Abstract
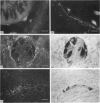
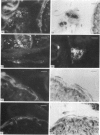
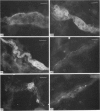
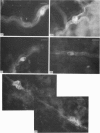
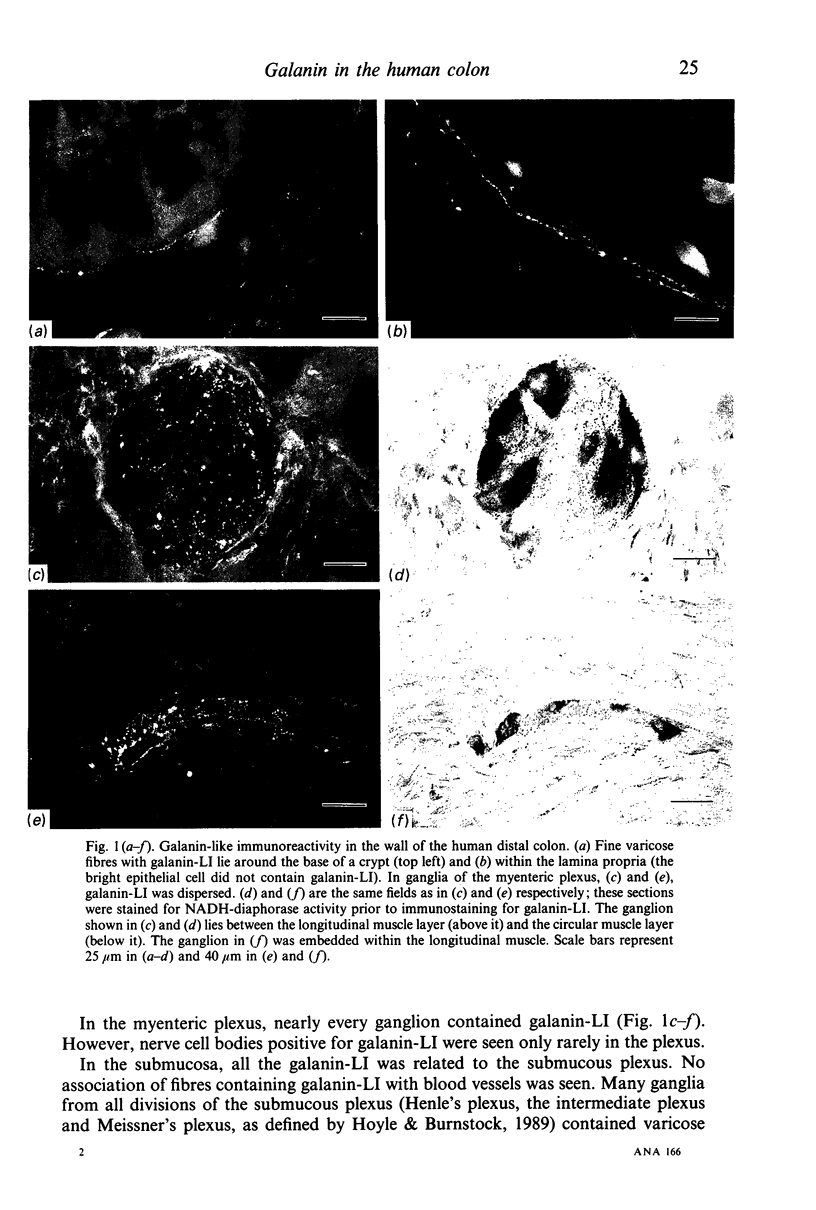
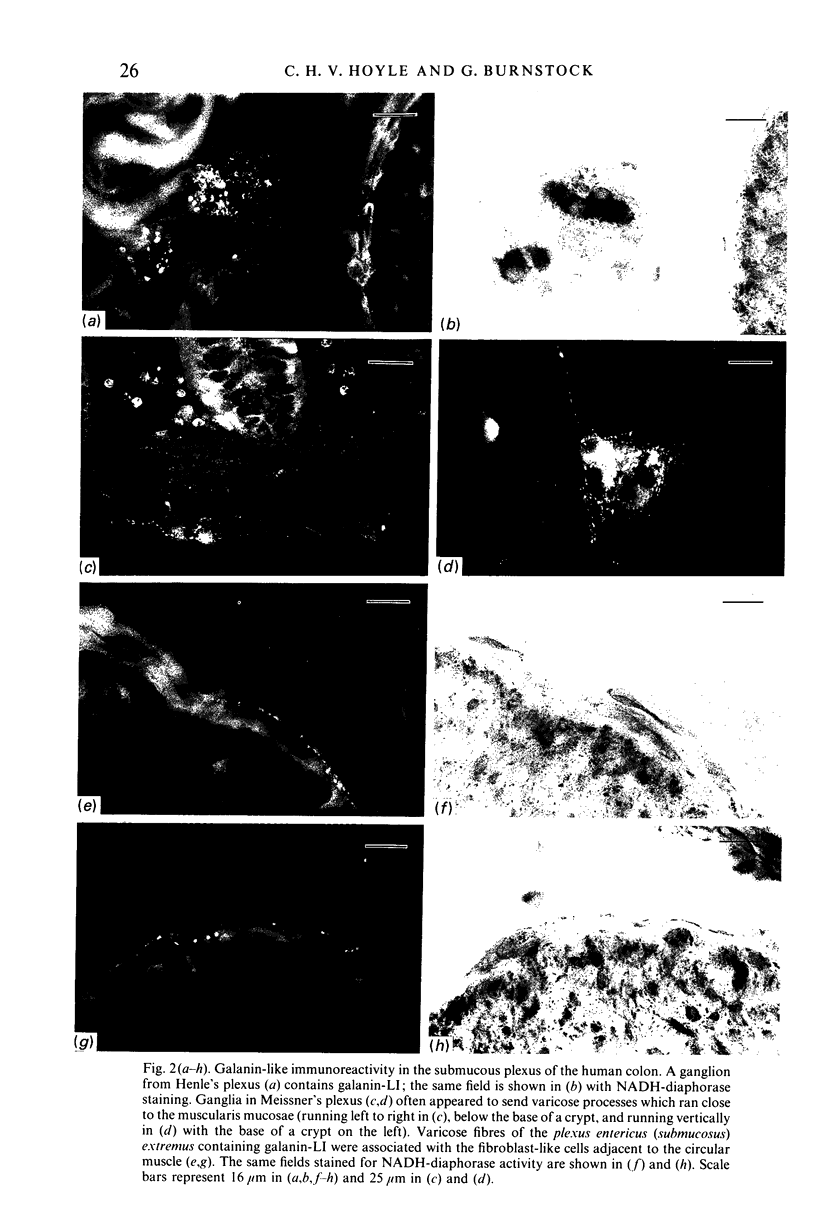
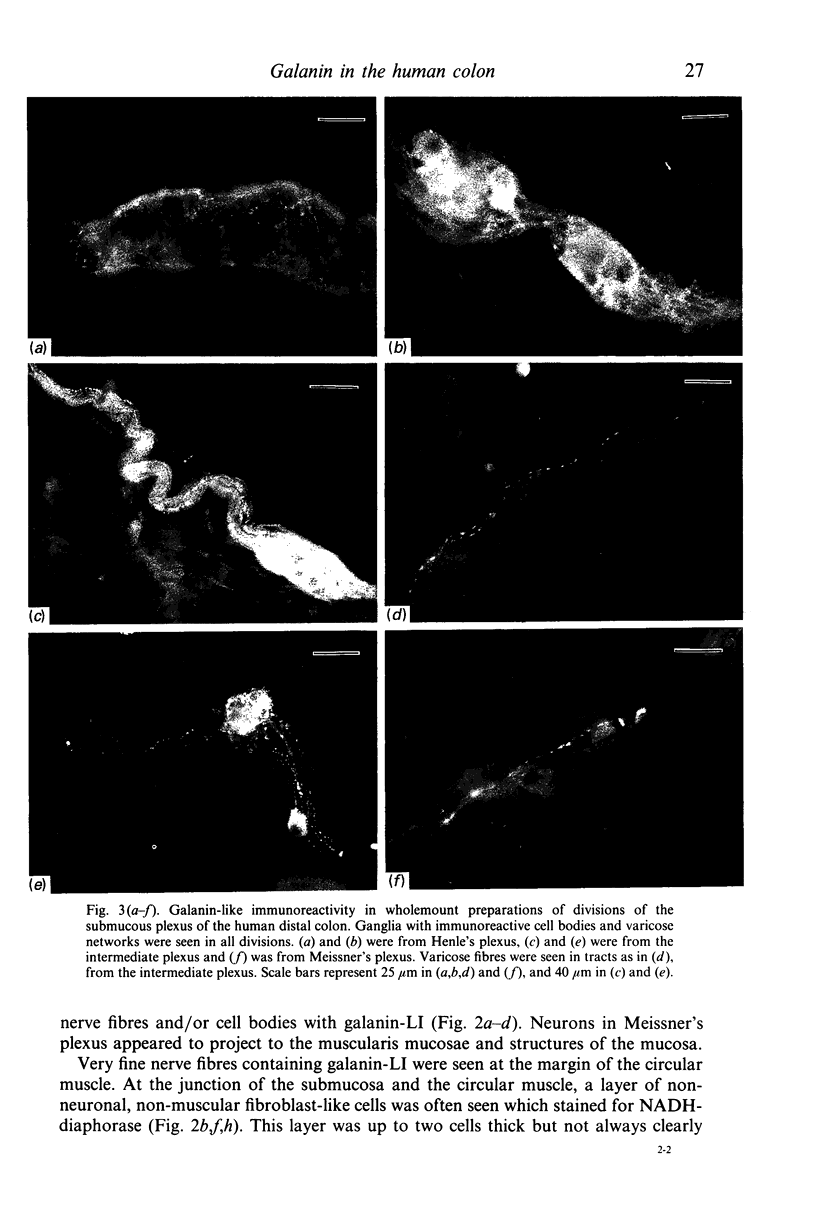
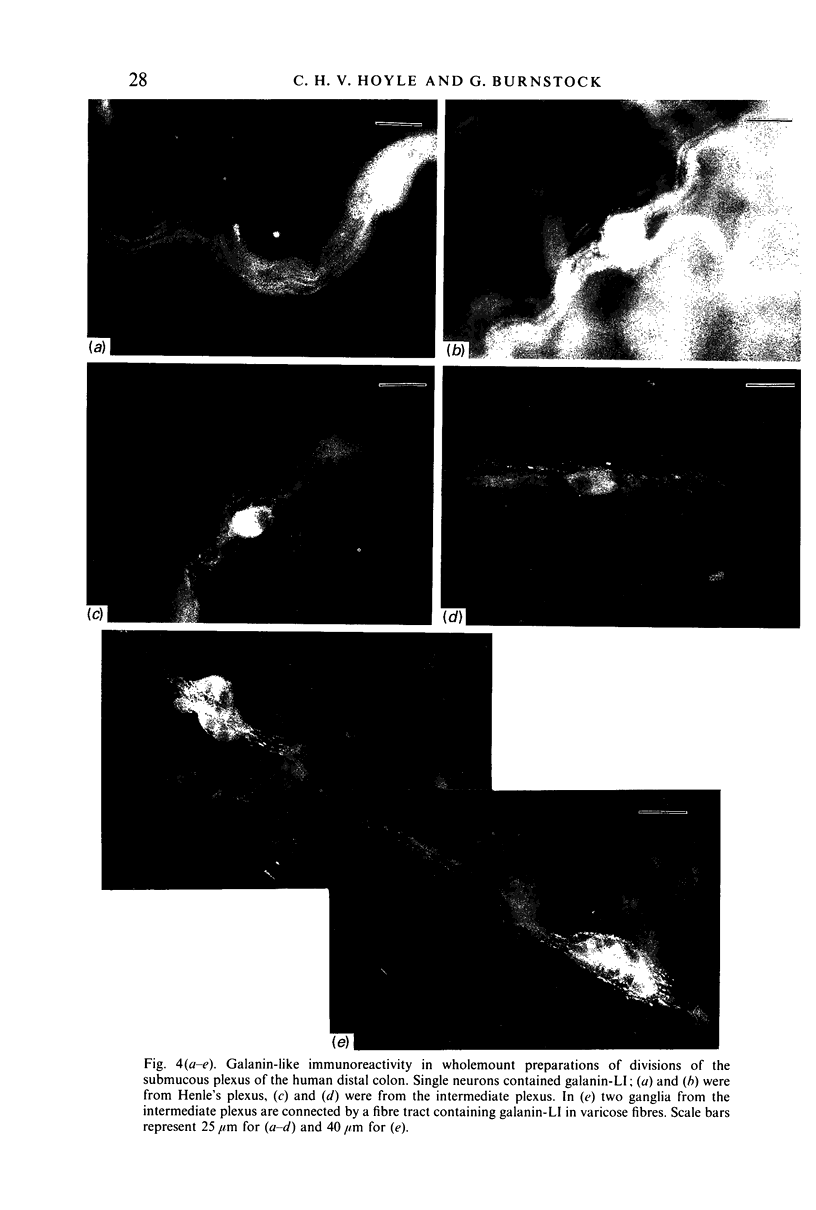
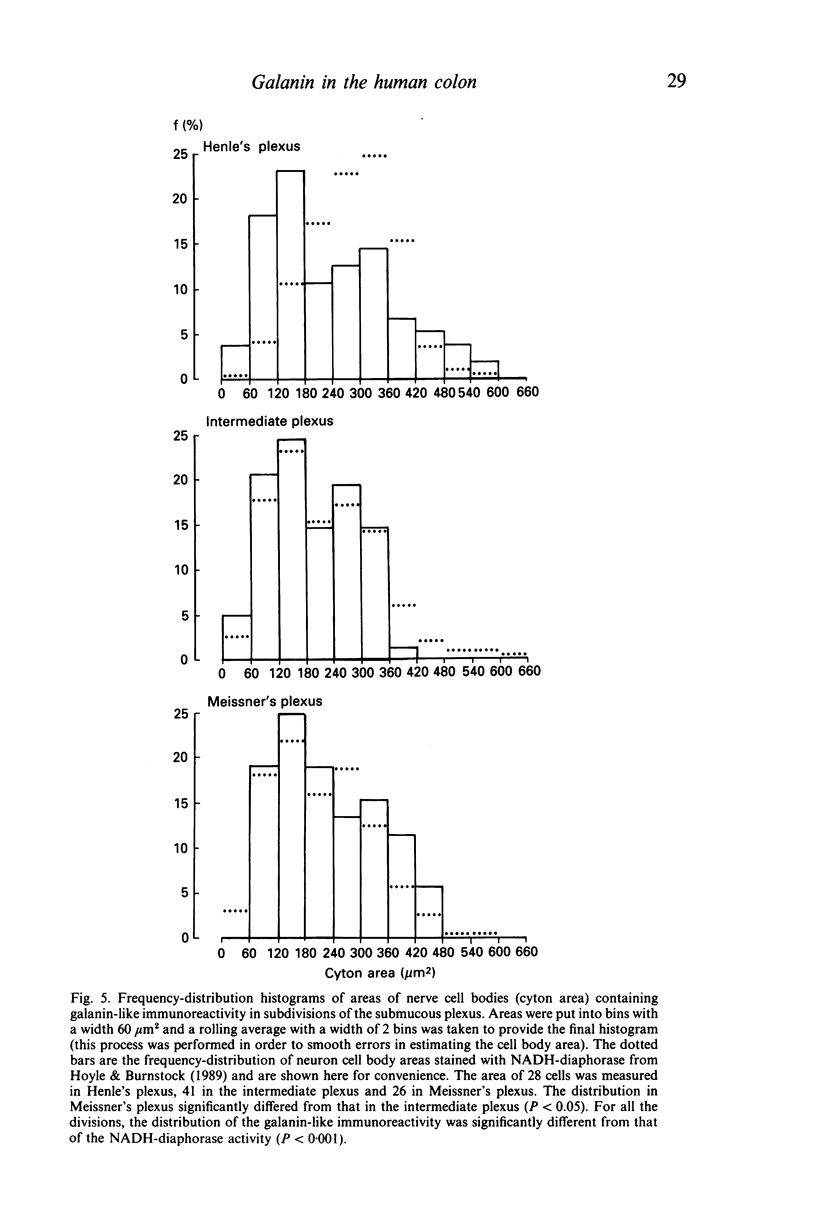
The distribution of galanin was investigated at the light microscopic level in the human distal colon using immunocytochemical techniques. Galanin-like immunoreactivity was seen in nerve cell bodies in ganglia of the myenteric and submucous plexuses and in nerve fibres innervating all the muscle layers of the colon, the lamina propria, and epithelial cells at the base of crypts or lining the colonic lumen. Immunoreactivity was more intense in the circular muscle than in the longitudinal muscle or the muscularis mucosae. Immunoreactive nerve cell bodies were much scarcer in the myenteric than in the submucous plexus. Within subdivisions of the submucous plexus, galanin-like immunoreactivity was heterogeneously distributed. In Henle's plexus and Meissner's plexus 82-83% of galanin-positive cell bodies were up to 360 microns 2 in profile-area, but in the intermediate plexus nearly all (99.8%) were below 360 microns 2. The frequency-distribution of cell body area of galanin-containing nerve cell bodies was similar for Henle's plexus and Meissner's plexus but these two plexuses contain different size-populations of neurons when stained for NADH-diaphorase activity. Galanin-like immunoreactive nerve fibres were found in the plexus entericus (submucosus) extremus, and this is the first report of neuropeptide in this location.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer F. E., Adrian T. E., Christofides N. D., Ferri G. L., Yanaihara N., Polak J. M., Bloom S. R. Distribution and molecular heterogeneity of galanin in human, pig, guinea pig, and rat gastrointestinal tracts. Gastroenterology. 1986 Oct;91(4):877–883. doi: 10.1016/0016-5085(86)90689-x. [DOI] [PubMed] [Google Scholar]
- Ch'ng J. L., Christofides N. D., Anand P., Gibson S. J., Allen Y. S., Su H. C., Tatemoto K., Morrison J. F., Polak J. M., Bloom S. R. Distribution of galanin immunoreactivity in the central nervous system and the responses of galanin-containing neuronal pathways to injury. Neuroscience. 1985 Oct;16(2):343–354. doi: 10.1016/0306-4522(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Christensen J., Rick G. A. Intrinsic nerves in the mammalian colon: confirmation of a plexus at the circular muscle-submucosal interface. J Auton Nerv Syst. 1987 Dec;21(2-3):223–231. doi: 10.1016/0165-1838(87)90025-7. [DOI] [PubMed] [Google Scholar]
- Couturier D., Roze C., Couturier-Turpin M. H., Debray C. Electromyography of the colon in situ. An experimental study in man and in the rabbit. Gastroenterology. 1969 Feb;56(2):317–322. [PubMed] [Google Scholar]
- Cowen T., Haven A. J., Burnstock G. Pontamine sky blue: a counterstain for background autofluorescence in fluorescence and immunofluorescence histochemistry. Histochemistry. 1985;82(3):205–208. doi: 10.1007/BF00501396. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Håkanson R., Sundler F., Wahlestedt C. Galanin: neuromodulatory and direct contractile effects on smooth muscle preparations. Br J Pharmacol. 1985 Sep;86(1):241–246. doi: 10.1111/j.1476-5381.1985.tb09455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E., Rökaeus A., Håkanson R., Sundler F. Galanin nerve fibers in the rat gut: distribution, origin and projections. Neuroscience. 1985 Oct;16(2):355–363. doi: 10.1016/0306-4522(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Faussone Pellegrini M. S., Cortesini C. Ultrastructural peculiarities of the inner portion of the circular layer of colon. I. Research in the human. Acta Anat (Basel) 1984;120(4):185–189. doi: 10.1159/000145918. [DOI] [PubMed] [Google Scholar]
- Faussone Pellegrini M. S. Ultrastructural peculiarities of the inner portion of the circular layer of the colon. II. Research on the mouse. Acta Anat (Basel) 1985;122(3):187–192. doi: 10.1159/000146000. [DOI] [PubMed] [Google Scholar]
- Fehér E., Burnstock G. Galanin-like immunoreactive nerve elements in the small intestine of the rat. An electron microscopic immunocytochemical study. Neurosci Lett. 1988 Oct 5;92(2):137–142. doi: 10.1016/0304-3940(88)90049-3. [DOI] [PubMed] [Google Scholar]
- Ferri G. L., Adrian T. E., Ghatei M. A., O'Shaughnessy D. J., Probert L., Lee Y. C., Buchan A. M., Polak J. M., Bloom S. R. Tissue localization and relative distribution of regulatory peptides in separated layers from the human bowel. Gastroenterology. 1983 Apr;84(4):777–786. [PubMed] [Google Scholar]
- Ferri G. L., Botti P. L., Vezzadini P., Biliotti G., Bloom S. R., Polak J. M. Peptide-containing innervation of the human intestinal mucosa. An immunocytochemical study on whole-mount preparations. Histochemistry. 1982;76(3):413–420. doi: 10.1007/BF00543961. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Rökaeus A., McDonald T. J., Brooks B. Galanin-immunoreactive neurons in the guinea-pig small intestine: their projections and relationships to other enteric neurons. Cell Tissue Res. 1987 Dec;250(3):607–615. doi: 10.1007/BF00218954. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Neuronal populations in the submucous plexus of the human colon. J Anat. 1989 Oct;166:7–22. [PMC free article] [PubMed] [Google Scholar]
- McDonald T. J., Dupre J., Tatemoto K., Greenberg G. R., Radziuk J., Mutt V. Galanin inhibits insulin secretion and induces hyperglycemia in dogs. Diabetes. 1985 Feb;34(2):192–196. doi: 10.2337/diab.34.2.192. [DOI] [PubMed] [Google Scholar]
- Melander T., Hökfelt T., Nilsson S., Brodin E. Visualization of galanin binding sites in the rat central nervous system. Eur J Pharmacol. 1986 May 27;124(3):381–382. doi: 10.1016/0014-2999(86)90247-5. [DOI] [PubMed] [Google Scholar]
- Melander T., Hökfelt T., Rökaeus A., Fahrenkrug J., Tatemoto K., Mutt V. Distribution of galanin-like immunoreactivity in the gastro-intestinal tract of several mammalian species. Cell Tissue Res. 1985;239(2):253–270. doi: 10.1007/BF00218003. [DOI] [PubMed] [Google Scholar]
- Narducci F., Bassotti G., Gaburri M., Morelli A. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut. 1987 Jan;28(1):17–25. doi: 10.1136/gut.28.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M., Schemann M., Tamura K., Wood J. D. Galanin mimics slow synaptic inhibition in myenteric neurons. Eur J Pharmacol. 1986 May 27;124(3):379–380. doi: 10.1016/0014-2999(86)90246-3. [DOI] [PubMed] [Google Scholar]
- Rökaeus A., Melander T., Hökfelt T., Lundberg J. M., Tatemoto K., Carlquist M., Mutt V. A galanin-like peptide in the central nervous system and intestine of the rat. Neurosci Lett. 1984 Jun 15;47(2):161–166. doi: 10.1016/0304-3940(84)90423-3. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D. M. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985 May-Jun;6(3):509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D. M. Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides. 1986 Jul-Aug;7(4):609–613. doi: 10.1016/0196-9781(86)90035-5. [DOI] [PubMed] [Google Scholar]
- Stach W. Der Plexus entericus extremus des Kickdarmes und seine Beziehungen zu den interstitiellen Zellen (Cajal. Z Mikrosk Anat Forsch. 1972;85(2):245–272. [PubMed] [Google Scholar]
- Tatemoto K., Rökaeus A., Jörnvall H., McDonald T. J., Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983 Nov 28;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- Taylor I., Duthie H. L., Smallwood R., Brown B. H., Linkens D. The effect of stimulation on the myoelectrical activity of the rectosigmoid in man. Gut. 1974 Aug;15(8):599–607. doi: 10.1136/gut.15.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanasin B., Ustach T. J., Schuster M. M. Electrical and motor activity of human and dog colon in vitro. Johns Hopkins Med J. 1974 Apr;134(4):201–210. [PubMed] [Google Scholar]
- Yau W. M., Dorsett J. A., Youther M. L. Evidence for galanin as an inhibitory neuropeptide on myenteric cholinergic neurons in the guinea pig small intestine. Neurosci Lett. 1986 Dec 23;72(3):305–308. doi: 10.1016/0304-3940(86)90531-8. [DOI] [PubMed] [Google Scholar]