Abstract
The import of proteins into the mitochondrial intermembrane space differs in various aspects from the classical import pathway into the matrix. Apocytochrome c defines one of several pathways known to reach the intermembrane space, yet the components and pathways involved in outer membrane translocation are poorly defined. Here, we report the reconstitution of the apocytochrome c import reaction using proteoliposomes harbouring purified components. Import specifically requires the protease-resistant part of the TOM complex and is driven by interactions of the apoprotein with internal parts of the complex (involving Tom40) and the ‘trans-side receptor’ cytochrome c haem lyase. Despite the necessity of TOM complex function, the translocation pathway of apocytochrome c does not overlap with that of presequence-containing preproteins. We conclude that the TOM complex is a universal preprotein translocase that mediates membrane passage of apocytochrome c and other preproteins along distinct pathways. Apocytochrome c may provide a paradigm for the import of other small proteins into the intermembrane space such as factors used in apoptosis and protection from stress.
Keywords: cytochrome c/intermembrane space/membrane translocation/porin/proteoliposomes
Introduction
Over the past two decades, it has become clear that the translocation of proteins into and across biological membranes requires the assistance of preprotein translocases (Schatz and Dobberstein, 1996). Generally, these translocases are organized as large complexes that contain surface-exposed preprotein receptors and membrane-embedded preprotein-conducting pores. Protein translocation into mitochondria is mediated by translocases in the outer (TOM complex) and inner (TIM complexes) membranes (Neupert, 1997; Koehler et al., 1999; Bauer et al., 2000; Pfanner and Geissler, 2001). The TOM complex is necessary and sufficient for insertion of outer membrane proteins and for translocation of constituents of the intermembrane space such as cytochrome haem lyases (Steiner et al., 1995; Lill and Neupert, 1996; Künkele et al., 1998a). During import of components of the inner membrane and the matrix, the TOM complex co-operates with the TIM complexes leading to simultaneous translocation of the polypeptide chain across both membranes. Translocation across the outer membrane occurs in at least two consecutive steps (Mayer et al., 1995c; Kanamori et al., 1999). First, the presequence interacts with the surface receptors Tom20/Tom22 (cis site). Then, the N-terminal part of the preprotein slides across the preprotein-conducting pore comprised by Tom40 to finally reach a binding site (trans site) on the internal face of the outer membrane (Bolliger et al., 1995; Mayer et al., 1995c; Moczko et al., 1997; Rapaport et al., 1997, 1998b; Kanamori et al., 1999).
The import of apocytochrome c into the mitochondrial intermembrane space differs from that of typical preproteins in several aspects (Stuart and Neupert, 1990; Dumont, 1996; Kranz et al., 1998). For instance, apocytochrome c lacks an N-terminal presequence and does not undergo proteolytic cleavage after import (Dumont et al., 1988; Nicholson et al., 1988). Import does not require external energy sources such as ATP hydrolysis and rather seems to be driven by the interaction of apocytochrome c with cytochrome c haem lyase (CCHL) acting as specific binding partner in the intermembrane space (termed ‘trans side receptor’) (Dumont et al., 1991, Mayer et al., 1995d). Furthermore, translocation occurs independently of protease-sensitive outer membrane proteins, in particular of the surface receptors of the TOM complex (Stuart et al., 1990; Mayer et al., 1995d). Translocation of apocytochrome c is unaffected by blocking the TOM complex with import-competent porin (Pfaller et al., 1988). Together, these findings have generally been taken to suggest that the apoprotein does not use the TOM complex as a translocase. Apocytochrome c possesses a high tendency to insert into lipid bilayers containing negatively charged phospholipids (de Kruijff et al., 1992; Jordi et al., 1992). Therefore, the apoprotein has been suspected to spontaneously pass across the membrane without assistance of membrane proteins. In fact, apocytochrome c is slowly degraded when added to liposomes containing enclosed trypsin (Rietveld and de Kruijff, 1984; Miao et al., 2001). This has been regarded as evidence that lipids suffice to achieve membrane passage of apocytochrome c. Recently the requirement of a protease-resistant outer membrane component for transport of apocytochrome c into the intermembrane space has been demonstrated using purified outer membrane vesicles (OMV) (Mayer et al., 1995d). Until now, the component mediating transport has not been identified.
The current investigation is aimed at (i) the identification of the translocase mediating apocytochrome c import and (ii) a better understanding of the translocation path way. We report the reconstitution of apocytochrome c translocation into proteoliposomes containing purified TOM complex and enclosed anti-apocytochrome c antibodies that served as high affinity trans-side receptors in the lumen of the proteoliposomes. Translocation of apocytochrome c was unaffected after blocking the default import pathway by addition of chemical amounts of preproteins. These results demonstrate that apocytoc uses the TOM complex for membrane passage. Yet, the protein follows a unique pathway that does not overlap with that used by other preproteins.
Results
Binding and import of apocytochrome c into outer membrane vesicles and into large unilamellar vesicles prepared by extrusion technology
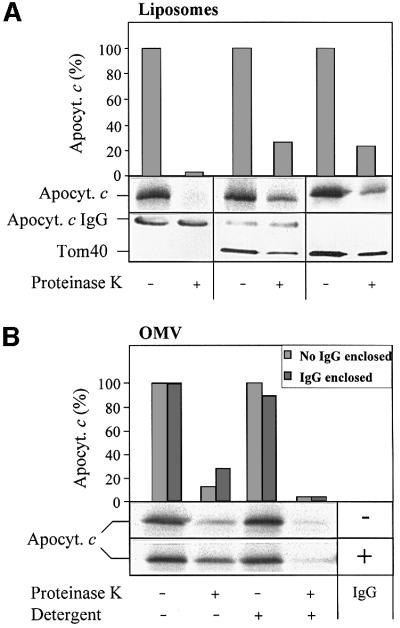
We first compared the ability of liposomes and purified OMV to bind and import apocytochrome c. To this end, large unilamellar vesicles comparable to OMV in both size and lipid composition (de Kroon et al., 1999) were prepared by an extrusion technique; these vesicles are the so-called large unilamellar vesicles prepared by extrusion technology (LUVET). In order to drive the translocation of the apoprotein across the membranes, IgGs specific for apocytochrome c were enclosed inside the LUVET and OMV by a freeze–thaw technique (Mayer et al., 1995a,d). IgG that was not entrapped in the lumen of the vesicles was removed by flotation centrifugation. Various amounts of the LUVET or OMV preparations were then used to analyse binding and import of [35S]methionine labelled apocytochrome c. To analyse the binding, material that was not associated with the vesicles was removed by membrane sedimentation. The resulting pellets were dissolved in buffer, proteins were precipitated by trichloroacetic acid (TCA) and the amount of bound apoprotein was analysed by SDS–PAGE and fluorography (Figure 1A and B, upper panels). Apocytochrome c bound efficiently to both LUVET and OMV. Quantification of binding by phosphorimager analysis shows that binding to LUVET was somewhat less efficient than that to OMV, but at higher lipid concentrations, a similar plateau of binding was reached with both vesicle types (Figure 1C). Binding was independent of the presence or absence of enclosed IgG (not shown).
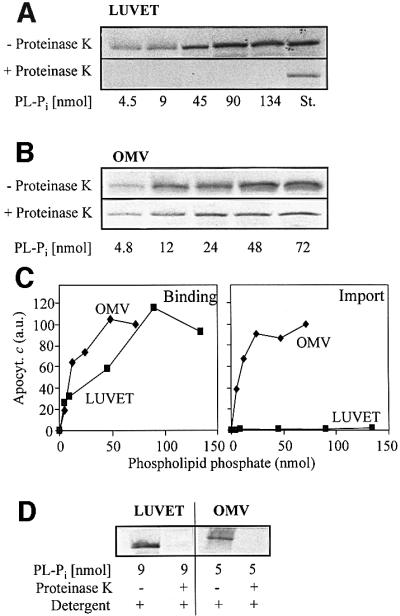
Fig. 1. Apocytochrome c binds to both LUVET and OMV, but is only imported into OMV. Anti-apocytochrome c IgGs were enclosed into LUVET or purified OMV by a freeze–thaw procedure. After flotation centrifugation to remove non-enclosed IgG, the indicated amounts of LUVET or OMV (based on phospholipid-bound phosphate, PL-Pi) were incubated with radiolabelled apocytochrome c for 10 min at 25°C. Half the samples were centrifuged to re-isolate the membranes (Binding). The other half was incubated for 20 min on ice with proteinase K (50 µg/ml) to assay the import of apocytochrome c. Protease digestion was halted by addition of 2 mM PMSF followed by TCA precipitation. The proteins were separated by SDS–PAGE. Bound or imported apocytochrome c was visualized by autoradiography of the gel (A and B) and quantitated by phosphorimager analysis. (C) Binding or import of apocytochrome c in OMV containing 72 nmol of PL-Pi was set to 100. (D) Control experiments were performed to verify that apocytochrome c was not aggregated and degraded by proteinase K after addition of 0.1% Triton X-100 (Detergent). St., a standard containing 50% of added apocytochrome c; a.u., arbitrary units.
Import of apocytochrome c was tested by treating the vesicles with proteinase K after the import reaction. No protease-resistant material was found associated with LUVET, not even at higher lipid concentrations (Figure 1A, lower panel). In contrast, about a third of the OMV-bound apoprotein was resistant to proteolytic attack indicating import into the OMV (Figure 1B, lower panel). Upon lysis of the OMV by detergent, apocytochrome c was completely degraded. This excludes the possibility that OMV-associated apocytochrome c was protease resistant due to aggregation (Figure 1D). These data demonstrate that apocytochrome c can efficiently bind to the surface of phospholipid membranes. For complete translocation across the lipid bilayer, however, a proteinaceous component of the mitochondrial outer membrane is necessary.
The TOM complex is necessary for import of apocytochrome c
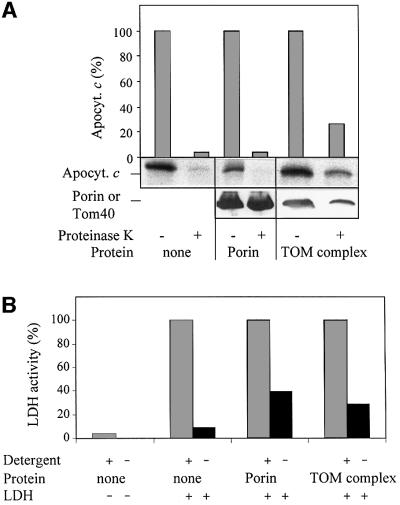
Which component of the outer membrane might be able to facilitate membrane passage of apocytochrome c? We tested the possible involvement of the TOM complex in mediating translocation of apocytochrome c. Proteo liposomes were formed by diluting detergent solutions of purified TOM core complex [containing Tom40, Tom22, Tom7 and Tom6 but depleted in Tom20 and Tom70 (Ahting et al., 1999, 2001)] and phospholipids below the critical micellar concentration (CMC). The proteoliposomes were harvested by centrifugation. IgG directed against apocytochrome c was enclosed into the proteoliposomes by a freeze–thaw technique; import was followed after adding radiolabelled apocytochrome c as described above. About 30% of total added apoprotein became resistant to proteolytic attack indicating that the TOM complex could support efficient import into the proteoliposomes (Figure 2A). The imported apoprotein was completely degraded upon lysis of the liposomes by the addition of detergent. In contrast, no significant amounts of protease-protected apocytochrome c were detectable when liposomes were prepared by detergent dilution in the absence of TOM complex (Figure 2B). Hence, the TOM complex is able to support membrane translocation of apocytochrome c.
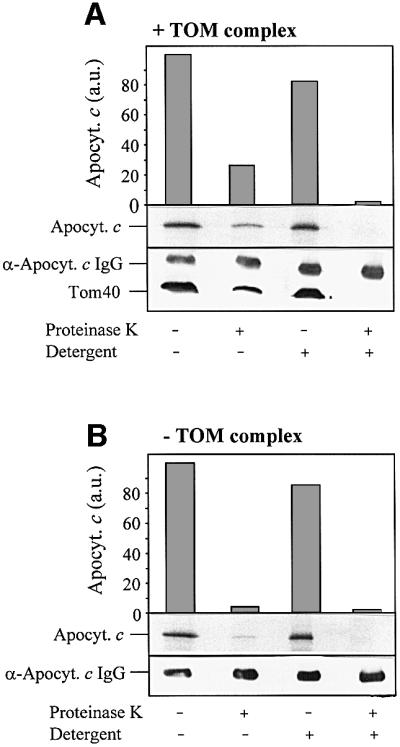
Fig. 2. The TOM complex is necessary for apocytochrome c translocation. (A) TOM complex-containing proteoliposomes. (B) Liposomes (each corresponding to 300 nmol phospholipid-bound phosphate) were prepared by detergent dilution; anti-apocytochrome c IgG was enclosed by a freeze–thaw technique. Radiolabelled apocytochrome c was added. After 10 min at 25°C, the samples were split and treated with proteinase K in the presence or absence of Triton X-100 (Detergent). Proteins were precipitated with TCA, separated by SDS–PAGE and blotted on to nitrocellulose. Quantitation was by phosphorimager analysis. Immunostaining was performed to visualize Tom40 (A) and the enclosed IgG. a.u., arbitrary units.
Import of apocytochrome c was reported to occur independently of protease-sensitive components of the mitochondrial outer membrane (Nicholson et al., 1988; Stuart et al., 1990; Mayer et al., 1995d). We therefore tested whether the preprotein receptor Tom22 is necessary for membrane translocation of apocytochrome c. After generation of the TOM complex-containing proteoliposomes and inclusion of anti-apocytochrome c IgG, the vesicles were treated with trypsin. The protease removes the surface-exposed parts of Tom22, Tom7 and Tom6, but leaves Tom40 intact (Figure 3, bottom; compare with Ahting et al., 1999). Import of apocytochrome c into these vesicles was as efficient as that of import into untreated proteoliposomes (Figure 3, top panel). The surface receptors, in particular Tom22, are apparently not required for TOM complex-mediated membrane translocation of apocytochrome c. Hence, our results using TOM complex-reconstituted proteoliposomes closely resemble the situation obtained for apocytochrome c translocation into intact mitochondria or OMV (see above; Mayer et al., 1995d).
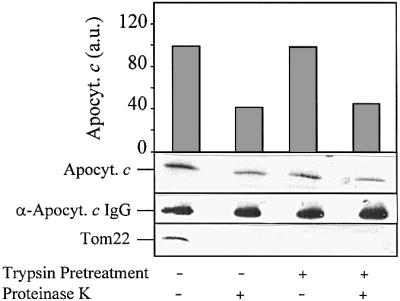
Fig. 3. Apocytochrome c translocation does not require protease-sensitive components of the TOM complex. After insertion of the TOM complex and enclosure of anti-apocytochrome c IgG, the proteoliposomes were incubated with 50 µg/ml trypsin for 15 min at 0°C. Soybean trypsin inhibitor (1 mg/ml) was added and the liposomes were re-isolated by flotation centrifugation. Import of apocytochrome c and further analysis was performed as in Figure 2. Anti-Tom22 antibodies were used for immunostaining. a.u., arbitrary units.
Porin does not mediate membrane translocation of apocytochrome c
The TOM complex contains a large preprotein-conducting pore that is formed by protease-resistant parts of the complex (Hill et al., 1998; Künkele et al., 1998a,b; Ahting et al., 2001). A pore of similar size is present in the mitochondrial outer membrane component porin (also termed VDAC) (Benz, 1994). We therefore asked whether a large pore such as porin might be sufficient for membrane translocation of apocytochrome c or whether the TOM complex is specifically required for import. To answer this question, purified porin was incorporated into proteoliposomes and anti-apocytochrome c IgG was enclosed. Import of apocytochrome c was not detectable in proteoliposomes containing porin (Figure 4A), and was only seen in vesicles harbouring the TOM complex.
Fig. 4. Membrane-embedded porin does not mediate translocation of apocytochrome c. (A) Liposomes were prepared by detergent dilution containing either no protein, purified porin or purified TOM complex, and anti-apocytochrome c antibodies were enclosed by a freeze–thaw technique. After an import reaction with radiolabelled apocytochrome c as in Figure 2, the samples were precipitated with TCA and subjected to SDS–PAGE. Proteins were blotted on to nitrocellulose and immunostained with antibodies against porin or Tom40. (B) LDH (50 µg/ml) was enclosed in the liposomes prepared as in (A) by a freeze–thaw technique. LDH activity was measured at 340 nm in SEM buffer (250 mM sucrose, 1 mM EDTA, 10 mM MOPS–KOH pH 7.0) by adding pyruvate (23 mM) and NADH (12 mM). To determine the total LDH activity per sample, the liposomes were lysed by adding 0.2% Triton X-100; this LDH activity was set to 100.
To verify the functionality of the purified porin preparation, we performed two control experiments. First, porin incorporated into black lipid membranes was found to be electrophysiologically active and formed the characteristic voltage-dependent channels (Benz, 1994; data not shown). Secondly, the porin- or TOM complex-containing vesicles were used for enclosure of lactate dehydrogenase (LDH) by a freeze–thaw technique. Non-enclosed enzyme was removed by flotation centrifugation. The resulting vesicles were used to test the accessibility of the enclosed enzyme for the externally added substrates nicotinamide adenine dinucleotide (NADH) and pyruvate. In the absence of added membrane proteins, hardly any LDH activity was detectable, indicating that the liposomes were tightly sealed and did not allow access of the substrates to the enclosed enzyme (Figure 4B). LDH was active after opening the liposomes by the addition of detergent.
When the liposomes contained porin, high LDH activity was measured (40% in comparison with the activity obtained after detergent lysis of the proteoliposomes; Figure 4B). This suggests strongly that porin was functionally reconstituted in the liposomes, i.e. mediating membrane passage of the low molecular mass substrates of LDH. A similar result was obtained with liposomes harbouring purified TOM complex, thus demonstrating that the preprotein-conducting pore of this complex is permeable for the enzyme substrates (Figure 4B). This result is in perfect agreement with a recent study demonstrating the importance of the TOM complex for metabolite trafficking in the absence of functional porin in intact mitochondria (Kmita and Budzinska, 2000). In conclusion, both the TOM complex and porin are capable of mediating membrane transport of small molecular mass compounds. However, only the TOM complex allows translocation of apocytochrome c across the lipid bilayer, demonstrating its specific function in membrane passage of apocytochrome c.
Interaction of apocytochrome c with the TOM complex is sufficient for its membrane passage
The function of the TOM complex in the translocation of apocytochrome c across the mitochondrial outer membrane suggests a direct interaction between these components. We therefore asked whether the TOM complex is able to mediate membrane passage of apocytochrome c in the absence of a trans-side receptor, i.e. IgG directed against apocytochrome c. To this end, liposomes formed with and without reconstituted TOM complex were loaded with anti-apocytochrome c IgG or left untreated and an import reaction was performed. Strikingly, even in the absence of enclosed IgG, a substantial amount of apocytochrome c became protease resistant (Figure 5A). The amount of protease-inaccessible material increased only slightly upon enclosure of IgG. Thus, the TOM complex appears to be sufficient for membrane translocation of apocytochrome c. These results support a direct interaction of apocytochrome c with the TOM complex preceding the association of the apoprotein with its trans-side receptor in the lumen of the vesicles (see below).
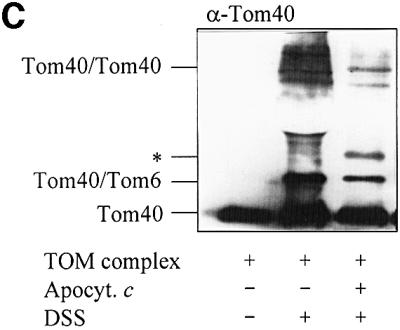
Fig. 5. The TOM complex is sufficient for membrane translocation of apocytochrome c. (A) Liposomes with or without reconstituted TOM complex and enclosed IgG were incubated with radiolabelled apocytochrome c; import was performed and analysed as described in Figure 2. (B) Purified OMV (15 µg/sample) with and without enclosed anti-apocytochrome c IgG were incubated with radiolabelled apocytochrome c and an import reaction was performed. Half the samples were lysed with Triton X-100 and import was analysed as in Figure 2. (C) Crosslinking of apocytochrome c with the TOM complex. Isolated TOM complex (6 µg) was incubated with or without apocytochrome c (10 µg) in the presence or absence of 0.25 mM DSS for 30 min on ice. The reaction was stopped by the addition of 5 mM Tris–HCl pH 7.0. The proteins were precipitated with TCA, separated by SDS–PAGE, blotted on to nitrocellulose and immunostained with anti-Tom40 antiserum. The crosslinking products are indicated (see Rapaport et al., 1998a). The asterisk indicates the crosslinking adduct between apocytochrome c and Tom40. The apparent molecular mass of this crosslinked product (∼52 kDa) fits well to an adduct between these two proteins. We tried to confirm the identity of apocytochrome c by immunostaining. However, the presence of a cross-reacting band (possibly a tetramer of apocytochrome c) in this molecular mass region (even in the absence of TOM complex) made this analysis impossible (data not shown).
Dependence of apocytochrome c translocation upon the presence of enclosed anti-apocytochrome c IgG has previously been reported for an OMV import system (Mayer et al., 1995d). These findings are at apparent variance with the results obtained above. We therefore re-examined the import of apocytochrome c into OMV that were or were not loaded with anti-apocytochrome c IgG. We were able to reproduce the previous findings with apocytochrome c of Neurospora crassa, i.e. import was dependent upon the enclosure of IgG (not shown; compare with Mayer et al., 1995d). With the standard precursor of this study, the apoprotein of Saccharomyces cerevisiae, however, two major differences were observed. First, its import efficiency was considerably higher than that of the N.crassa precursor (30–40% versus 10%, respectively, relative to OMV-bound material; Figure 5B). Secondly, significant import of the yeast apoprotein was detected even in the absence of enclosed anti-apocytochrome c antibodies. Nevertheless, the presence of IgG increased the efficiency of import 2- to 3-fold, indicating that the apoprotein was pulled into the lumen of the vesicles by consecutive interactions with the TOM complex and with anti-apocytochrome c IgG.
We examined the putative interaction between apocytochrome c and the TOM complex by a crosslinking approach. Purified TOM complex was incubated with or without chemical amounts of apocytochrome c in the presence of the homo-bifunctional crosslinker disuccinyldisuberate (DSS). After crosslinking, the samples were analysed by immunostaining for Tom40. In the absence of apocytochrome c, characteristic crosslinking products between Tom40 and Tom6 as well as between two Tom40 molecules were formed as described previously (Figure 5C; Rapaport et al., 1998a). Addition of apocytochrome c characteristically altered the crosslinking pattern, indicating an influence of added apocytochrome c on the interactions between the TOM complex components. A strikingly similar behaviour has been reported for the interaction of a presequence-containing preprotein or a presequence peptide with the TOM complex (Rapaport et al., 1998a). Thus, the TOM complex seems to undergo dynamic changes upon interaction with apocytochrome c. The vicinity of apocytochrome c and Tom40 is indicated by the presence of a Tom40 crosslinking band observed only upon addition of apocytochrome c (Figure 5C, *). Its molecular mass fits well with an adduct between Tom40 and apocytochrome c. We conclude from these data that apocytochrome c interacts with the TOM complex via Tom40. Protease resistance of the imported apoprotein indicates that the interaction takes place either within the outer membrane or on the intermembrane space side of the outer membrane. Consequently, mitochondrial import of apocytochrome c may be driven by consecutive interactions with (i) the TOM complex mediating membrane passage and (ii) anti-apocytochrome c antibodies serving as a trans-side receptor.
The import pathways of apocytochrome c and of presequence-containing preproteins do not overlap
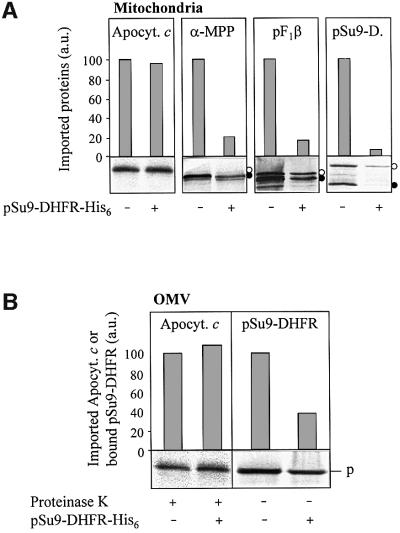
Does apocytochrome c use a pathway similar to or distinct from that taken by presequence-containing preproteins? To distinguish between the two possibilities, we purified chemical amounts of a presequence-containing preprotein (pSu9-DHFR-His6) that undergoes a specific, high-affinity interaction with the TOM complex (Stan et al., 2000). The preprotein was used to block the import sites of either intact isolated mitochondria or of OMV (Mayer et al., 1995b; Rapaport et al., 1998b); the residual import efficiency of radiolabelled apocytochrome c was then tested. No significant reduction of the import efficiency of apocytochrome c was detectable in the presence of the preprotein (Figure 6). To verify the successful occupancy of the import sites by pSu9-DHFR-His6, various radiolabelled preproteins were imported in parallel experiments. Import of these preproteins into intact mitochondria (Figure 6A; see Künkele et al., 1998a; Rapaport and Neupert, 1999) and presequence translocation of pSu9-DHFR in OMV (Figure 6B; see Mayer et al., 1995c) was strongly inhibited by the addition of pSu9-DHFR-His6, demonstrating that the import sites were occupied to a large extent by this preprotein. Evidently, apocytochrome c follows an import pathway that does not detectably overlap with that of canonical mitochondrial preproteins, even though both types of preproteins depend on TOM complex function for membrane translocation. We conclude that the TOM complex offers more than one pathway to move preproteins across the mitochondrial outer membrane.
Fig. 6. Chemical amounts of pSu9-DHFR-His6 do not inhibit apocytochrome c translocation. (A) Isolated mitochondria (50 µg/sample) or (B) purified OMV (15 µg/sample) were incubated with radiolabelled apocytochrome c, the precursors (open circles in the autoradiographs) of matrix processing peptidase α-subunit (α-MPP), F1-ATPase β-subunit (pF1β) or Su9-DHFR as indicated, in the presence or absence of chemical amounts of pSu9-DHFR-His6 (4 µM final concentration; Künkele et al., 1998a). After 6 min at 25°C, mitochondria and OMV were re-isolated by centrifugation. Following proteinase K treatment (for apocytochrome c), the proteins were precipitated with TCA, analysed by SDS–PAGE, blotted on to nitrocellulose and subjected to phosphorimager analysis and autoradiography. Import into mitochondria (A) was estimated from the amounts of protease-resistant apocytochrome c and of the processed (mature) forms of the preproteins (closed circles). The signal in the absence of added pSu9-DHFR-His6 was set to 100. Translocation of the presequence of pSu9-DHFR in OMV (B) was taken from the amount of bound preprotein after pelleting the membranes in the presence of 100 mM NaCl (trans site binding; Mayer et al., 1995c). p, precursor; a.u., arbitrary units.
Discussion
In this investigation, we have reconstituted the import of apocytochrome c into the mitochondrial intermembrane space using proteoliposomes containing purified components. We show that apocytochrome c can interact efficiently with the lipid surface, but it is not translocated across the bilayer. For full membrane translocation, the protease-resistant part of the TOM complex is both necessary and sufficient. Entry into the lumen of the vesicles is driven by binding of the translocating apoprotein to the enclosed anti-apocytochrome c antibodies. In vivo, this trans-side receptor function is provided by CCHL (Dumont et al., 1991; Mayer et al., 1995d). The reconstitution of the import reaction of apocytochrome c solves the long-debated question as to which membrane constituents are required for translocation of the apoprotein (de Kruijff et al., 1992; Dumont, 1996; Kranz et al., 1998). Apocytochrome c has been suspected to be transferred across the mitochondrial outer membrane without the help of proteinaceous factors, but instead using negatively charged phospholipids for membrane translocation. This proposal was based on a number of observations including the strong tendency of the apoprotein to insert into the lipid bilayer (see for example Snel et al., 1994), the fact that import into mitochondria was not affected by proteolytic treatment of the organelles (Nicholson et al., 1988; Mayer et al., 1995d) and the finding that trypsin enclosed inside liposomes could promote digestion of apocytochrome c added on the outside (Rietveld and de Kruijff, 1984). In the present study, we succeeded in identifying the protease-resistant portion of the TOM complex as a proteinaceous factor facilitating complete translocation into the intermembrane space.
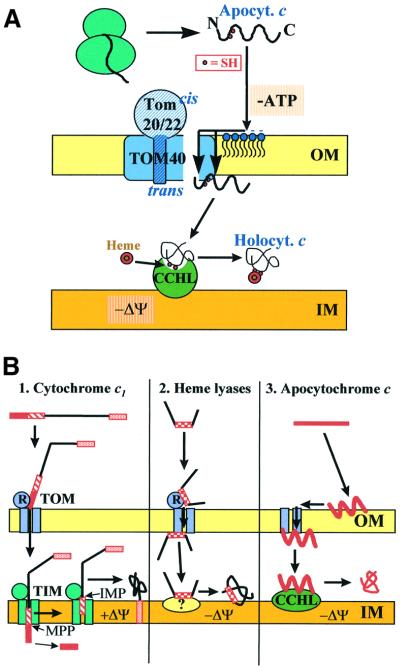
These data, in combination with earlier findings on the mitochondrial import of apocytochrome c, allow us to propose a detailed model for the mechanism of outer membrane translocation of this protein (Figure 7A). After its synthesis on cytosolic ribosomes, apocytochrome c interacts with the negatively charged phospholipids at the outer face of the outer membrane. This interaction is followed by insertion into the membrane. The membrane interaction is unlikely to provide selectivity for targeting to the mitochondrial surface, since the acidic lipid concentration of the mitochondrial outer membrane differs only marginally from that of most other cellular membranes (de Kroon et al., 1999). Nevertheless, the rather high lipid/protein mass ratio of this membrane (70:30) may support preferential binding to acidic lipids at the mitochondrial surface. Membrane insertion will then be stabilized by specific interaction of the apoprotein with the TOM complex, which mediates its movement across the outer membrane by providing a binding site for apocytochrome c (Figure 7A). At this site, the apoprotein is no longer accessible to protease from the cytosolic side of the membrane. At least in part, this binding site is comprised by Tom40. Even though striking similarities are evident between this interaction and the association of the presequence part of preproteins with the trans site of the TOM complex (Mayer et al., 1995c; Lill and Neupert, 1996), there is no apparent overlap between the two binding sites. The trans site can be occupied by preproteins without affecting the import efficiency of apocytochrome c. As shown here, the association at the apocytochrome c binding site of the TOM complex can drive membrane translocation. This becomes particularly evident when TOM complex is present in high concentrations in the membranes of reconstituted proteoliposomes. Entry of apocytochrome c into the intermembrane space is facilitated by engaging an interaction with CCHL, which is located at the outer face of the mitochondrial inner membrane (Figure 7A). Complex formation between the two proteins is both stable and strong, and may ensure shifting the equilibrium to completion of import (Dumont et al., 1991; Mayer et al., 1995d). Finally, the process of translocation is rendered irreversible by covalent attachment of haem to the apoprotein, folding to the native conformation and dissociation from CCHL. Holocyto chrome c will now be able to perform its function as a diffusible electron carrier of the mitochondrial electron transfer chain.
Fig. 7. Working models for the import of proteins into the mitochondrial intermembrane space. (A) Hypothetical mechanism for translocation of apocytochrome c (Apocyt. c) and maturation to holocytochrome c (Holocyt. c). Apocytochrome c requires the TOM complex and cytochrome c haem lyase (CCHL) for translocation (for detailed explanation see Discussion). (B) Three distinct modes of translocation of various intermembrane space constituents across the outer membrane are compared. Whereas cytochrome c1 uses both the TOM and TIM17/23 (TIM) complexes for import and requires a membrane potential (ΔΨ), haem lyases and apocytochrome c use only the TOM complex for membrane translocation. In the latter two cases, import may be driven by an interaction with a trans-side receptor (CCHL in the case of apocytochrome c import; unknown for import of haem lyases). The different targeting signals of these proteins are depicted in red. For details, see Discussion. OM, outer membrane; IM, inner membrane; R, surface receptors; IMP, inner membrane proteases; MPP, matrix processing peptidase.
As mentioned earlier, the translocation mechanism of apocytochrome c is unique and differs in several aspects from that of mitochondrial preproteins. A striking finding underlining this notion is our observation that the import pathways of apocytochrome c and presequence-containing preproteins do not overlap. In our competition experiments with chemical amounts of preprotein, virtually all sites of interaction during translocation of the presequence- containing preprotein were occupied. Nevertheless, import of apocytochrome c was not detectably hampered. This raises the interesting question of how the TOM complex facilitates membrane translocation of apocytochrome c. The complex contains two large ion-conducting pores through which preproteins are thought to permeate (Künkele et al., 1998a; Ahting et al., 1999). Our results render it rather unlikely that, for membrane passage, apocytochrome c utilizes the same pore as canonical preproteins. It is possible, however, that apocytochrome c slides across the second pore. An attractive alternative model could be that the protein may move across the bilayer at the TOM protein/lipid interface (Figure 7A). The conspicuous property of apocytochrome c to interact with negatively charged phospholipids may provide the molecular basis for such a putative pathway. Future biophysical experiments utilizing our reconstituted TOM complex-containing proteoliposomes will have to unravel these mechanistic aspects of membrane passage.
Numerous proteins of the mitochondrial intermembrane space have been identified in the past 4 years. These proteins include a number of factors with a role in apoptosis: e.g. AIP; caspases (see e.g. Martinou and Green, 2001); protein transport, e.g. the small Tim proteins (Koehler et al., 1999; Bauer et al., 2000; Pfanner and Geissler, 2001); and components serving in protection from oxidative stress, Sod1 and Lys7 (Sturtz et al., 2001) or from heavy metal toxicity [metallothionein (Ye et al., 2001)]. Together, some 20 proteins of the intermembrane space have been identified to date. The mode of how they reach their native localization has been unravelled for only four of these proteins in some detail. It appears from these studies that rather diverse mechanisms underlie correct protein targeting to this compartment (Figure 7B). The best-studied pathways of intermembrane space-located proteins are those of the cytochromes b2 and c1 (see for example Glick et al., 1992; Gärtner et al., 1995; Stuart and Neupert, 1996; Arnold et al., 1998). These proteins use a typical N-terminal presequence and the TOM and TIM17/23 complexes for import (Figure 7B, left panel). Translocation is driven mainly by the inner membrane potential (ΔΨ) facilitating passage of the N-terminal part of the targeting sequence across the membrane. Thus, the initial steps of translocation resemble those utilized by typical matrix-targeted preproteins (Neupert, 1997; Pfanner and Geissler, 2001). The second part of the targeting sequence, the ‘sorting signal’, is recognized by the TIM17/23 complex and serves to localize the protein in the intermembrane space in a manner that is not entirely understood. Cleavage of the sorting signal by the inner membrane proteases (Imp1/2) leads to the release of the mature proteins into the intermembrane space. In the case of cytochrome c1 (Figure 7B, left panel), the C-terminal hydrophobic segment harbours additional targeting information and assures insertion into the inner membrane (Arnold et al., 1998).
In contrast, translocation of cytochrome haem lyases depends on the help of the surface receptors and the pore of the TOM complex, but does not involve any of the TIM complexes or an inner membrane potential, ΔΨ (Figure 7B, middle panel; Lill et al., 1992; Steiner et al., 1995). Targeting is mediated by a short internal stretch of 60 amino acid residues that are strikingly different in their chemical character from typical presequences (Diekert et al., 1999). The driving force for import has been proposed to be provided by trans site binding at the TOM complex followed by high affinity interaction of haem lyases with a partner protein at the inner membrane (Steiner et al., 1995), but the latter speculation has not yet been confirmed experimentally. The targeting of apocytochrome c as described in this study involves the protease-resistant part of the TOM complex and CCHL as a trans-side receptor at the outer face of the inner membrane (Figure 7B, right panel). Thus, the import pathway differs from that proposed for haem lyases with respect to the involvement of the surface receptors of the TOM complex and the targeting pathway, yet the principle of the driving force for import may be similar.
The import mechanisms depicted in Figure 7B make use of a number of targeting signals (drawn in red), which differ in both chemical character and location within the protein. These signals may well be present in other preproteins and thus the three pathways known to date might provide paradigms for the import of other constituents into the intermembrane space. In fact, import of Cu/Zn superoxide dismutase (Sod1) may be driven by its interaction with the metallo-chaperone Lys7 in the intermembrane space (Sturtz et al., 2001). These proteins do not contain cleaved N-terminal presequences and therefore may employ mechanisms used by haem lyases or by apocytochrome c. However, it has not been experimentally proven yet which, if any, of these pathways is used for translocation. Future mechanistic studies on the import pathways utilized by these and other resident proteins of the intermembrane space will clarify the interesting question of which of the three known pathways may be followed. It is tempting to speculate that additional modes of entry into this compartment may yet be discovered.
Materials and methods
General biochemical methods
Published methods were used for the following procedures: isolation of mitochondria from S.cerevisiae (Daum et al., 1982); purification of OMV from S.cerevisiae (de Kroon et al., 1999) and N.crassa (Mayer et al., 1993); transcription and translation reactions using reticulocyte lysate (Promega) and [35S]methionine (ICN Radiochemicals) as radioactive label (Söllner et al., 1991); SDS–PAGE and autoradiography of the resulting gels (Mayer et al., 1993); raising antisera and purification of IgG (Mayer et al., 1995c). Blotting of proteins on to nitrocellulose and immunostaining of blotted proteins using the ECL chemiluminescence detection system (Amersham) were performed according to the supplier’s instructions. The amounts of radioactive protein on nitrocellulose membranes were quantitated by analysis with a Fuji Bioimager. Phospholipid phosphorus was determined as described previously (Fiske and Subbarow, 1925) after destruction of lipids with perchloric acid. Protein concentrations were measured by the Coomassie dye binding assay (Bio-Rad).
The TOM complex of N.crassa mitochondria was purified as TOM core complex as described previously (Ahting et al., 1999, 2001). Chemical amounts of apocytochrome c (Fisher et al., 1973) and of pSu9-DHFR-His6 (a fusion protein of the presequence of subunit 9 of F0-ATPase and dihydrofolate reductase carrying a His6 tag; Rapaport et al., 1998a) was prepared as described earlier.
Preparation of LUVET, liposomes and proteoliposomes
LUVET were prepared as described by Hope et al. (1985). Phospholipids (from Avanti Polar Lipids Inc., Alabaster, AL) were mixed in chloroform in the following molar ratios: 45% 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 37% 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 18% l-α-phosphatidylinositol (PI, from soybean, sodium salt). The phospholipid mixture was dried under a stream of nitrogen. The resulting lipid film was left in a desiccator overnight. The dried lipid film, corresponding to 5 µmol phospholipid phosphorus, was hydrated in 1 ml of NEM buffer (20 mM NaCl, 1 mM EDTA, 10 mM MOPS–KOH pH 7.2) and vortexed with a glass marble (0.4 cm diameter). The samples were subjected to 10 freeze–thaw cycles in liquid nitrogen and warm water. LUVETs were then formed by passing the suspension 10 times through polycarbonate membrane filters (400 nm pore size) as described by Hope et al. (1985). The resulting suspension was centrifuged in a TLA 45 rotor (Beckman) at 45 000 r.p.m. The pellet was dissolved in 400 µl of ICB buffer [5 mg/ml fatty acid-free bovine serum albumin (BSA), 10 mM MOPS–KOH pH 6.5] for inclusion of IgG.
Proteoliposomes were prepared by reconstitution of the TOM complex or porin in the phospholipid mixture described above by detergent dilution (van der Does et al., 1998). The lipid film was hydrated in 800 µl of reconstitution buffer (50 mM KOAc, 50 mM KCl, 50 mM MOPS– KOH pH 7.0). Two hundred microlitres of this lipid suspension were mixed with 20 µl of n-octyl-β-D-glucopyranoside [12.5% (w/v) stock solution] and 5–20 µg of purified TOM complex or porin (pre-incubated with ergosterol as described below). After incubation for 5 min on ice in a reaction tube, the solution was transferred into a Ti70 centrifuge tube. A total of 4 ml of reconstitution buffer was added in four aliquots and incubation was continued for 5 min on ice. The solution was centrifuged for 30 min at 4°C at 50 000 r.p.m. in a Ti70 rotor (Beckman). The proteoliposome pellet was resuspended in 200 µl of ICB buffer or EMK buffer (1 mM EDTA, 10 mM MOPS–KOH pH 7.2, 150 mM KCl).
Inclusion of proteins into the lumen of (proteo)liposomes, LUVET and OMV
Solutions containing (proteo)liposomes or LUVET in ICB buffer (100 µl; lipid phosphate content of 300 nmol for four samples) were added to 20 µl of purified IgG or lactate dehydrogenase (final concentration 5 mg/ml). The mixture was immediately frozen in liquid nitrogen and placed in a metal block in an ice/water bath to permit slow thawing (as described by Mayer et al., 1995a). This usually took 40–60 min. After addition of one-fifth volume of 100 mM MOPS–KOH pH 7.5, the mixture was incubated for 5 min at 25°C and adjusted to a sucrose concentration of 45% by addition of 70% (w/v) sucrose in EMK buffer. The protein-loaded (proteo)liposomes or LUVET were recovered from this mixture by flotation centrifugation (45 min at 140 000 g) through a sucrose gradient consisting of 500 µl steps of 45 and 40% sucrose (both in EMK buffer), and 35 and 0% sucrose (both in EM buffer, i.e. EMK buffer without KCl) in a Beckman SW60 rotor. The (proteo)liposomes or LUVET were harvested from the 0–35% sucrose interface and diluted to the desired lipid concentration (30–40 nmol/sample) with SEM buffer (250 mM sucrose in EM buffer). Enclosure of IgG into OMV (50 µg protein in 100 µl ICB buffer per sample) was performed and the protein-loaded OMV treated as described above.
Pre-incubation of porin with ergosterol
An aqueous ergosterol solution was prepared by evaporating a chloroform solution of ergosterol and then suspending the ergosterol at a concentration of 1% (w/v) in 2% n-octyl-β-d-glucopyranoside, 1 mM EDTA, 10 mM potassium phosphate pH 7 (Popp et al., 1995). One volume (10 µl) of porin solution (10 mg/ml) was mixed with 1 vol. of a 1% (w/v) ergosterol solution and vortexed for 10 s. Before use, the samples were stored on ice for at least 5 min.
Import of apocytochrome c and other preproteins into mitochondria, OMV, (proteo)liposomes and LUVET
[35S]methionine radiolabelled apocytochrome c of S.cerevisiae or, in some experiments, N.crassa was synthesized in reticulocyte lysate, precipitated with ammonium sulfate (66% saturation) and resuspended in the same volume of EM buffer. This treatment removes most of the haemoglobin, which interferes with the electrophoretic analysis of the translocation experiments. For import, 4 µl of the resuspended solution were incubated with mitochondria (50 µg/sample), OMV (10– 20 µg/sample), (proteo)liposomes or LUVET (typically 30–40 nmol lipid-bound phosphate/sample) for 10 min at 25°C in SEM buffer in a total volume of 100 µl. Import reactions were chilled on ice and proteinase K added to a final concentration of 50 µg/ml. Protease digestion was stopped after 20 min at 0°C by addition of 2 mM phenylmethylsulfonyl fluoride (PMSF). The proteins were precipitated with 1 vol. of 25% TCA and subjected to SDS–PAGE and autoradiography. Import of other preproteins into mitochondria was described earlier (Diekert et al., 1999).
Acknowledgments
Acknowledgements
The expert technical assistance of M.Braun, U.Staudinger and M.C.Koorengevel is gratefully acknowledged. We thank Dr R.Benz for help with the reconstitution of porin. Our work was supported by grants of the Sonderforschungsbereiche 184 and 286 of the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie and the European Science Foundation.
References
- Ahting U., Thun,C., Hegerl,R., Typke,D., Nargang,F.E., Neupert,W. and Nussberger,S. (1999) The TOM core complex: the general protein import pore of the outer membrane of mitochondria. J. Cell Biol., 147, 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahting U., Thieffry,M., Engelhardt,H., Hegerl,R., Neupert,W. and Nussberger,S. (2001) Tom40, the pore-forming component of the protein-conducting TOM channel in the outer membrane of mitochondria. J. Cell Biol., 153, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold I., Folsch,H., Neupert,W. and Stuart,R.A. (1998) Two distinct and independent mitochondrial targeting signals function in the sorting of an inner membrane protein, cytochrome c1. J. Biol. Chem., 273, 1469–1476. [DOI] [PubMed] [Google Scholar]
- Bauer M.F., Hofmann,S., Neupert,W. and Brunner,M. (2000) Protein translocation into mitochondria: the role of the TIM complexes. Trends Cell Biol., 10, 25–31. [DOI] [PubMed] [Google Scholar]
- Benz R. (1994) Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim. Biophys. Acta, 1197, 167–196. [DOI] [PubMed] [Google Scholar]
- Bolliger L., Junne,T., Schatz,G. and Lithgow,T. (1995) Acidic receptor domains on both sides of the outer membrane mediate translocation of precursor proteins into yeast mitochondria. EMBO J., 14, 6318–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Böhni,P.C. and Schatz,G. (1982) Import of proteins into mitochondria: cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem., 257, 13028–13033. [PubMed] [Google Scholar]
- de Kroon A.I.P.M., Koorengevel,M.C., Goerdayal,S.S., Mulders,P.C., Janssen,M.J. and de Kruijff,B. (1999) Isolation and characterization of highly purified mitochondrial outer membranes of the yeast Saccharomyces cerevisiae. Mol. Membr. Biol., 16, 205–211. [DOI] [PubMed] [Google Scholar]
- de Kruijff B. et al. (1992) Lipid involvement in protein translocation. In Neupert,W. and Lill,R. (eds), Lipid Involvement in Protein Translocation. Elsevier Science Publishers, Amsterdam, The Netherlands, pp. 85–101.
- Diekert K., Kispal,G., Guiard,B. and Lill,R. (1999) An internal targeting signal directing proteins into the mitochondrial intermembrane space. Proc. Natl Acad. Sci. USA, 96, 11746–11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M.E. (1996) Lipid involvement in protein translocation. In Hartl,F.U. (ed.), Mitochondrial Import of Cytochrome c. Vol. 17. JAI Press, Greenwich, CT, pp. 103–126.
- Dumont M.E., Ernst,J.F. and Sherman,F. (1988) Coupling of haem attachment to import of cytochrome c into yeast mitochondria. J. Biol. Chem., 263, 15928–15937. [PubMed] [Google Scholar]
- Dumont M.E., Cardillo,T.S., Hayes,M.K. and Sherman,F. (1991) Role of cytochrome c haem lyase in mitochondrial import and accumulation of cytochrome c in S. cerevisiae. Mol. Cell. Biol., 11, 5487–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher W.R., Taniuchi,H. and Anfinsen,C.B. (1973) On the role of haem in the formation of the structure of cytochrome c. J. Biol. Chem., 248, 3188–3195. [PubMed] [Google Scholar]
- Fiske L.M. and Subbarow,Y. (1925) The colorimetric determination of phosphorus. J. Biol. Chem., 66, 375–389. [Google Scholar]
- Gärtner F., Bömer,U., Guiard,B. and Pfanner,N. (1995) The sorting signal of cytochrome b2 promotes early divergence from the general mitochondrial import pathway and restricts the unfoldase activity of matrix Hsp70. EMBO J., 14, 6043–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B.S., Brandt,A., Cunningham,K., Müller,S., Hallberg,R.L. and Schatz,G. (1992) Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell, 69, 809–822. [DOI] [PubMed] [Google Scholar]
- Hill K., Model,K., Ryan,M.T., Dietmeier,K., Martin,F., Wagner,R. and Pfanner,N. (1998) Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature, 395, 516–521. [DOI] [PubMed] [Google Scholar]
- Hope M.J., Bally,M.B., Webb,G. and Cullis,P.R. (1985) Production of large unilamellar vesicles by a rapid extrusion technique. Biochim. Biophys. Acta, 812, 55–65. [DOI] [PubMed] [Google Scholar]
- Jordi W., Hergersberg,C. and de Kruijff,B. (1992) Bilayer-penetrating properties enable apocytochrome c to follow a special import pathway into mitochondria. Eur. J. Biochem., 204, 841–846. [DOI] [PubMed] [Google Scholar]
- Kanamori T., Nishikawa,S., Nakai,M., Shin,I., Schultz,P.G. and Endo,T. (1999) Uncoupling of transfer of the presequence and unfolding of the mature domain in precursor translocation across the mitochondrial outer membrane. Proc. Natl Acad. Sci. USA, 96, 3634–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmita H. and Budzinska,M. (2000) Involvement of the TOM complex in external NADH transport into yeast mitochondria depleted of mitochondrial porin1. Biochim. Biophys. Acta, 1509, 86–94. [DOI] [PubMed] [Google Scholar]
- Koehler C.M., Merchant,S. and Schatz,G. (1999) How membrane proteins travel across the mitochondrial intermembrane space. Trends Biochem. Sci., 24, 428–432. [DOI] [PubMed] [Google Scholar]
- Kranz R., Lill,R., Goldman,B., Bonnard,G. and Merchant,S. (1998) Molecular mechanisms of cytochrome c biogenesis: three distinct systems. Mol. Microbiol., 29, 383–396. [DOI] [PubMed] [Google Scholar]
- Künkele K.P., Heins,S., Dembowski,M., Nargang,F.E., Benz,R., Thieffry,M., Walz,J., Lill,R., Nussberger,S. and Neupert,W. (1998a) The preprotein translocation channel of the outer membrane of mitochondria. Cell, 93, 1009–1019. [DOI] [PubMed] [Google Scholar]
- Künkele K.P., Juin,P., Pompa,C., Nargang,F.E., Henry,J.P., Neupert,W., Lill,R. and Thieffry,M. (1998b) The isolated complex of the translocase of the outer membrane of mitochondria. Characterization of the cation-selective and voltage-gated preprotein-conducting pore. J. Biol. Chem., 273, 31032–31039. [DOI] [PubMed] [Google Scholar]
- Lill R. and Neupert,W. (1996) Mechanisms of protein import across the mitochondrial outer membrane. Trends Cell Biol., 6, 56–61. [DOI] [PubMed] [Google Scholar]
- Lill R., Stuart,R.A., Drygas,M.E., Nargang,F.E. and Neupert,W. (1992) Import of cytochrome c haem lyase into mitochondria: a novel pathway into the intermembrane space. EMBO J., 11, 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou J.C. and Green,D.R. (2001) Breaking the mitochondrial barrier. Nature Rev. Mol. Cell Biol., 2, 63–67. [DOI] [PubMed] [Google Scholar]
- Mayer A., Lill,R. and Neupert,W. (1993) Translocation and insertion of precursor proteins into isolated outer membranes of mitochondria. J. Cell Biol., 121, 1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Driessen,A., Neupert,W. and Lill,R. (1995a) Purified and protein-loaded mitochondrial outer membrane vesicles for functional analysis of preprotein transport. Methods Enzymol., 260, 252–263. [DOI] [PubMed] [Google Scholar]
- Mayer A., Nargang,F.E., Neupert,W. and Lill,R. (1995b) MOM22 is a receptor for mitochondrial targeting sequences and cooperates with MOM19. EMBO J., 14, 4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Neupert,W. and Lill,R. (1995c) Mitochondrial protein import: Reversible binding of the presequence at the trans side of the outer membrane drives partial translocation and unfolding. Cell, 80, 127–137. [DOI] [PubMed] [Google Scholar]
- Mayer A., Neupert,W. and Lill,R. (1995d) Translocation of apocytochrome c across the outer membrane of mitochondria. J. Biol. Chem., 270, 12390–12397. [DOI] [PubMed] [Google Scholar]
- Miao Q., Han,X. and Yang,F. (2001) Phosphatidic acid-phosphatidylethanolamine interaction and apocytochrome c translocation across model membranes. Biochem. J., 354, 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczko M., Bomer,U., Kubrich,M., Zufall,N., Honlinger,A. and Pfanner,N. (1997) The intermembrane space domain of mitochondrial Tom22 functions as a trans binding site for preproteins with N-terminal targeting sequences. Mol. Cell. Biol., 17, 6574–6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert W. (1997) Protein import into mitochondria. Annu. Rev. Biochem., 66, 861–915. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W., Hergersberg,C. and Neupert,W. (1988) Role of cytochrome c haem lyase in the import of cytochrome c into mitochondria. J. Biol. Chem., 263, 19034–19042. [PubMed] [Google Scholar]
- Pfaller R., Steger,H.F., Rassow,J., Pfanner,N. and Neupert,W. (1988) Import pathways of precursor proteins into mitochondria: Multiple receptor sites are followed by a common membrane insertion site. J. Cell Biol., 107, 2483–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N. and Geissler,A. (2001) Versatility of the mitochondrial protein import machinery. Nature Rev. Mol. Cell Biol., 2, 339–349. [DOI] [PubMed] [Google Scholar]
- Popp B., Schmid,A. and Benz,R. (1995) Role of sterols in the functional reconstitution of water-soluble mitochondrial porins from different organisms. Biochemistry, 34, 3352–3361. [DOI] [PubMed] [Google Scholar]
- Rapaport D. and Neupert,W. (1999) Biogenesis of Tom40, core component of the TOM complex of mitochondria. J. Cell Biol., 146, 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D., Neupert,W. and Lill,R. (1997) Mitochondrial protein import: Tom40 plays a major role in targeting and translocation of preproteins by forming a specific binding site for the presequence. J. Biol. Chem., 272, 18725–18731. [DOI] [PubMed] [Google Scholar]
- Rapaport D., Künkele,K.P., Dembowski,M., Ahting,U., Nargang,F.E., Neupert,W. and Lill,R. (1998a) Dynamics of the TOM complex of mitochondria during binding and translocation of preproteins. Mol. Cell. Biol., 18, 5256–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D., Mayer,A., Neupert,W. and Lill,R. (1998b) Cis and trans sites of the TOM complex in unfolding and translocation of preproteins across the outer membrane of mitochondria. J. Biol. Chem., 273, 8806–8813. [DOI] [PubMed] [Google Scholar]
- Rietveld A. and de Kruijff,B. (1984) Is the mitochondrial precursor protein apocytochrome c able to pass a lipid barrier? J. Biol. Chem., 259, 6704–6707. [PubMed] [Google Scholar]
- Schatz G. and Dobberstein,B. (1996) Common principles of protein translocation across membranes. Science, 271, 1519–1526. [DOI] [PubMed] [Google Scholar]
- Snel M.M., de Kruijff,B. and Marsh,D. (1994) Membrane location of spin-labelled apocytochrome c and cytochrome c determined by paramagnetic relaxation agents. Biochemistry, 33, 11150–11157. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow,J. and Pfanner,N. (1991) Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol., 34, 345–358. [DOI] [PubMed] [Google Scholar]
- Stan T., Ahting,U., Dembowski,M., Künkele,K.P., Nussberger,S., Neupert,W. and Rapaport,D. (2000) Recognition of preproteins by the isolated TOM complex of mitochondria. EMBO J., 19, 4895–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H., Zollner,A., Haid,A., Neupert,W. and Lill,R. (1995) Biogenesis of mitochondrial haem lyases in yeast. Import and folding in the intermembrane space. J. Biol. Chem., 270, 22842–22849. [DOI] [PubMed] [Google Scholar]
- Stuart R.A. and Neupert,W. (1990) Apocytochrome c: An exceptional mitochondrial precursor protein using an exceptional import pathway. Biochimie, 72, 115–121. [DOI] [PubMed] [Google Scholar]
- Stuart R.A. and Neupert,W. (1996) Topogenesis of inner membrane proteins of mitochondria. Trends Biochem. Sci., 21, 261–267. [PubMed] [Google Scholar]
- Stuart R.A., Nicholson,D.W. and Neupert,W. (1990) Early steps in mitochondrial protein import: receptor functions can be substituted by the membrane insertion activity of apocytochrome c.Cell, 60, 31–43. [DOI] [PubMed] [Google Scholar]
- Sturtz L.A., Diekert,K., Jensen,L.T., Lill,R. and Culotta,V.C. (2001) A fraction of yeast Cu/Zn superoxide dismutase and its metallo chaperone, CCS, localize to the intermembrane space of mitochondria: a physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem., in press. [DOI] [PubMed] [Google Scholar]
- van der Does C., Manting,E.H., Kaufmann,A., Lutz,M. and Driessen,A.J. (1998) Interaction between SecA and SecYEG in micellar solution and formation of the membrane-inserted state. Biochemistry, 37, 201–210. [DOI] [PubMed] [Google Scholar]
- Ye B., Maret,W. and Vallee,B.L. (2001) Zinc metallothionein imported into liver mitochondria modulates respiration. Proc. Natl Acad. Sci. USA, 98, 2317–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]