Abstract
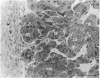
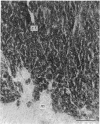
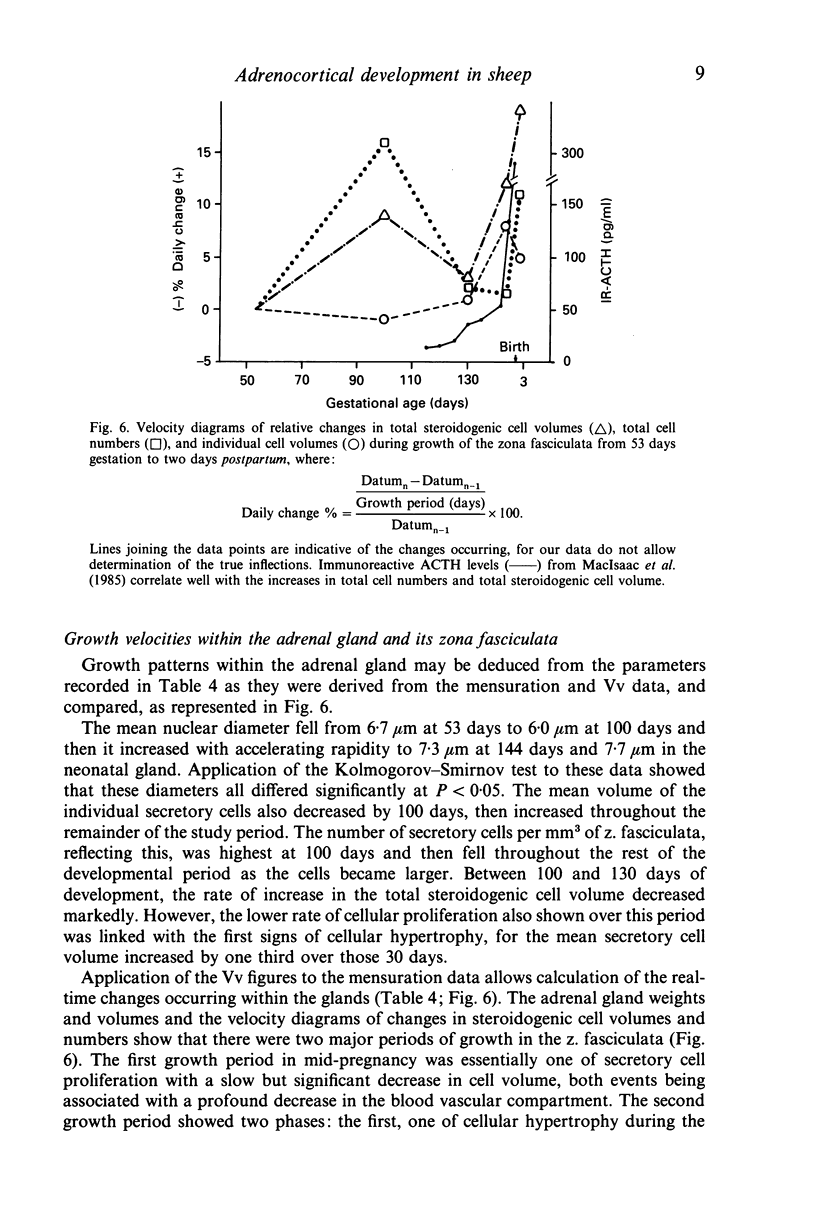
This, the first linear morphometric analysis of the epigenesis of the fetal mammalian adrenal cortex, has shown that in the fetal sheep during the latter two thirds of gestation and in the newborn lamb, there are two periods of rapid growth separated by a period of much reduced growth. The fetal ages studies were 53 days (0.36 gestation), a period when the fetal adrenal cortex is actively steroidogenic; 100 days (0.68 gestation), a period of adrenocortical quiescence; 130 days (0.88 gestation), the period of increasing responsiveness to ACTH and cortisol production; 144 days (0.98 gestation), the period of maximal adrenocortical steroidogenesis; and 2 days postpartum, when cortisol production is normally maintained. The first adrenocortical growth period extends to mid-gestation, then growth slows to 0.85 gestation when the second growth period begins. The changes between the first growth period (0.36 gestation) and the period of quiescence (0.68 gestation) are characterised by the attainment of normal adrenocortical zonation and the separation of the medulla. The rate of adrenocortical cell division slows and the zona fasciculata cells become smaller in size. The volume density of the adrenocortical blood sinusoids decreases significantly. The onset of the second growth phase is associated with the previously reported increased levels of fetal plasma ACTH at 0.85 gestation and is expressed initially as a hypertrophic response. Cellular hypertrophy increases from 0.88 gestation to 0.98 gestation and then declines over the birth period. The rate of adrenocortical cell division increases from 0.88 gestation and maintains a maximal rate from 0.98 gestation to 2 days postpartum. These interactions of cellular hypertrophy and hyperplasia, which result in adrenocortical growth, may be explained as a response to fetal ACTH, which has the ability to stimulate the production of peptide growth and differentiation factors, e.g. IGF-II, and cortisol, which then control adrenocortical development in an autocrine and paracrine fashion.
Full text
PDF


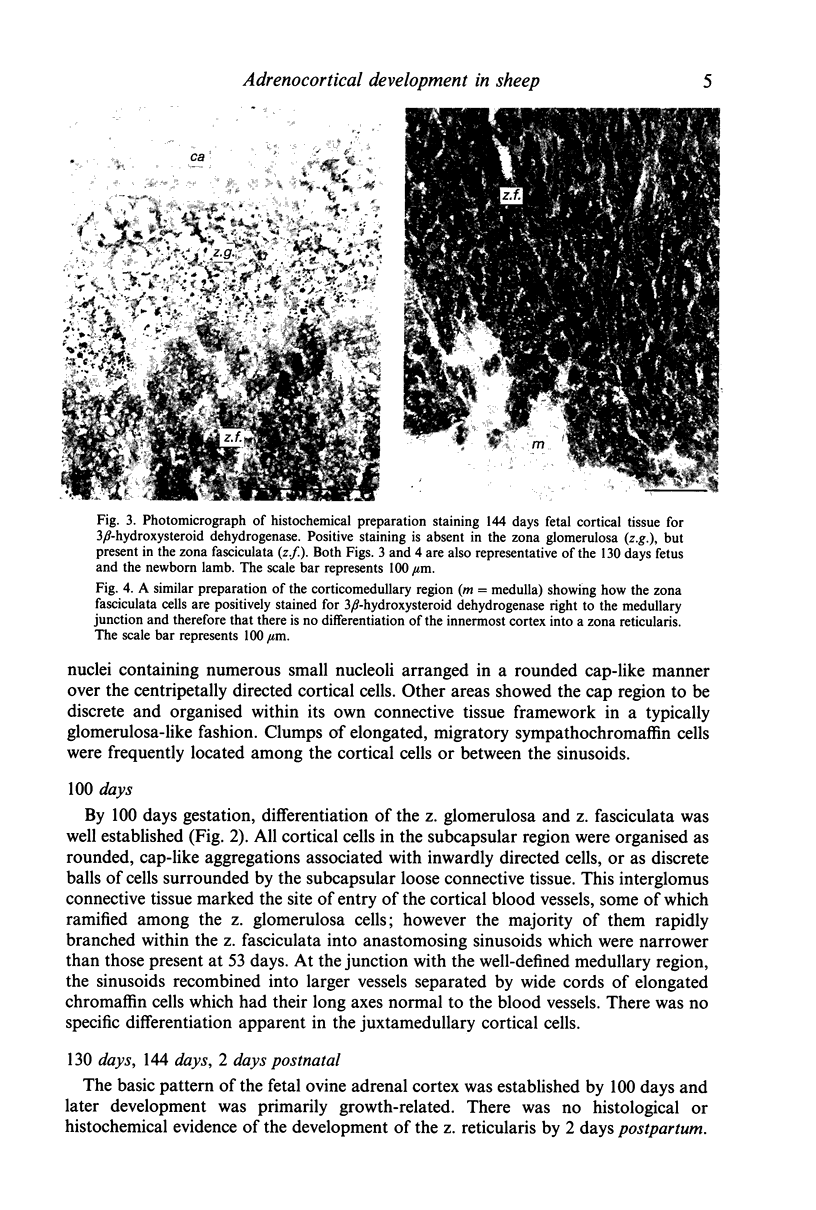
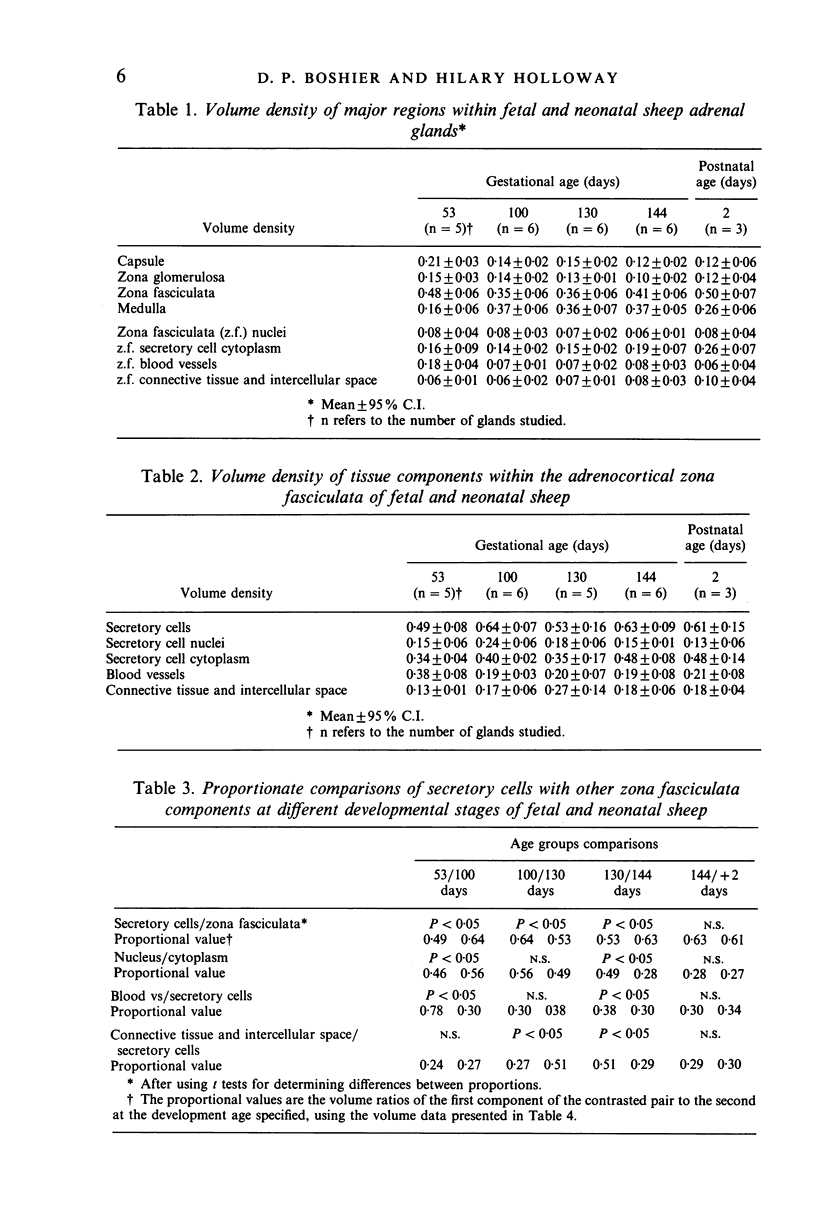
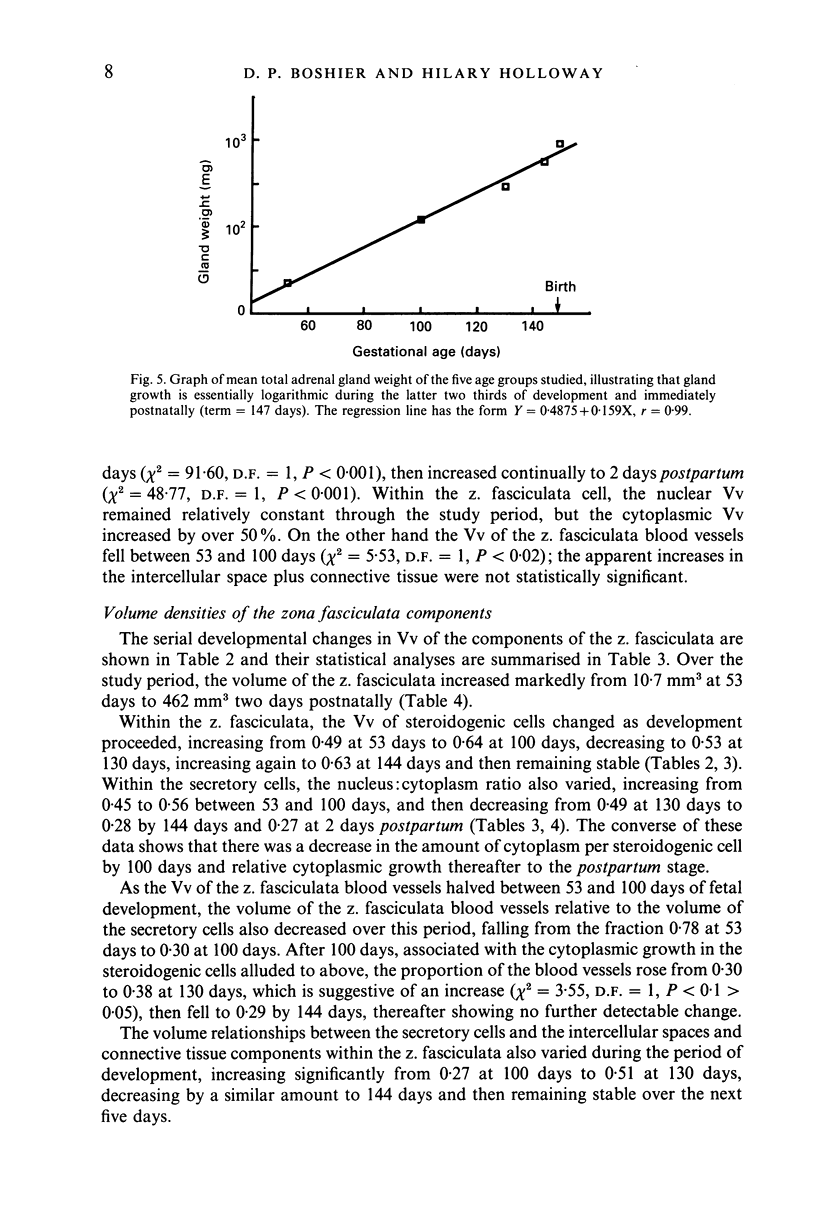





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard P. L. Glucocorticoids and differentiation. Monogr Endocrinol. 1979;12:493–515. doi: 10.1007/978-3-642-81265-1_26. [DOI] [PubMed] [Google Scholar]
- Bassett J. M., Thorburn G. D. Foetal plasma corticosteroids and the initiation of parturition in sheep. J Endocrinol. 1969 Jun;44(2):285–286. doi: 10.1677/joe.0.0440285. [DOI] [PubMed] [Google Scholar]
- Boshier D. P., Holloway H., Liggins G. C. Effects of cortisol and ACTH on adrenocortical growth and cytodifferentiation in the hypophysectomized fetal sheep. J Dev Physiol. 1981 Dec;3(6):355–373. [PubMed] [Google Scholar]
- Boshier D. P., Holloway H., Liggins G. C. Growth and cytodifferentiation of the fetal lamb adrenal cortex prior to parturition. J Anat. 1980 Jan;130(Pt 1):97–111. [PMC free article] [PubMed] [Google Scholar]
- Bousquet J., Lye S. J., Challis J. R. Comparison of leucine enkephalin and adrenocorticotrophin effects on adrenal function in fetal and adult sheep. J Reprod Fertil. 1984 Mar;70(2):499–506. doi: 10.1530/jrf.0.0700499. [DOI] [PubMed] [Google Scholar]
- COMLINE R. S., SILVER M. The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. J Physiol. 1961 May;156:424–444. doi: 10.1113/jphysiol.1961.sp006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis J. R., Mitchell B. F., Lye S. J. Activation of fetal adrenal function. J Dev Physiol. 1984 Feb;6(1):93–105. [PubMed] [Google Scholar]
- D'Ercole A. J. Somatomedins/insulin-like growth factors and fetal growth. J Dev Physiol. 1987 Dec;9(6):481–495. [PubMed] [Google Scholar]
- Dallman M. F. Control of adrenocortical growth in vivo. Endocr Res. 1984;10(3-4):213–242. doi: 10.1080/07435808409036499. [DOI] [PubMed] [Google Scholar]
- Durand P. ACTH receptor levels in lamb adrenals at late gestation and early neonatal stages. Biol Reprod. 1979 May;20(4):837–845. doi: 10.1095/biolreprod20.4.837. [DOI] [PubMed] [Google Scholar]
- Durand P., Bosc M., Nicolle A. Croissance des surrénales de foetus ovin en fin de gestation: évolution de l'ADN et des protéines membranaires. C R Acad Sci Hebd Seances Acad Sci D. 1978 Sep 11;287(4):297–300. [PubMed] [Google Scholar]
- Glickman J. A., Challis J. R. The changing response pattern of sheep fetal adrenal cells throughout the course of gestation. Endocrinology. 1980 May;106(5):1371–1376. doi: 10.1210/endo-106-5-1371. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Hornsby P. J., Gill G. N. Control of bovine adrenal cortical cell proliferation by fibroblast growth factor. Lack of effect of epidermal growth factor. Endocrinology. 1977 Apr;100(4):1080–1089. doi: 10.1210/endo-100-4-1080. [DOI] [PubMed] [Google Scholar]
- Han V. K., D'Ercole A. J., Lund P. K. Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science. 1987 Apr 10;236(4798):193–197. doi: 10.1126/science.3563497. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Oliver I. T. Plasma corticosterone concentrations in the perinatal rat. Biochem J. 1968 Jun;108(2):339–341. doi: 10.1042/bj1080339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiba N., Nomura K., Hizuka N., Takano K., Demura H., Shizume K. Effects of IGF-I on proliferation and steroidogenesis of cultured adrenal zona glomerulosa cells. Endocrinol Jpn. 1987 Aug;34(4):611–614. doi: 10.1507/endocrj1954.34.611. [DOI] [PubMed] [Google Scholar]
- Hornsby P. J., Gill G. N. Hormonal control of adrenocortical cell proliferation. Desensitization to ACTH and interaction between ACTH and fibroblast growth factor in bovine adrenocortical cell cultures. J Clin Invest. 1977 Aug;60(2):342–352. doi: 10.1172/JCI108782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins G. C. Premature parturition after infusion of corticotrophin or cortisol into foetal lambs. J Endocrinol. 1968 Oct;42(2):323–329. doi: 10.1677/joe.0.0420323. [DOI] [PubMed] [Google Scholar]
- Lohse J. K., First N. L. Development of the porcine fetal adrenal in late gestation. Biol Reprod. 1981 Aug;25(1):181–190. doi: 10.1095/biolreprod25.1.181. [DOI] [PubMed] [Google Scholar]
- MacIsaac R. J., Bell R. J., McDougall J. G., Tregear G. W., Wang X., Wintour E. M. Development of the hypothalamic-pituitary-axis in the ovine fetus: ontogeny of action of ovine corticotropin-releasing factor. J Dev Physiol. 1985 Oct;7(5):329–338. [PubMed] [Google Scholar]
- Madill D., Bassett J. M. Corticosteroid release by adrenal tissue from foetal and newborn lambs in response to corticotrophin stimulation in a perifusion system in vitro. J Endocrinol. 1973 Jul;58(1):78–87. doi: 10.1677/joe.0.0580075. [DOI] [PubMed] [Google Scholar]
- Malendowicz K. A correlated stereological and functional studies on the long-term effects of ACTH on rat adrenal cortex. Folia Histochem Cytobiol. 1986;24(3):203–211. [PubMed] [Google Scholar]
- Mathieu O., Claassen H., Weibel E. R. Differential effect of glutaraldehyde and buffer osmolarity on cell dimensions: a study on lung tissue. J Ultrastruct Res. 1978 Apr;63(1):20–34. doi: 10.1016/s0022-5320(78)80041-0. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Human fetal serum cortisol levels related to gestational age: evidence of a midgestational fall and a steep late gestational rise, independent of sex or mode of delivery. Am J Obstet Gynecol. 1982 Oct 1;144(3):276–282. doi: 10.1016/0002-9378(82)90579-8. [DOI] [PubMed] [Google Scholar]
- Norman L. J., Lye S. J., Wlodek M. E., Challis J. R. Changes in pituitary responses to synthetic ovine corticotrophin releasing factor in fetal sheep. Can J Physiol Pharmacol. 1985 Nov;63(11):1398–1403. doi: 10.1139/y85-230. [DOI] [PubMed] [Google Scholar]
- Nussdorfer G. G. Cytophysiology of the adrenal cortex. Int Rev Cytol. 1986;98:1–405. [PubMed] [Google Scholar]
- Robinson P. M., Rowe E. J., Wintour E. M. The histogenesis of the adrenal cortex in the foetal sheep. Acta Endocrinol (Copenh) 1979 May;91(1):134–149. doi: 10.1530/acta.0.0910134. [DOI] [PubMed] [Google Scholar]
- Samsoondar J., Kudlow J. E. Partial purification of an adrenal growth factor produced by normal bovine anterior pituitary cells in culture. Endocrinology. 1987 Mar;120(3):929–935. doi: 10.1210/endo-120-3-929. [DOI] [PubMed] [Google Scholar]
- Scott J., Cowell J., Robertson M. E., Priestley L. M., Wadey R., Hopkins B., Pritchard J., Bell G. I., Rall L. B., Graham C. F. Insulin-like growth factor-II gene expression in Wilms' tumour and embryonic tissues. Nature. 1985 Sep 19;317(6034):260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- Simonian M. H., Capp M. W., Templeman M. C., Chang E. C. Placental-derived mitogenic factor for human fetal adrenocortical cell cultures. In Vitro Cell Dev Biol. 1987 Jan;23(1):57–62. doi: 10.1007/BF02623494. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintour E. M., Brown E. H., Denton D. A., Hardy K. J., McDougall J. G., Oddie C. J., Whipp G. T. The ontogeny and regulation of corticosteroid secretion by the ovine foetal adrenal. Acta Endocrinol (Copenh) 1975 Jun;79(2):301–316. doi: 10.1530/acta.0.0790301. [DOI] [PubMed] [Google Scholar]
- Yamauchi S. Histological development of the equine fetal adrenal gland. J Reprod Fertil Suppl. 1979;(27):487–491. [PubMed] [Google Scholar]