Abstract
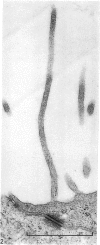
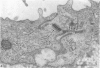
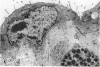
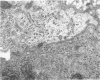
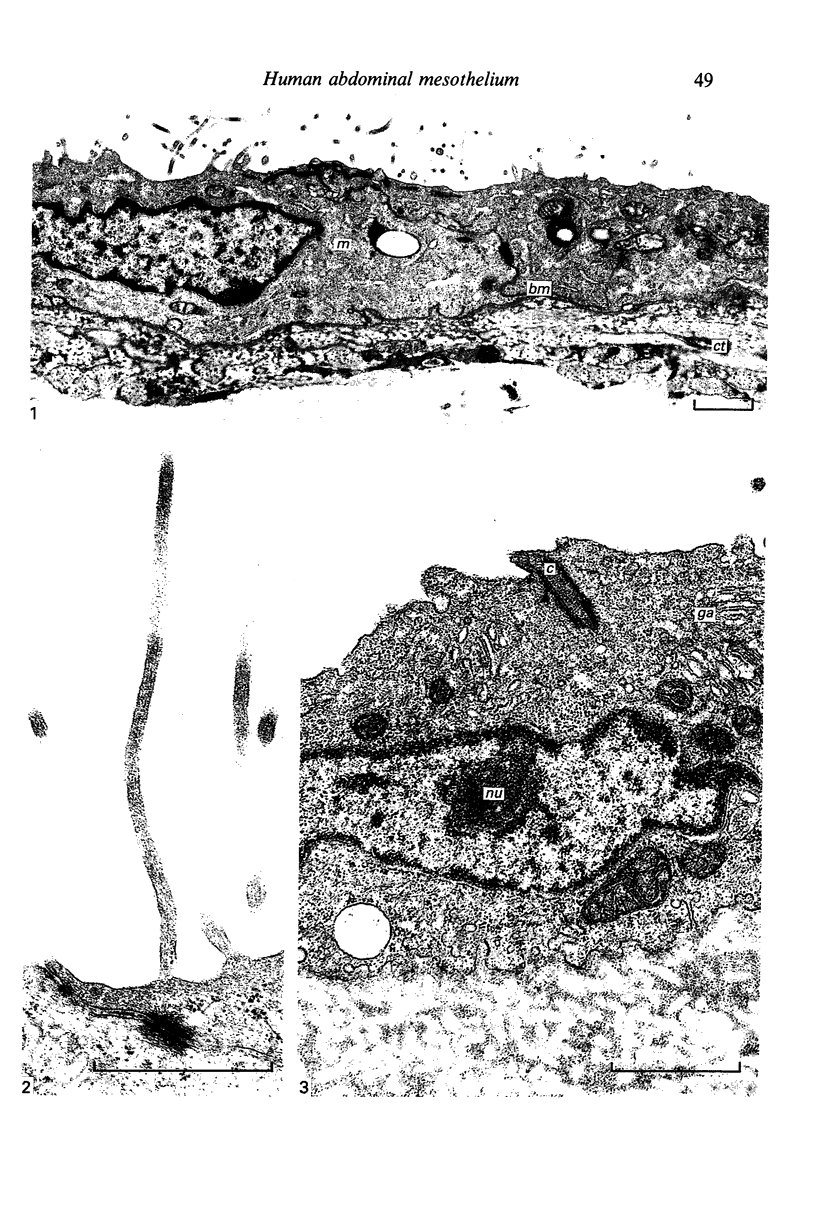
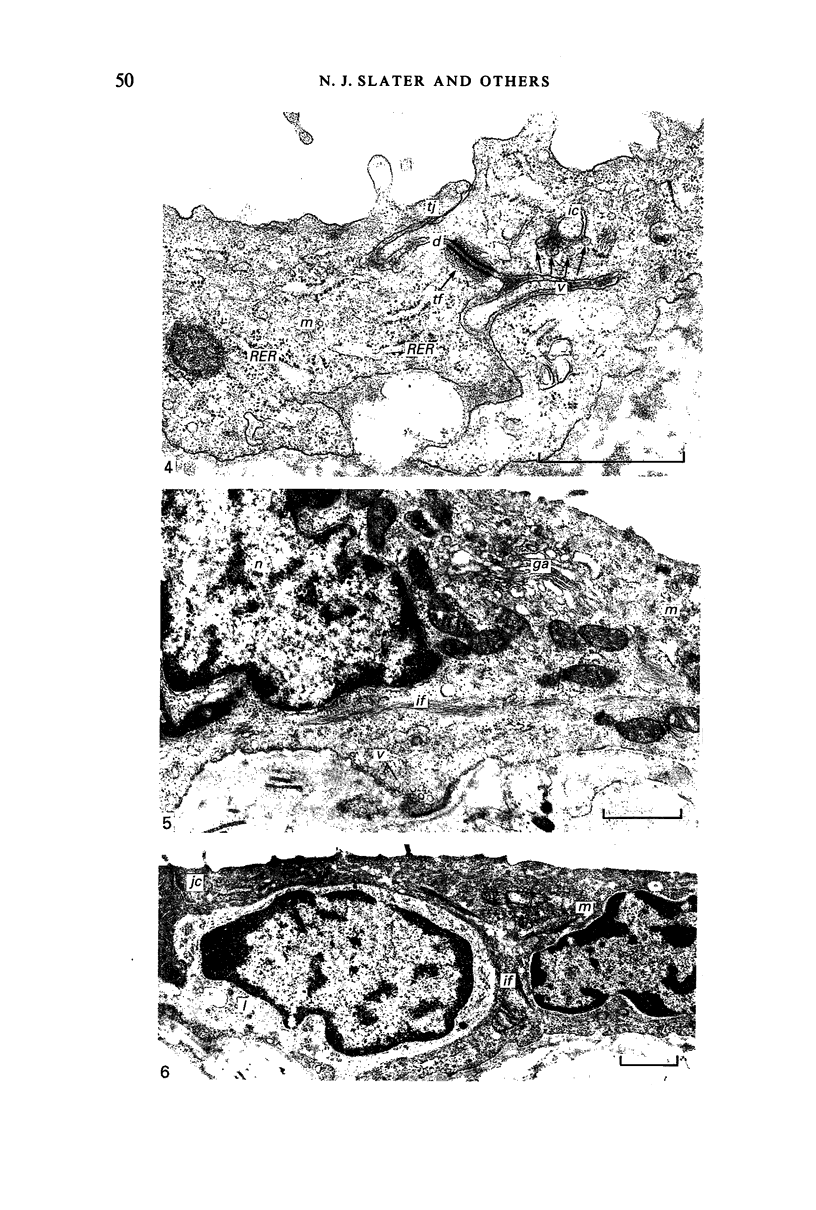
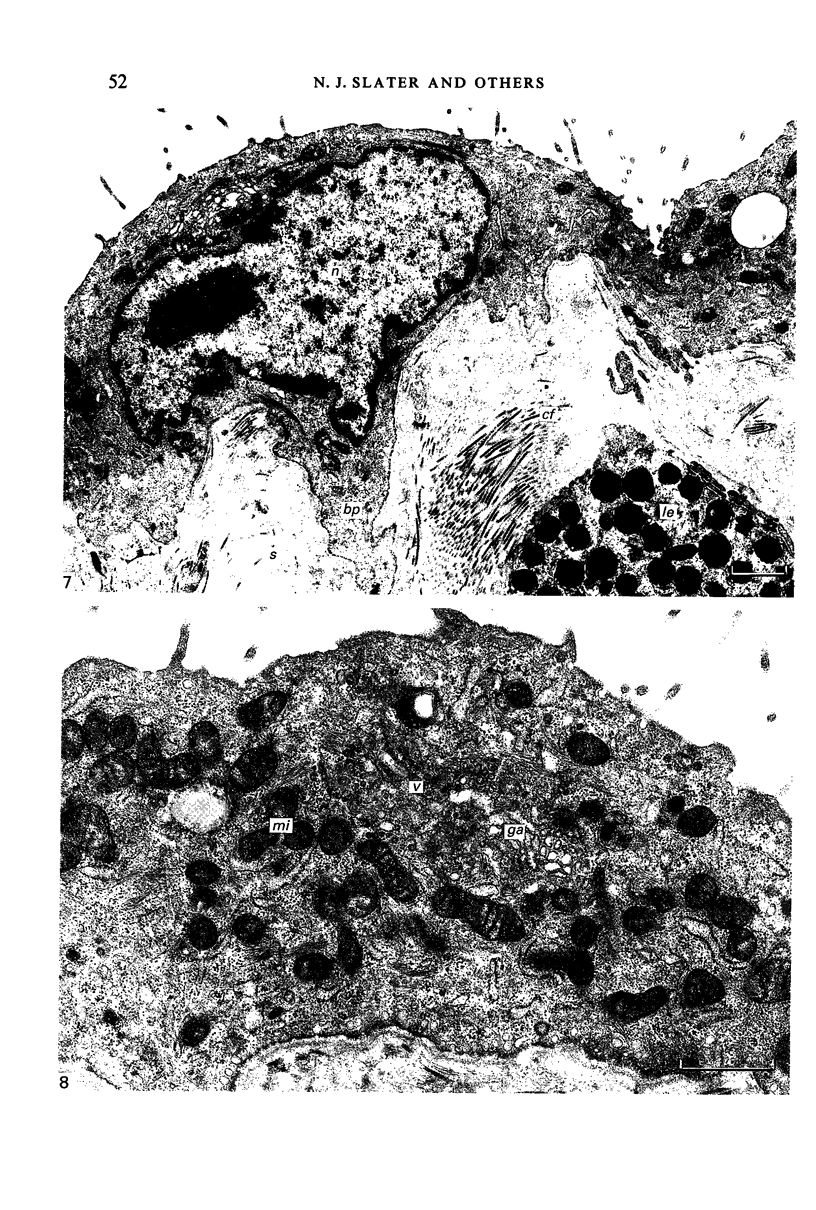
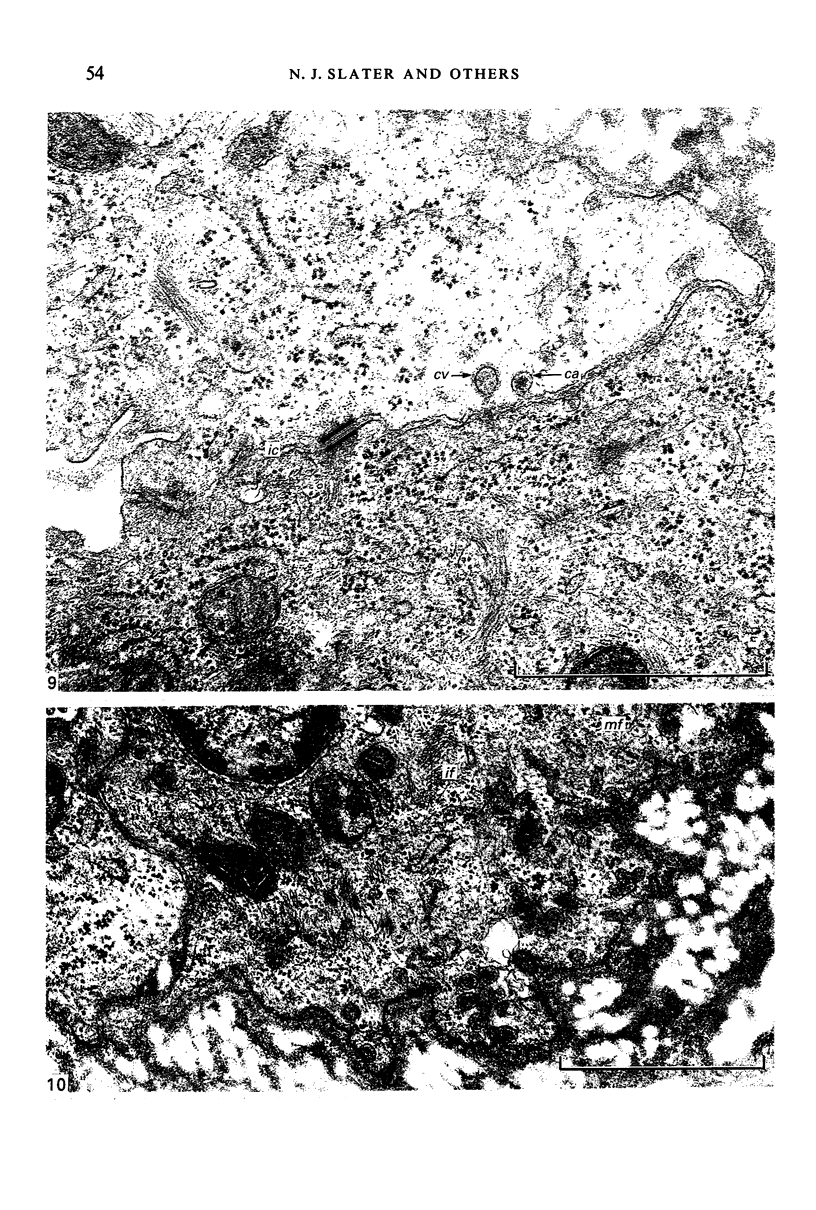
Fresh specimens of human peritoneum collected from heart-beating cadaver organ donors have been examined by transmission electron microscopy. Samples were taken from the anterior abdominal wall and from the surfaces of the liver, stomach and diaphragm. The mesothelium consisted of a single layer of flattened cells generally 2.5 microns to 3 microns thick. These were joined by tight junctions and desmosomes to form a continuous sheet. The cells rested on a prominent basement membrane deep to which was a layer of fibrous connective tissue. This layer was more compact under the mesothelium from the abdominal wall and liver than elsewhere. Long microvilli projected from apical surface of the cells. In many cases these covered the entire surface but sometimes they were more profuse at the edges of the cells near the intercellular junctions. The cells possessed a well-developed cytoskeleton of intermediate filaments which coursed through the cytoplasm in thick bundles. The cells also had a well-developed rough endoplasmic reticulum and Golgi apparatus. Numerous smooth-surfaced and coated vesicles could be seen adjacent to the plasmalemma at all surfaces, providing evidence of considerable pinocytotic activity. There was little regional variation in the structure of the mesothelium. We found no evidence of pores passing through the layer although, on the liver, cisternae were present between the cells and these were often occupied by lymphocytes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. M., Porter K. R. The ultrastructural morphology and possible functional significance of mesothelial microvilli. Anat Rec. 1973 Nov;177(3):409–426. doi: 10.1002/ar.1091770307. [DOI] [PubMed] [Google Scholar]
- Baradi A. F., Rao S. N. A scanning electron microscope study of mouse peritoneal mesothelium. Tissue Cell. 1976;8(1):159–162. doi: 10.1016/0040-8166(76)90027-6. [DOI] [PubMed] [Google Scholar]
- Barberini F., Carpino F., Renda T., Motta P. Etude au microscope électronique à balayage du péritoine du rat. Anat Anz. 1977;142(5):486–496. [PubMed] [Google Scholar]
- Casley-Smith J. R. An electron microscopical study of the passage of ions through the endothelium of lymphatic and blood capillaries, and through the mesothelium. Q J Exp Physiol Cogn Med Sci. 1967 Apr;52(2):105–113. doi: 10.1113/expphysiol.1967.sp001892. [DOI] [PubMed] [Google Scholar]
- Cotran R. S., Karnovsky M. J. Ultrastructural studies on the permeability of the mesothelium to horseradish peroxidase. J Cell Biol. 1968 Apr;37(1):123–137. doi: 10.1083/jcb.37.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Nicca C. The intercellular localization of cations in mesothelium. A light and electron microscopic study. Lab Invest. 1968 Apr;18(4):407–415. [PubMed] [Google Scholar]
- Digenis G. E., Rabinovich S., Medline A., Rodella H., Wu G., Oreopoulos D. G. Electron microscopic study of the peritoneal kinetics of iron dextran during peritoneal dialysis in the rabbit. Nephron. 1984;37(2):108–112. doi: 10.1159/000183224. [DOI] [PubMed] [Google Scholar]
- Dobbie J. W., Zaki M., Wilson L. Ultrastructural studies on the peritoneum with special reference to chronic ambulatory peritoneal dialysis. Scott Med J. 1981 Jul;26(3):213–223. doi: 10.1177/003693308102600305. [DOI] [PubMed] [Google Scholar]
- Gokal R., Ramos J. M., Francis D. M., Ferner R. E., Goodship T. H., Proud G., Bint A. J., Ward M. K., Kerr D. N. Peritonitis in continuous ambulatory peritoneal dialysis. Laboratory and clinical studies. Lancet. 1982 Dec 18;2(8312):1388–1391. doi: 10.1016/s0140-6736(82)91282-x. [DOI] [PubMed] [Google Scholar]
- Gotloib L., Digenis G. E., Rabinovich S., Medline A., Oreopoulos D. G. Ultrastructure of normal rabbit mesentery. Nephron. 1983;34(4):248–255. doi: 10.1159/000183024. [DOI] [PubMed] [Google Scholar]
- Madison L. D., Bergstrom-Porter B., Torres A. R., Shelton E. Regulation of surface topography of mouse peritoneal cells. Formation of microvilli and vesiculated pits on omental mesothelial cells by serum and other proteins. J Cell Biol. 1979 Sep;82(3):783–797. doi: 10.1083/jcb.82.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrhe-Jensen O., Larsen S. B., Astrup T. Fibrinolytic activity in serosal and synovial membranes. Rats, guinea pigs, and rabbits. Arch Pathol. 1969 Dec;88(6):623–630. [PubMed] [Google Scholar]
- ODOR D. L. Observations of the rat mesothelium with the electron and phase microscopes. Am J Anat. 1954 Nov;95(3):433–465. doi: 10.1002/aja.1000950304. [DOI] [PubMed] [Google Scholar]
- Raftery A. T. Effect of peritoneal trauma on peritoneal fibrinolytic activity and intraperitoneal adhesion formation. An experimental study in the rat. Eur Surg Res. 1981;13(6):397–401. doi: 10.1159/000128208. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N. Organization of cell junctions in the peritoneal mesothelium. J Cell Biol. 1977 Jul;74(1):98–110. doi: 10.1083/jcb.74.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N. Cellular aspects of transcapillary exchange. Physiol Rev. 1983 Oct;63(4):1536–1579. doi: 10.1152/physrev.1983.63.4.1536. [DOI] [PubMed] [Google Scholar]
- Tormey J. M., Diamond J. M. The ultrastructural route of fluid transport in rabbit gall bladder. J Gen Physiol. 1967 Sep;50(8):2031–2060. doi: 10.1085/jgp.50.8.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger C., Luger A., Moore H. L., Nolph K. D. Acute changes in peritoneal morphology and transport properties with infectious peritonitis and mechanical injury. Kidney Int. 1983 Jun;23(6):823–831. doi: 10.1038/ki.1983.101. [DOI] [PubMed] [Google Scholar]