Abstract
Rho GTPases, which control polarized cell growth through cytoskeletal reorganization, have recently been implicated in the control of endo- and exocytosis. We now report that both Rho1p and Cdc42p have a direct role in mediating the docking stage of homotypic vacuole fusion. Vacuoles prepared from strains with temperature-sensitive alleles of either Rho1p or Cdc42p are thermolabile for fusion. RhoGDI (Rdi1p), which extracts Rho1p and Cdc42p from the vacuole membrane, blocks vacuole fusion. The Rho GTPases can not fulfill their function as long as priming and Ypt7p-dependent tethering are inhibited. However, reactions that are reversibly blocked after docking by the calcium chelator BAPTA have passed the point of sensitivity to Rdi1p. Extraction and removal of Ypt7p, Rho1p and Cdc42p from docked vacuoles (by Gdi1p, Gyp7p and Rdi1p) does not impede subsequent membrane fusion, which is still sensitive to GTPγS. Thus, multiple GTPases act in a defined sequence to regulate the docking steps of vacuole fusion.
Keywords: Cdc42p/GTPase/membrane fusion/Rho1p/Saccharomyces cerevisiae
Introduction
Small GTPases of the Rab, Arf and Rho families regulate several key steps of vesicle trafficking (Bourne et al., 1991; Matozaki et al., 2000). These 20–30 kDa monomeric proteins bind and hydrolyze GTP and bind membranes via lipid modifications. They act as molecular switches that cycle between active (GTP-bound) and inactive (GDP-bound) states. Effector complexes associate with the active state and are responsible for transducing downstream effects. The bound-nucleotide state is regulated by guanine-nucleotide exchange factors (GEFs), which exchange GTP for GDP, and the subsequent hydrolysis of GTP is accelerated by GTPase-activating proteins (GAPs). Membrane association is controlled by a guanine-dissociation inhibitor (GDI), which extracts the GDP-bound form of the protein and may aid in its recycling.
Each family of GTPases has a characteristic function in trafficking. Arf proteins regulate vesicle generation (Chavier and Goud, 1999). Vesicle budding requires Arf-GTP and coat complexes, while subsequent uncoating requires Arf-GTP hydrolysis. On uncoated membranes, activated Rab proteins associate with effector complexes to drive the early tethering stages of vesicle docking and help to establish docking specificity. The Rho family of GTPases transduce signals for cytoskeletal reorganizaton and cell polarity (Drubin and Nelson, 1996; Cabib et al., 1998; Hall, 1998). This is necessary for the spatial targeting of vesicles within cells (Govindan et al., 1995; Pruyne et al., 1998; Guo et al., 2001). Rho proteins are also implicated in the regulation of endo- and exocytosis. For example, addition of rhoGDI to permeabilized mast cells blocks stimulated secretion, suggesting that a crucial Rho protein has been extracted (Brown et al., 1998; Hong-Geller and Cerione, 2000). Activated Cdc42p regulates cytoskeletal assembly, which controls antigen endocytosis in dendritic cells and basolateral endocytosis in MDCK cells (Kroschewski et al., 1999; Garrett et al., 2000). Endosome dynamics is governed by RhoD, which catalyzes actin cytoskeleton disassembly (Murphy et al., 1996). Finally, membrane fusion can be stimulated by actin disassembly (Vitale et al., 1991; Muallem et al., 1995) or cycles of actin polymerization and depolymerization (Vitale et al., 1995; Bernstein et al., 1998; Lang et al., 2000), which may result in the transient removal of a physical actin barrier. Each of these reactions may be controlled by Rho proteins (Norman et al., 1994; Hall, 1998). Not all studies support the ‘actin barrier’ model, since actin polymerization or stabilizing agents act as positive effectors of membrane fusion in some systems (Koffer et al., 1990; Jahraus et al., 2001). Other effects of Rho proteins on vesicular traffic may be independent of cytoskeletal reorganization. For example, Rho3p interacts with the exocytosis machinery in yeast, and several subunits of the exocyst complex, including the rab GTPase Sec4, can act as multicopy suppressors of rho3Δ (Imai et al., 1996; Adamo et al., 1999; Robinson et al., 1999). However, for all of these trafficking events, it is not clear whether Rho proteins act on membrane fusion per se or merely regulate an actin barrier or the cytoskeletal ‘tracks’ for motor-driven vesicle movement to docking sites.
Homotypic fusion of yeast vacuoles can occur in vitro without added cytosol, allowing a detailed examination of those GTPase-regulated events that occur in the absence of cytoplasmic cytoskeletal meshwork. This reaction occurs in three sequential stages: priming, docking and membrane fusion (see Wickner and Haas, 2000). We now report that docking requires not only the Rab GTPase Ypt7p, but also two Rho GTPases, Rho1p and Cdc42p. These Rho proteins can only function after Ypt7p (Rab) function has been fulfilled, yet extraction of Ypt7p, Rho1p and Cdc42p from vacuoles that have completed docking does not impede subsequent fusion. This last stage of the reaction is still sensitive to the addition of GTPγS, although the molecular identity of its target is not yet established.
Results
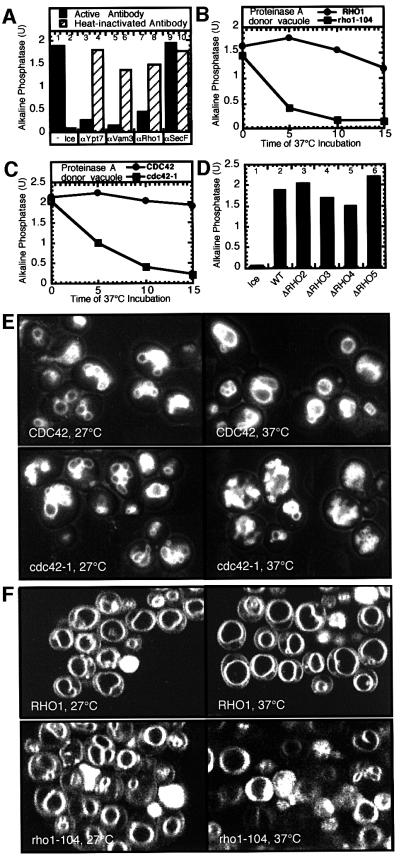
Ypt7p is required for vacuole docking (Mayer and Wickner, 1997). Once docking is complete, the final stage of membrane fusion is not affected by the extraction of Ypt7p yet remains sensitive to GTPγS (Eitzen et al., 2000). Our current studies began with a search for a late acting GTPase. The yeast genome encodes five RHO genes and CDC42; antibodies to Rho1p inhibited fusion (Figure 1A, lane 7). To further test whether Rho1p is required for fusion, the alkaline phosphatase gene (PHO8) was deleted from strain NHY21, which contains a temperature-sensitive allele of the essential gene RHO1, and from the wild-type parental strain ONHY1 (Yamochi et al., 1994). Vacuoles isolated from these strains were fused with vacuoles isolated from a strain in which the proteinase A gene, PEP4, is deleted and which therefore contain the catalytically inactive pro-alkaline phosphatase. Fusion, as measured by maturation of the pro-alkaline phosphatase by proteinase A donated from rho1-ts or wild-type vacuoles, occurred normally when vacuoles were mixed and incubated at 27°C; however, pre-incubation of vacuoles bearing a temperature-sensitive Rho1p at 37°C inhibited their capacity for fusion (Figure 1B, solid squares) whereas vacuoles with wild-type Rho1p were not thermolabile (solid circles). Similarly, vacuoles from a strain with a cdc42-ts allele were also thermolabile for fusion (Figure 1C, solid squares). Vacuoles from strains with deletions in the non-essential RHO genes (RHO2–5) were not defective for fusion (Figure 1D). Therefore, the two essential Rho GTPases, Rho1p and Cdc42p, are required for in vitro vacuole fusion.
Fig. 1. Rho1p and Cdc42p are essential for vacuole fusion. (A) Anti-Rho1p antibodies inhibit vacuole fusion. Anti-Vam3p, anti-Rho1p or anti-SecF IgG fraction or affinity-purified anti-Ypt7p antibodies were added to standard fusion reactions. Aliquots were heated to 95°C for 10 min for heat inactivation. (B and C) Vacuole-containing rho1-ts or cdc42-ts proteins are thermolabile for fusion. Vacuoles isolated from strain (B) GEY6048 (RHO1) (circles) and GEY1658 (rho1-104) (squares) or (C) GEY6038 (CDC42) (circles) and GEY2298 (cdc42-1) (squares) were incubated at 37°C for the indicated times before adding to a fusion reaction with vacuoles from strain BJ3505. (D) Deletion of the RHO2, 3, 4 and 5 genes does not affect vacuole fusion. Vacuoles isolated from strains GEY6028 (WT), GEY0908 (ΔRHO2), GEY2288 (ΔRHO3) and GEY1808 (ΔRHO5), which lack alkaline phosphatase, were tested for fusion with vacuoles from strain BJ3505. Vacuoles isolated from strain GEY0554 (ΔRHO4), which lacks proteinase A, were tested for fusion with vacuoles from strain DKY6281. (E and F) In vivo vacuole morphology in RHO mutant and parental strains. The vacuoles from strains TD4 (CDC42), DJTD2-16A (cdc42-1), ONHY1 (RHO1) and NHY21 (rho1-104) were stained with FM4-64. Cells were grown at 27°C to an OD600 ∼ 1. An aliquot of each was removed and incubated at 37°C for 90 min prior to observation.
To corroborate the role of Cdc42p in vacuole fusion, we examined the vacuole morphology in cdc42-ts cells. Several large round vacuoles were seen in cells at the permissive temperature in cdc42-ts (Figure 1E, cdc42-1) and parental strains (Figure 1E, CDC42). However, after a brief incubation at the non-permissive temperature (37°C), there was increased vacuole fragmentation in cells with a cdc42-ts allele. The rho1-ts strain also has fragmented vacuoles when incubated at the non-permissive temperature (Figure 1F). Antibodies to a peptide fragment of Cdc42p also inhibit vacuole fusion (Müller et al., 2001).
Rdi1p extracts Rho1p and Cdc42p from vacuoles and blocks fusion
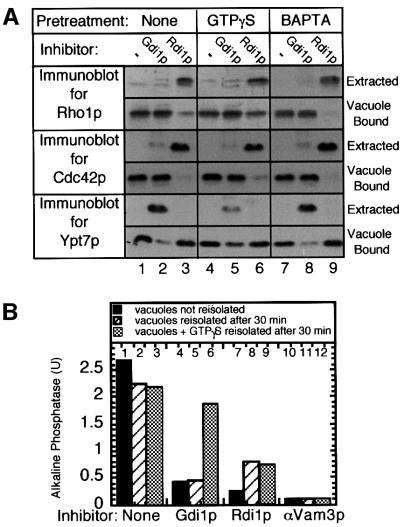
Rdi1p blocks vacuole fusion (Figure 2A) by extracting membrane-bound Rho proteins such as Rho1p and Cdc42p (Masuda et al., 1994). A soluble lumenal enzyme, proteinase A, is not released from vacuoles during fusion reactions with Rdi1p, demonstrating that inhibition is not due to vacuolar lysis (Figure 2B).
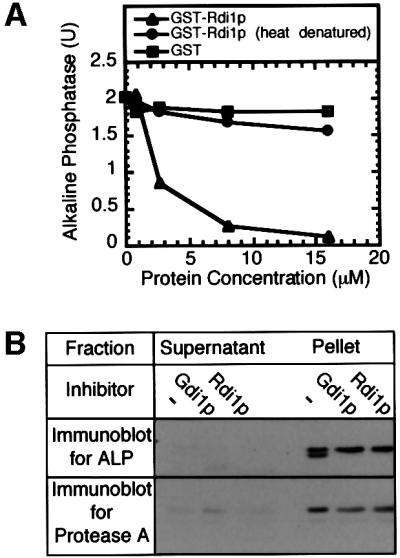
Fig. 2. Rdi1p (RhoGDI) blocks fusion. (A) Addition of GST-tagged Rdi1p (triangles), heat-denatured (95°C incubation for 10 min) GST–Rdi1p (circles) or GST (squares) to standard fusion reactions. (B) Inhibition by Rdi1p of pro-alkaline phosphatase processing is not due to vacuole lysis. Standard fusion reactions were incubated at 27°C for 90 min in the absence or presence of Gdi1p or Rdi1p. Reactions were centrifuged (5 min, 10 000 g), supernatants were removed from pelleted vacuoles, and equal fractions were analyzed by immunoblotting for proteinase A and alkaline phosphatase.
Both Rdi1p and Gdi1p extract lipid-anchored GTPases from membranes (Garrett et al., 1994; Masuda et al., 1994). Their actions are distinct, as Rdi1p extracts Rho1p and Cdc42p from the vacuole but does not extract Ypt7p, while Gdi1p extracts Ypt7p but not Rho1p or Cdc42p (Figure 3A, lanes 1–3). Pre-incubation of vacuoles with GTPγS may affect extraction by Rdi1p or Gdi1p since only the GDP-bound form of each GTPase is extractable. The extraction of Ypt7p and Rho1p is inhibited when vacuoles are pre-incubated with GTPγS, although there is no change in the efficiency of Cdc42p extraction (Figure 3A, lanes 4–6). Pre-incubation with the calcium chelator BAPTA, another late stage inhibitor, does not affect GTPase extraction (Figure 3A, lanes 7–9), underscoring the specificity of Gdi1p and Rdi1p action. Pre-incubation of vacuoles with Rdi1p or Gdi1p irreversibly blocks fusion (Figure 3B, lanes 2, 5 and 8). Co-incubation of vacuoles with GTPγS and Gdi1p inhibits Ypt7p extraction (Figure 3A, lane 5) and thereby allows the reaction to resume when these inhibitors are removed after 30 min (Figure 3B, lane 6). Thus, the inhibitory effect of Gdi1p is overcome by locking Ypt7p in the GTP-bound form. However, incubation of vacuoles with GTPγS prevents neither the Rdi1p-mediated extraction of Cdc42p (Figure 3A, lane 9) nor the consequent irreversible loss of fusion (Figure 3B, lanes 7–9), underscoring the importance of Cdc42p for the fusion reaction.
Fig. 3. Rdi1p extracts Rho1p and Cdc42p from vacuoles. (A) GTPase extractions. Standard fusion reactions (90 µl) with no inhibitor (None), 3 mM GTPγS or 3 mM BAPTA were incubated for 30 min at 27°C. Aliquots (30 µl) then received PS buffer (–), Gdi1p or Rdi1p and were further incubated for 60 min at 27°C. Reactions were centrifuged (5 min, 10 000 g) and equal fractions of supernatants and vacuole pellets were analyzed by immunoblotting for Rho1p, Cdc42p and Ypt7p. (B) GTPγS blocks Gdi1p but not Rdi1p inhibition. Fusion reactions were incubated with no inhibitor, Gdi1p, Rdi1p or anti-Vam3p antibodies in the absence (striped bars) or presence (gray bars) of GTPγS. After 30 min at 27°C, vacuoles were re-isolated, supplied with fresh reaction buffer without inhibitors and incubated for 60 min. Control samples without GTPγS (black bars) were not re-isolated.
Rho proteins act after Ypt/Rab proteins
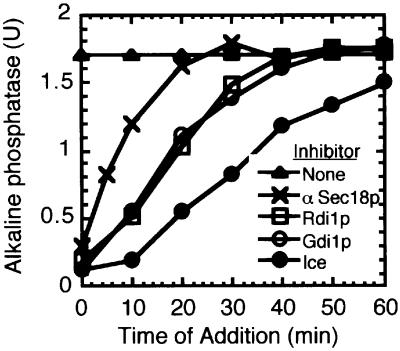
In vitro vacuole fusion can be kinetically dissected into distinct stages of priming, docking and fusion. The stage affected by each inhibitor can be inferred from the time that it takes for the reaction to become insensitive to inhibitor addition. The fusion reaction becomes insensitive to the addition of Rdi1p after 30–40 min (Figure 4) with the same kinetics as for Gdi1p, an established inhibitor of docking (Mayer and Wickner, 1997; Eitzen et al., 2000). This curve of inhibition falls between priming, defined by the relative sensitivity to Sec18p antibodies, and the completion of fusion, measured by the mature, active alkaline phosphatase in samples transferred to ice at various times.
Fig. 4. Kinetic analysis of Rdi1p inhibition. Fusion reactions (270 µl) were incubated at 27°C. At the indicated times, aliquots (30 µl) were removed and added to tubes on ice or containing PS buffer, anti-Sec18p antibodies, Gdi1p or Rdi1p. Reactions were incubated for 90 min (total) and then assayed for alkaline phosphatase.
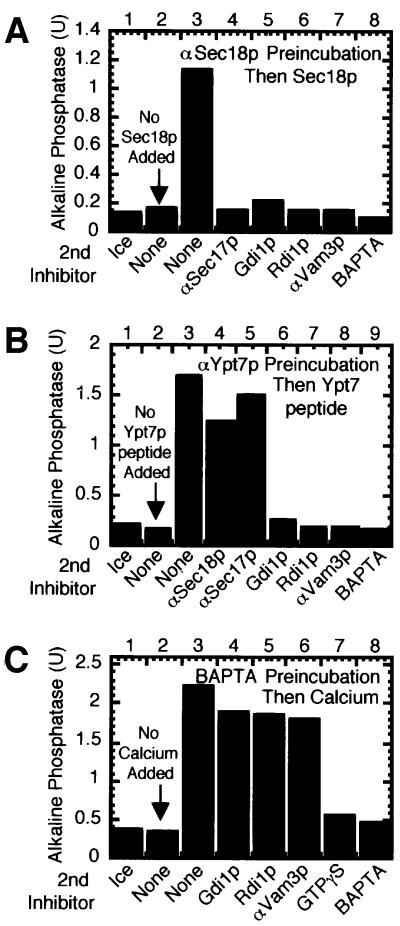
The in vitro reaction can also be blocked at specific stages by reversible inhibitors. We have employed this strategy, in three experiments, to show that the Rho GTPases can only act after Sec18p and Ypt7p and that these Rho GTPases have fulfilled their function before Ca2+-triggered fusion. In one experiment, priming was blocked by incubation with Sec18p antibodies for 30 min. This block was reversed (Mayer et al., 1996) upon addition of active recombinant Sec18p (Figure 5A, compare lanes 2 and 3). Although uninhibited reactions are normally resistant to Rdi1p addition by 30 min (Figure 4), reactions blocked for 30 min by anti-Sec18p remain sensitive to docking and fusion inhibitors, including Rdi1p (lane 6), when the block is reversed by Sec18p addition. Priming must therefore be completed before the Rho proteins can complete their function. In a similar manner, the reaction is blocked by incubation with antibodies to a synthetic Ypt7p peptide (Figure 5B, lane 2). Inhibition can be reversed after 30 min by the addition of the cognate Ypt7p peptide (lane 3). These reactions are no longer sensitive to the addition of priming inhibitors (lanes 4 and 5) but remain sensitive to docking and fusion inhibitors including Rdi1p (lanes 6–9). The Ypt7p function must therefore also be completed prior to satisfying the Rho function. Finally, the fusion stage of the reaction can be blocked by the calcium chelator BAPTA (Figure 5C, lane 2). After 40 min this block can be reversed by the addition of calcium (lane 3). These reactions are no longer sensitive to priming or docking inhibitors, including Rdi1p (lanes 4–6). The Rho proteins therefore complete their function prior to the calcium-triggered initiation of the membrane fusion step.
Fig. 5. Rho GTPases can only act after Sec18p and Ypt7p but vacuoles blocked for fusion with the calcium chelator BAPTA are past Rho function. Fusion reactions were incubated at 27°C in the presence of (A) anti-Sec18p antibodies, (B) anti-Ypt7 peptide antibodies or (C) BAPTA. After 30 min the inhibition was reversed by the addition of (A) His6-Sec18p, (B) Ypt7p peptide or (C) calcium, in the presence of a second inhibitor or PS buffer (none) as indicated. Lane 2, no reversal of inhibition. Reactions were incubated for an additional 60 min before assaying for alkaline phosphatase.
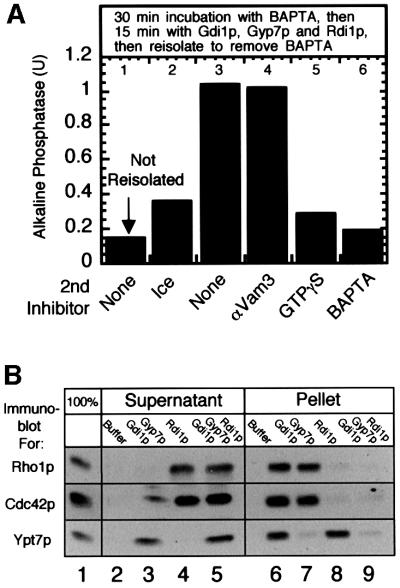
When docked, BAPTA-blocked vacuoles are incubated with Rdi1p (to extract Rho1p and Cdc42p), Gdi1p and Gyp7p (to extract Ypt7p) or a mixture of Rdi1p, Gdi1p and Gyp7p (to extract all three GTPases) and then re-isolated, the fusion step remains sensitive to GTPγS (Figure 6A). Immunoblot analysis showed that these vacuoles were indeed depleted of Ypt7p, Rho1p and Cdc42p by these extractions (Figure 6B). Therefore, there is presumbably a unique GTPase or other GTPγS-senstive target that functions within the fusion step and remains to be identified.
Fig. 6. Docked vacuoles, stripped of Ypt7p, Rho1p and Cdc42p, are still sensitive to GTPγS. (A) Docked vacuoles no longer require Ypt7p, Rho1p or Cdc42p yet are still sensitive to GTPγS. Fusion reactions (180 µl) were incubated at 27°C in the presence of BATPA for 30 min (docked vacuoles). Lane 1, 30 µl was removed and incubated for 60 min at 27°C. Twenty-five microliters of Gdi1p, 5 µl of Gyp7p and 30 µl of Rdi1p were added to the rest of the reaction and incubated for 15 min at 27°C. The reaction was centrifuged (5 min, 10 000 g) and the vacuole pellet was resuspended in 150 µl of PS buffer supplemented with 125 mM KCl, 5 mM MgCl2, 30 µM Ca2+, 10 µM CoA. Aliquots (30 µl) were either placed on ice or added to tubes containing PS buffer, anti-Vam3p IgG, GTPγS or BAPTA and incubated at 27°C for 60 min prior to assaying for alkaline phosphatase. (B) Ypt7p, Rho1p and Cdc42p are extracted from docked vacuoles by Gdi1p, Gyp7p and Rdi1p. Fusion reactions (120 µl) were incubated at 27°C in the presence of BAPTA for 30 min (docked vacuoles). Samples (30 µl) were added to tubes containing 6 µl of PS buffer, 5 µl of Gdi1p and 1 µl of Gyp7p, 6 µl of Rdi1p or 5 µl of Gdi1p, 1 µl of Gyp7p and 6 µl of Rdi1p, and incubated at 27°C for 15 min. Samples were centrifuged for 5 min at 10 000 g and equal portions of the supernatant and pellet were analyzed by immunoblot analysis.
Discussion
Previous studies indicated that, in addition to the Rab GTPase Ypt7p, vacuole fusion requires a late-acting, GTPγS-sensitive factor (Eitzen et al., 2000). We have now identified two additional GTPases, Rho1p and Cdc42p, which are required for homotypic vacuole fusion, although each acts before the GTPγS-sensitive step. Four lines of evidence prove that both Rho1p and Cdc42p are required for the fusion reaction: (i) Rho1p and Cdc42p antibodies inhibit vacuole fusion (Figure 1A; Müller et al., 2001); (ii) Rdi1p treatment, which extracts Rho1p and Cdc42p from vacuole membranes, blocks vacuole fusion (Figures 2A and 3A); (iii) vacuoles isolated from RHO1 and CDC42 temperature-sensitive strains are thermolabile for fusion (Figure 1B and C); and (iv) these strains show vacuole fragmentation after a brief incubation at the non-permissive temperature (Figure 1E and F).
Our results and those of Müller et al. (2001) show that Cdc42p is required for vacuole docking, functioning between Ypt7p-mediated tethering and trans-SNARE pairing. Müller et al. (2001) have identified a specific site-directed cdc42-ts allele, cdc42-123, which affects vacuole fusion, whereas another, cdc42-124, does not. This also demonstrates that Rho proteins are capable of mediating multiple downstream effects. Additionally, Cdc42p interacts genetically with Nrf1p, which is involved in vacuole trafficking (Murray and Johnson, 2000, 2001). Nrf1p is a subunit of the VTC complex that interacts with the V-ATPase (Cohen et al., 1999), which has an important role in membrane fusion (Peters et al., 2001). Rho1p also functions to regulate the targeting of Sec3p, a component of the exocytic machinery, to the plasma membrane (Guo et al., 2001).
The Rho family of GTPases are thought to have a central role in vesicular trafficking pathways by controlling the organization of the actin cytoskeleton to spatially direct the transport of vesicles and to regulate endocytosis (Murphy et al., 1996; Kroschewski et al., 1999; Merrifield et al., 1999; Guo et al., 2001). However, our current study shows that Rho GTPases can also function to promote the fusion of isolated vacuoles in the absence of cytoskeleton or cytosol.
There are several possible roles for Rho proteins within our reaction. As was shown for Rho3p (Adamo et al., 1999; Robinson et al., 1999), Rho1p interacts with tethering complexes (Guo et al., 2001). Vacuole tethering complexes form specialized membrane docking domains (L.Wang, A.Merz, S.Seeley and W.Wickner, manuscript submitted), and Rho1p might be required for the maintenance of these complexes. Rho GTPases also regulate phosphatidylinositol 4,5-bisphosphate (PIP2) synthesis (Chong et al., 1994; Weernink et al., 2000). Vacuole docking needs PIP2 and this lipid is synthesized from PI by isolated vacuoles (Mayer et al., 2000). Rho proteins can also be stimulated by PIP2 to control actin dynamics (Higgs and Pollard, 2000; Rohatgi et al., 2000). Localized actin remodeling may be necessary as F-actin can either block or promote membrane fusion (Muallem et al., 1995; Ayscough, 2000; Jahraus et al., 2001).
Rho GTPases control actin structure through effector cascades (Hall, 1998). Cdc42p-regulated kinases (Cla4p and Ste20p in Saccharomyces cerevisiae) act on type I myosins (Myo3p and Myo5p in yeast) and regulate the complex of Wiskott–Aldrich syndrome proteins (Bee1p and Vrp1p) and Arp2/3p that controls actin polymerization and branching (Naqvi et al., 1998; Madania et al., 1999; Winter et al., 1999; Higgs and Pollard, 2000). Studies that exploit vacuole fragmentation as a phenotype of defective vacuole fusion have shown vacuole fragmentation in cells with deletions in the genes for Cla4p and Arc18p, as well as for Sac2p and Sac6p, which also modulate actin structure. Actin is found on the surface of our isolated vacuoles (P.Slusarewicz, A.Merz and W.Wickner, unpublished) and undergoes G to F conversion in conditions of our fusion reaction. Further studies will be needed to establish whether actin participates in vacuole docking and whether Rho GTPases regulate PIP2 biosynthesis and actin polymerization on vacuoles.
Materials and methods
Yeast strains and genetic modifications
Yeast strains used in this study are listed in Table I. To create PHO8 or PEP4 gene deletions, strains were grown in YPD to an OD600 ∼ 1.5 and transformed by the lithium acetate method (Gietz and Schiestl, 1995) using a linear DNA fragment containing the URA3 or HIS3 gene for PHO8 and the LEU2 gene for PEP4 flanked by 40 nucleotides of homology to the upstream and downstream region of the PHO8 or PEP4 genes. The oligonucleotides CCAGCATTACGGGACATTATTTGAACGCGCATTAGCAGCAAGATTGTACTGAGAGTGCAC and ATTAAATAATATGTGAAAAAAGAGGGAGAGTTAGATAGGACTGT GCGGTATTTCACACCG were used to PCR amplify the URA3 gene from plasmid pRS406 and the HIS3 gene from plasmid pRS403, and the oligonucleotides ACCTAGTATTTAATCCAAATAAAATTCAAACAAAAACCAAAGATTGTACTGAGAGTGCAC and TAGATGGCAGAAAAGGATAGGGCGGAGAAGTAAGAAAAGTCTGTGCGGTATTTCACACCG were used to PCR amplify the LEU2 gene from plasmid pRS405 (Brachmann et al., 1998). Transformants were selected on SD –ura, –his or –leu (Bio101) agar plates and screened for proper integration by PCR, using primers TAGCGATAAGCTTCGCGC and CGCGGATCCACGTGTCATGCGGTTAG for PHO8 and TGAGAAGCCTACCACGTA and AGCAGCATAGAACAATGGA for PEP4, which anneal to the upstream and downstream regions, respectively. Vacuoles were visualized in vivo by FM4-64 staining (Vida and Emr, 1995) with a Zeiss fluorescence microscope and Tmax 400 film (Kodak) used at an exposure index of 1600.
Table I. Yeast strains.
| Strain | Genotype | Source |
|---|---|---|
| BJ3505 | Mat α, ura3, trp1, his3, lys2, gal2, can, prb1-Δ1.6R, pep4::HIS3 | Elizabeth Jones |
| DKY6281 | Mat α, ura3, leu2, trp1, his3, lys2, suc2, pho8::TRP1 | Dan Klionsky |
| ONHY1 | Mat a, ura3, leu2, trp1, his3, ade2 | Enrico Cabib |
| NHY21 | Mat a, ura3, leu2, trp1, his3, ade2, rho1-104 | Enrico Cabib |
| TD4 | Mat a, ura3, leu2, trp1, his4, can | Gerald Fink |
| DJTD216A | Mat a, ura3, leu2, trp1, his4, gal2, cdc42-1 | ATCC |
| BY4742 | Mat α, his3Δ1, leu2Δ0, ura3Δ0, lys2Δ0 | Research Genetics |
| GEY6048 | ONHY1, pho8::URA3 | this study |
| GEY1658 | NHY21, pho8::URA3 | this study |
| GEY6038 | TD4, pho8::URA3 | this study |
| GEY2298 | DJTD2-16A, pho8::URA3 | this study |
| GEY6028 | BY4742, pho8::URA3 | this study |
| GEY0908 | BY4742, rho2::kanMX, pho8::HIS3 | this study |
| GEY1188 | BY4742, rho3::kanMX, pho8::URA3 | this study |
| GEY0558 | BY4742, rho4::kanMX, pep4::LEU2 | this study |
| GEY1808 | BY4742, rho5::kanMX, pho8::URA3 | this study |
Biochemicals
All biochemical reagents were dissolved or dialyzed in PS buffer (20 mM PIPES–KOH pH 6.8, 200 mM sorbitol). Anti-Rho1p (Drgonova et al., 1999), anti-Vam3p, anti-Sec17p, anti-Sec18p and anti-SecF antibody inhibition experiments used IgG, purified as previously described (Mayer et al., 1996). Anti-Ypt7p antibody was affinity purified. Anti-Cdc42p antibodies were purchased from Santa Cruz Biochemicals. Inhibitors were used at the following final concentrations: affinity-purified anti-Ypt7p antibodies (50 µg/ml), anti-Vam3p IgG (150 µg/ml), anti-Sec18p IgG (250 µg/ml), anti-Sec17p IgG (250 µg/ml), anti-Rho1p IgG (280 µg/ml), Gdi1p (100 µg/ml), Gyp7p (30 µg/ml), Rdi1p (500 µg/ml), Mg-GTPγS (3 mM) and BAPTA (3 mM).
Synthesis and purification of recombinant proteins
His6-Sec18p, Gyp7p and Gdi1p were purified as previously described (Garrett et al., 1994; Haas and Wickner, 1996; Eitzen et al., 2000). The RDI1 gene was PCR amplified from yeast genomic DNA using primers CGCGGATCCGCCGAAGAAAGTACCGACTT and CCGGAATTCATTAGTGATCTACTAGCTAAATC. The amplification product was digested with BamHI and EcoRI and cloned into pGEX-4T1 (Pharmacia) to create plasmid pGST-RDI. pGST-RDI was used to transform Escherichia coli BL21 and glutathione S-transferase (GST)–Rdi1p was purified (Ausubel et al., 1997). Proteins were buffer exchanged with P6 columns (Bio-Rad) into PS buffer.
Vacuole fusion reactions and reversible inhibition
Vacuoles were isolated from strains grown in YPD (plus 20 µg/ml adenine for ade2 strains) to an OD600 ∼ 0.9 (Haas, 1995). Standard vacuole fusion reactions contain 3 µg of vacuoles without proteinase A, 3 µg of vacuoles without alkaline phosphatase, 1 mM ATP, 40 mM creatine phosphate, 0.5 mg/ml creatine kinase, 125 mM KCl, 6 mM MgCl2, 10 µM CoA, 200 mM sorbitol, 20 mM PIPES–KOH pH 6.8 in 30 µl. Reactions were incubated for 90 min at 27°C prior to assaying for alkaline phosphatase activity (Haas, 1995). For reversible blocks of the priming, docking and fusion stages, 200 µg/ml anti-Sec18p IgG, 64 µg/ml anti-Ypt7p antibodies (raised against the peptide TEAFEDDYNDAINIRC) or 3 mM BAPTA was added, respectively. To reverse these blocks, 0.1 µg/ml His6-Sec18p, 67 µg/ml Ypt7 peptide or 3.5 mM CaCl2 was added after 30 min from 30× concentrated stock solutions.
Acknowledgments
Acknowledgements
We thank Scott Seeley and Alex Merz for helpful suggestions, Drs Enrico Cabib and Gerald Fink for providing strains, and Dr Cabib for providing Rho1p antibodies. This work was supported by a grant from the National Institute of General Medical Sciences.
References
- Adamo J.E., Rossi,G. and Brennwald,P. (1999) The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell, 10, 4121–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel F., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (1997) Short Protocols in Molecular Biology, 3rd edn. Section 16.8. John Wiley and Sons, Inc., New York, NY.
- Ayscough K.R. (2000) Endocytosis and the development of cell polarity in yeast require a dynamic F-actin cytoskeleton. Curr. Biol., 10, 1587–1590. [DOI] [PubMed] [Google Scholar]
- Bernstein B.W., DeWitt,M. and Bamburg,J.R. (1998) Actin disassembles reversibly during electrically induced recycling of synaptic vesicles in cultured neurons. Brain Res. Mol. Brain Res., 53, 236–251. [DOI] [PubMed] [Google Scholar]
- Bourne H.R., Sanders,D.A. and McCormick,F. (1991) The GTPase superfamily: conserved structure and molecular mechanism. Nature, 349, 117–127. [DOI] [PubMed] [Google Scholar]
- Brachmann C.B., Davies,A., Cost,G.J., Caputo,E., Li,J., Hieter,P. and Boeke,J.D. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast, 14, 115–132. [DOI] [PubMed] [Google Scholar]
- Brown A.M., O’Sullivan,A.J. and Gomperts,B.D. (1998) Induction of exocytosis from permeabilized mast cells by the guanosine triphosphatases Rac and Cdc42. Mol. Biol. Cell, 9, 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Drgonova,J. and Drgon,T. (1998) Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem., 67, 307–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavier P. and Goud,B. (1999) The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol., 11, 466–475. [DOI] [PubMed] [Google Scholar]
- Chong L.D., Traynor-Kaplan,A., Bokoch,G.M. and Schwartz,M.A. (1994) The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell, 79, 507–513. [DOI] [PubMed] [Google Scholar]
- Cohen A., Perzov,N., Nelson,H. and Nelson,N. (1999) A novel family of yeast chaperons involved in the distribution of V-ATPase and other membrane proteins. J. Biol. Chem., 274, 26885–26893. [DOI] [PubMed] [Google Scholar]
- Drgonova J., Drgon,T., Roh,D. and Cabib,E. (1999) The GTP-binding protein Rho1p is required for cell cycle progression and polarization of the yeast cell. J. Cell Biol., 146, 373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D.G. and Nelson,W.J. (1996) Origins of cell polarity. Cell, 84, 335–344. [DOI] [PubMed] [Google Scholar]
- Eitzen G., Will,E., Gallwitz,D., Haas,A. and Wickner,W. (2000) Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J., 19, 6713–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett M.D., Zahner,J.E., Cheney,C.M. and Novick,P.J. (1994) GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J., 13, 1718–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett W.S., Chen,L., Kroschewski,R., Ebersold,M., Turley,S., Trombetta,S., Galan,J.E. and Mellman,I. (2000) Developmental control of endocytosis in dendritic cells by Cdc42. Cell, 102, 325–334. [DOI] [PubMed] [Google Scholar]
- Gietz R.D. and Schiestl,R.H. (1995) Transforming yeast with DNA. Methods Mol. Cell. Biol., 5, 255–269. [Google Scholar]
- Govindan B., Bowser,R. and Novick,P. (1995) The role of Myo2p, a yeast class V myosin, in vesicular transport. J. Cell Biol., 128, 1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Tamanoi,F. and Novick,P. (2001) Spatial regulation of the exocyst complex by Rho1 GTPase. Nature Cell Biol., 3, 353–360. [DOI] [PubMed] [Google Scholar]
- Haas A. (1995) A quantitative assay to measure homotypic fusion in vitro. Methods Cell Sci., 17, 283–294. [Google Scholar]
- Haas A. and Wickner,W. (1996) Homotypic vacuole fusion requires Sec17p (yeast a-SNAP) and Sec18p (yeast NSF). EMBO J., 15, 3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Higgs H.N. and Pollard,T.D. (2000) Activation by Cdc42 and PIP(2) of Wiskott–Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol., 150, 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Geller E. and Cerione,R.A. (2000) Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP3/calcium pathway in RBL-2H3 mast cells. J. Cell Biol., 148, 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai J., Toh-E,A. and Matsui,Y. (1996) Genetic analysis of the Saccharomyces cerevisiae RHO3 gene, encoding a rho-type small GTPase, provides evidence for a role in bud formation. Genetics, 142, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahraus A. et al. (2001) ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol. Biol. Cell, 12, 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffer A., Tatham,P.E.R. and Gomperts,B.D. (1990) Changes in the state of actin during the exocytotic reaction of permeabilized rat mast cells. J. Cell Biol., 111, 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R., Hall,A. and Mellman,I. (1999) Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nature Cell Biol., 1, 8–13. [DOI] [PubMed] [Google Scholar]
- Lang T., Wacker,I., Wunderlich,I., Rohrbach,A., Giese,G., Soldati,T. and Almers,W. (2000) Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys. J., 78, 2863–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madania A., Dumoulin,P., Grava,S., Kitamoto,H., ScharerBrodbeck,C., Soulard,A., Moreau,V. and Winsor,B. (1999) The Saccharomyces cerevisiae homologue of human Wiskott–Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol. Biol. Cell, 10, 3521–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T., Tanaka,K., Nonaka,H., Yamochi,W., Maeda,A. and Takai,Y. (1994) Molecular cloning and characterization of yeast rho GDP dissociation inhibitor. J. Biol. Chem., 269, 19713–19718. [PubMed] [Google Scholar]
- Matozaki T., Nakanishi,H. and Takai,Y. (2000) Small G-protein networks: their crosstalk and signal cascades. Cell. Signal., 12, 515–524. [DOI] [PubMed] [Google Scholar]
- Mayer A. and Wickner,W. (1997) Docking of yeast vacuoles is catalysed by the ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF). J. Cell Biol., 136, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner,W. and Haas,A. (1996) Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell, 85, 83–94. [DOI] [PubMed] [Google Scholar]
- Mayer A., Scheglmann,D., Dove,S., Glatz,A., Wickner,W. and Haas,A. (2000) Phosphatidylinositol-(4,5)-bisphosphate regulates two steps of homotypic vacuole fusion. Mol. Biol. Cell, 11, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield C.J., Moss,S.E., Ballestrem,C., Imhof,B.A., Giese,G., Wunderlich,I. and Almers,W. (1999) Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nature Cell Biol., 1, 72–74. [DOI] [PubMed] [Google Scholar]
- Muallem S., Kwiatkowska,K., Xu,X. and Yin,H.L. (1995) Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J. Cell Biol., 128, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller O., Johnson,D.I. and Mayer,A. (2001) Cdc42p functions at the docking stage of yeast vacuole membrane fusion. EMBO J., 20, 5657–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. et al. (1996) Endosome dynamics regulated by a Rho protein. Nature, 384, 427–432. [DOI] [PubMed] [Google Scholar]
- Murray J.M. and Johnson,D.I. (2000) Isolation and characterization of Nrf1, a novel regulator of the Cdc42p GTPase in Schizosaccharo myces pombe. Genetics, 154, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.M. and Johnson,D.I. (2001) The Cdc42p GTPase and its regulators Nrf1p and Scd1p are involved in endocytic trafficking in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem., 276, 3004–3009. [DOI] [PubMed] [Google Scholar]
- Naqvi S.N., Zahn,R., Mitchell,D.A., Stevenson,B.J. and Munn,A.L. (1998) The WASp homologue Las17p functions with the WIP homologue End5p/verprolin and is essential for endocytosis in yeast. Curr. Biol., 8, 959–962. [DOI] [PubMed] [Google Scholar]
- Norman J.C., Price,L.S., Ridley,A.J., Hall,A. and Koffer,A. (1994) Actin filament organization in activated mast cells is regulated by heterotrimeric and small GTP-binding proteins. J. Cell Biol., 126, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C., Bayer,M.J., Buhler,S., Andersen,J.S., Mann,M. and Mayer,A. (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature, 409, 581–588. [DOI] [PubMed] [Google Scholar]
- Pruyne D.W., Schott,D.H. and Bretscher,A. (1998) Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol., 143, 1931–1945. [DOI] [PubMed] [Google Scholar]
- Robinson N.G., Guo,L., Imai,J., Toh-e,A., Matsui,Y. and Tamanoi,F. (1999) Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell. Biol., 19, 3580–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R., Ho,H.Y. and Kirschner,M.W. (2000) Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J. Cell Biol., 150, 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida T.A. and Emr,S.D. (1995) A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol., 128, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale M.L., Rodrigues del Castillo,A., Tchakarov,L. and Trifaro,J.-M. (1991) Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis, a phenomenon not exhibited by gelsolin. J. Cell Biol., 113, 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale M.L., Seward,E.P. and Trifaro,J.-M. (1995) Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron, 14, 353–363. [DOI] [PubMed] [Google Scholar]
- Weernink P.A., Guo,Y., Zhang,C., Schmidt,M., Eichel-Streiber,C. and Jakobs,K.H. (2000) Control of cellular phosphatidylinositol 4,5-bisphosphate levels by adhesion signals and rho GTPases in NIH 3T3 fibroblasts involvement of both phosphatidylinositol-4-phosphate 5-kinase and phospholipase C. Eur. J. Biochem., 267, 5237–5246. [DOI] [PubMed] [Google Scholar]
- Wickner W. and Haas,A. (2000) Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms. Annu. Rev. Biochem., 69, 247–275. [DOI] [PubMed] [Google Scholar]
- Winter D., Lechler,T. and Li,R. (1999) Activation of the yeast Arp2/3 complex by Bee1p, a WASP-family protein. Curr. Biol., 9, 501–504. [DOI] [PubMed] [Google Scholar]
- Yamochi W., Tanaka,K., Nonaka,H., Maeda,A., Musha,T. and Takai,Y. (1994) Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol., 124, 1077–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]