Abstract
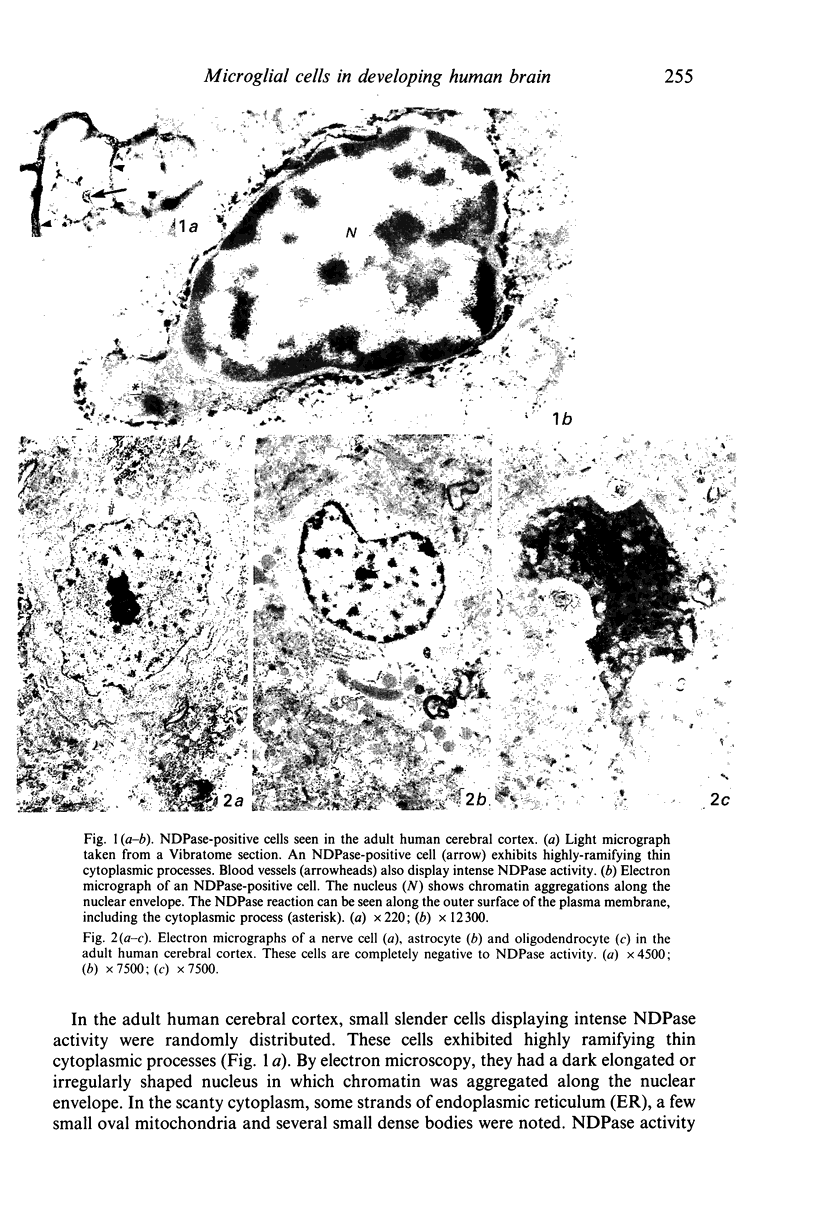
Applying nucleoside diphosphatase (NDPase) histochemistry, the appearance and differentiation of microglial cells in the developing human cerebral hemispheres were investigated by light and electron microscopy. In the pallium of the 38 days old human embryo, a few round NDPase-positive cells (round cells) were observed in the expanding zone. Although distinct blood vessels had not yet formed within the wall of the pallium, some cellular elements resembling haemopoietic cells were noticed in the expanding zone. In the 51 days old fetus, blood vessels displaying NDPase activity were seen in the mantle and marginal layers, and some invaded the matrix. Several round NDPase-positive cells were distributed, mainly around the vascular sprouts (primitive blood vessels) in the matrix. In the marginal layer, NDPase-positive cells exhibiting short cytoplasmic processes were encountered (poorly ramifying cells). In the 58, 66 and 82 days old fetuses, the round NDPase-positive cells were seen mainly in the matrix or subcortical layer where vascular sprouts were conspicuous and the poorly ramifying cells were in the subcortical and marginal layers. In the two latter fetuses, NDPase-positive cells showing long highly ramifying cytoplasmic processes (highly ramifying cells) were noted mainly in the marginal layer and sometimes in the subcortical layer. In the 5 months old fetuses, numerous NDPase-positive cells were distributed in the mantle, subcortical and marginal layers, and most of them appeared to belong to the populations of the poorly or highly ramifying cells. On the basis of the ultrastructural features, the round cells and highly ramifying cells were regarded as amoeboid cells and microglial cells, respectively. These findings suggest that at least some amoeboid cells are transformed into microglial cells via the stages of poorly ramifying microglial cells, and also that, in the human cerebral hemispheres, appearance of the microglial elements is closely related with vascularisation, especially in the early developmental stages.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsopp G., Gamble H. J. Light and electron microscopic observations on the development of the blood vascular system of the human brain. J Anat. 1979 May;128(Pt 3):461–477. [PMC free article] [PubMed] [Google Scholar]
- Barón M., Gallego A. The relation of the microglia with the pericytes in the cat cerebral cortex. Z Zellforsch Mikrosk Anat. 1972;128(1):42–57. doi: 10.1007/BF00306887. [DOI] [PubMed] [Google Scholar]
- Booz K. H., Felsing T. Uber ein transitorisches, perinatales subependymales Zellsystem der weissen Ratte. Z Anat Entwicklungsgesch. 1973;141(3):275–288. [PubMed] [Google Scholar]
- Boya J., Calvo J., Prado A. The origin of microglial cells. J Anat. 1979 Aug;129(Pt 1):177–186. [PMC free article] [PubMed] [Google Scholar]
- Choi B. H. Hematogenous cells in the central nervous system of developing human embryos and fetuses. J Comp Neurol. 1981 Mar 10;196(4):683–694. doi: 10.1002/cne.901960412. [DOI] [PubMed] [Google Scholar]
- FIELD E. J. Observations on the development of microglia together with a note on the influence of cortisone. J Anat. 1955 Apr;89(2):201–208. [PMC free article] [PubMed] [Google Scholar]
- Ferrer I., Sarmiento J. Nascent microglia in the developing brain. Acta Neuropathol. 1980;50(1):61–67. doi: 10.1007/BF00688537. [DOI] [PubMed] [Google Scholar]
- Fujimoto E., Miki A., Mizoguti H. Histochemical studies of the differentiation of microglial cells in the cerebral hemispheres of chick embryos and chicks. Histochemistry. 1987;87(3):209–216. doi: 10.1007/BF00492411. [DOI] [PubMed] [Google Scholar]
- Fujita S., Kitamura T. Origin of brain macrophages and the nature of the so-called microglia. Acta Neuropathol Suppl. 1975;Suppl 6:291–296. doi: 10.1007/978-3-662-08456-4_51. [DOI] [PubMed] [Google Scholar]
- Imamoto K., Fujiwara R., Nagai T., Maeda T. Distribution and fate of macrophagic ameboid cells in the rat brain. Arch Histol Jpn. 1982 Dec;45(5):505–518. doi: 10.1679/aohc.45.505. [DOI] [PubMed] [Google Scholar]
- Imamoto K., Leblond C. P. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol. 1978 Jul 1;180(1):139–163. doi: 10.1002/cne.901800109. [DOI] [PubMed] [Google Scholar]
- Imamoto K. Origin of microglia: cell transformation from blood monocytes into macrophagic ameboid cells and microglia. Prog Clin Biol Res. 1981;59A:125–139. [PubMed] [Google Scholar]
- Innocenti G. M., Clarke S., Koppel H. Transitory macrophages in the white matter of the developing visual cortex. II. Development and relations with axonal pathways. Brain Res. 1983 Dec;313(1):55–66. doi: 10.1016/0165-3806(83)90201-8. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M., Koppel H., Clarke S. Transitory macrophages in the white matter of the developing visual cortex. I. Light and electron microscopic characteristics and distribution. Brain Res. 1983 Dec;313(1):39–53. doi: 10.1016/0165-3806(83)90200-6. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Labelling of amoeboid microglial cells in rats of various ages following an intravenous injection of horseradish peroxidase. Acta Anat (Basel) 1986;125(2):132–137. doi: 10.1159/000146150. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Transformation of amoeboid microglial cells into microglia in the corpus callosum of the postnatal rat brain. An electron microscopical study. Arch Histol Jpn. 1985 Feb;48(1):17–25. doi: 10.1679/aohc.48.17. [DOI] [PubMed] [Google Scholar]
- Kitamura T. Dynamic aspects of glial reactions in altered brains. Pathol Res Pract. 1980;168(4):301–343. doi: 10.1016/S0344-0338(80)80271-8. [DOI] [PubMed] [Google Scholar]
- Kitamura T. The origin of brain macrophages--some considerations on the microglia theory of Del Rio-Hortega. Acta Pathol Jpn. 1973 Feb;23(1):11–26. doi: 10.1111/j.1440-1827.1973.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Leong S. K., Shieh J. Y., Ling E. A., Wong W. C. Labelling of amoeboid microglial cells in the supraventricular corpus callosum following the application of horseradish peroxidase in the cerebrum and spinal cord in rats. J Anat. 1983 Mar;136(Pt 2):367–377. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A. Cytochemical localization of peroxidase in amoeboid cells in the corpus callosum in postnatal rats. Arch Histol Jpn. 1980 Oct;43(4):305–310. doi: 10.1679/aohc1950.43.305. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Kaur C., Wong W. C. Light and electron microscopic demonstration of non-specific esterase in amoeboid microglial cells in the corpus callosum in postnatal rats: a cytochemical link to monocytes. J Anat. 1982 Sep;135(Pt 2):385–394. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A. Light and electron microscopic demonstration of some lysosomal enzymes in the amoeboid microglia in neonatal rat brain. J Anat. 1977 Jul;123(Pt 3):637–648. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Penney D., Leblond C. P. Use of carbon labeling to demonstrate the role of blood monocytes as precursors of the 'ameboid cells' present in the corpus callosum of postnatal rats. J Comp Neurol. 1980 Oct 1;193(3):631–657. doi: 10.1002/cne.901930304. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Transformation of monocytes into amoeboid microglia in the corpus callosum of postnatal rats, as shown by labelling monocytes by carbon particles. J Anat. 1979 Jun;128(Pt 4):847–858. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Ikuta F. Appearance and distribution of fetal brain macrophages in mice. Immunohistochemical study with a monoclonal antibody. Cell Tissue Res. 1985;239(2):271–278. doi: 10.1007/BF00218004. [DOI] [PubMed] [Google Scholar]
- Miyake T., Tsuchihashi Y., Kitamura T., Fujita S. Immunohistochemical studies of blood monocytes infiltrating into the neonatal rat brain. Acta Neuropathol. 1984;62(4):291–297. doi: 10.1007/BF00687611. [DOI] [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Identification of microglia in light and electron microscopy. J Comp Neurol. 1969 Jan;135(1):57–80. doi: 10.1002/cne.901350104. [DOI] [PubMed] [Google Scholar]
- Murabe Y., Sano Y. Morphological studies on neuroglia. V. Microglial cells in the cerebral cortex of the rat, with special reference to their possible involvement in synaptic function. Cell Tissue Res. 1982;223(3):493–506. doi: 10.1007/BF00218471. [DOI] [PubMed] [Google Scholar]
- Murabe Y., Sano Y. Morphological studies on neuroglia. VI. Postnatal development of microglial cells. Cell Tissue Res. 1982;225(3):469–485. doi: 10.1007/BF00214798. [DOI] [PubMed] [Google Scholar]
- Murabe Y., Sano Y. Thiaminepyrophosphatase activity in the plasma membrane of microglia. Histochemistry. 1981;71(1):45–52. doi: 10.1007/BF00592569. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., GOLDFISCHER S. Nucleosidediphosphatase activity in the Golgi apparatus and its usefulness for cytological studies. Proc Natl Acad Sci U S A. 1961 Jun 15;47:802–810. doi: 10.1073/pnas.47.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehmichen M. Are resting and/or reactive microglia macrophages? Immunobiology. 1982 Apr;161(3-4):246–254. doi: 10.1016/S0171-2985(82)80080-6. [DOI] [PubMed] [Google Scholar]
- Oehmichen M., Wiethölter H., Greaves M. F. Immunological analysis of human microglia: lack of monocytic and lymphoid membrane differentiation antigens. J Neuropathol Exp Neurol. 1979 Mar;38(2):99–103. doi: 10.1097/00005072-197903000-00002. [DOI] [PubMed] [Google Scholar]
- Schelper R. L., Adrian E. K., Jr Monocytes become macrophages; they do not become microglia: a light and electron microscopic autoradiographic study using 125-iododeoxyuridine. J Neuropathol Exp Neurol. 1986 Jan;45(1):1–19. doi: 10.1097/00005072-198601000-00001. [DOI] [PubMed] [Google Scholar]
- Schelper R. L., Adrian E. K., Jr Non-specific esterase activity in reactive cells in injured nervous tissue labeled with 3H-thymidine or 125iododeoxyuridine injected before injury. J Comp Neurol. 1980 Dec 15;194(4):829–844. doi: 10.1002/cne.901940408. [DOI] [PubMed] [Google Scholar]
- Stensaas L. J., Reichert W. H. Round and amoeboid microglial cells in the neonatal rabbit brain. Z Zellforsch Mikrosk Anat. 1971;119(2):147–163. doi: 10.1007/BF00324517. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. A developmental study of epiplexus cells and supraependymal cells and their possible relationship to microglia. Neuropathol Appl Neurobiol. 1978 Sep-Oct;4(5):307–322. doi: 10.1111/j.1365-2990.1978.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. Microglia in the human embryonic optic nerve. J Anat. 1984 Aug;139(Pt 1):81–91. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. Microglia in the prenatal mouse neostriatum and spinal cord. J Anat. 1981 Dec;133(Pt 4):499–512. [PMC free article] [PubMed] [Google Scholar]
- Tseng C. Y., Ling E. A., Wong W. C. Light and electron microscopic and cytochemical identification of amoeboid microglial cells in the brain of prenatal rats. J Anat. 1983 Jun;136(Pt 4):837–849. [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi Y., Kitamura T., Fujita S. Immunofluorescence studies of the monocytes in the injured rat brain. Acta Neuropathol. 1981;53(3):213–219. doi: 10.1007/BF00688024. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Gollahon K. A., Tilzer S. A., Vats T., Morantz R. A. The failure of microglia in normal brain to exhibit mononuclear phagocyte markers. J Neuropathol Exp Neurol. 1979 Jul;38(4):369–376. doi: 10.1097/00005072-197907000-00002. [DOI] [PubMed] [Google Scholar]








