction with insulin therapy. J Neurol Sci. 1988 Jun;85(2):131–147. doi: 10.1016/0022-510x(88)90151-7. [DOI] [PubMed] [Google Scholar]
- Boyne R., Fell B. F., Robb I. The surface area of the intestinal mucosa in the lactating rat. J Physiol. 1966 Apr;183(3):570–575. doi: 10.1113/jphysiol.1966.sp007884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary W. F. Effect of insulin and experimental diabetes mellitus on the digestive-absorptive function of the small intestine. Digestion. 1973 Oct;9(3):248–263. doi: 10.1159/000197452. [DOI] [PubMed] [Google Scholar]
- Cheng H., Bjerknes M. Whole population cell kinetics of mouse duodenal, jejunal, ileal, and colonic epithelia as determined by radioautography and flow cytometry. Anat Rec. 1982 Jun;203(2):251–264. doi: 10.1002/ar.1092030207. [DOI] [PubMed] [Google Scholar]
- Clarke R. M. Mucosal architecture and epithelial cell production rate in the small intestine of the albino rat. J Anat. 1970 Nov;107(Pt 3):519–529. [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M. The effect of growth and of fasting on the number of villi and crypts in the small intestine of the albino rat. J Anat. 1972 May;112(Pt 1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M. The effects of age on mucosal morphology and epithelial cell production in rat small intestine. J Anat. 1977 Jul;123(Pt 3):805–811. [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Karasov W. H., Cary C., Enders D., Yung R. Effect of dietary carbohydrate on monosaccharide uptake by mouse small intestine in vitro. J Physiol. 1984 Apr;349:419–440. doi: 10.1113/jphysiol.1984.sp015165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. B., PARSONS D. S. The gradient of mucosal surface area in the small intestine of the rat. J Anat. 1950 Jul;84(3):272–282. [PMC free article] [PubMed] [Google Scholar]
- Ferraris R. P., Diamond J. M. Use of phlorizin binding to demonstrate induction of intestinal glucose transporters. J Membr Biol. 1986;94(1):77–82. doi: 10.1007/BF01901015. [DOI] [PubMed] [Google Scholar]
- Forgue-Lafitte M. E., Marescot M. R., Chamblier M. C., Rosselin G. Evidence for the presence of insulin binding sites in isolated rat intestinal epithelial cells. Diabetologia. 1980 Oct;19(4):373–378. doi: 10.1007/BF00280523. [DOI] [PubMed] [Google Scholar]
- Forrester J. M. The number of villi in rat's jejunum and ileum: effect of normal growth, partial enterectomy, and tube feeding. J Anat. 1972 Feb;111(Pt 2):283–291. [PMC free article] [PubMed] [Google Scholar]
- Hromádková V., Skála I. Factors influencing the assessment of size of the mucosal surface and length of the small intestine in rats. Digestion. 1968;1(3):149–158. doi: 10.1159/000196847. [DOI] [PubMed] [Google Scholar]
- Jervis E. L., Levin R. J. Anatomic adaptation of the alimentary tract of the rat to the hyperphagia of chronic alloxan-diabetes. Nature. 1966 Apr 23;210(5034):391–393. doi: 10.1038/210391a0. [DOI] [PubMed] [Google Scholar]
- Karasov W. H., Diamond J. M. Adaptive regulation of sugar and amino acid transport by vertebrate intestine. Am J Physiol. 1983 Oct;245(4):G443–G462. doi: 10.1152/ajpgi.1983.245.4.G443. [DOI] [PubMed] [Google Scholar]
- Keelan M., Walker K., Thomson A. B. Intestinal brush border membrane marker enzymes, lipid composition and villus morphology: effect of fasting and diabetes mellitus in rats. Comp Biochem Physiol A Comp Physiol. 1985;82(1):83–89. doi: 10.1016/0300-9629(85)90708-x. [DOI] [PubMed] [Google Scholar]
- Lal D., Schedl H. P. Intestinal adaptation in diabetes: amino acid absorption. Am J Physiol. 1974 Oct;227(4):827–831. doi: 10.1152/ajplegacy.1974.227.4.827. [DOI] [PubMed] [Google Scholar]
- Leblond C. P. The life history of cells in renewing systems. Am J Anat. 1981 Feb;160(2):114–158. doi: 10.1002/aja.1001600202. [DOI] [PubMed] [Google Scholar]
- Lorenz-Meyer H., Köhn R., Riecken E. O. Vergleich verschiedener morphometrischer Methoden zur Erfassung der Schleimhautoberfläche des Rattendünndarms und deren Beziehung zur Funktion. Histochemistry. 1976 Oct 22;49(2):123–129. doi: 10.1007/BF00495676. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M. A geometric model for estimating villous surface area in rat small bowel is justified by unbiased estimates obtained using vertical sections. J Anat. 1988 Dec;161:187–193. [PMC free article] [PubMed] [Google Scholar]
- Mayhew T. M. Geometric model of the rat intestinal mucosa for stereological evaluation of villus amplification factors. J Microsc. 1984 Sep;135(Pt 3):337–346. doi: 10.1111/j.1365-2818.1984.tb02538.x. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M., Middleton C. Crypts, villi and microvilli in the small intestine of the rat. A stereological study of their variability within and between animals. J Anat. 1985 Aug;141:1–17. [PMC free article] [PubMed] [Google Scholar]
- Mayhew T. M. Quantitative ultrastructural study on the responses of microvilli along the small bowel to fasting. J Anat. 1987 Oct;154:237–243. [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Hanson W., Schedl H. P., Osborne J. W. Proliferation rate and transit time of mucosal cells in small intestine of the diabetic rat. Gastroenterology. 1977 Dec;73(6):1326–1332. [PubMed] [Google Scholar]
- Miller D. S., Rahman M. A., Tanner R., Mathan V. I., Baker S. J. The vascular architecture of the different forms of small intestinal villi in the rat (Rattus norvegicus). Scand J Gastroenterol. 1969;4(6):477–482. doi: 10.3109/00365526909180637. [DOI] [PubMed] [Google Scholar]
- Nakabou Y., Ishikawa Y., Misake A., Hagihira H. Effect of food intake on intestinal absorption and mucosal hydrolases in alloxan diabetic rats. Metabolism. 1980 Feb;29(2):181–185. doi: 10.1016/0026-0495(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Nakabou Y., Okita C., Takano Y., Hagihira H. Hyperplastic and hypertrophic changes of the small intestine in alloxan diabetic rats. J Nutr Sci Vitaminol (Tokyo) 1974;20(3):227–234. doi: 10.3177/jnsv.20.227. [DOI] [PubMed] [Google Scholar]
- Olsen W. A., Korsmo H. Enhancement of intestinal sucrase activity in experimental diabetes: the role of intraluminal factors. J Lab Clin Med. 1975 May;85(5):832–837. [PubMed] [Google Scholar]
- Olsen W. A., Rosenberg I. H. Intestinal transport of sugars and amino acids in diabetic rats. J Clin Invest. 1970 Jan;49(1):96–105. doi: 10.1172/JCI106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothier P., Hugon J. S. Characterization of isolated villus and crypt cells from the small intestine of the adult mouse. Cell Tissue Res. 1980;211(3):405–418. doi: 10.1007/BF00234396. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. A comprehensive model of the crypts of the small intestine of the mouse provides insight into the mechanisms of cell migration and the proliferation hierarchy. J Theor Biol. 1987 Aug 21;127(4):381–391. doi: 10.1016/s0022-5193(87)80136-4. [DOI] [PubMed] [Google Scholar]
- Pénzes L., Skála I. Changes in the mucosal surface area of the small gut of rats of different ages. J Anat. 1977 Sep;124(Pt 1):217–222. [PMC free article] [PubMed] [Google Scholar]
- Ross G. A., Mayhew T. M. Effects of fasting on mucosal dimensions in the duodenum, jejunum and ileum of the rat. J Anat. 1985 Oct;142:191–200. [PMC free article] [PubMed] [Google Scholar]
- Ross G. A., Mayhew T. M. Effects of fasting on villi along the small intestine: a stereological approach to the problem of quantifying villus 'shape'. Experientia. 1984 Aug 15;40(8):856–858. doi: 10.1007/BF01951993. [DOI] [PubMed] [Google Scholar]
- Schedl H. P., Wilson H. D. Effects of diabetes on intestinal growth and hexose transport in the rat. Am J Physiol. 1971 Jun;220(6):1739–1745. doi: 10.1152/ajplegacy.1971.220.6.1739. [DOI] [PubMed] [Google Scholar]
- Stenling R., Helander H. F. Stereological studies on the small intestinal epithelium of the rat. 1. The absorptive cells of the normal duodenum and jejunum. Cell Tissue Res. 1981;217(1):11–21. doi: 10.1007/BF00233821. [DOI] [PubMed] [Google Scholar]
- Stenling R., Hägg E., Falkmer S. Stereological studies on the rat small intestinal epithelium. III. Effects of short-term alloxan diabetes. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;47(3):263–270. doi: 10.1007/BF02890209. [DOI] [PubMed] [Google Scholar]
- van Dongen J. M., Visser W. J., Daems W. T., Galjaard H. The relation between cell proliferation, differentiation and ultrastructural development in rat intestinal epithelium. Cell Tissue Res. 1976 Oct 29;174(2):183–199. doi: 10.1007/BF00222158. [DOI] [PubMed] [Google Scholar]


