Abstract
Apoptosis signal-regulating kinase 1 (ASK1) is a MAP kinase kinase kinase (MAPKKK) that activates the JNK and p38 MAP kinase cascades and is activated in response to oxidative stress such as hydrogen peroxide (H2O2). A yeast two-hybrid screening identified a serine/threonine protein phosphatase 5 (PP5) as a binding partner of ASK1. PP5 directly dephosphorylated an essential phospho-threonine residue within the kinase domain of ASK1 and thereby inactivated ASK1 activity in vitro and in vivo. The interaction between PP5 and ASK1 was induced by H2O2 treatment and was followed by the decrease in ASK1 activity. PP5 inhibited not only H2O2-induced sustained activation of ASK1 but also ASK1-dependent apoptosis. Thus, PP5 appears to act as a physiological inhibitor of ASK1–JNK/p38 pathways by negative feedback.
Keywords: apoptosis/ASK1/MAP kinase/oxidative stress/PP5
Introduction
The mitogen-activated protein kinase (MAPK) signaling cascades are well conserved in cells from yeast to human and are composed of three sequentially activating protein kinases which are referred to as MAPK, MAPK kinase (MAPKK) and MAPKK kinase (MAPKKK). Once activated, MAPKKK phosphorylates and thereby activates specific MAPKKs, which then phosphorylates and activates specific MAPKs. Two mammalian MAPKs, c-Jun N-terminal kinase (JNK) and p38 MAPK, are known to be activated by various environmental stresses and regulate diverse cellular functions including cytokine production, differentiation and apoptosis (Nishida and Gotoh, 1993; Xia et al., 1995; Kyriakis and Avruch, 1996; Ichijo, 1999; Widmann et al., 1999; Davis, 2000; Ono and Han, 2000; Matsuzawa and Ichijo, 2001). In addition to being activated by stresses such as oxidative stress, high osmolarity, UV and endoplasmic reticulum stress, the JNK and p38 can also be activated by pro-inflammatory cytokines such as tumor necrosis factor (TNF), Fas-ligand and IL-1. JNK is activated by MAPKKs SEK1 (also known as MKK4) or MKK7, and p38 is activated by MKK3 or MKK6. Numerous candidates for MAPKKKs that activate SEK1/MKK4, MKK7, MKK3 and/or MKK6 have been reported (Ichijo, 1999; Davis, 2000).
Apoptosis signal-regulating kinase (ASK) 1, a mammalian MAPKKK, activates the JNK and p38 pathways and is activated in response to various cytotoxic stresses, including hydrogen peroxide (H2O2), Fas ligation, TNF, serum withdrawal and anti-tumor reagents (Ichijo et al., 1997; Tobiume et al., 1997; Chang et al., 1998; Gotoh and Cooper, 1998; Nishitoh et al., 1998; Saitoh et al., 1998; Wang,T.H. et al., 1998, 1999). Overexpression of ASK1 in epithelial cells in low serum conditions induced apoptosis (Ichijo et al., 1997), and ASK1-deficient cells were resistant to H2O2- and TNF-induced apoptosis (Tobiume et al., 2001), indicating that ASK1 plays a pivotal role in stress-induced apoptosis. On the other hand, moderate expression of a constitutively active form of ASK1 induced neuronal differentiation in PC12 cells (Takeda et al., 2000). In addition, low and high expression of exogenous ASK1 in keratinocytes induced differentiation and apoptosis, respectively (Sayama et al., 2000). These results suggest that ASK1 has a broad range of biological activities depending on cell-types, cellular context or the extent of ASK1 activation. The kinase activity of ASK1 is tightly regulated within cells; under non-stressed conditions, ASK1 is inhibited by association with its physiological inhibitor, thioredoxin (Trx). When cells are exposed to H2O2 or TNF, reactive oxygen species (ROS)-dependent oxidation of Trx occurs, which results in dissociation of Trx from ASK1 and thereby activation of ASK1 (Saitoh et al., 1998; Liu et al., 2000). Oligomerization-dependent autophosphorylation appears to be the next step required for full activation of ASK1 after the release from Trx (Gotoh and Cooper, 1998; Liu et al., 2000; K.Tobiume, M.Saitoh and H.Ichijo, submitted for publication). On the other hand, mechanisms of how the activated ASK1 returns to an inactive form has not been elucidated.
Many protein phosphatases that directly dephosphorylate and thereby inactivate JNK or p38 have been identified, which include VHR (Ishibashi et al., 1994), CL100 (MKP1) (Charles et al., 1992; Keyse and Emslie, 1992; Alessi et al., 1993; Sun et al., 1993), PAC1 (Rohan et al., 1993; Ward et al., 1994), MKP2 (hVH2, TYP1) (Guan and Butch, 1995; King et al., 1995; Misra-Press et al., 1995), hVH5 (M3/6) (Martell et al., 1995; Theodosiou et al., 1996), Pyst2 (Dowd et al., 1998) and MKP5 (Tanoue et al., 1999). Serine/threonine protein phosphatase (PP) 2Cα inactivates the stress-responsive MAPK pathways at the level of either MAPKK or MAPK (Takekawa et al., 1998). Moreover, it was recently shown that PP2Cβ inactivated a MAPKKK, TAK1, through direct dephosphorylation (Hanada et al., 2001).
Protein phosphatase 5 (PP5) is a member of the serine/threonine protein phosphatase family which includes PP1, PP2A, PP2B, PP2C, PP4 and PP7. PP5 possesses four tetratricopeptide repeat (TPR) domains in its N-terminus (Chen et al., 1994), which are implicated in protein– protein interactions (Blatch and Lassle, 1999). PP5 has been suggested to negatively regulate the functions of p53 and glucocorticoid receptor (GR) (Chen,M.S. et al., 1996; Silverstein et al., 1997; Zuo et al., 1998, 1999; Russell et al., 1999). PP5 interacts with various molecules including CDC16, CDC27 (Ollendorff and Donoghue, 1997), hCRY2 (Zhao and Sancar, 1997) and the HSP90–GR complex (Chen,M.S. et al., 1996; Silverstein et al., 1997; Russell et al., 1999), and the catalytic activity of PP5 was reported to be inhibited or activated in vitro by okadaic acid (Chen et al., 1994) and arachidonic acid (Chen and Cohen, 1997; Skinner et al., 1997), respectively. However, physiological substrates of PP5 have not been identified.
Here we report that PP5 directly binds to ASK1 and inhibits ASK1 activity in a negative feedback manner. The interaction of PP5 and ASK1 was induced by the treatment of cells with H2O2. PP5 dephosphorylates a critical phospho-threonine residue within the activation loop of ASK1 and thereby inactivated H2O2-induced ASK1 activity. Moreover, PP5 inhibited H2O2-induced sustained activation of ASK1 and ASK1-dependent apoptosis.
Results
PP5 directly interacts with ASK1
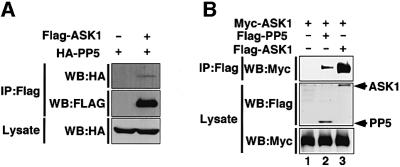
During the course of two-hybrid screening for ASK1 binding proteins, we found that PP5 interacted with ASK1 in yeast (see Materials and methods). We thus examined whether PP5 and ASK1 interact in mammalian cells by a co-immunoprecipitation analysis. When Flag-tagged ASK1 and hemagglutinin (HA)-tagged PP5 were co-transfected in 293 cells, HA-PP5 was co-immunoprecipitated with Flag-ASK1 (Figure 1A). When Myc-ASK1 was co-transfected with Flag-PP5 or Flag-ASK1, PP5–ASK1 and ASK1–ASK1 interactions were clearly observed (Figure 1B). These results indicated that PP5 interacts with ASK1 in vivo.
Fig 1. Interaction of PP5 with ASK1 in non-stressed cells. (A and B) 293 cells were transiently co-transfected with the indicated plasmids. Lysates were divided and immunoprecipitated with anti-Flag antibody (M2 gel). Immunoprecipitates were subjected to immunoblot analysis with anti-HA antibody (A, top) or with anti-Myc antibody (B, top). The presence of expressed proteins in the same lysates was verified by the indicated combination of immunoprecipitation (IP) and immunoblotting (WB).
H2O2- and TNF-induced interaction of PP5 and ASK1 in vivo
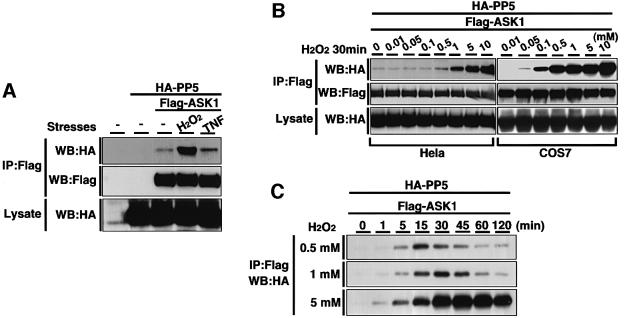
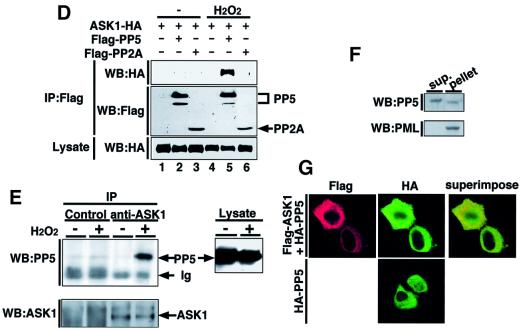
Because the observed interaction between PP5 and ASK1 in non-stressed cells was much weaker than the homo-oligomeric interaction of ASK1 (Figure 1B), we asked whether cell stimulation may alter this interaction. We overexpressed Flag-ASK1 and HA-PP5 in HeLa cells and subjected them to a co-immunoprecipitation analysis after stimulating the cells with certain stresses. Treatment with H2O2, one of the most potent activators of ASK1, dramatically increased the association between PP5 and ASK1 (Figure 2A). A slight increase of interaction was also observed by TNF treatment (Figure 2A), which activates ASK1 through a ROS-dependent manner (Saitoh et al., 1998; Liu et al., 2000). We analyzed the dose- and time-dependent effects of H2O2 on the interaction of PP5 and ASK1 in HeLa cells. H2O2-induced association was observed from 0.5 mM H2O2 and increased in a dose-dependent manner (Figure 2B). Similar results were observed in COS7 cells, in which H2O2-induced association was observed from as low as 0.05 mM H2O2 (Figure 2B). The PP5–ASK1 interaction in HeLa cells was detected within 1 min after treatment with 0.5 mM H2O2, peaked at 15 min and decreased thereafter (Figure 2C). More sustained and stronger interaction of PP5 and ASK1 was detected by the treatment with 1 or 5 mM H2O2 (Figure 2C). The PP5–ASK1 interaction appeared to be specific since PP2A was unable to bind ASK1 even after treatment of cells with H2O2 (Figure 2D). To confirm the observed PP5–ASK1 interaction under more physiological conditions, we examined the endogenous association of PP5 and ASK1 in non-transfected cells. Lysates from H2O2-treated A549 cells were immunoprecipitated with normal rabbit IgG or anti-ASK1 polyclonal antibody, and the immunoprecipitates were analyzed by immunoblotting with anti-PP5 antibody. The interaction was clearly induced by H2O2 treatment (Figure 2E). TNF-dependent interaction of endogenous PP5 with overexpressed ASK1 was also observed in mouse L929 cells (data not shown).
Fig. 2. Oxidative stress enhances the interaction between PP5 and ASK1. (A) H2O2- and TNF-induced interaction of PP5 and ASK1. HeLa cells were transiently co-transfected with HA-PP5 and Flag-ASK1. Thirty-six hours later, the cells were treated with 1 mM H2O2 or 200 ng/ml TNF for 20 min, and lysates were subjected to co-immunoprecipitation analysis as described in Figure 1A. (B) H2O2 dose-dependent interaction of PP5 and ASK1. HeLa cells and COS7 were transfected as in (A), treated with increasing concentrations of H2O2 for 30 min and analyzed by co-immunoprecipitation analysis. (C) Time course of the H2O2-induced interaction of PP5 and ASK1. HeLa cells were transfected as in (A), treated with indicated concentrations of H2O2 for the indicated periods and analyzed by co-immunoprecipitation analysis. (D) Specific interaction of ASK1 with PP5 but not PP2A. 293 cells were transiently co-transfected with ASK1-HA and Flag-PP5 or Flag-PP2A. Cells were treated with 5 mM H2O2 for 30 min, and lysates were subjected to co-immunoprecipitation analysis as described in Figure 1A. (E) Interaction of endogenous PP5 and ASK1. Approximately 5 × 107 of A549 cells were treated with 5 mM H2O2 for 30 min. Cell lysates were divided and immunoprecipitated with normal rabbit IgG or anti-ASK1 polyclonal antibody (DAV) and were immunoblotted with anti-PP5 monoclonal antibody. The presence of ASK1 and PP5 in the same lysates was verified by immunoblotting (WB). Ig indicates non-specific reactions derived from rabbit IgG. (F) Subcellular localization of endogenous PP5 in A549 cells. A subcellular fractionation was performed as described in Materials and methods, and PP5 was detected by immunoblotting. Anti-PML antibody was used as a positive control for the nuclear protein. (G) Subcellular localization of transfected ASK1 and PP5 in HeLa cells. HeLa cells were transfected with Flag-ASK1 and HA-PP5, or with HA-PP5 alone, and the cells were subjected to an immunofluorescence staining as described in Materials and methods.
PP5 has been reported to exist mainly in the nucleus (Chen et al., 1994), whereas ASK1 occurs exclusively in the cytoplasm (see below). To examine a topological rationale to the observed association of PP5 and ASK1, we determined the subcellular localization of PP5. A simple subcellular fractionation using a sucrose-containing buffer divides cellular components into two major fractions: a supernatant which contains mainly cytoplasmic proteins, and a pellet which contains nuclear, cytoskeletal and mitochondrial proteins and large fragments of cellular membranes. Immunoblot analysis revealed that while promyelocyte (PML), a positive control for nuclear protein, was detected only in the pellet, PP5 can be detected not only in the pellet but also in the supernatant (Figure 2F). This result suggests that a substantial amount of endogenous PP5 exists in the cytoplasm. We further confirmed the cytoplasmic localization of PP5 by an immunofluorescence staining of HeLa cells which were transiently transfected with Flag-ASK1 and HA-PP5. HA-PP5 was detected both in the cytoplasm and nucleus, whereas Flag-ASK1 was found mainly in the cytoplasm (Figure 2G). In addition, subcellular localization of PP5 was unaffected by the overexpression of ASK1 or H2O2 treatment (Figure 2G and data not shown). These results indicate that PP5 meets ASK1 mainly in the cytoplasm.
PP5 inhibits ASK1 activity in vivo
To explore a potential effect of PP5 on ASK1 in vivo, we determined the kinase activity of ASK1 by an immunocomplex kinase assay after transfection of ASK1 with or without PP5 in HeLa cells. Co-expression of PP5 partially inhibited the basal kinase activity of ASK1 (Figure 3A, lane 3). This partial inhibition may reflect the weak interaction of PP5 and ASK1 in non-stressed cells (Figure 1B and see below). ASK1 activates JNK and p38 pathways but not the extracellular signal-regulated kinase (ERK) pathway (Ichijo et al., 1997). We thus tested whether PP5 specifically inhibits ASK1-dependent JNK and p38 pathways. Figure 3B shows that expression of PP5 reduced ASK1-induced activation of JNK in a dose-dependent manner. ASK1-induced p38 activation was also reduced by PP5 (data not shown). On the other hand, PP5 had little effect on an MEKK1-induced activation of JNK (Figure 3C) or a serum-induced activation of ERK (Figure 3D), indicating that PP5 selectively inhibits ASK1 and its downstream targets, JNK and p38, in vivo.
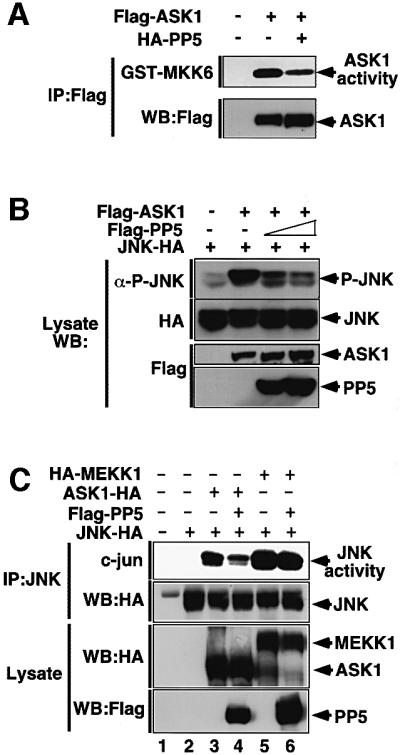
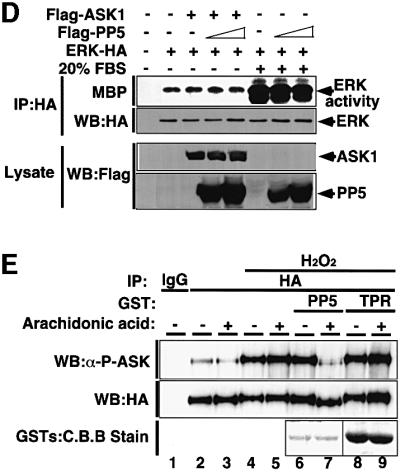
Fig. 3. PP5 dephosphorylates and inactivates ASK1 in vivo and in vitro. (A) PP5 reduces ASK1 activity in vivo. HeLa cells were transfected with the indicated plasmids. Thirty-six h later, immunocomplex kinase assay for ASK1 was performed as described in Materials and methods. ASK1 activity was measured using GST–MKK6 as a substrate (top). Consistent expression of Flag-ASK1 was confirmed by immunoblotting (bottom). (B) PP5 reduces ASK1-induced activation of JNK. 293 cells were transfected with the indicated plasmids. JNK activity was measured by immunoblotting using phospho-specific antibody to SAPK/JNK (Thr183/Tyr185). Expression of JNK-HA, Flag-ASK1 and Flag-PP5 was confirmed by immunoblotting using the indicated antibodies. (C) PP5 does not inhibit MEKK1-induced activation of JNK. 293 cells were transfected with the indicated plasmids. Lysates were immunoprecipitated with anti-JNK antibody, and JNK activity was measured by immunocomplex kinase assay using GST–c-jun as a substrate. Expression of JNK-HA, ASK1-HA, HA-MEKK1 and Flag-PP5 was confirmed by immunoblotting using the indicated antibodies. (D) PP5 does not inhibit the ERK pathway. 293 cells were transfected with the indicated plasmids. Cells were then stimulated with 20% FBS for 20 min, and ERK activity was measured by immunecomplex kinase assay using MBP as a substrate (top). Expression of transfected plasmids were confirmed by immunoblotting using the indicated antibodies. (E) PP5 directly dephosphorylates a critical phospho-threonine residue of ASK1. PAE cells stably transfected with ASK1-HA were treated with 1 mM H2O2 for 30 min. ASK1 was immunoprecipitated with anti-HA, incubated with recombinant full-length PP5 or with truncated PP5 (TPR domain only) for 20 min in the presence (+) or absence (–) of arachidonic acid. The samples were subjected to immunoblotting analysis with anti-phospho-ASK1 antibody (P-ASK). The presence of ASK1 and GST fusion proteins was verified by immunoblotting (WB) and staining with Coomassie Brilliant Blue dye (C.B.B stain), respectively.
ASK1 is a direct substrate for PP5
PP5 negatively regulates GR- and/or p53-signaling pathways (Chen,M.S. et al., 1996; Silverstein et al., 1997; Zuo et al., 1998, 1999; Russell et al., 1999); however, a direct substrate for PP5 has not been identified. Moreover, although the above results indicate that PP5 interacts with and inactivates ASK1 in vivo, the mechanism of inactivation of ASK1 is unknown. We thus examined whether PP5 can directly dephosphorylate and thereby inactivate ASK1 in vitro. To this end, immunoprecipitated ASK1-HA was incubated with recombinant glutathione S-transferase (GST) fusion proteins of PP5 (GST–PP5) or the TPR domain only (GST–TPR), and the phosphorylation status of ASK1 was monitored by an immunoblot analysis using a phospho-specific antibody to a critical phospho-threonine residue (Thr845) within the activation loop of ASK1 (K.Tobiume, M.Saitoh and H.Ichijo, submitted for publication). Phosphorylation of Thr845 of ASK1, which represents an activation status of ASK1 (K.Tobiume, M.Saitoh and H.Ichijo, submitted for publication), was induced by H2O2 treatment (Figure 3E, top panel, compare lanes 2 and 4). GST–PP5 but not GST–TPR, dephosphorylated the Thr845 of ASK1 only in the presence of arachidonic acid, a specific activator of PP5 (Figure 3E, top panel, lane 7). Moreover, ASK1 incubated with active PP5 was found to migrate faster on SDS–PAGE (Figure 3E, middle panel, lane 7), also suggesting a dephosphorylation of ASK1. These results indicate that PP5 can directly dephosphorylate at least Thr845 of ASK1 and thereby inactivate ASK1.
PP5 inhibits ASK1 in a negative feedback manner in vivo
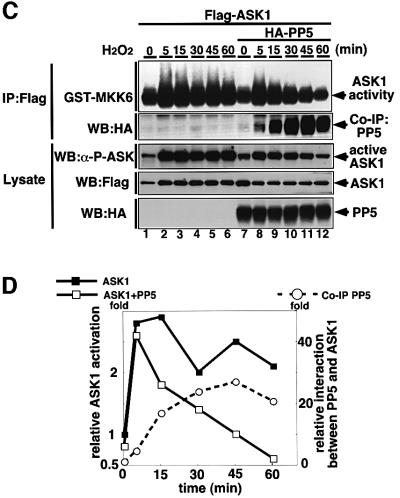
Trx has been identified as a physiological inhibitor of ASK1 under non-stressed conditions (Saitoh et al., 1998). It was of interest to compare the modes of inhibitory action between PP5 and Trx. We first examined whether PP5 can participate in the complex with ASK1 and Trx. HA-PP5 was co-immunoprecipitated with Flag-Trx in 293 cells only in the presence of ASK1 (Figure 4A), indicating that a Trx–ASK1–PP5 ternary complex can be formed in non-stressed conditions. This also suggests that PP5 may play a role in keeping ASK1 inactive together with Trx under non-stressed conditions; however, since the interaction between PP5 and ASK1 was much stronger in H2O2-treated cells (Figure 2), the effects of PP5 on ASK1 are likely to be exerted mainly in stressed conditions. We thus analyzed the stoichiometry of interaction between PP5–ASK1 and Trx–ASK1 in the cells treated with or without H2O2 (Figure 4B). Although the interaction of PP5 and ASK1 was much weaker than that of Trx and ASK1 in non-stressed cells (Figure 4B, lanes 2 and 3), H2O2 clearly induced the dissociation of Trx from ASK1, and reciprocally induced the association of PP5 with ASK1 (Figure 4B, lanes 5 and 6). These results suggest that these two ASK1 inhibitors may play different roles in ASK1 inhibition; PP5 appears to mainly target and inactivate the activated form of ASK1. We next examined the kinetics of the PP5–ASK1 interaction and that of activation and phosphorylation states of ASK1 in H2O2-stimulated cells (Figure 4C). Without co-transfection of PP5 (Figure 4C, lanes 1–6), ASK1 activity (Figure 4C, top panel; Figure 4D) and activating phosphorylation of ASK1 (Figure 4C, third panel) were induced by H2O2 within 5 min and sustained for at least 60 min. In contrast, when PP5 was co-transfected (Figure 4C, lanes 7–12), H2O2-induced activation as well as phosphorylation of ASK1 peaked at 5 min and decreased thereafter. Reciprocally, PP5 started to bind to ASK1 after 5 min, and the interaction was increased with time (Figure 4C, second panel; Figure 4D). This inverse correlation between PP5–ASK1 complex formation and activation/phosphorylation of ASK1 strongly suggests that PP5 specifically targets the activated form of ASK1 by negative feedback.
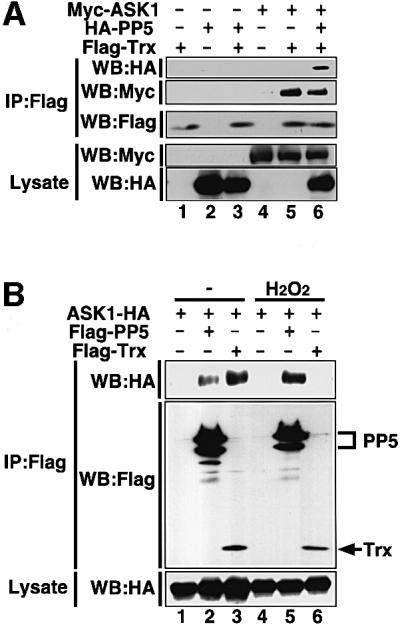
Fig. 4. PP5 inhibits H2O2-induced sustained activation of ASK1 in vivo. (A) PP5 can participate in the complex of ASK1 and Trx. 293 cells were transiently co-transfected with the indicated plasmids. Lysates were divided and immunoprecipitated with anti-Flag antibody (M2 gel). Immunoprecipitates were subjected to immunoblot analysis with anti-HA antibody (top panel) or with anti-Myc antibody (second panel). The presence of expressed proteins in the same lysates was verified by the indicated combination of immunoprecipitation (IP) and immunoblotting (WB). (B) Opposing effects of H2O2 on the interactions of ASK1 with PP5 and Trx. 293 cells were transfected with the indicated plasmids, treated with 5 mM H2O2 for 30 min and analyzed by co-immunoprecipitation analysis. (C) PP5 inhibits H2O2-induced sustained activation of ASK1. Flag-ASK1 was transiently transfected with or without HA-PP5 into HeLa cells. Thirty-six h later, the cells were treated with 5 mM H2O2 for the indicated periods. Lysates were divided, immunoprecipitated or immunoblotted, and the kinase activity of ASK1 (top panel), phosphorylation status of Thr845 of ASK1 (third panel) and co-immunoprecipitated HA-PP5 (second panel) were analyzed. The presence of HA-PP5 and Flag-ASK1 in the same lysates was verified by immunoblotting (WB). (D) The intensity of GST–MKK6 phosphorylation (scale in the left) and the amount of co-immunoprecipitated PP5 (scale in the right) in (C) were quantified and represented by a graph. Relative values of activation and interaction were calculated by dividing the intensities of phosphorylated GST–MKK6 or co-immunoprecipitated HA-PP5 (Co-IP PP5) at different time points by those at time zero.
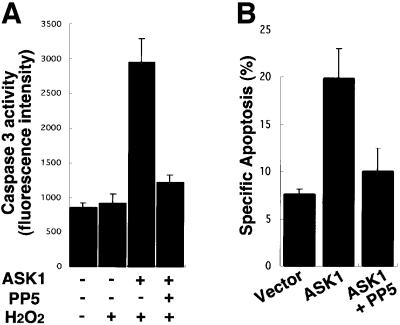
PP5 inhibits ASK1-dependent apoptosis
We have recently shown by deleting ASK1 in mice that H2O2-induced sustained activations of JNK and p38 are lost in ASK1–/– embryonic fibroblasts, and that ASK1–/– cells are resistant to H2O2-induced apoptosis (Tobiume et al., 2001). Thus, H2O2-induced sustained activation of JNK/p38, which resulted from sustained activation of ASK1, strongly correlated with apoptosis. These findings suggest that duration of ASK1 activation may directly link to the determination of cell fate (survival or apoptosis). Since PP5 inhibited only sustained but not transient ASK1 activity induced by H2O2 (Figure 4C and D), we examined whether PP5 inhibits H2O2-induced ASK1-dependent apoptosis. ASK1 was transfected into 293 cells with or without PP5, and H2O2-induced apoptosis was assessed by caspase-3 activity. While ASK1 enhanced H2O2-induced caspase-3 activation, co-expressed PP5 suppressed the ASK1-dependent apoptosis (Figure 5A). The inhibitory effect of PP5 on ASK1-dependent apoptosis was also confirmed in HeLa cells as determined by cell morphology (Figure 5B). Collectively, PP5 negatively regulates H2O2-induced sustained activation of ASK1–JNK/p38 pathways, and thereby inhibits ASK1-dependent apoptosis by negative feedback.
Fig. 5. PP5 inhibits ASK1-dependent apoptosis. (A) PP5 inhibits ASK1-dependent activation of caspase-3-like protease. Indicated plasmids were transiently transfected into 293 cells and caspase-3-like protease activity was measured as described in Materials and methods. Results are the means of duplicate determinations ± SE from one of more than three representative experiments. (B) PP5 inhibits ASK1-dependent cell death. The indicated plasmids were transiently transfected into HeLa cells with pEGFP, and apoptotic cell death was determined by a morphological analysis as described in Materials and methods. Results are the means of duplicate determinations ± SE from one of two representative experiments.
Discussion
Transient and persistent activations of MAPK are known to lead to different cell fates (Marshall, 1995); early and transient activation of ERK induces proliferation of PC12 cells, whereas prolonged activation of ERK induces neuronal differentiation. Early/transient and late/sustained activations of JNK induced by TNF (Guo et al., 1998; Roulston et al., 1998), UV-C or gamma-radiation (Chen,Y.R. et al., 1996) have been reported to correlate with survival and apoptosis, respectively. However, the mechanism by which duration of MAPK activation is regulated has not been fully elucidated. In the present study, we found that PP5 directly interacts with and inactivates activated ASK1 in a negative feedback manner and thereby inhibits ASK1-dependent sustained activations of JNK/p38 and apoptosis. Such a negative feedback system may be useful for cells to determine their fates (survival or apoptosis) in response to exposed stresses depending on their dose or duration. The ratio of expression levels between ASK1 and PP5 may be an important determinant of cellular sensitivity to oxidative stresses.
In this study, we found that PP5 specifically targets active form(s) of ASK1. However, which part of active configuration of ASK1 is recognized by PP5 is unknown. Although H2O2-induced phosphorylation of Thr845 returned to the basal level after 60 min (Figure 4C, third panel, compare lanes 7 and 12), ASK1 still bound a substantial amount of PP5 (Figure 4C, second panel, compare lanes 7 and 12). These results suggest that phosphorylation of Thr845 itself is unlikely to give rise to a site recognized by PP5. A fine mapping of interaction sites between PP5 and ASK1 may answer this question.
PP5 interacts not only with ASK1 but also with GR complex (Chen,M.S. et al., 1996; Silverstein et al., 1997; Russell et al., 1999), CDC16, CDC27 (Ollendorff and Donoghue, 1997) and hCRY2 (Zhao and Sancar, 1997). Thus, it is formally possible that the anti-apoptotic activity of PP5 observed in this study may not be solely due to its inhibitory action on ASK1. Interestingly, antisense oligonucleotide-mediated inhibition of PP5 has been reported to activate transcriptional activity of p53, a potent inducer of apoptosis, suggesting that PP5 inhibits p53 function in vivo (Zuo et al., 1998). On the other hand, p53 is reported to be activated by JNK or p38 via phosphorylation or stabilization of p53 protein (Fuchs et al., 1998; Bulavin et al., 1999; Huang et al., 1999; Potapova et al., 2000; She et al., 2000). We do not know yet whether ASK1-induced apoptosis requires JNK/p38-dependent p53 modification. Such a study will disclose an exact mechanism of how oxidative stress induces apoptosis through the ASK1–MAPK cascade.
Materials and methods
Yeast two-hybrid system
To analyze the regulatory mechanisms of ASK1, we employed the yeast two-hybrid system to search for proteins that bind to ASK1 by using LexA DNA binding domain–ASK1–K709R (kinase inactive mutant form of ASK1) as a bait (Saitoh et al., 1998). We identified several positive clones which were closely related to ASK1, and designated this gene as ASK2 [K.Takeda and H.Ichijo, manuscript in preparation; also called MAPKKK6 (Wang,X.S. et al., 1998)]. A human fibroblast cDNA library in the pJG4-5 prey plasmid (a gift from Roger Brent) was screened for proteins that interact with mouse ASK2/MAPKKK6 using the EGY48 yeast reporter strain and the pSH18-34 reporter plasmid as described (Kawabata et al., 1995). The bait plasmid expressing ASK2 protein was constructed in-frame with the LexA DNA-binding domain of the pEG202 bait plasmid. Plasmids of positive clones were recovered, and the cDNA inserts were sequenced. DNA sequencing analysis of the ASK2-interacting clones revealed that one of them encoded a full-length cDNA of PP5. To assay the interaction between ASK1 and PP5, ASK1 and PP5 constructs were co-transformed along with the pSH18-34 reporter plasmid into EGY48 yeast strain. Transformants were tested on 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal; Calbiochem) containing plates. PP5 interacted with not only ASK2 but also ASK1 in yeast (data not shown).
Cell culture, expression vectors and transfections
HeLa, A549 and COS7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). DMEM containing a higher concentration of glucose (4.5 mg/ml) was used for 293 cells. Porcine aortic endothelial (PAE) cells stably transfected with ASK1-HA were cultured in HAM’s F12 medium supplemented with 10% FCS and 200 µg/ml G418. All cells were cultured with 100 units/ml penicillin G in a 5% CO2 atmosphere at 37°C. A Flag tag was inserted at the N-termini of human ASK1 (Flag-ASK1), rat PP2Aα catalytic subunit (a gift from Dr Hitoshi Nakagama; Flag-PP2A), human Trx (Flag-Trx) and human PP5 (Flag-PP5) in pcDNA3.0 (Invitrogen). An HA tag was inserted at the N-termini of human PP5 (HA-PP5) and human MEKK1 (a gift from Dr Gary Johnson; HA-MEKK1), and at the C-termini of human ASK1 (ASK1-HA), mouse JNK3-1 (JNK-HA) and Xenopus MAPK (ERK-HA) in pcDNA3.0. Six copies of the Myc tag was inserted at the N-terminus of human ASK1 (Myc-ASK1) in pcDNA3.1. Transfection was performed with Fugene 6 (Roche) according to the manufacturer’s instructions.
Antibodies and reagents
Monoclonal antibodies to the HA tag (clone 3F10 and 12CA5) were purchased from Roche Molecular Biochemicals. Monoclonal antibodies to PP5 and to JNK3 (MAPKp49) were purchased from Transduction Laboratories. Rabbit polyclonal antisera to ASK1 antibody (DAV) (Saitoh et al., 1998) and phospho-specific polyclonal antibody to ASK1 (P-ASK; K.Tobiume, M.Saitoh and H.Ichijo, submitted for publication) was as described. Phospho-specific polyclonal antibody to SAPK/JNK (Thr183/Tyr185) was purchased from New England Biolabs. Anti-PML monoclonal antibody (clone PG-M3) and normal rabbit IgG were purchased from Santa Cruz Biotechnology Inc. The anti-Flag monoclonal antibodies (M2, biotinated-M2 and M2 gel), myelin basic protein (MBP) and arachidonic acid were purchased from Sigma. Human recombinant TNF-α was purchased from Pepro Tech EC Ltd. The anti-Myc monoclonal antibody (clone 9E10) was purchased from Calbiochem.
Co-immunoprecipitation assay and immunoblotting
Cells were lysed in a lysis buffer containing 150 mM NaCl, 20 mM Tris–HCl pH 7.5, 5 mM EGTA, 1% Triton X-100, 1% deoxycholate, 12 mM β-glycerophosphate, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1.5% aprotinin. Cell extracts were clarified by centrifugation, and the supernatants were immunoprecipitated with anti-Flag antibody gel (M2 gel) or anti-ASK1 antibody (DAV) using protein A–Sepharose (Zymed). The beads were washed twice with the washing buffer A (1% Triton X-100, 500 mM NaCl, 20 mM Tris–HCl pH 7.5, 5 mM EGTA, 1 mM DTT), and twice with the washing buffer B (150 mM NaCl, 20 mM Tris–HCl pH 7.5, 5 mM EGTA, 1 mM DTT). The beads were subjected to SDS–PAGE followed by electroblotting onto PVDF membranes. After blocking with 5% skim milk in TBS-T (150 mM NaCl, 50 mM Tris–HCl pH 8.0, 0.05% Tween 20) for 1 h, the membranes were probed with antibodies. The antibody–antigen complexes were detected using the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech). Aliquots of whole-cell lysates were subjected to immunoblotting analysis to confirm appropriate expression of proteins. The amount of co-immunoprecipitated HA-PP5 were quantitated by Quantity One® (PDI), and were presented in fold amount of co-immunoprecipitated HA-PP5 compared with untreated cells.
Immunocomplex kinase assay
GST constructs for MKK6 kinase negative mutant (GST–MKK6) and c-jun (1–79) (GST–c-jun) were prepared in pGEX-4T-1 vector (Amersham Pharmacia Biotech) by PCR. Cells were lysed and immunoprecipitated as described above. The beads were incubated with 1 µg of GST–MKK6 as a substrate for ASK1 for 20 min at 30°C in a final volume of 30 µl of kinase buffer (20 mM Tris–HCl pH 8.0, 20 mM MgCl2 and 0.3 µCi of [γ-32P]ATP). GST–c-jun and MBP were used as substrates for JNK and ERK, respectively. Kinase reactions were stopped by adding SDS sample buffer. The beads were resolved on SDS–PAGE followed by immunoblotting analysis with ECL. Phosphorylation of GST–MKK6 or MBP was analyzed by a Fuji BAS2000 Image Analyzer. Aliquots of whole-cell lysates were subjected to immunoblotting analysis to confirm appropriate expression of proteins.
In vitro phosphatase assay
GST constructs for full-length PP5 (GST–PP5) and TPR domain of PP5 (GST–TPR) were prepared in pGEX-4T-1 vector (Amersham Pharmacia Biotech). GST fusion proteins were purified as described (Skinner et al., 1997) using glutathione–Sepharose beads (Amersham Pharmacia Biotech). GST fusion proteins were eluted with a buffer containing 50 mM Tris–HCl pH 8.0, 4 mM MnCl2, 0.1% 2-mercaptoethanol, 10 mM glutathione and stored at –20°C in 50% glycerol. PAE cells stably transfected with ASK1-HA were treated with 1 mM H2O2 for 30 min, and cell lysate was prepared by lysis buffer. Aliquots of the lysates were immunoprecipitated with anti-HA antibodies (12CA5) and protein A–Sepharose beads (Zymed), and the beads were washed twice with washing buffer B. The beads were then added with GST–PP5 or GST–TPR in the presence or absence of 100 µM arachidonic acid, and reaction mixtures were incubated at 30°C for 20 min. Phosphatase reaction was stopped by adding SDS sample buffer. The beads were then resolved on SDS–PAGE followed by electroblotting onto PVDF membranes and analyzed by immunoblotting with ECL.
Measurement of caspase-3 activity
293 cells were transiently transfected with vector alone, Flag-ASK1, or Flag-ASK1 plus Flag-PP5. Forty-eight h later, the cells were washed with phosphate-buffered saline (PBS) and refed with serum-free medium. Cells were stimulated with or without 0.1 mM H2O2 plus 25 mM aminotriazole (catalase inhibitor) for 4 h. Caspase-3-like activity was measured by a CPP32/caspase-3 fluorometric protease assay kit (MBL) in which a fluorogenic synthetic peptide DEVD-7-amino-4-trifluoromethyl coumarine (AFC) was used as a substrate. The fluorescence of the released AFC was measured with an excitation wavelength of 360 nm and an emission wavelength of 530 nm. To confirm appropriate expressions of transfected plasmids, the same lysates of duplicate wells were combined and verified by immunoblot analysis.
Cell death assay
HeLa cells were transiently transfected with vector alone, Flag-ASK1, or Flag-ASK1 plus Flag-PP5 along with pEGFP C1 (Clontech). Thirty-six h later, the cells were washed with PBS and refed with serum-free medium. Cells were then treated with or without 0.5 mM H2O2 plus 25 mM aminotriazole (catalase inhibitor) for 8 h. Cell morphology and green fluorescence protein (GFP) signals were analyzed using a microscope. The ratio of apoptotic cells was determined by dividing the number of GFP-positive cells showing apoptotic morphologies, e.g. membrane blebbing and cell shrinkage, etc., by the total GFP-positive cell number (∼500 cells). Specific apoptosis was calculated as the percentage of apoptotic cells in each experimental condition minus the percentage of apoptotic cells of the vector control.
Subcellular fractionation
A549 cells were washed three times in ice-cold PBS, scraped and harvested by centrifugation at 2000 g for 10 min at 4°C. The pellet was resuspended in a buffer containing 250 mM sucrose, 20 mM HEPES–KOH pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 0.5 mM DTT. After incubating on ice for 10 min, cells were homogenized with 20 strokes of a Dounce homogenizer (Wheaton). The homogenate was centrifuged at 2000 g for 10 min at 4°C and the supernatant was collected as the cytoplasmic fraction (sup.). The pellet was washed in ice-cold PBS and lysed in a lysis buffer containing 150 mM NaCl, 20 mM Tris–HCl, pH 7.5, 5 mM EGTA, 1% Triton X-100, 1% deoxycholate, 12 mM β-glycerophosphate, 1 mM DTT, 1 mM PMSF, 1.5% aprotinin. Each subcellular fraction was resolved on SDS–PAGE and analyzed by immunoblotting using anti-PP5 antibody. Anti-PML antibody was used as a positive control of the nuclear protein.
Immunofluorescence staining of cells
HeLa cells were plated onto Lab-Tek Chamber Slide (Nalge Nunc International), transiently transfected with HA-PP5 and Flag-ASK1, or with HA-PP5 alone, and cultured overnight. After a brief wash at room temperature (RT) in PBS, the cells were fixed in 4% paraformaldehyde in PBS for 30 min at RT. After several washes in PBS, the cells were permeabilized and blocked in PBS containing 0.2% Triton X-100 and 3% bovine serum albumin (BSA) for 30 min at RT. They were then incubated with anti-HA (clone 3F10; rat) and anti-Flag (clone M2; mouse) antibodies in PBS containing 0.2% Triton X-100 and 3% BSA (dilution buffer) for 1 h at RT, followed by three washes with PBS. Samples were incubated for 45 min with fluorescein isothiocyanate-conjugated anti-rat IgG and Texas Red® dye-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.) in the dilution buffer, washed three times with PBS, and the slides were mounted in 90% glycerol. Samples were then analyzed and recorded by an Olympus Fluoview microscope.
Acknowledgments
Acknowledgements
We thank Shinri Tamura, Kohsuke Takeda and Atsushi Matsuzawa for valuable discussion. We also thank all the members of Cell Signaling Laboratory for their critical comments. This work was supported by Grants-in-Aid for scientific research from the Ministry of Education, Science, Culture of Japan.
References
- Alessi D.R., Smythe,C. and Keyse,S.M. (1993) The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene, 8, 2015–2020. [PubMed] [Google Scholar]
- Blatch G.L. and Lassle,M. (1999) The tetratricopeptide repeat: a structural motif mediating protein–protein interactions. BioEssays, 21, 932–939. [DOI] [PubMed] [Google Scholar]
- Bulavin D.V., Saito,S., Hollander,M.C., Sakaguchi,K., Anderson,C.W., Appella,E. and Fornace,A.J.,Jr (1999) Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J., 18, 6845–6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.Y., Nishitoh,H., Yang,X., Ichijo,H. and Baltimore,D. (1998) Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science, 281, 1860–1863. [DOI] [PubMed] [Google Scholar]
- Charles C.H., Abler,A.S. and Lau,L.F. (1992) cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene, 7, 187–190. [PubMed] [Google Scholar]
- Chen M.X. and Cohen,P.T. (1997) Activation of protein phosphatase 5 by limited proteolysis or the binding of polyunsaturated fatty acids to the TPR domain. FEBS Lett., 400, 136–140. [DOI] [PubMed] [Google Scholar]
- Chen M.X., McPartlin,A.E., Brown,L., Chen,Y.H., Barker,H.M. and Cohen,P.T. (1994) A novel human protein serine/threonine phosphatase, which possesses four tetratricopeptide repeat motifs and localizes to the nucleus. EMBO J., 13, 4278–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.S., Silverstein,A.M., Pratt,W.B. and Chinkers,M. (1996) The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J. Biol. Chem., 271, 32315–32320. [DOI] [PubMed] [Google Scholar]
- Chen Y.R., Wang,X., Templeton,D., Davis,R.J. and Tan,T.H. (1996) The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem., 271, 31929–31936. [DOI] [PubMed] [Google Scholar]
- Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Dowd S., Sneddon,A.A. and Keyse,S.M. (1998) Isolation of the human genes encoding the pyst1 and Pyst2 phosphatases: characterisation of Pyst2 as a cytosolic dual-specificity MAP kinase phosphatase and its catalytic activation by both MAP and SAP kinases. J. Cell Sci., 111, 3389–3399. [DOI] [PubMed] [Google Scholar]
- Fuchs S.Y., Adler,V., Buschmann,T., Yin,Z., Wu,X., Jones,S.N. and Ronai,Z. (1998) JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev., 12, 2658–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y. and Cooper,J.A. (1998) Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-α signal transduction. J. Biol. Chem., 273, 17477–17482. [DOI] [PubMed] [Google Scholar]
- Guan K.L. and Butch,E. (1995) Isolation and characterization of a novel dual specific phosphatase, HVH2, which selectively dephosphorylates the mitogen-activated protein kinase. J. Biol. Chem., 270, 7197–7203. [DOI] [PubMed] [Google Scholar]
- Guo Y.L., Baysal,K., Kang,B., Yang,L.J. and Williamson,J.R. (1998) Correlation between sustained c-Jun N-terminal protein kinase activation and apoptosis induced by tumor necrosis factor-α in rat mesangial cells. J. Biol. Chem., 273, 4027–4034. [DOI] [PubMed] [Google Scholar]
- Hanada M., Ninomiya-Tsuji,J., Komaki,K., Ohnishi,M., Katsura,K., Kanamaru,R., Matsumoto,K. and Tamura,S. (2001) Regulation of the TAK1 signaling pathway by protein phosphatase 2C. J. Biol. Chem., 276, 5753–5759. [DOI] [PubMed] [Google Scholar]
- Huang C., Ma,W.Y., Maxiner,A., Sun,Y. and Dong,Z. (1999) p38 kinase mediates UV-induced phosphorylation of p53 protein at serine 389. J. Biol. Chem., 274, 12229–12235. [DOI] [PubMed] [Google Scholar]
- Ichijo H. (1999) From receptors to stress-activated MAP kinases. Oncogene, 18, 6087–6093. [DOI] [PubMed] [Google Scholar]
- Ichijo H. et al. (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science, 275, 90–94. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Bottaro,D.P., Michieli,P., Kelley,C.A. and Aaronson,S.A. (1994) A novel dual specificity phosphatase induced by serum stimulation and heat shock. J. Biol. Chem., 269, 29897–29902. [PubMed] [Google Scholar]
- Kawabata M., Chytil,A. and Moses,H.L. (1995) Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-beta receptor. J. Biol. Chem., 270, 5625–5630. [DOI] [PubMed] [Google Scholar]
- Keyse S.M. and Emslie,E.A. (1992) Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature, 359, 644–647. [DOI] [PubMed] [Google Scholar]
- King A.G., Ozanne,B.W., Smythe,C. and Ashworth,A. (1995) Isolation and characterisation of a uniquely regulated threonine, tyrosine phosphatase (TYP 1) which inactivates ERK2 and p54jnk. Oncogene, 11, 2553–2563. [PubMed] [Google Scholar]
- Kyriakis J.M. and Avruch,J. (1996) Protein kinase cascades activated by stress and inflammatory cytokines. BioEssays, 18, 567–577. [DOI] [PubMed] [Google Scholar]
- Liu H., Nishitoh,H., Ichijo,H. and Kyriakis,J.M. (2000) Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell. Biol., 20, 2198–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C.J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80, 179–185. [DOI] [PubMed] [Google Scholar]
- Martell K.J., Seasholtz,A.F., Kwak,S.P., Clemens,K.K. and Dixon,J.E. (1995) hVH-5: a protein tyrosine phosphatase abundant in brain that inactivates mitogen-activated protein kinase. J. Neurochem., 65, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A. and Ichijo,H. (2001) Molecular mechanisms of the decision between life and death: regulation of apoptosis by apoptosis signal-regulating kinase 1. J. Biochem. (Tokyo), 130, 1–8. [DOI] [PubMed] [Google Scholar]
- Misra-Press A., Rim,C.S., Yao,H., Roberson,M.S. and Stork,P.J. (1995) A novel mitogen-activated protein kinase phosphatase. Structure, expression and regulation. J. Biol. Chem., 270, 14587–14596. [DOI] [PubMed] [Google Scholar]
- Nishida E. and Gotoh,Y. (1993) The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci., 18, 128–131. [DOI] [PubMed] [Google Scholar]
- Nishitoh H., Saitoh,M., Mochida,Y., Takeda,K., Nakano,H., Rothe,M., Miyazono,K. and Ichijo,H. (1998) ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell, 2, 389–395. [DOI] [PubMed] [Google Scholar]
- Ollendorff V. and Donoghue,D.J. (1997) The serine/threonine phosphatase PP5 interacts with CDC16 and CDC27, two tetratricopeptide repeat-containing subunits of the anaphase-promoting complex. J. Biol. Chem., 272, 32011–32018. [DOI] [PubMed] [Google Scholar]
- Ono K. and Han,J. (2000) The p38 signal transduction pathway: activation and function. Cell Signal, 12, 1–13. [DOI] [PubMed] [Google Scholar]
- Potapova O., Gorospe,M., Dougherty,R.H., Dean,N.M., Gaarde,W.A. and Holbrook,N.J. (2000) Inhibition of c-Jun N-terminal kinase 2 expression suppresses growth and induces apoptosis of human tumor cells in a p53-dependent manner. Mol. Cell. Biol., 20, 1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan P.J., Davis,P., Moskaluk,C.A., Kearns,M., Krutzsch,H., Siebenlist,U. and Kelly,K. (1993) PAC-1: a mitogen-induced nuclear protein tyrosine phosphatase. Science, 259, 1763–1766. [DOI] [PubMed] [Google Scholar]
- Roulston A., Reinhard,C., Amiri,P. and Williams,L.T. (1998) Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor α. J. Biol. Chem., 273, 10232–10239. [DOI] [PubMed] [Google Scholar]
- Russell L.C., Whitt,S.R., Chen,M.S. and Chinkers,M. (1999) Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J. Biol. Chem., 274, 20060–20063. [DOI] [PubMed] [Google Scholar]
- Saitoh M., Nishitoh,H., Fujii,M., Takeda,K., Tobiume,K., Sawada,Y., Kawabata,M., Miyazono,K. and Ichijo,H. (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J., 17, 2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayama K., Hanakawa,Y., Shirakata,Y., Yamasaki,K., Sawada,Y., Sun,L., Yamanishi,K., Ichijo,H. and Hashimoto,K. (2001) Apoptosis signal regulating kinase 1 (ASK1) is an intracellular inducer of keratinocyte differentiation. J. Biol. Chem., 276, 999–1004. [DOI] [PubMed] [Google Scholar]
- She Q.B., Chen,N. and Dong,Z. (2000) ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J. Biol. Chem., 275, 20444–20449. [DOI] [PubMed] [Google Scholar]
- Silverstein A.M., Galigniana,M.D., Chen,M.S., Owens-Grillo,J.K., Chinkers,M. and Pratt,W.B. (1997) Protein phosphatase 5 is a major component of glucocorticoid receptor.hsp90 complexes with properties of an FK506-binding immunophilin. J. Biol. Chem., 272, 16224–16230. [DOI] [PubMed] [Google Scholar]
- Skinner J., Sinclair,C., Romeo,C., Armstrong,D., Charbonneau,H. and Rossie,S. (1997) Purification of a fatty acid-stimulated protein-serine/threonine phosphatase from bovine brain and its identification as a homolog of protein phosphatase 5. J. Biol. Chem., 272, 22464–22471. [DOI] [PubMed] [Google Scholar]
- Sun H., Charles,C.H., Lau,L.F. and Tonks,N.K. (1993) MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell, 75, 487–493. [DOI] [PubMed] [Google Scholar]
- Takeda K., Hatai,T., Hamazaki,T.S., Nishitoh,H., Saitoh,M. and Ichijo,H. (2000) Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J. Biol. Chem., 275, 9805–9813. [DOI] [PubMed] [Google Scholar]
- Takekawa M., Maeda,T. and Saito,H. (1998) Protein phosphatase 2Cα inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J., 17, 4744–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T., Moriguchi,T. and Nishida,E. (1999) Molecular cloning and characterization of a novel dual specificity phosphatase, MKP-5. J. Biol. Chem., 274, 19949–19956. [DOI] [PubMed] [Google Scholar]
- Theodosiou A.M. et al. (1996) A member of the MAP kinase phosphatase gene family in mouse containing a complex trinucleotide repeat in the coding region. Hum. Mol. Genet., 5, 675–684. [DOI] [PubMed] [Google Scholar]
- Tobiume K., Inage,T., Takeda,K., Enomoto,S., Miyazono,K. and Ichijo,H. (1997) Molecular cloning and characterization of the mouse apoptosis signal-regulating kinase 1. Biochem. Biophys. Res. Commun., 239, 905–910. [DOI] [PubMed] [Google Scholar]
- Tobiume K. et al. (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep., 2, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.H., Wang,H.S., Ichijo,H., Giannakakou,P., Foster,J.S., Fojo,T. and Wimalasena,J. (1998) Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal-regulating kinase pathways. J. Biol. Chem., 273, 4928–4936. [DOI] [PubMed] [Google Scholar]
- Wang T.H., Popp,D.M., Wang,H.S., Saitoh,M., Mural,J.G., Henley,D.C., Ichijo,H. and Wimalasena,J. (1999) Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK)-dependent and -independent pathways in ovarian cancer cells. J. Biol. Chem., 274, 8208–8216. [DOI] [PubMed] [Google Scholar]
- Wang X.S., Diener,K., Tan,T.H. and Yao,Z. (1998) MAPKKK6, a novel mitogen-activated protein kinase kinase kinase, that associates with MAPKKK5. Biochem. Biophys. Res. Commun., 253, 33–37. [DOI] [PubMed] [Google Scholar]
- Ward Y., Gupta,S., Jensen,P., Wartmann,M., Davis,R.J. and Kelly,K. (1994) Control of MAP kinase activation by the mitogen-induced threonine/tyrosine phosphatase PAC1. Nature, 367, 651–654. [DOI] [PubMed] [Google Scholar]
- Widmann C., Gibson,S., Jarpe,M.B. and Johnson,G.L. (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev., 79, 143–180. [DOI] [PubMed] [Google Scholar]
- Xia Z., Dickens,M., Raingeaud,J., Davis,R.J. and Greenberg,M.E. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science, 270, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Zhao S. and Sancar,A. (1997) Human blue-light photoreceptor hCRY2 specifically interacts with protein serine/threonine phosphatase 5 and modulates its activity. Photochem. Photobiol., 66, 727–731. [DOI] [PubMed] [Google Scholar]
- Zuo Z., Dean,N.M. and Honkanen,R.E. (1998) Serine/threonine protein phosphatase type 5 acts upstream of p53 to regulate the induction of p21(WAF1/Cip1) and mediate growth arrest. J. Biol. Chem., 273, 12250–12258. [DOI] [PubMed] [Google Scholar]
- Zuo Z., Urban,G., Scammell,J.G., Dean,N.M., McLean,T.K., Aragon,I. and Honkanen,R.E. (1999) Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry, 38, 8849–8857. [DOI] [PubMed] [Google Scholar]