Abstract
The role of herpes simplex virus ICP27 protein in mRNA export is investigated by microinjection into Xenopus laevis oocytes. ICP27 dramatically stimulates the export of intronless viral mRNAs, but has no effect on the export of cellular mRNAs, U snRNAs or tRNA. Use of inhibitors shows, in contrast to previous suggestions, that ICP27 neither shuttles nor exports viral mRNA via the CRM1 pathway. Instead, ICP27-mediated viral RNA export requires REF and TAP/NXF1, factors involved in cellular mRNA export. ICP27 binds directly to REF and complexes containing ICP27, REF and TAP are found in vitro and in virally infected cells. A mutant ICP27 that does not interact with REF is inactive in viral mRNA export. We propose that ICP27 associates with viral mRNAs and recruits TAP/NXF1 via its interaction with REF proteins, allowing the otherwise inefficiently exported viral mRNAs to access the TAP-mediated export pathway. This represents a novel mechanism for export of viral mRNAs.
Keywords: ICP27/mRNA export/REF/TAP/NXF1
Introduction
ICP27 (IE63) is a herpes simplex virus-1 (HSV-1) protein that binds RNA (Ingram et al., 1996; Mears and Rice, 1996) and shuttles between the nucleus and the cytoplasm (Phelan and Clements, 1997; Soliman et al., 1997; Mears and Rice, 1998; Sandri-Goldin, 1998). ICP27 is essential for viral replication and well-conserved homologues are present in all herpesviruses sequenced to date. During lytic infection, HSV-1 genes are classified as immediate early (IE), early or late. ICP27 is an IE viral protein.
Viruses mutant in ICP27 show reduced levels of viral mRNAs, are defective in viral DNA replication (Rice and Knipe, 1988; Sekulovich et al., 1988; McCarthy et al., 1989; Rice and Knipe, 1990) and fail to efficiently suppress cellular gene expression (Hardwicke and Sandri-Goldin, 1994). ICP27 appears to affect host pre-mRNA splicing (Hardy and Sandri-Goldin, 1994; Sandri-Goldin and Hibbard, 1996) and promotes RNA 3′ processing at weak viral poly(A) sites (McLauchlan et al., 1989; McGregor et al., 1996). ICP27 has been reported to shuttle between the nucleus and the cytoplasm and to bind intronless viral mRNAs but not spliced viral transcripts (Sandri-Goldin, 1998). It has also been proposed to affect nuclear export of viral RNAs via CRM1-dependent and -independent pathways (Soliman and Silverstein, 2000). However, no direct role for ICP27 in viral mRNA export has been demonstrated.
CRM1 mediates the nuclear export of a variety of protein and RNA substrates. These include incompletely spliced human immunodeficiency virus type 1 (HIV-1) mRNAs, via the Rev protein (Malim et al., 1989; Fischer et al., 1995; Fornerod et al., 1997), and cellular U-rich small nuclear RNAs (snRNAs). CRM1 is dispensable for export of tRNA and most cellular mRNAs (Fornerod et al., 1997). Simple retroviruses such as simian virus type D have a constitutive transport element (CTE) that promotes CRM1-independent export of unspliced viral mRNA by interaction with TAP/NXF1 (Grüter et al., 1998; Braun et al., 1999; Kang and Cullen, 1999). TAP has also been implicated in the export of cellular mRNA (Pasquinelli et al., 1997; Saavedra et al., 1997; Braun et al., 2001). Although TAP binds the viral CTE specifically (Braun et al., 1999) the interaction of TAP and its yeast homologue Mex67p with cellular RNAs is thought to be mediated by a conserved family of proteins called REFs (RNA and export factor binding proteins) that are required for mRNA export (Strässer and Hurt, 2000; Stutz et al., 2000; Rodrigues et al., 2001).
REFs have sequence similarities to heterogeneous nuclear ribonucleoproteins (hnRNPs), and bind RNA non-specifically (Stutz et al., 2000). REFs associate with cellular mRNA, either in a sequence-independent manner by being deposited during the process of splicing as components of a larger complex (Le Hir et al., 2000, 2001; Zhou et al., 2000), or independent of splicing in a manner that is influenced by both the sequence and the length of the transcript (Rodrigues et al., 2001). REFs are thought to participate in mRNA export by recruiting TAP/Mex67p to cellular mRNPs (Le Hir et al., 2000, 2001; Strässer and Hurt, 2000; Stutz et al., 2000).
We investigated the role of ICP27 in HSV-1 viral mRNA export by microinjection into Xenopus laevis oocytes. Nuclear export of the viral RNAs studied is dramatically stimulated in the presence of microinjected recombinant ICP27. ICP27 binds directly to REF and to RNA, and a mutant defective in REF binding is inactive in stimulating viral RNA export. ICP27 is present in a complex with REF and TAP in virus-infected cells and stimulation of viral mRNA export in oocytes requires REF and TAP. We propose that ICP27 binds viral mRNAs and recruits REF to them directly and thus TAP/NXF1 indirectly. Therefore, ICP27 acts to stimulate export of intonless viral mRNAs that are otherwise inefficiently exported from the nucleus by accessing the TAP-mediated export pathway.
Results
ICP27 interacts with REF in vitro and in vivo
In order to identify cellular partners of ICP27 we used the yeast two-hybrid assay to screen a HeLa cell cDNA library. From a total of 2.3 × 106 transformants screened, 82 clones fulfilled the criteria for interaction and were sequenced and checked against the GenBank database. Four clones of similar size were identified as human REF1-I/ALY (Bruhn et al., 1997; Stutz et al., 2000).
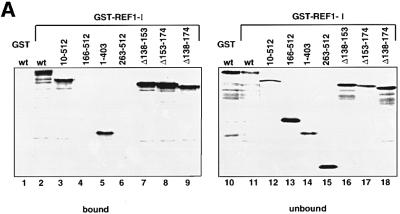
To confirm the binding of ICP27 to REF, [35S]methionine-labelled ICP27 and various ICP27 fragments were synthesized in vitro, and assayed for binding to beads carrying recombinant REF1-I. Full-length ICP27 (residues 1–512) bound to beads carrying REF1-I (Figure 1A, lane 2), REF1-II or REF2-II (data not shown). Fragments of ICP27 that contain residues 10–512 (lane 3), 1–403 (lane 5) or fragments that lack residues 138–152 (lane 7), 153–174 (lane 8) or 138–174 (lane 9) also bind REF. Neither the 166–512 nor the 263–512 fragment of ICP27 exhibited detectable REF binding (lanes 4, 6 and 13, 15). These results indicate that an N-terminal region of ICP27, including amino acids lying between positions 10 and 138, is involved in binding to REF proteins.
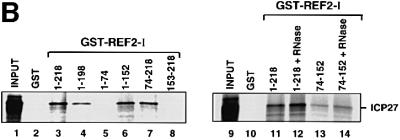
Fig. 1. ICP27 interacts with REF in vitro. (A) [35S]methionine-labelled ICP27 (wt), and fragments of ICP27 were incubated with GST–REF1-I beads or GST beads as indicated. The bound (lanes 1–9) and unbound fractions (lanes 10–18) were analysed by SDS–PAGE followed by fluorography. (B) ICP27 associates with the central domain (RBD) of REF. ICP27 synthesized in vitro was incubated with beads pre-coated with GST–REF2-I (wt) or the truncations indicated. One-tenth of the input (lanes 1 and 9) and one-third of the bound fractions (lanes 2–8 and 10–14) were analysed by SDS–PAGE. RNase (10 µg/ml) was added to the reactions shown in lanes 12 and 14.
We also tested the specificity of ICP27 binding to REF by using [35S]methionine-labelled ICP27 binding to beads loaded with recombinant REF2-I (Stutz et al., 2000), or with the deletions indicated. REF2-I full-length (residues 1–218) (Figure 1B, lane 3), or fragments of REF2-I that contain residues 1–198 (lane 4), 1–152 (lane 6) or 74–218 (lane 7) bound to ICP27 protein whereas fragments containing residues 1–74 (lane 5) or 153–218 (lane 8) did not. This indicates that the central domain of REF is required for interaction with ICP27. Indeed, a REF2-I fragment containing residues 74–152, which resembles an RNP-type RNA binding domain (Rodrigues et al., 2001), could still bind weakly to ICP27 (Figure 1B, lane 13). RNase treatment (lane 14) did not disrupt this interaction.
To determine whether ICP27 can interact with REF in infected cells, anti-ICP27 immunoprecipitations were performed in extracts of infected HeLa cells (Figure 2A). ICP27 co-immunoprecipitated with REF from extracts of cells infected with wt HSV-1 (KOS strain) but not from extracts of mock-infected cells or cells infected with the ICP27 null mutant virus 27-lacZ (Figure 2A, lanes 2–4; Smith et al., 1992). Control western blot analysis of extracts from KOS-infected HeLa cells (Figure 2A, lane 1) showed no cross-reactivity of the anti-REF antibodies with ICP27. Since REF proteins have been shown to interact with the TAP/NXF1 RNA export receptor (Strässer and Hurt, 2000; Stutz et al., 2000; Rodrigues et al., 2001), we also tested for the presence of TAP in this complex. ICP27 co-immunoprecipitated with TAP protein from extracts of infected cells (Figure 2A, lanes 2–4; lower panel). RNase treatment (lane 5) did not disrupt these interactions.
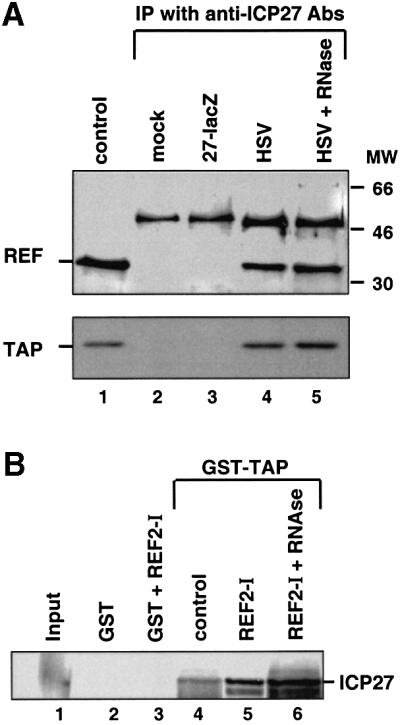
Fig. 2. ICP27 forms a complex with REF and TAP proteins. (A) HeLa cells were mock-infected or infected with wt HSV-1 virus (KOS) or the 27-lacZ mutant virus and immunoprecipitation was performed using the monoclonal anti-ICP27 antibodies H1119 and H1113. Western blot analysis was performed using anti-REF and anti-TAP polyclonal antibodies. The control lane represents western blot analysis of KOS-infected HeLa cells. In the upper panel, the ∼50 kDa bands correspond to the heavy chain of the antibody used for immunoprecipitation. (B) ICP27 synthesized in vitro was incubated with 5 µg of immobilized GST or GST–TAP. Five micrograms of histidine-tagged REF2-I, histidine-tagged thioredoxin (as control) or RNase were added to the binding reactions as indicated. Bound proteins were eluted and one-tenth of the input (lane 1) or one-third of the bound fractions (lanes 2–6) were analysed by SDS–PAGE.
To investigate whether TAP binds directly or indirectly to ICP27, the ability of both REF2-I and TAP to bind to GST–ICP27 was tested. As expected from Figure 1B, recombinant REF2-I did bind to GST–ICP27, while recombinant TAP did not, irrespective of the presence of REF (data not shown). Next, we tested the ability of [35S]methionine-labelled ICP27 synthesized in vitro in rabbit reticulocyte lysate to bind to recombinant GST–TAP (Figure 2B). In the absence of REF, a weak interaction of ICP27 with TAP was observed (Figure 2B, lane 4). The addition of recombinant REF stimulated the recruitment of ICP27 to GST–TAP (Figure 2B, lane 5). This suggests that ICP27 can form a complex that contains REF and TAP proteins. It is possible that endogenous REF proteins present in the lysate mediate the interaction between TAP and ICP27 seen in lane 4. RNase treatment did not abolish complex formation (Figure 2B, lane 6). The fact that a ternary complex was not observed using Escherichia coli-derived TAP, REF2-I and GST–ICP27 (data not shown) might reflect a requirement for protein modification and/or additional components of the lysate for complex formation. Trivial explanations, such as a lack of proper folding of the recombinant proteins or an inhibitory effect of the GST fusion partner are, however, also possible.
ICP27 can shuttle in Xenopus laevis oocytes
ICP27 protein binds intronless viral mRNAs (Sandri-Goldin, 1998) and has been proposed to mediate export of viral mRNAs via CRM1-dependent and -independent pathways (Soliman and Silverstein, 2000). The fact that ICP27 interacts with REF proteins further suggested a role in RNA export. We therefore investigated the role of ICP27 in viral mRNA export by microinjection into X.laevis oocytes. To verify that ICP27 shuttles in oocytes, as it does in mammalian cells (Phelan and Clements, 1997; Soliman et al., 1997; Sandri-Goldin, 1998), a mixture of [35S]methionine-labelled ICP27, CBP80 (the large subunit of the nuclear cap binding protein complex, CBC; Izaurralde et al., 1994) and GST–M10 proteins were injected into the oocyte cytoplasm and assayed for their import into the nucleus. GST–M10 remains in the injection compartment and serves as an injection and dissection control. Immediately after injection, all proteins were found in the cytoplasmic fraction (Figure 3A, lanes 1 and 2). Following a 6 h incubation, ∼50% of ICP27 had moved into the nucleus (Figure 3A, lanes 3 and 4). Nuclear localization of ICP27 in mammalian cells is mediated mostly through a bipartite nuclear localization signal (NLS) (Mears et al., 1995). If ICP27 is also imported via the importin β pathway in oocytes, the importin β binding (IBB) domain of importin α should specifically block the import of ICP27 (Görlich et al., 1996; Weis et al., 1996). Oocytes were pre-injected in the cytoplasm with either truncated or full-length IBB proteins, followed by cytoplasmic injection of [35S]methionine-labelled CBP80, ICP27 and GST–M10 proteins. In oocytes injected with the full-length IBB, import of both ICP27 and the CBP80 control was significantly reduced (Figure 3A, lanes 7 and 8). The truncated IBB had no effect on CBP80 or ICP27 protein accumulation in the nucleus (Figure 3A, lanes 5 and 6). This suggests that importin β mediates ICP27 protein import into the nucleus.
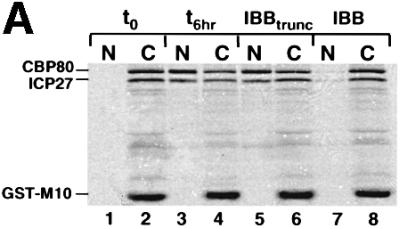
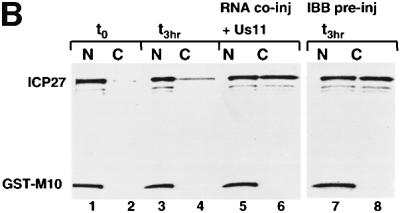
Fig. 3. ICP27 protein shuttles in Xenopus oocytes. (A) Xenopus laevis oocytes were microinjected into the cytoplasm with a mix of [35S]methionine-labelled CBP80, ICP27 and GST–M10 proteins. Oocytes were either pre-injected into the cytoplasm with truncated (30 mg/ml, lanes 5 and 6) or full-length (30 mg/ml, lanes 7 and 8) IBB. Protein samples from nuclear (N) and cytoplasmic (C) fractions were collected immediately (lanes 1 and 2) or after 6 h incubation (lanes 3–8). Labelled proteins were visualized by SDS–PAGE and fluorography. CBP80, ICP27 and GST–M10 are indicated. (B) A mix of [35S]methionine-labelled ICP27 and GST–M10 proteins was microinjected into oocyte nuclei alone (lanes 1–4, 7 and 8) or in the presence of 7.5 µM Us11 mRNA (lanes 5 and 6). In lanes 7 and 8 the protein mix was injected into oocytes pre-injected into the cytoplasm with IBB. Protein samples from nuclear (N) and cytoplasmic (C) fractions were collected immediately (lanes 1 and 2) or after 3 h incubation (lanes 3–10).
To assay nuclear export of ICP27, a mix of [35S]methionine-labelled ICP27 and GST–M10 proteins was injected into oocyte nuclei. Immediately after injection, both proteins were found in the nuclear fraction (Figure 3B, lanes 1 and 2). After a 3 h incubation ICP27 remained predominantly in the nucleus (Figure 3B, lanes 3 and 4). No increase in cytoplasmic accumulation was observed even after >18 h of incubation (data not shown), suggesting that this distribution reflected a steady state between export and re-import. Consistent with this explanation, increased accumulation of ICP27 in the cytoplasm was observed when re-import of the protein was blocked by IBB injection (Figure 3B, lanes 7 and 8).
To test whether the presence of a substrate RNA alters the distribution of ICP27, an intronless late viral RNA (Us11) was co-injected into the nucleus with ICP27 protein. Following a 3 h incubation, ∼50% of the protein accumulated in the cytoplasm in the presence of Us11 RNA (Figure 3B, lanes 5 and 6). In summary, these results indicate that ICP27 is a shuttling protein in Xenopus oocytes and when substrate RNAs are limiting, the rate of import is greater than the rate of export.
ICP27 specifically stimulates nuclear export of viral but not cellular mRNAs in Xenopus laevis oocytes
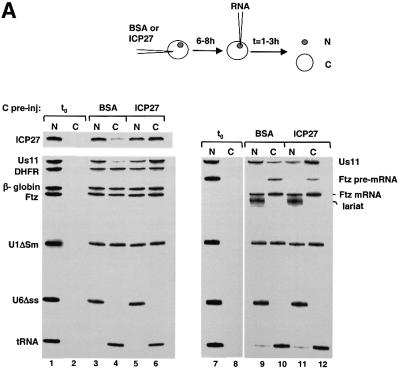
To test whether recombinant ICP27 protein might stimulate mRNA export we pre-injected either bovine serum albumin (BSA) or a GST–ICP27 fusion protein into the oocyte cytoplasm, incubated for 6 h to allow nuclear import, then injected a mix of 32P-labelled RNAs into the nucleus. The RNAs tested were viral and cellular mRNAs, U1ΔSm snRNA and tRNA, which are all exported from the nucleus, and U6Δss snRNA, which remains in the nucleus after injection (Jarmolowski et al., 1994). The presence of ICP27 had a dramatic stimulatory effect on the export of two viral mRNAs, the IE transcript ICP27 and the late transcript Us11 (Figure 4A). Without ICP27 the viral mRNAs were poor export substrates (11% export) compared with cellular dihydrofolate reductase (DHFR), β-globin or Fushi tarazu (Ftz) mRNAs (∼50% export; Figure 4A, lanes 3 and 4). In contrast, in the presence of recombinant ICP27 protein export of both viral mRNAs was stimulated, as ∼65% of these mRNAs was detected in the cytoplasm (Figure 4A, lanes 5 and 6). This represents an average of 6-fold export stimulation (results from five independent experiments). No general stimulation or significant inhibition of export of the other RNAs was observed in the presence of ICP27 (Figure 4A, lanes 3–6). Double nuclear injections of first ICP27 protein followed by the RNA mix gave identical results (data not shown).
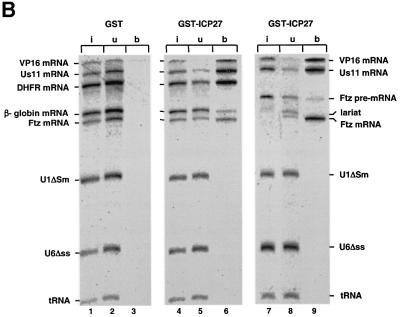
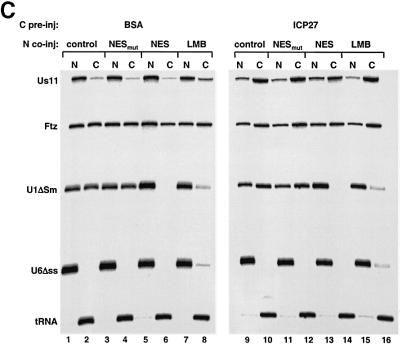
Fig. 4. ICP27 protein stimulates viral mRNA export. (A) Oocytes were pre-injected into the cytoplasm with purified recombinant GST–ICP27–his (5.5 µM) or BSA and incubated for 6–8 h at 18°C. Subsequently, a mix of 32P-labelled RNAs was injected into the nuclei, as indicated. Oocytes were dissected into nuclear (N) and cytoplasmic (C) fractions immediately (lanes 1, 2 and 7, 8) or 2 h after injection (lanes 3–6 and 9–12). RNAs were purified and analysed on denaturing 8% polyacrylamide gels followed by autoradiography. The injected RNAs as well as the intron lariat and spliced product of the Ftz pre-mRNA are indicated. (B) Recombinant GST–ICP27–his (lanes 4–9) or GST alone (lanes 1–3) was injected into oocyte nuclei. Subsequently, a mix containing either VP16 and Us11 viral RNAs, DHFR, β-globin and Ftz mRNAs, U1ΔSm, U6Δss and tRNA (lanes 1–6), or VP16, Us11, Ftz pre-mRNA, U1ΔSm, U6Δss and tRNA was injected into the oocyte nuclei (lanes 7–9). RNA samples from total oocytes were collected 20 min after injection and analysed directly (input, i, lanes 1, 4 and 7), or immunoprecipitated using anti-GST antibodies: unbound fractions (u, lanes 2, 5 and 8) and bound fractions (b, lanes 3, 6 and 9). (C) Oocytes were pre-injected with BSA (lanes 1–8) or recombinant ICP27 (lanes 9–16), followed by nuclear injection of RNA mix. The RNA mix was injected alone (lanes 1, 2, 7–10, 15 and 16), in the presence of 5 mg/ml mutant NES conjugate (NESmut) (lanes 3, 4 and 11, 12) or 5 mg/ml wt NES conjugate (NES) (lanes 5, 6 and 13, 14), or oocytes were pre-incubated in the presence of 200 nM LMB for 2 h, followed by nuclear injection of the RNAs (lanes 7, 8, 15 and 16). RNA samples from nuclear (N) and cytoplasmic (C) fractions were collected 2 h after injection.
ICP27 has been implicated in inhibition of host cell splicing (Hardy and Sandri-Goldin, 1994; Sandri-Goldin and Hibbard, 1996). We thus performed the same injections as described above except that a Ftz pre-mRNA was included in the RNA mix. This substrate was spliced and exported efficiently in either the absence or presence of recombinant ICP27 protein (Figure 4A, lanes 9–12). Under these conditions ICP27 had no effect on the splicing of the pre-mRNA or export of the spliced mRNA.
In order to test whether the export stimulation of the viral RNAs is a result of selective binding of ICP27, we analysed the interaction of RNA with ICP27 by co-immunoprecipitation. Recombinant ICP27 was pre- injected followed by injection of two different RNA mixes. As a control, GST protein was pre-injected. Following RNA injection, the oocytes were incubated for 20 min and subjected to immunoprecipitation using GST antibodies (Figure 4B). In oocytes injected with ICP27 the GST antibodies immunoprecipitated both Us11 and VP16 viral mRNAs (VP16 mRNA export is stimulated by ICP27, data not shown) as well as a variable fraction of the cellular DHFR, β-globin and Ftz mRNAs (Figure 4B, lanes 4–6). In contrast, U1ΔSm, U6Δss or tRNA were not immunoprecipitated (Figure 4B, lanes 4–9). When Ftz pre-mRNA was tested, ICP27 immunoprecipitated the spliced mRNA but not the precursor or the intron lariat (Figure 4B, lanes 7–9). Recombinant ICP27 protein exhibited similar binding specificity for naked RNAs in vitro (data not shown). Thus, ICP27 associates with both viral and cellular mRNAs in vitro and in vivo, but not with other classes of RNA.
It has been proposed that ICP27 mediates the export of some late HSV-1 viral mRNAs via CRM1 (Soliman and Silverstein, 2000). To test whether ICP27-stimulated Us11 RNA export is via CRM1/Xpo1, we used either nuclear export signal (NES) peptides conjugated to BSA, which saturate CRM1 and inhibit export of its substrates (Fischer et al., 1995), or leptomycin B (LMB), a specific inhibitor of CRM1 (Fornerod et al., 1997). Peptides corresponding to the wild-type protein kinase A inhibitor (PKI) NES or the P10 mutant sequence (Wen et al., 1995) were conjugated to BSA and co-injected with a mix of RNAs into the nuclei of oocytes pre-injected with BSA or ICP27 (Figure 4C). There was no effect of the BSA–NES conjugates on ICP27-stimulated Us11 RNA export (Figure 4C lanes 9–14), although CRM1-dependent U1ΔSm export was blocked (Figure 4C lanes 5, 6, 13 and 14). Similarly, LMB had no inhibitory effect on Us11 RNA export in the control or in the ICP27 pre-injected oocytes, while it significantly reduced the export of U1ΔSm (Figure 4C lanes 7, 8, 15 and 16). We conclude that CRM1 is not involved in the ICP27-stimulated Us11 RNA nuclear export.
REF and TAP/NXF1 proteins mediate nuclear export of viral mRNA
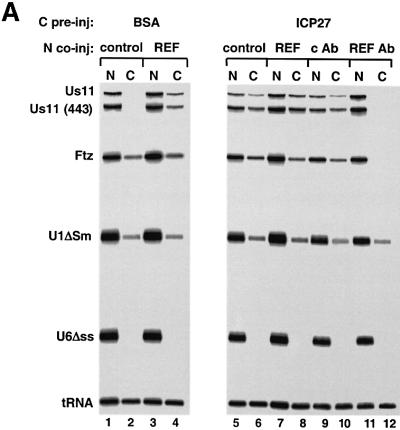
We next investigated whether REF is involved with ICP27 in the export of viral mRNAs. Oocytes pre-injected with BSA or ICP27 in the cytoplasm were subsequently co-injected in the nucleus with purified recombinant REF protein and a mix of RNAs including full-length Us11 RNA and a 3′ C-terminal fragment of Us11 of 443 nt (Figure 5A). After short incubation there was no detectable export of viral Us11 RNAs in the control oocytes while in the ICP27 pre-injected oocytes a significant amount of both Us11 and (443) Us11 RNA was in the cytoplasm (Figure 5A, lanes 1, 2, 5 and 6). Co-injection of recombinant REF2-II with the RNA mix stimulated export of Us11 RNA in the absence of ICP27, while no significant effect on Ftz mRNA export was observed (Figure 5A, lanes 3 and 4). In contrast, REF2-II had no further stimulatory effect on the export of Us11 RNA in the presence of ICP27 (Figure 5A, lanes 7 and 8). Recombinant REF2-II was used instead of REF1-II, as it has been reported to have a greater effect on mRNA export (Rodrigues et al., 2001).
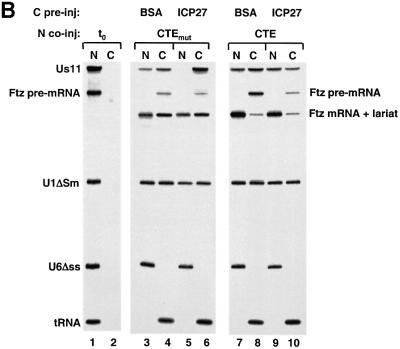
Fig. 5. REF and TAP are involved in viral mRNA export. (A) Oocytes were pre-injected with BSA (lanes 1–4) or ICP27 (lanes 5–12) followed by nuclear injection of RNA mix. The RNA mix was injected alone (lanes 1, 2, 5 and 6), with 14 µM GST–REF (lanes 3, 4, 7 and 8) or with affinity-purified anti-REF antibodies (5 mg/ml) (lanes 11 and 12) or control antibodies (lanes 9 and 10). RNA samples from nuclear (N) and cytoplasmic (C) fractions were collected 1 h after injection. (B) Oocytes were pre-injected with BSA (lanes 3, 4, 7 and 8) or ICP27 (lanes 5, 6, 9 and 10), followed by nuclear injection of RNAs in the presence of 0.1 pmol CTE M36 mutant (lanes 3–6) or 0.1 pmol wild-type CTE RNA (lanes 7–10). RNA samples from nuclear (N) and cytoplasmic (C) fractions were collected immediately (lanes 1 and 2) or 3.5 h after injection (lanes 3–10). In this gel (10%), the lariat and the Ftz mRNA co-migrate.
These results are consistent with previous observations that REFs only stimulate the nuclear exit of inefficiently exported mRNAs (Rodrigues et al., 2001) and could indicate that ICP27 functionally replaces REF. To test this we used antibodies raised against the RBD of REFs that prevent their binding to RNA but not to TAP and have been used to inhibit nuclear export of intronless and spliced mRNAs (Rodrigues et al., 2001). Affinity purified anti-REF antibodies were co-injected with the same mix of RNAs in ICP27 pre-injected oocytes. Microinjection of anti-REF antibodies resulted in export inhibition of the Us11 RNAs and Ftz mRNA (Figure 5A, lanes 11 and 12) while there was no significant inhibition of the export of U1ΔSm or tRNA, as described previously (Rodrigues et al., 2001). Microinjection of control antibodies had no effect on export of any of the injected RNAs (Figure 5A, lanes 9 and 10). This suggests that ICP27 does not replace REF protein function but rather acts together with REF to efficiently export Us11 RNA.
REF proteins are thought to recruit TAP/NXF1 to mRNAs. Injection of excess of CTE RNA (see Introduction), which binds TAP, inhibits TAP-dependent RNA export (Grüter et al., 1998). To investigate the involvement of TAP in ICP27-mediated RNA export, competitor CTE RNA was co-injected with RNA mix into the nuclei of oocytes pre-injected with either BSA or ICP27. As a control, a mutant CTE (M36) that does not bind TAP was used (Grüter et al., 1998). Following 3.5 h incubation, export of the Us11 RNA was complete in the ICP27 pre-injected oocytes, and the presence of wild-type (wt), but not mutant, CTE considerably reduced export (Figure 5B, compare lanes 5 and 6 with 9 and 10). The inefficient Us11 RNA export in the BSA pre-injected oocytes was only slightly reduced by CTE (Figure 5B, compare lanes 3 and 4 with 7 and 8). However, higher concentrations of CTE competitor RNA almost completely blocked ICP27-independent Us11 RNA export (data not shown).
mRNA-mediated ICP27 protein export is inhibited by CTE
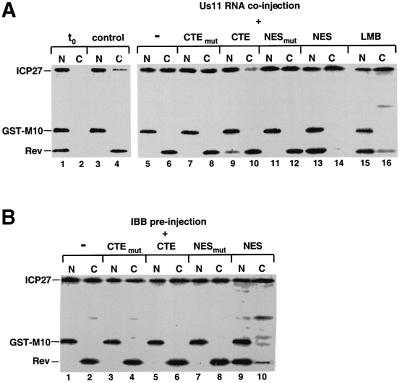
At steady state, ICP27 is a nuclear protein in oocytes, but excess of viral RNA causes the protein to accumulate in the cytoplasm (Figure 3B). To investigate whether export of ICP27 protein under these conditions requires the same factors as ICP27-mediated viral RNA export, the inhibitors used above for the RNA export were tested on RNA-induced ICP27 export. A mix of [35S]methionine-labelled ICP27, GST–M10 and Rev proteins was co-injected into oocyte nuclei in the presence or the absence of Us11 RNA (Figure 6A). The Rev protein contains a leucine-rich NES and was used as a control for CRM1-mediated export. Under conditions where Rev export was drastically reduced either by co-injection of BSA–NES conjugates (Figure 6A, lanes 13 and 14) or by incubation in the presence of LMB (Figure 6A, lanes 15 and 16), there was no inhibition of RNA-mediated ICP27 protein accumulation in the cytoplasm. However, co-injection of excess CTE, which blocks viral mRNA export (Figure 5B), inhibited ICP27 protein accumulation in the cytoplasm (Figure 6A, lanes 9 and 10). These results suggest that ICP27 moves to the cytoplasm as a complex with the viral mRNA.
Fig. 6. ICP27 protein is exported via TAP/NXF1 in the presence of viral mRNA. (A) Labelled ICP27, GST–M10 and Rev proteins were injected into oocyte nuclei alone (lanes 1–6, 15 and 16), in the presence of 0.1 pmol M36 mutant CTE or wt CTE RNA (lanes 7–10) or 5 mg/ml mutant or wt NES conjugate (lanes 11–14). In lanes 15 and 16, the oocytes were pre-incubated in the presence of 200 nM LMB. Oocytes were dissected into nuclear (N) and cytoplasmic (C) fractions immediately (lanes 1 and 2) or after a 3 h incubation (lanes 3–16). (B) Oocytes pre-injected into the cytoplasm with IBB, were injected into the nuclei with labelled ICP27, GST–M10 and Rev proteins alone (lanes 1 and 2), in the presence of 0.1 pmol M36 mutant CTE or wt CTE (lanes 3–6), or 5 mg/ml mutant or wt NES conjugate (lanes 7–10). Oocytes were dissected into nuclear (N) and cytoplasmic (C) fractions after a 3 h incubation.
It is interesting to note that when re-import of ICP27 protein was blocked by excess of IBB, the protein accumulated in the cytoplasm although no viral RNA was present (Figures 3B and 6B lanes 1 and 2). Cytoplasmic accumulation of the protein under these conditions was not inhibited by blocking CRM1 or TAP (Figure 6B, lanes 5, 6, 9 and 10). Thus, ICP27 protein export in the absence of viral RNA does not involve either of these export factors.
REF binding to ICP27 is required for viral RNA export stimulation
We next tested whether binding of REF was necessary for ICP27 action in RNA export. ICP27 co-immunoprecipitated with REF from extracts of cells infected with wt HSV-1 (Figures 2A and 7A, lane 4). However, when anti-ICP27 immunoprecipitations were performed in extracts of HeLa cells infected with an ICP27 viral mutant that lacks part of the binding site defined earlier (Figure 1A), amino acids 109–138 (HSV d3-4; Mears et al., 1995), REF protein was not co-immunoprecipitated (Figure 7A, lane 5), although similar levels of mutant and the wild-type protein were produced (Figure 7A, lower panel).
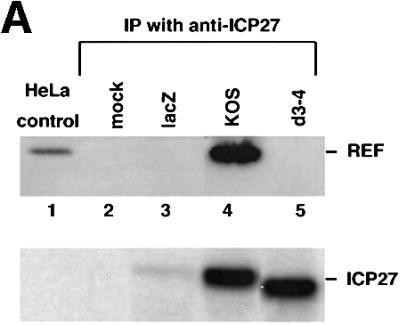
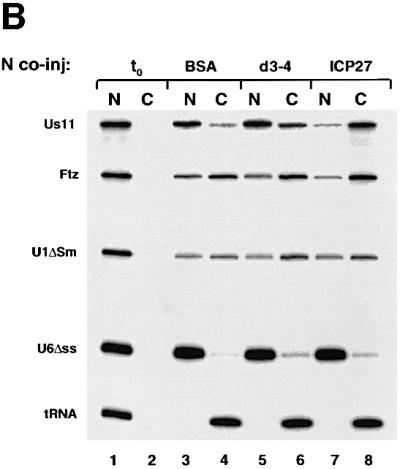
Fig. 7. REF binding to ICP27 is required for viral RNA export stimulation. (A) HeLa cells were mock-infected or infected with wt HSV-1 (KOS), the 27-lacZ mutant or the d3-4 ICP27 deletion mutant virus, and immunoprecipitation was performed using the mAb H1119. Western blot analysis was performed using anti-REF polyclonal antibodies (upper panel), or the anti-ICP27 mAb H1119 (lower panel). (B) Oocytes were injected into the nucleus with BSA (lanes 3 and 4), d3-4 mutant ICP27 (lanes 5 and 6) or wt ICP27 (lanes 7 and 8), followed by nuclear injection of the RNA mix. RNA samples from nuclear (N) and cytoplasmic (C) fractions were collected 3 h after injection.
To test the activity of the d3-4 mutant in RNA export, we performed double nuclear injections by injecting recombinant d3-4 mutant or wt ICP27 into the nucleus, followed by RNA mix. After a 3 h incubation the d3-4 mutant ICP27 protein failed to stimulate Us11 viral mRNA export (Figure 7B, lanes 5 and 6), while wt ICP27 stimulated export of Us11 as expected (Figure 7B, lanes 7 and 8). Since the d3-4 mutant ICP27 protein has lost the ability to bind REF proteins but still contains the RGG box and binds RNA (Mears and Rice, 1996; our unpublished observations), binding of ICP27 to REF proteins is necessary for viral RNA export stimulation.
Discussion
Our data demonstrate that the IE HSV-1 ICP27 protein, essential for virus viability, is an RNA export factor that specifically stimulates viral but not cellular mRNA nuclear export. Shuttling of ICP27 is linked to viral mRNA export, suggesting co-export with viral RNAs to the cytoplasm. ICP27 interacts directly with REF and forms a complex with REF and TAP proteins in vitro and in HSV-1-infected cells. We provide evidence that both REF and TAP are involved in ICP27-mediated viral mRNA export. Furthermore, an ICP27 mutant that does not bind REF failed to stimulate viral mRNA export. These results are consistent with a model in which the association of endogenous REF with viral mRNAs is weak but is enhanced by ICP27, such that the RNAs become efficient export substrates. Thus, we propose that ICP27 recruits REF proteins to intronless viral RNAs and escorts them to the cytoplasm using the same pathway proposed for cellular mRNAs, via the interaction of REF with TAP (Stutz et al., 2000; Zhou et al., 2000; Rodrigues et al., 2001).
It is interesting to note that ICP27 contains a region at the N-terminus that has been proposed to function as a leucine-rich NES (Sandri-Goldin, 1998), and prolonged treatment of HSV-1-infected cells with LMB inhibits shuttling of the protein (Soliman and Silverstein, 2000). However, we found that in oocytes ICP27 is exported via the TAP-mediated pathway in the presence of viral RNA. In addition, LMB treatment of HSV-1-infected cells for up to 5 h did not inhibit ICP27 protein shuttling, although it did inhibit shuttling of HIV-1 Rev (our unpublished observations). In oocytes, ICP27 protein export in the absence of viral RNA can be observed if re-import of the protein is inhibited. This export requires neither CRM1 nor TAP and we are currently investigating the requirements for RNA-independent ICP27 export. Similarly, we could not detect evidence for a CRM1 requirement in ICP27-stimulated mRNA export. It seems possible that the inhibition of ICP27 protein shuttling by LMB observed previously was due to non-specific effects of long incubation periods. In this context it is of note that the Epstein–Barr virus homologue of ICP27, EB2 protein, even though it also contains a region that matches the leucine-rich NES consensus, exports unspliced RNAs via a CRM1-independent pathway (Boyle et al., 1999; Farjot et al., 2000).
Since cytoplasmically located ICP27 is apparent late in HSV-1 infection but not at early times, it has been suggested that the presence of late RNAs could regulate shuttling of the protein (Soliman et al., 1997; Soliman and Silverstein, 2000). Similarly, we show that the presence of viral mRNA stimulates cytoplasmic accumulation of ICP27 protein in oocytes. Interestingly, co-injection of a cellular mRNA, DHFR, did not result in cytoplasmic accumulation of ICP27 (data not shown), reflecting the situation in non-infected cells expressing ICP27, and suggesting that ICP27 displacement from viral and cellular mRNAs in the cytoplasm is not identical.
We show that ICP27 can stimulate export of not only late viral mRNAs (Us11), but also an IE mRNA (ICP27). This suggests that ICP27 might stimulate export of all temporal classes of viral RNAs throughout HSV-1 infection and further, the consequent accumulation of ICP27 in the cytoplasm late in infection may simply reflect the high abundance of RNAs expressed from replicated viral genomes at this time.
HSV infection inhibits host cell pre-mRNA splicing (Hardy and Sandri-Goldin, 1994), and ICP27 has been implicated in this process (Hardwicke and Sandri-Goldin, 1994). However, the effect of ICP27 on splicing does not seem to be critical for virus replication (Soliman et al., 1997). In accordance with this, it has been shown that ICP27-dependent cytoplasmic accumulation of unspliced α-globin RNA can be uncoupled from the inhibitory effects of ICP27 on splicing (Ellison et al., 2000). In this study, we did not observe any inhibition of splicing or export of the cellular transcripts examined in Xenopus oocytes. Although we cannot exclude that ICP27 may inhibit splicing in other cell types or when present in higher amounts, it seems probable that ICP27 may not affect α-globin pre-mRNA splicing directly (Ellison et al., 2000), but may rather stimulate export of the pre-mRNA by a mechanism similar to that seen here for viral mRNAs.
The mode of action of ICP27 on viral mRNAs is mechanistically interesting although, at least superficially, counter-intuitive. ICP27 was not found to associate specifically with viral mRNAs but rather, although with somewhat variable efficiency, with all the viral or cellular mRNAs tested. ICP27 did not detectably interact with U snRNA, tRNA or the Ftz intron lariat or pre-mRNA, indicating that its RNA binding properties are not completely non-specific, but rather are reminiscent of cellular hnRNP proteins, which bind to many mRNAs with somewhat variable affinity (Bennett et al., 1992; Matunis et al., 1993; see review by Dreyfuss et al., 1993).
Why then does ICP27 only stimulate export of viral, but not cellular mRNAs? Our data indicate that ICP27 acts by recruiting REF to viral mRNAs. This suggests that these mRNAs do not, in contrast to many cellular RNAs (see Introduction), efficiently interact with REF in the absence of ICP27. By overcoming this lack of interaction, ICP27, rather than providing viral mRNAs with an absolute advantage over cellular mRNAs would act to remove the competitive disadvantage of viral mRNAs, which bind poorly to REF in its absence. This is consistent with the observed specific stimulation of export of viral RNAs, since the presence of ICP27 would not confer any advantage to cellular mRNAs, which already bind well to REF proteins.
Although REF proteins have been implicated in the export of cellular mRNAs, it is still unclear whether direct binding of REF to mRNA occurs. REF is part of a complex that is recruited to mRNAs during splicing (Le Hir et al., 2000, 2001; Zhou et al., 2000), but it can also associate with intronless mRNAs, with variable efficiency (Rodrigues et al., 2000). ICP27 may be the prototype for a class of cellular proteins that would modulate the efficiency of the REF association with cellular mRNAs.
HSV-1 virus uses the same RNA export receptor as the simian type D retroviruses, the cellular protein TAP/NXF1. However, the two viruses access the same pathway in a different manner. The retroviruses promote export of unspliced viral mRNAs by direct binding of TAP to a cis-acting RNA structure, the CTE (Grüter et al., 1998; Braun et al., 1999; Kang and Cullen, 1999), while HSV-1 utilizes ICP27 to target intronless viral RNAs to the same pathway via its interaction with REF proteins. Complex retroviruses, like HIV-1, also promote export of incompletely spliced HIV-1 RNAs via a viral export factor, the Rev protein (Malim et al., 1989; Fischer et al., 1995). Rev, however, accesses a different cellular pathway, mediated by CRM1 (Fischer et al., 1995; Fornerod et al., 1997). Thus, we describe here a novel way by which intronless viral RNAs can access a conserved cellular mechanism to promote viral mRNA export.
Why HSV-1 has evolved poorly exported intronless mRNAs that require a specific viral protein for their export is unclear. This situation is analogous to one described previously for this virus. 3′ end formation on several HSV-1 mRNAs, like nuclear export of the mRNAs produced, is inefficient in the absence of ICP27 (McLauchlan et al., 1989, 1992; McGregor et al., 1996). It seems as though, in addition to its role in transcription of some late genes (Jean et al., 2001), ICP27 may generally be employed at the post-transcriptional level by HSV-1 to switch viral gene expression from an inefficient to an efficient mode and thus to mark a transition or transitions in the viral life cycle.
Materials and methods
Plasmids
GST–ICP27–his plasmid was constructed from the pGEX27plasmid (Mears and Rice, 1996), by inserting a C-terminal histidine tag. GST–d3-4–his plasmid was constructed by exchanging the DraIII–NsiI fragment of pMd3-4 (Mears et al., 1995) into pGST–ICP27–his. Plasmids pCITE-27, pCITE-d4-5 (deletion of ICP27 residues 138–153), pCITE- d5-6 (deletion of residues 153–174), pCITE-d4-6 (deletion of residues 138–174) and pCITE-262C (ICP27 residues 262–512), have been described previously (Mears and Rice, 1996). PTriEx-10–512, pTriEx-166–512 and pTriEx-1–403 were constructed by inserting a KpnI–XhoI PCR amplified fragment of ICP27 with the corresponding residues, into the KpnI–XhoI sites of the pTriEx-1 vector (Novagen). The two-hybrid screen with ICP27 amino acids 10–512 as bait and a HeLa cell prey cDNA library (Clontech) was as described (Wadd et al., 1999).
Protein expression in E.coli
Expression of GST–REF recombinant proteins (Rodrigues et al., 2001) and GST–TAP (Braun et al., 1999) has been described. GST–ICP27–His and GST–d3-4–His recombinant proteins were expressed in E.coli TOPP or BL21 cells, respectively (Stratagene) and purified on Ni+-NTA–Sepharose fast flow (Qiagen). Histidine-tagged thioredoxin from pET32a and TAP from pET9-TapHis were expressed in BL21 DE3 and purified using Talon resin (Clontech). Histidine-tagged REF2-I was initially purified on Talon resin, then dialysed against buffer A (50 mM sodium phosphate pH 7.4, 50 mM NaCl, 1 mM EDTA, 1 mM DTT) and further purified on a SP-Sepharose column using a gradient of buffer A + 1 M NaCl.
In vitro GST pull-downs
[35S]methionine-labelled ICP27 from pCITE-ICP27 was produced by using the transcription/translation TNT kit (Promega). For GST–REF1-I, binding reactions were carried out in 500 µl of: 10 mM Tris–HCl pH 8, 150 mM NaCl, 1% Triton X-100, 10% glycerol buffer, using ∼5 µg of GST fusion bound to 20 µl of glutathione–agarose, together with 5 µl of labelled ICP27. For GST–TAP, binding reactions were carried out in 500 µl of PBS. In some experiments 5 µg of REF2-1, thioredoxin or RNase was added to the reactions. Proteins were eluted from the resin using elution buffer (50 mM Tris–HCl pH 8.0, 20 mM glutathione) and analysed by SDS–PAGE.
Xenopus oocyte microinjections and immunoprecipitation
Oocyte microinjections and analysis of microinjected RNA by denaturing gel electrophoresis and autoradiography were as described previously (Jarmolowski et al., 1994). Recombinant ICP27 (5.5 µM) was either pre-injected into the oocyte cytoplasm followed by a 6–8 h incubation at 18°C, or injected into the oocyte nucleus and incubated for 30 min before injection of RNA mix. Both procedures gave identical results. In control experiments 6 µM BSA was injected.
DNA templates for labelled cellular RNAs have been described (Jarmolowski et al., 1994; Arts et al., 1998; Rodrigues et al., 2001). Identities of the Ftz pre-mRNA and mRNA were as described (Rodrigues et al., 2001). For the viral transcripts, the ICP27 sequence from HSV-1 KOS strain was inserted into the pGEM-3 vector (T7-ICP27), the VP16 sequence into pGEM-2 (Ace et al., 1988), the Us11 sequence into pBluescript (T3-Us11) and the C-terminal 443 nt of Us11 into pBluescript (T3-Us11–443). Plasmids encoding the SRV1 CTE and the mutant M36 have been described (Grüter et al., 1998). Isolation of protein from oocytes and SDS–PAGE was as described (Kambach and Mattaj, 1992).
Where indicated, the injection mix included 5 mg/ml of the PKI BSA–NES (CELALKLAGLDIN) or the BSA–NES mutant P10 (CELALKAAGADIN) peptide conjugates. Conjugation was performed as described (Fischer et al., 1995).
For immunoprecipitations, nuclear and cytoplasmic fractions from 20 injected oocytes were collected, homogenized and subjected to immunoprecipitation using GST antibodies as described previously (Braun et al., 1999).
Cell infection and viruses
HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. Sub-confluent cell monolayers were infected with either wild-type or mutant HSV-1 KOS at a multiplicity of infection of 10 p.f.u./cell. The ICP27 insertion mutant HSV-1 27-lacZ (Smith et al., 1992) and the d3-4 deletion mutant (Mears et al., 1995) have been described.
Immunoprecipitation of infected extracts
For immunoprecipitations, infected HeLa cells were harvested from a 60 mm dish, scraped and resuspended in 300 µl of binding buffer (10 mM Tris–HCl pH 8, 100 mM NaCl, 1 mM EDTA) containing a protease inhibitor mix (Roche) and passed through a syringe with a 26-gauge needle five times. Cell debris was removed by centrifugation at 13 000 g. Cell extracts were mixed with the monoclonal antibodies anti-ICP27 H1119 and H1113 (Goodwin Institute, Plantation, FL) for 2 h in binding buffer and protein A/G–Sepharose was added for another 2 h. The precipitates were washed five times with cold IPP150 (10 mM Tris–HCl pH 8, 150 mM NaCl, 1% Triton X-100). Western blot analysis was performed using rabbit polyclonal antibodies to murine REF (KJ70, raised against the recombinant RBD of REF1-II (Rodrigues et al., 2001) and to TAP (Braun et al., 1999), diluted at 1:2000.
Acknowledgments
Acknowledgements
We are grateful to Dr S.A.Rice, University of Minnesota Medical School, Minneapolis, for the gift of ICP27 constructs, to Ms Melis Niyage, Department of Biomolecular Sciences, UMIST, Manchester, for communicating unpublished data and Mr James E.Scott for technical assistance. We thank Mutsuhito Ohno, Ludwig Englmeier and Sheila V.Graham for critical reading of the manuscript.
References
- Ace C.I., Dalrymple,M.A., Ramsay,F.H., Preston,V.G. and Preston,C.M. (1988) Mutational analysis of the herpes simplex virus type 1 trans-inducing factor Vmw65. J. Gen. Virol., 69, 2595–2605. [DOI] [PubMed] [Google Scholar]
- Arts G.J., Fornerod,M. and Mattaj,I.W. (1998) Identification of a nuclear export receptor for tRNA. Curr. Biol., 8, 305–314. [DOI] [PubMed] [Google Scholar]
- Bennett M., Pinol-Roma,S., Staknis,D., Dreyfuss,G. and Reed,R. (1992) Differential binding of heterogeneous nuclear ribonucleoproteins to mRNA precursors prior to spliceosome assembly in vitro. Mol. Cell. Biol., 12, 3165–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S.M., Ruvolo,V., Gupta,A.K. and Swaminathan,S. (1999) Association with the cellular export receptor CRM1 mediates function and intracellular localization of Epstein–Barr virus SM protein, a regulator of gene expression. J. Virol., 73, 6872–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun I.C., Rohrbach,E., Schmitt,C. and Izaurralde,E. (1999) TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO J., 18, 1953–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun I.C., Herold,A., Rode,M., Conti,E. and Izaurralde,E. (2001) Overexpression of TAP/p15 heterodimers bypasses nuclear retention and stimulates nuclear mRNA export. J. Biol. Chem., 276, 20536–20543. [DOI] [PubMed] [Google Scholar]
- Bruhn L., Munnerlyn,A. and Grosschedl,R. (1997) ALY, a context-dependent coactivator of LEF-1 and AML-1, is required for TCR α enhancer function. Genes Dev., 11, 640–653. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Matunis,M.J., Pinol-Roma,S. and Burd,C.G. (1993) hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem., 62, 289–321. [DOI] [PubMed] [Google Scholar]
- Ellison K.S., Rice,S.A., Verity R. and Smiley J. (2000) Processing of α-globin and ICP0 mRNA in cells infected with herpes simplex virus type 1 ICP27 mutants. J. Virol., 74, 7307–7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjot G., Buisson,M., Duc Dodon,M., Gazzolo,L., Sergeant,A. and Mikaelian,I. (2000) Epstein–Barr virus EB2 protein exports unspliced RNA via a Crm-1-independent pathway. J. Virol., 74, 6068–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U., Huber,J., Boelens,W.C., Mattaj,I.W. and Lührmann,R. (1995) The HIV-1 Rev activation domain is a nuclear export signal that acesses an export pathway used by specific cellular RNAs. Cell, 82, 475–483. [DOI] [PubMed] [Google Scholar]
- Fornerod M., Ohno,M., Yoshida,M. and Mattaj,I.W. (1997) CRM1 is an export receptor for leucine-rich nuclear export signals. Cell, 90, 1051–1060. [DOI] [PubMed] [Google Scholar]
- Görlich D., Henklein,P., Laskey,R.A. and Hartmann,E. (1996) A 41 amino acid motif in importin α confers binding to importin beta and hence transit into the nucleus. EMBO J., 15, 1810–1817. [PMC free article] [PubMed] [Google Scholar]
- Grüter P., Tabernero,C., von Kobbe,C., Schmitt,C., Saavedra,C., Bachi,A., Wilm,M., Felber,B.K. and Izaurralde,E. (1998) TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell, 1, 649–659. [DOI] [PubMed] [Google Scholar]
- Hardwicke M.A. and Sandri-Goldin,R.M. (1994) The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J. Virol., 68, 4797–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W.R. and Sandri-Goldin,R.M. (1994) Herpes simplex virus inhibits host cell splicing and regulatory protein ICP27 is required for this effect. J. Virol., 68, 7790–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram A., Phelan,A., Dunlop,J. and Clements,J.B. (1996) Immediate early protein IE63 of herpes simplex virus type 1 binds RNA directly. J. Gen. Virol., 77, 1847–1851. [DOI] [PubMed] [Google Scholar]
- Izaurralde E., Lewis,J., McGuigan,C., Jankowska,M., Darzynkiewicz,E. and Mattaj,I.W. (1994) A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell, 78, 657–668. [DOI] [PubMed] [Google Scholar]
- Jarmolowski A., Boelens,W.C., Izaurralde,E. and Mattaj I.W. (1994) Nuclear export of different classes of RNA is mediated by specific factors. J. Cell Biol., 124, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean S., LeVan,K.M., Song,B., Levine,M. and Knipe,D.M. (2001) Herpes simplex virus 1 ICP27 is required for transcription of two viral late (γ2) genes in infected cells. Virology, 283, 273–284. [DOI] [PubMed] [Google Scholar]
- Kambach C. and Mattaj,I.W. (1992) Intracellular distribution of the U1A protein depends on active transport and nuclear binding to U1 snRNA. J. Cell Biol., 118, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. and Cullen,B.R. (1999) The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences Genes Dev., 13, 1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hir H., Izaurralde,E., Maquat,L.E. and Moore,M.J. (2000) The splicesome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon–exon junctions. EMBO J., 19, 6860–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hir H., Gatfield,D., Izaurralde,E. and Moore,M.J. (2001) The exon–exon junction complex provides a binding platform for factors involved in mRNA export and NMD. EMBO J., 20, 4987–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A.M., McMahan,L. and Schaffer,P.A. (1989) Herpes simplex virus type 1 ICP27 deletion mutant exhibits altered patterns of transcription and are DNA deficient. J. Virol., 63, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor F., Phelan,A., Dunlop,J. and Clements,J.B. (1996) Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE63 in the early–late switch. J. Virol., 70, 1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J., Simpson,S. and Clements,J.B. (1989) Herpes simplex virus induces a processing factor that stimulates poly(A) site usage. Cell, 59, 1093–1105. [DOI] [PubMed] [Google Scholar]
- McLauchlan J., Phelan,A., Loney,C., Sandri-Goldin,R.M. and Clements, J.B. (1992) Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J. Virol., 66, 6939–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M.H., Hauber,J., Le S.-Y., Maizel J.V. and Cullen B.R. (1989) The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature, 338, 254–257. [DOI] [PubMed] [Google Scholar]
- Matunis E.L., Matunis,M.J. and Dreyfuss,G. (1993) Association of individual hnRNP proteins and snRNPs with nascent transcripts. J. Cell Biol., 121, 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears W.E. and Rice,S.A. (1996) The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol., 70, 7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears W.E. and Rice,S.A. (1998) The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology, 242, 128–137. [DOI] [PubMed] [Google Scholar]
- Mears W.E., Lam,V. and Rice,S.A. (1995) Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J. Virol., 69, 935–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli A.E., Ernst,R.K., Lund,E., Grimm,C., Zapp,M.L., Rekosh,D., Hammarskjold,M.L. and Dahlberg,J.E. (1997) The constitutive transport element (CTE) of Mason–Pfizer monkey virus (MPMV) accesses a cellular mRNA export pathway. EMBO J., 16, 7500–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan A. and Clements,J.B. (1997) Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J. Gen. Virol., 78, 3327–3331. [DOI] [PubMed] [Google Scholar]
- Rice S.A. and Knipe,D.M. (1988) Gene-specific transactivation by herpes simplex virus type 1 α protein ICP27. J. Virol., 62, 3814–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S.A. and Knipe,D.M. (1990) Genetic evidence for two distinct trans-activation functions of the herpes simplex virus α protein ICP27. J. Virol., 64, 1704–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues J.P., Rode M., Gatfield,D., Blencowe,B.,J., Carmo-Fonseca,M. and Izaurralde,E. (2001) REF proteins mediate the export of spliced and unspliced mRNAs from the nucleus. Proc. Natl Acad. Sci. USA, 98, 1030–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra C.,B. Felber and E. Izaurralde. (1997) The simian retrovirus-1 constitutive transport element, unlike the HIV-1 RRE, utilises factors required for the export of cellular mRNAs. Curr. Biol., 7, 619–628 [DOI] [PubMed] [Google Scholar]
- Sandri-Goldin R.M. (1998) ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev., 12, 868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri-Goldin R.M. and Hibbard,M.K. (1996) The herpes simplex virus type 1 regulatory protein ICP27 coimmunoprecipitates with anti-Sm anti-serum and the C terminus appears to be required for this interaction. J. Virol., 70, 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulovich R.E., Leary,K. and Sandri-Goldin,R.M. (1988) The herpes simplex virus type 1 protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J. Virol., 62, 4510–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I.L., Hardwicke,M.A. and Sandri-Goldin,R.M. (1992) Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology, 186, 74–86. [DOI] [PubMed] [Google Scholar]
- Soliman T.M. and Silverstein S.J. (2000) Herpesvirus mRNAs are sorted for export via Crm1-dependent and -independent pathways. J. Virol., 74, 2814–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman T.M., Sandri-Goldin,R.M. and Silverstein,S.J. (1997) Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol., 71, 9188–9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strässer K. and Hurt,E. (2000) Yra1p, a conserved nuclear RNA binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J., 19, 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F., Bachi,A., Doerks,T., Braun,I.C., Seraphin,B., Wilm,M., Bork,P. and Izaurralde,E. (2000) REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA, 6, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadd S., Bryant,H.E., Filhol,O., Scott,J.E., Hsieh,T., Everett,R. and Clements J.B. (1999) The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J. Biol. Chem., 274, 28991–28998. [DOI] [PubMed] [Google Scholar]
- Weis K., Ryder,U. and Lamond,A.I. (1996) The conserved amino terminal domain of hSRP1 is essential for nuclear protein import. EMBO J., 15, 1818–1825. [PMC free article] [PubMed] [Google Scholar]
- Wen W., Meinkoth,J.L., Tsien,Y.R. and Taylor,S.S. (1995) Identification of a signal for rapid export of proteins from the nucleus. Cell, 82, 463–473. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Luo,M.-J., Strasser,K., Katahira,J., Hurt,E. and Reed,R. (2000) The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature, 407, 401–405. [DOI] [PubMed] [Google Scholar]