Abstract
Adenomatous polyposis coli (APC) is mutated in most colorectal cancers. APC downregulates nuclear β-catenin, which is thought to be critical for its tumour suppressor function. However, APC may have additional and separate functions at the cell periphery. Here, we examine polarized MDCK and WIF-B hepatoma cells and find that APC is associated with their lateral plasma membranes. This depends on the actin cytoskeleton but not on microtubules, and drug wash-out experiments suggest that APC is delivered continuously to the plasma membrane by a dynamic actin-dependent process. In polarized MDCK cells, APC also clusters at microtubule tips in their basal-most regions. Microtubule depolymerization causes APC to relocalize from these tips to the plasma membrane, indicating two distinct peripheral APC pools that are in equilibrium with each other in these cells. Truncations of APC such as those found in APC mutant cancer cells can neither associate with the plasma membrane nor with microtubule tips. The ability of APC to reach the cell periphery may thus contribute to its tumour suppressor function in the intestinal epithelium.
Keywords: actin cytoskeleton/APC tumour suppressor/colorectal cancer cells/lateral plasma membrane/microtubules
Introduction
Mutations in the adenomatous polyposis coli (APC) tumour suppressor gene are common in tumours of the human colorectum, and APC loss is an early, if not initiating, event during tumorigenesis in this tissue (Kinzler and Vogelstein, 1996). In the mouse, carriers of heterozygous Apc mutations inevitably develop multiple polyps in the intestinal tract, each of which shows inactivation of both Apc alleles (Su et al., 1992; Oshima et al., 1995, 1997). Interestingly, the earliest detectable consequence of Apc loss in the intestinal epithelium is an abnormal tissue architecture rather than an increase in cell proliferation (Oshima et al., 1995, 1997). Early adenoma cells appear to lag behind in the proliferative crypt stem cell compartment rather than leaving it at the normal rate to become differentiated, which may reflect a mis-specification of cell fate (Korinek et al., 1998), or a failure in cell migration or adhesion (Näthke et al., 1996; Barth et al., 1997; Pollack et al., 1997; reviewed by Bienz and Clevers, 2000).
APC proteins promote the destabilization of cytoplasmic β-catenin, an effector of the Wnt pathway, by binding to the Axin complex. This complex earmarks β-catenin for degradation by the ubiquitin pathway (Polakis, 2000). Furthermore, APC harbours a nuclear export function that is deleted by most APC colorectal tumour mutations (Rosin-Arbesfeld et al., 2000). As a consequence, colorectal cancer cells show high levels of nuclear β-catenin that, together with the transcription factor TCF (T cell factor), activate the transcription of Wnt target genes (Korinek et al., 1997). The ability of APC to exit from the nucleus and to bind to the cytoplasmic Axin complex is thought to be critical for its tumour suppressor function (Smits et al., 1999; Rosin-Arbesfeld et al., 2000; von Kries et al., 2000).
APC proteins are also concentrated at peripheral subcellular sites. Most strikingly, APC accumulates at the distal tips of microtubules in cellular protrusions of motile cells (Näthke et al., 1996). This clustering of APC at microtubule tips results from the ability of APC to track along microtubules (Mimori-Kiyosue et al., 2000). Evidence suggests that these APC clusters may be functionally relevant for cellular migration and adhesion (Barth et al., 1997; Pollack et al., 1997). Furthermore, in mitotic cells, the association of APC with microtubule plus ends appears to affect the fidelity of chromosomal segregation (Fodde et al., 2001; Kaplan et al., 2001).
The Drosophila homologue E-APC/dAPC2 is associated with the apicolateral adherens junctions of embryonic and larval epithelia. The junctional association of E-APC is critical for its function to destabilize Armadillo, the Drosophila homologue of β-catenin (McCartney et al., 1999; Yu et al., 1999). This association may thus reflect E-APC’s binding to the Axin complex which appears to be localized underneath the plasma membrane (Zeng et al., 1997; Bienz, 1999; Fagotto et al., 1999). It could also reflect a separate function of E-APC in the maintenance of junctional integrity (Townsley and Bienz, 2000).
Little is known about the subcellular distribution of the human APC tumour suppressor in polarized epithelial cells. We chose two polarized cell models to examine this: Madin–Darby canine kidney (MDCK) cells, a widely used cell line that readily polarizes under appropriate culture conditions (Simons and Fuller, 1985; Rodriguez-Boulan and Nelson, 1989), and WIF-B cells, a hybrid cell line derived from rat hepatoma cells and human fibroblasts that exhibits a hepatic morphology and attains a high degree of polarization upon prolonged culture (Ihrke et al., 1993; Decaens et al., 1996). We found that endogenous APC is associated with the lateral plasma membrane of both polarized cell types. This association depends on the actin cytoskeleton but not on microtubules. In addition, we also observed microtubule-dependent clusters of APC in the basal-most regions of polarized MDCK cells. Our evidence indicates that these cells contain two distinct peripheral pools of APC that are in equilibrium with each other: actin-dependent membrane-associated APC and microtubule-dependent APC clusters. Finally, we show that truncations of APC such as those typically found in APC-mutant cancer cells have lost the ability to reach either of these peripheral locations.
Results
Two distinct peripheral pools of APC in MDCK cells
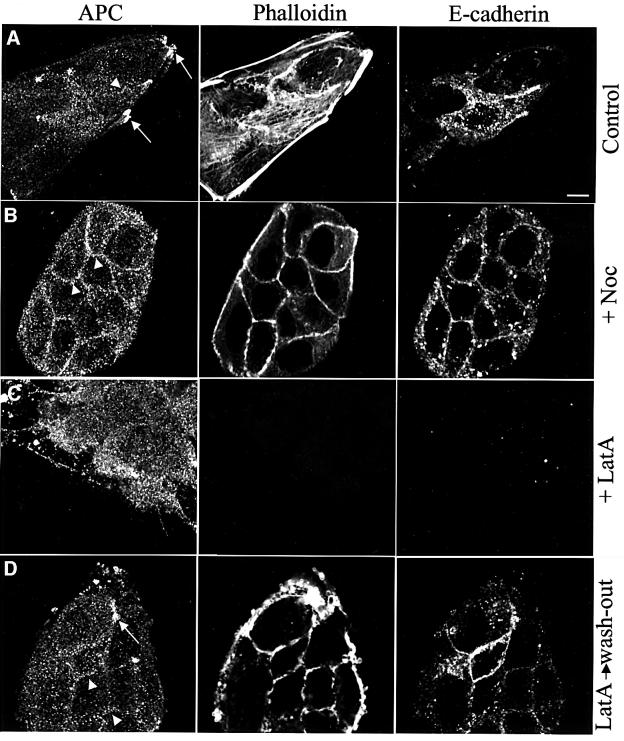
To examine the subcellular distribution of endogenous APC in MDCK cells, we stained subconfluent cells with an antibody raised against a central fragment of human APC (Näthke et al., 1996). Subconfluent cells show some cytoplasmic staining, characteristically grainy. This graininess is not due to the secondary antibody (not shown), and is absent from APC-mutant cancer cells that do not express detectable APC protein (see Supplementary data available at The EMBO Journal Online). Grainy staining is also observed in other mammalian cells (see below), as well as in Drosophila cells stained for E-APC (Yu et al., 1999). In addition to the grainy cytoplasmic staining, subconfluent MDCK cells show numerous bright clusters of APC associated with microtubule tips at the cell periphery (Näthke et al., 1996) (Figure 1A, arrows). However, these clusters not only seemed to coincide with microtubule tips, but also with phalloidin staining (Figure 1A), an observation that was confirmed in COS cells transfected with APC tagged with green fluorescent protein (GFP): these large spread-out cells allowed direct observation of microtubule-associated APC–GFP clusters coinciding with tips of actin filaments (not shown). We thus re-examined the dependence of the peripheral APC clusters on the cytoskeleton, using depolymerizing drugs.
Fig. 1. Two distinct peripheral locations of APC in subconfluent MDCK cells. Confocal sections through subconfluent MDCK cells after paraformaldehyde fixation, co-stained as indicated above the panels, drug-treated as indicated on the right (D, recovery after latrunculin A treatment). (A) Basal-most plane, providing optimal visualization of microtubule tip clusters (arrows); these disappear after treatment with microtubule- (B) or actin-depolymerizing drugs (C), but are restored after drug removal (D, arrow; not shown). Arrowheads point to membrane-associated APC staining (optimal visualization ∼2.5 µm above basal-most plane, B–D) which disappears after actin depolymerization (C), reappears 15 min after drug removal (D), and which is considerably increased after microtubule depolymerization (B). Scale bar, 10 µm (in this and all subsequent figures).
As previously shown (Näthke et al., 1996), the peripheral APC clusters disappeared completely after treatment with nocodazole (Figure 1B). This drug caused thorough depolymerization of microtubules (not shown), but had no major effects on phalloidin or E-cadherin staining. Interestingly, in cell cultures that were nearly confluent, nocodazole treatment also resulted in a striking association of APC with the plasma membrane (Figure 1B, arrowheads). Some membrane-associated APC was also observed in untreated cells, especially at plasma membrane stretches that stained strongly for E-cadherin (Figure 1A, arrowhead). Nevertheless, the microtubule depolymerization caused a considerable redistribution of APC from microtubule tips to the plasma membrane. It seems that this alternative peripheral destination of APC revealed by the drug experiment is normally overshadowed by a preference of APC to cluster at microtubule tips in these cells.
Treatment of MDCK cells with cytochalasin D did not significantly affect the peripheral APC clusters (Näthke et al., 1996 and data not shown). We thus applied latrunculin A, whose effect on actin depolymerization is more potent than that of cytochalasin D (Ayscough, 1998). This revealed a considerable effect on the APC clusters, most of which disappeared after latrunculin A treatment (Figure 1C). Also, the membrane-associated APC staining was no longer detectable, paralleling the disappearance of E-cadherin staining from the plasma membrane (Figure 1C). Concomitantly, the levels of cytoplasmic APC were increased in the drug-treated cells (Figure 1C, compare with A).
We asked whether these effects of latrunculin A were reversible. We thus conducted drug wash-out experiments, replacing the drug solution with fresh medium and fixing cells at various intervals of recovery. This revealed that APC began to re-associate with the plasma membrane after several minutes, along with actin repolymerization and re-appearance of membrane-associated E-cadherin staining. APC staining along the plasma membrane became maximal after 15 min of recovery, and tended to be more pronounced than in cells that had not been treated with the drug (Figure 1D, arrowheads, compare with A). Microtubule tip clusters of APC also recovered, albeit at a slower rate (Figure 1D, arrow).
We conclude that there are two distinct peripheral locations of APC in MDCK cells: membrane-associated APC and microtubule tip clusters of APC. The former depends on the actin cytoskeleton but not on microtubules. The clusters depend on microtubules and only partly on the actin cytoskeleton. This partial dependence indicates that the actin cytoskeleton may contribute less directly than microtubules to the formation of the peripheral APC clusters. Finally, the drug experiments indicate that APC can shift from one peripheral location to the other.
Membrane-associated APC and microtubule-dependent APC clusters in polarized MDCK cells
MDCK cells can be induced to polarize and form an epithelial-like monolayer when grown on micropore filters (Simons and Fuller, 1985; Rodriguez-Boulan and Nelson, 1989). We thus examined polarized MDCK cells to see whether the process of polarization affected the subcellular distribution of APC.
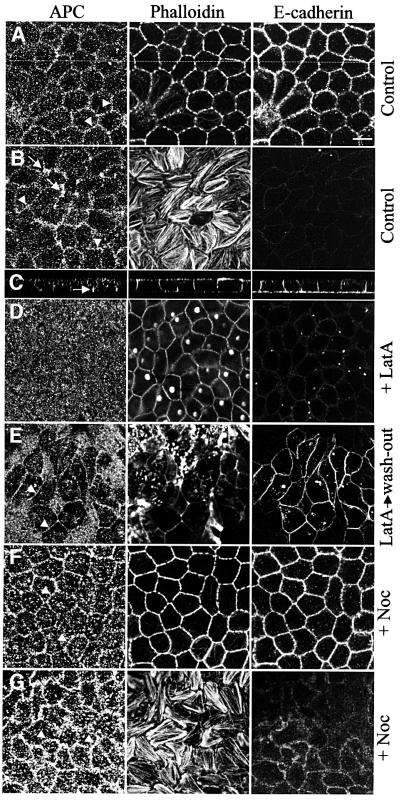
We grew MDCK cells on filters for 4–6 days (see Materials and methods) after which time they formed a tight monolayer, with a height of cells ranging from 8–15 µm. In these monolayer cells, E-cadherin staining was predominantly in the basolateral plasma membranes, with very little staining along the apical plasma membrane (Figure 2A–C), as previously shown (Le Bivic et al., 1990; Shore and Nelson, 1991), indicating their polarization. Polarized cells showed grainy cytoplasmic staining of APC, as well as some APC staining along the lateral plasma membranes (Figure 2A, arrowheads). This membrane-associated staining was very prominent in the basal-most zones of the lateral plasma membranes (Figure 2B, arrowheads). In addition, these confocal sections through the basal zones of cells showed numerous clusters of APC staining, typically in the vertices of cells (Figure 2B and C, arrows). These basal clusters seemed to coincide with the tips of actin stress fibres, but we did not observe any significant co-localization with the focal adhesion protein vinculin (not shown). There was no detectable concentration of APC along the apical plasma membrane.
Fig. 2. Peripheral locations of APC in polarized MDCK cells. Confocal sections through polarized MDCK cells after paraformaldehyde fixation, co-stained as indicated above the panels, drug-treated as indicated on the right (E, recovery after latrunculin A treatment); (A, D–F) ∼8 µm above the basal-most plane; (B and G) basal-most plane, showing actin stress fibres and basal APC clusters (arrows) which disappear after microtubule depolymerization; (C) z-section along the line indicated in (A), revealing E-cadherin staining predominantly along the basolateral plasma membrane (arrow indicates basal APC cluster). Association of APC with lateral plasma membranes (A, B, arrowheads) disappears after actin depolymerization (D), reappears after drug removal (E), and increases somewhat after microtubule depolymerization (F, G).
We exposed polarized MDCK cells to latrunculin A to determine whether the membrane association of APC requires the actin cytoskeleton. This was the case, although the drug-treated cells still showed phalloidin staining in the cellular cortex, presumably reflecting residual cortical actin filaments. Also there was no longer any APC staining associated with the lateral plasma membrane and the levels of cytoplasmic APC staining seemed to have increased (Figure 2D). This drug treatment also reduced the levels of E-cadherin staining of the lateral plasma membranes (Figure 2D). However, after washing out the drug, the membrane-associated APC staining recovered in a large fraction of the cells (Figure 2E, arrowheads). At the same time, the cytoplasmic APC levels became reduced, and recovery of membrane-associated E-cadherin staining was observed (Figure 2E). This demonstrated that the drug-induced dissociation of APC from the plasma membrane is reversible. We noticed that the numbers of basal APC clusters were also significantly reduced in latrunculin-treated cells; complete restoration of these clusters was slow, requiring at least 16 h post drug removal (not shown).
Exposure of polarized MDCK cells to nocodazole caused thorough depolymerization of the microtubules (not shown), but this treatment neither affected significantly the E-cadherin nor the phalloidin staining (Figure 2F, compare with A). However, the basal APC clusters completely disappeared after the drug treatment (Figure 2G, compare with B, arrows), while the membrane-associated APC staining was somewhat increased under these conditions (Figure 2F and G compare with A and B, arrowheads), indicating a re-localization of APC from the former to the latter. Prolonged recovery from drug treatment led to the restoration of the basal APC clusters (not shown).
We conclude that MDCK cells retain two interconnected pools of peripheral APC protein after polarization: an actin-dependent APC pool associated with the lateral plasma membrane and basal APC clusters that depend predominantly on microtubules. Furthermore, given that the membrane association of APC is more pronounced in polarized MDCK cells compared with unpolarized cells, this indicates that the process of cell polarization may cause an equilibrium shift of the peripheral APC from microtubule tips to the lateral plasma membrane.
Actin-dependent membrane association of APC in polarized WIF-B cells
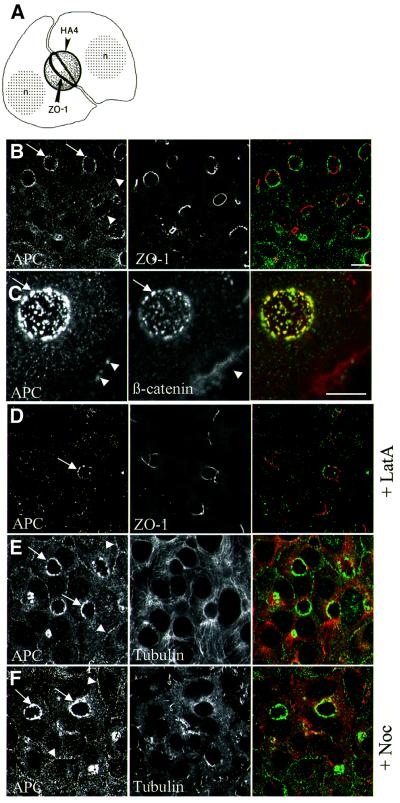
To determine whether our findings in MDCK cells also applied to other epithelial cells, we turned to WIF-B cells. These hepatic cells undergo two successive steps of polarization, attaining first a simple epithelial polarization, similar to that of polarized MDCK cells, followed by a secondary hepatic polarization that recapitulates some aspects of bile canaliculi differentiation (Ihrke et al., 1993; Decaens et al., 1996). After culture for 10–15 days, WIF-B cells form closed ovoid-shaped extracellular spaces between them that resemble bile canaliculi. Apical markers (such as HA4) segregate into the plasma membranes enclosing these ‘ovoids’, whereas basolateral markers are excluded from them (Figure 3A). Furthermore, the ovoids are lined with a belt of ZO-1 (Ihrke et al., 1993) (Figure 3A and B), a constituent of tight junctions that separate the apical from the basolateral plasma membranes in polarized cells.
Fig. 3. Actin-dependent membrane association of APC in polarized WIF-B cells. (A) Schematic representation of two adjacent polarized WIF-B cells forming a bile canaliculus-like structure between them, i.e. an ovoid of extracellular space enclosed by apical plasma membranes (HA4, apical marker); a belt of ZO-1 demarcates the junction between apical and basolateral plasma membranes (n, nucleus; from Ihrke et al., 1993, reproduced from The Journal of Cell Biology, 1993, 123, pp. 1761–1775 by copyright permission of The Rockerfeller University Press). (B–F) Confocal sections through polarized WIF-B cells after methanol fixation, ∼5 µm from the basal-most level, co-stained as indicated within the panels (merges show APC in green, ZO-1, β-catenin or α-tubulin in red), drug-treated as indicated on the right. Arrows point to membrane-associated APC staining, arrowheads to apical APC puncta [co-localizing with β-catenin puncta, as shown in the higher magnification images in (C) in which an ovoid is grazed tangentially]. Membrane-associated APC and apical APC puncta disappear after actin depolymerization (D; arrow marks an ovoid with residual apical APC puncta, as occasionally observed); both are unaffected by microtubule depolymerization (F). Note also that neither drug affects the integrity of the ovoids [as revealed by normal ZO-1 staining (D) and not shown].
In polarized WIF-B cells, we observed grainy cytoplasmic APC staining as well as a considerable level of APC staining along the lateral plasma membranes (Figure 3B, arrowheads). In addition, there were discrete APC puncta in the apical cortex (Figure 3B, arrows). These cortical APC puncta coincided with similar puncta of β-catenin staining that were associated with the ovoids (Figure 3C, arrows) although there was no E-cadherin staining along the ovoids (Decaens et al., 1996; data not shown).
Exposure of polarized WIF-B cells to latrunculin A resulted in thorough depolymerization of actin filaments as revealed by phalloidin staining (not shown), but did not affect the frequency nor the shapes of the ovoids, as judged by the normal ZO-1 staining pattern (Figure 3D). However, APC was largely detached from the ovoids and from the lateral plasma membranes after this drug treatment (Figure 3D), with only residual APC puncta remaining associated with occasional ovoids (Figure 3D, arrow). Essentially the same was observed after exposure to cytochalasin D (not shown). In contrast, exposure of cells to nocodazole neither affected the cortical APC puncta nor the association of APC with the lateral plasma membrane, despite causing extensive disruption of the microtubules as revealed by α-tubulin staining (Figure 3F, compare with E). Thus, intact actin filaments are required for association of APC with the plasma membrane in WIF-B cells, whereas microtubules are dispensable for this association.
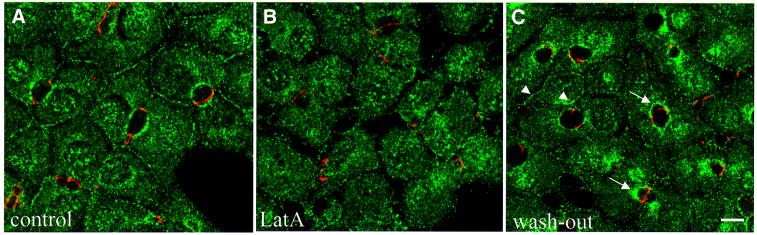
We asked whether the dissociation of APC from the plasma membrane in drug-treated cells was reversible. Indeed, APC staining began to re-associate rapidly with lateral plasma membranes and ovoids after drug removal, and membrane-associated APC staining was restored to nearly normal after 15 min (Figure 4C, compare with A and B). These experiments confirmed that the association of APC with the plasma membrane depends on a continuous function of intact actin filaments.
Fig. 4. Reversible membrane association of APC in polarized WIF-B cells. Merged images of polarized WIF-B cells, controls (A) or treated with latrunculin A before (B) or 6–7 min after drug removal (C), co-stained with antibodies against APC (green) and ZO-1 (red). Note the restoration of apical APC puncta (arrows) and of membrane-associated APC staining (arrowheads) after recovery from drug treatment (C).
APC truncations cannot reach peripheral locations
In an attempt to identify the domains that target APC to the plasma membrane or to microtubule tips, we generated GFP-tagged versions of full-length APC and of three APC fragments. One of these spans the central third of the protein (MAPC–GFP) which is capable of complementing APC’s nuclear export function in APC-mutant cancer cells (Rosin-Arbesfeld et al., 2000). The second one spanned the C-terminus (CAPC–GFP), a construct similar to one made by Mimori-Kiyosue et al. (2000) which is capable of decorating microtubules. The third one spanned the N-terminal half of the protein (NAPC–GFP), and mimics truncations that are typically found in APC-mutant cancer cells (Rowan et al., 2000). Since transfection of WIF-B cells was highly inefficient, we tested these constructs in transiently transfected MDCK cells before and after polarization.
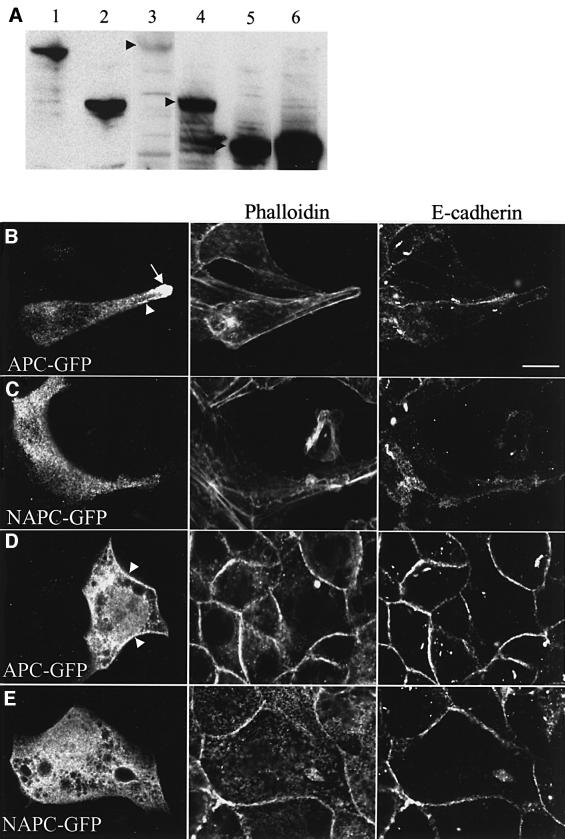
We found that CAPC–GFP and MAPC–GFP were expressed at high levels in transiently transfected MDCK cells, as revealed by western blot analysis of total cellular protein (Figure 5A, lanes 5 and 6, respectively). In contrast, NAPC–GFP was readily detectable only at early time points (e.g. at 18 h after transfection; Figure 5A, lane 4); however, 24 h after transfection, this fusion protein became undetectable by western blot analysis (see Materials and methods). This was due to gradual rounding off, detachment and, ultimately, lysis of transfected cells (not shown), indicating a noxious effect of this construct on cells. Similarly, full-length APC–GFP protein was only expressed at low levels, and was readily detectable by western blot analysis early on (18 h after transfection; Figure 5A, lane 3), fading away later, reflecting a gradual loss of transfected cells from the culture. This parallels earlier observations by Morin et al. (1996) who found that inducible overexpression of full-length APC in colorectal cancer cells caused the transfected cells to undergo apoptosis. Consistent with the western blot analysis, the frequency of transfected MDCK cells was high in the case of MAPC–GFP and CAPC–GFP, even at later time points, but was much lower in the case of APC–GFP and NAPC–GFP, and many of the cells transfected with the latter constructs looked somewhat unhealthy, especially after polarization (Figure 5D and E).
Fig. 5. Expression of APC–GFP fusion proteins in transfected MDCK cells. (A) Western blot analysis of APC fusion proteins transiently expressed in transfected subconfluent MDCK cells (lane 3, GFP–APC; lane 4, NAPC–GFP; lane 5, CAPC–GFP; lane 6, MAPC–GFP), probed with anti-GFP antibody; arrowheads indicate bands of the correct sizes expected for the different GFP fusion proteins (for comparison, endogenous full-length APC protein in HCT116 cells colorectal cancer cells, lane 1, and truncated APC protein in DLD-1 cells, lane 2, probed with anti-M-APC). (B–E) Confocal sections through subconfluent MDCK cells (B and C; basal-most plane) or confluent MDCK monolayer cells (D and E; ∼8 µm above basal-most plane), transfected with APC–GFP or NAPC–GFP (B and C, fluorescence; D and E, anti-GFP antibody), fixed with paraformaldehyde and co-stained as indicated above panels; representative examples are shown. Arrow in (B) points to a microtubule tip cluster formed by APC–GFP; arrowheads in panels B and D indicate membrane-associated APC–GFP. Neither of these is ever observed with NAPC–GFP, which shows an even cytoplasmic distribution (C and E).
To assess the subcellular distributions of these GFP fusion proteins, we restricted our analysis to relatively healthy looking cells with low or moderate levels of expression. We found that, in transfected subconfluent MDCK cells, the green fluorescence due to full-length APC–GFP was very similar to the endogenous APC staining pattern: in many cells, we saw conspicuous clusters of APC–GFP at the cell periphery (Figure 5B, arrow; see also Supplementary data, Figure 3A), apparently associated with microtubule tips (not shown). Furthermore, we also observed cells in which some green fluorescence was associated with the plasma membrane (Figure 5B, arrowhead) in addition to variable levels of cytoplasmic APC–GFP. As mentioned above, a similar subcellular distribution of APC–GFP was observed in transiently transfected COS cells in which conspicuous APC–GFP clusters at peripheral microtubule tips were observed.
This contrasted with the subcellular distribution of the GFP-tagged APC fragments which looked virtually indistinguishable from each other in transfected MDCK cells. Namely, none of the APC fragments showed any microtubule tip clusters nor any green fluorescence along the plasma membrane. Instead, each fragment produced a green fluorescence that was spread throughout the cytoplasm (Figure 5C, and data not shown). This was true even after nocodazole treatment which emphasizes the membrane-associated APC (see above): we never observed any lining of the plasma membrane with any of the APC fragments, whereas a significant proportion of the cells transfected with full-length APC–GFP showed membrane-associated green fluorescence after the drug treatment (not shown). Similarly, in transfected COS cells, we never observed any microtubule tip clusters with any of these GFP-tagged APC fragments (not shown). However, we did observe decoration of microtubules by CAPC–GFP, albeit not by MAPC–GFP nor by NAPC–GFP, in transfected COS cells, in agreement with the results of Mimori-Kiyosue et al. (2000) who showed that a C-terminal fragment of APC exhibited microtubule decoration in transfected Xenopus kidney epithelial cells. Note that the microtubule network is much more conspicuous, and readily detectable by immunofluorescence, in COS cells compared with MDCK cells, which may explain why the fluorescence pattern of CAPC–GFP was not detectably different from that of the other APC fragments in the latter.
Essentially the same was found in transfected MDCK cells that were transferred to filters to allow monolayer formation and polarization. Namely, a significant fraction of cells transfected with full-length APC–GFP showed cytoplasmic GFP staining as well as distinct lining of the plasma membrane (Figure 5D, arrowheads), while none of the APC fragments produced GFP staining along the plasma membranes (Figure 5E). Furthermore, we observed occasional basal clusters in cell vertices only with full-length APC–GFP, but never with any of the APC fragments (not shown).
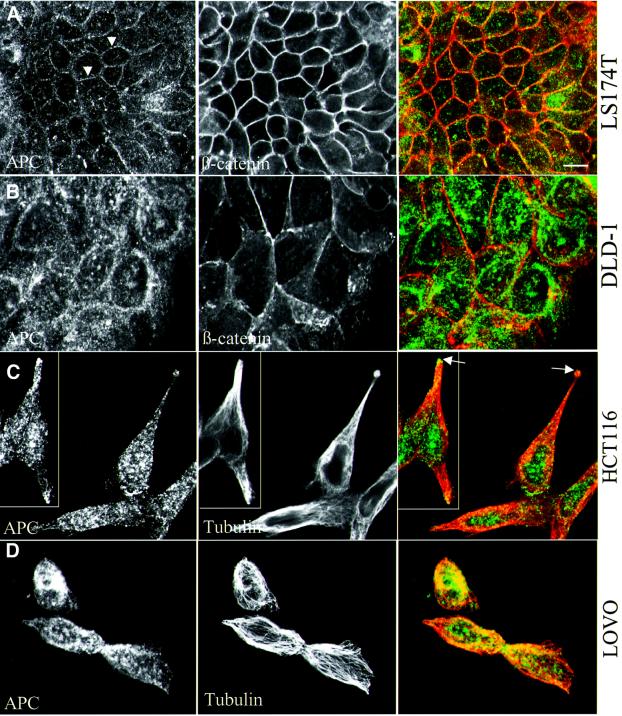
These results indicate that neither the microtubule-dependent clusters of APC nor its membrane association is mediated by a single domain of APC. Furthermore, they imply that the APC truncations typically found in colorectal cancer cells would not be capable of reaching either of these peripheral locations. To test this, we examined the subcellular distribution of endogenous APC in a number of colorectal cancer cell lines, wild-type or mutant for APC. Indeed, cells that express wild-type APC (HCT116, LS174T; Figure 5A, lane 1) showed relatively low levels of cytoplasmic APC staining, characteristically grainy, but significant association of this staining with the plasma membrane, coinciding with β-catenin staining (Figure 6A) (see also Rosin-Arbesfeld et al., 2000). In contrast, APC-mutant cells that express truncated APC, for example DLD-1 or LOVO cells (Figure 5A, lane 2), showed moderately high levels of cytoplasmic as well as nuclear APC staining, but never any staining associated with the plasma membrane (Figure 6B; see also Supplementary data). Essentially the same was found in other APC mutant cells, e.g. in SW480 cells (Rosin-Arbesfeld et al., 2000) and in T84, GP5D and HT55 cells (to be described elsewhere; note that the nuclei are not visible in most of the focal planes shown here). This parallels our results from the MDCK cell transfections and demonstrates for endogenous protein that, in this case too, only full-length APC but not APC truncations can associate with the plasma membrane.
Fig. 6. Subcellular distribution of APC in APC wild-type and mutant colorectal cancer cells. Colorectal cancer cells (LS174T, HCT116, APC wild type; LOVO, DLD-1 APC mutant) after methanol fixation, co-stained as indicated in the panels (merges show APC in green, β-catenin or α-tubulin in red). (A and B) Subconfluent cells, sectioned ∼3 µm above basal-most plane, allowing optimal visualization of membrane-associated APC in APC wild-type cells (arrowheads in A); membrane-associated APC is not detectable in APC mutant cells (B, and not shown; see text). (C and D) Individual spread-out cells, sectioned at their basal planes, allowing optimal visualization of APC clusters in cellular protrusions (co-localizing with microtubule tips, arrows in C); no such clusters are observed in APC mutant cells (D, and not shown) which show high levels of even cytoplasmic APC (B and D) as well as nuclear APC (above focal planes shown; see also text).
The cell morphology and growth patterns vary considerably between these different cancer cell lines. Some of the lines show clusters and lumps of small rounded cells, whereas others show larger fibroblast-like cells that spread in culture and that can be observed as individuals. Focusing on HCT116 cells which belong to the latter class, we noticed distinct peripheral clusters of APC in cells that were detached from others or that occupied positions at the periphery of cell clusters, in addition to membrane-associated APC (Figure 6C). These clusters resembled those seen in MDCK cells, being located predominantly in cellular protrusions and coinciding with microtubule tips (Figure 6C, arrows). Significantly, we could never detect such clusters in individually spread-out cells in any of the APC-mutant cell lines, for example in LOVO cells (Figure 6D), SW480 or DLD-1 cells (not shown). These observations confirm that endogenous APC truncations, like exogenous ones (see Figure 5), have lost the ability to cluster at microtubule tips.
Discussion
Actin-dependent association of APC with the plasma membrane in polarized epithelial cells
The purpose of our study was to examine the subcellular distribution of APC in polarized mammalian epithelial cells. We used two polarized cell models, WIF-B and MDCK cells, and we found APC associated with the lateral plasma membrane in both cell types. These lateral plasma membranes, at least in the case of MDCK cells, are known to engage in cellular adhesion by virtue of their cadherin/catenin complexes (Boulan-Rodriguez and Nelson, 1989). The association of APC with the lateral plasma membrane depends on the actin cytoskeleton but not on microtubules. This parallels our findings in Drosophila tissues in which E-APC associates in an actin-dependent way with adherens junctions and with E-cadherin-containing plasma membranes in multiple tissues (Townsley and Bienz, 2000).
Polarized WIF-B cells also showed a conspicuous concentration of APC in discrete puncta along the apical plasma membrane. These APC puncta may reflect membrane-anchored Axin complexes (see Introduction) since they also contain β-catenin but no E-cadherin. However, neither polarized MDCK cells nor colorectal cancer cells showed any apical concentration of APC. Similarly, APC has been observed along lateral plasma membranes in epithelial cells of the mouse intestine (Miyashiro et al., 1995; Senda et al., 1996), but shows no obvious apical concentration in either the murine or the human intestinal epithelium (Näthke et al., 1996; Midgley et al., 1997). Perhaps, the apical APC/β-catenin puncta in polarized WIF-B cells reflect a unique hepatic differentiation feature of these cells. The only other known example of apically concentrated APC are the cortical actin caps in Drosophila blastoderm cells (McCartney et al., 1999; Yu and Bienz, 1999) which are unique and transient subcellular structures found in these early embryonic cells.
Although the apical APC puncta in WIF-B cells and the actin caps in Drosophila embryos appear to be specialized cases, they nevertheless depend on the actin cytoskeleton, like the membrane-associated APC. They may thus reflect the same underlying actin-dependent targeting mechanism. Furthermore, we found that transiently transfected E-APC–GFP also forms apical puncta associated with the ovoids of polarized WIF-B cells (not shown). Therefore, the ability of APC proteins to utilize the actin cytoskeleton to associate with the plasma membrane appears to be a fundamental and conserved property of these proteins. Indeed, preliminary results from actin sedimentation assays (Pope et al., 2000) revealed that a central domain of APC and one of E-APC that span the highly conserved β-catenin-binding motifs specifically bind to F-actin (R.Rosin-Arbesfield, A.Cliffe, S.Yeoh and M.Bienz, unpublished observations).
During the final stages of our manuscript preparation, another study of the subcellular distribution of APC in polarized mammalian cells was published (Reinacher-Schick and Gumbiner, 2001). These authors reported a conspicuous apical concentration of APC in all cell types examined, including polarized MDCK cells, wild-type and APC-mutant colorectal cancer cells, and mouse intestinal epithelium, in stark contrast to our own results. We noted that these authors used a different antibody (called N-15, raised against an N-terminal peptide of APC; Santa Cruz Biotechnology, Inc.). We thus undertook a systematic comparison of the two antibodies and fixation conditions (see Supplementary data). This confirmed that the two antisera produce utterly different staining patterns when compared side by side. We further established that (i) the N-15 antiserum did not detect transfected APC–GFP protein while the anti-M-APC antiserum used by us did so reliably and specifically, and (ii) the N-15 produced strong apical staining in HCA46 cells which do not contain any detectable APC protein (Rowan et al., 2000), while anti-M-APC only produced background staining of these cells. Note also that M.M.Mogensen, J.B.Tucker, J.B.Mackie, A.R.Prescott and I.S.Náthke (submitted) demonstrated that the majority of the bands detected by N-15 on western blots do not correspond to APC protein, while anti-M-APC shows a high specificity in detecting APC by this method (see also Figure 5A). Thus, the N-15 antiserum is highly unreliable in detecting APC, and the apical staining reported by Reinacher-Schick and Gumbiner (2001) is likely to reflect a ubiquitous cross-reacting antigen.
A dynamic process mediating association of APC with the plasma membrane
How does the actin cytoskeleton mediate the membrane association of APC? The underlying targeting mechanism could be a static or a dynamic one. For example, APC could be trapped, and anchored, by the meshwork of actin filaments that are typically found associated with the cortex of epithelial cells. Alternatively, APC could track along actin filaments that are linked to cadherin/catenin complexes to reach adhesive membranes (Bienz, 1999). Such a tracking mechanism seems a possibility, given the observed microtubule-tracking behaviour of APC–GFP (Mimori-Kiyosue et al., 2000).
Our results do not allow us to decide definitively between these possibilities. However, the drug wash-out experiments argue for a dynamic mechanism: they showed that the membrane association of APC could be re-established within minutes of recovery from actin depolymerization. This indicates that the process of actin-dependent membrane association of APC requires the actin cytoskeleton continuously, consistent with continuous and directed delivery of APC to the plasma membrane.
Two distinct peripheral pools of APC
Perhaps our most interesting observation was that nocodazole treatment of MDCK cells shifted APC from microtubule-dependent clusters to the plasma membrane. This illustrated that these two peripheral locations of APC differ in terms of their cytoskeletal requirements. It provided evidence for the existence of two distinct pools of APC that are in equilibrium with each other.
What are the factors that determine the choice of APC between these peripheral pools? Correlative evidence suggests that this choice depends on whether a cell is freely motile or part of an epithelial tissue: the cells that we have examined fall broadly into two groups, showing either predominantly microtubule-dependent APC clusters (motile MDCK and COS cells), or predominantly actin-dependent membrane-associated APC (polarized WIF-B and MDCK cells, colorectal cancer cells derived from the intestinal epithelium, and native Drosophila epithelia and tissues; Townsley and Bienz, 2000). It thus seems that, in motile cells, APC prefers a microtubule-dependent mode to reach peripheral sites, whereas in epithelial tissue, APC prefers an actin-dependent mode to reach the plasma membrane. Perhaps, microtubules are more suitable than actin filaments for fast and transient movement of APC over relatively large distances in motile cells. On the other hand, actin filaments may be suitable for continuous delivery of APC to adhesive plasma membranes, given that these filaments are permanently connected to the cadherin/catenin complexes in these membranes (Takeichi, 1991; Tepass, 1997).
APC cancer truncations cannot reach the cell periphery
We were unable to identify a single APC domain sufficient to confer APC targeting to the cell periphery, suggesting that the targeting mechanism is complex and requires multiple protein interactions. This is consistent with the results of Mimori-Kiyosue et al. (2000) whose APC truncations also failed to exhibit the normal tracking and clustering behaviour of full-length APC. Importantly, our studies showed that the N-terminal half of APC is neither capable of localizing to microtubule tips nor to the plasma membrane. Indeed, APC truncations such as those commonly found in colorectal tumours (which typically consist of the N-terminal half) are evenly distributed throughout the cytoplasm, and are also found in the nuclei of APC-mutant cancer cells as a consequence of their loss of nuclear export signals (Rosin-Arbesfeld et al., 2000). Thus, we expect the function(s) of peripherally located APC to be lost in most APC-mutant cancer cells.
What could these functions be? In motile cells, the clustering of APC at microtubule tips may affect cellular migration and adhesion (Näthke et al., 1996; Barth et al., 1997; Pollack et al., 1997). On the other hand, the membrane-associated E-APC in Drosophila may have a function in the maintenance of cellular adhesion (Townsley and Bienz, 2000). These suggestions await confirmation by genetic analysis of APC-mutant cells and tissues. Whatever the case, it is conceivable that the putative functions of APC in cellular migration and adhesion, if lost in APC-mutant cells, could promote not only the initial formation of adenomas, but also the acquisition of invasiveness during tumour progression (Bienz and Clevers, 2000).
Materials and methods
Plasmids
Human APC was tagged with GFP at the N-terminus by subcloning the full-length cDNA into pEGFP-C1 (between BglII and SalI sites) containing the cytomegalovirus promoter. MAPC–GFP encodes a GFP-tagged central fragment of human APC (residues 1379–2080), as described (Rosin-Arbesfeld et al., 2000). Two further N-terminally-tagged GFP constructs of human APC were also generated in pEGFP-C1: NAPC, mimicking the mutation found in SW480 cells (amino acids 1–1338; Rowan et al., 2000), and CAPC encompassing the C-terminus (amino acids 2068–2843).
Cell lines, tissue culture and transfections
MDCK II cells were grown on glass coverslips in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), and analysed 24 h after seeding. For polarization, 5 × 105 cells were seeded into the apical chamber of 6.5 mm-diameter Transwell polycarbonate filters (Costar) and grown typically for 6 days prior to analysis.
MDCK II cells were transfected with LipofectAMINE (Gibco-BRL); 3–5 µg of plasmid DNA (Qiagen Miniprep Kit) were used per 35 mm culture well. Typically, subconfluent cells were fixed and stained 36 h after transfection. Alternatively, cells were trypsinized 24 h after transfection, spun down and seeded into the apical chamber of 6.5 mm-diameter Transwell polycarbonate filters, as described above. Monolayer cells were examined 4–6 days after transfection.
To check the integrity of the expressed GFP constructs and to determine their expression levels, transiently transfected MDCK cells were analysed by western blot analysis as described (Rosin-Arbesfeld et al., 2000). Full-length APC–GFP and NAPC–GFP proved to be difficult to detect by this method, and optimal expression times had to be determined. To do this, cells were inspected for green fluorescence at various time points between 18–36 h after transfection, to assess the frequency of transfected cells and the subcellular distribution of constructs; aliquots of cells were harvested and lysed at these time points.
WIF-B cells were cultured as described with minor modifications (Ihrke et al., 1993). Cells were grown in modified Ham’s F12 medium (Gibco-BRL) supplemented with HAT and 4% FCS. Typically, 10–12 days after sowing, the cultures exhibited a high degree of polarization after plating onto glass coverslips at 1 × 104 cells/cm2, forming numerous ovoids.
Simian COS cells were grown in DMEM supplemented with 10% FCS, and transfected with FuGENE 6.
The following APC wild-type and mutant colorectal cancer cells were used (genetic lesions described by Rowan et al., 2000): HCT116, LS174T, LOVO, DLD-1, T84, GP5D, HT55, HCA46. These cells were grown on glass coverslips, HCA46 in Iscove’s modified Dulbecco’s medium, all others in DMEM, supplemented with 10% FCS.
Drug treatments and immunofluorescence
Polarized MDCK or WIF-B cells were incubated for 60 min at 37°C with 12.5 µm or 5 µm latrunculin A (Molecular Probes), or with 3 µg/ml or 1 µg/ml cytochalasin D (Sigma), respectively. Subconfluent MDCK cells were exposed to 1 µm latrunculin A or 1 µg/ml cytochalasin D for 60 min at 37°C. For microtubule depolymerization, WIF-B or MDCK cells were incubated for 15 min on ice in 33 µm nocodazole followed by 60 min at 37°C in drug-containing medium. Control cells were incubated in solvent-containing medium (< 0.25% dimethyl sulfoxide). For drug wash-out experiments, the drug solution was replaced with fresh medium, and cells were fixed typically after 5–15 min, but occasionally after ∼16 h at 37°C (see Results).
Following drug treatment, cells were rinsed twice in phosphate buffered saline (PBS) and fixed for 30 min in 4% paraformaldehyde (freshly prepared in PBS) or for 10 min with chilled methanol at –20°C. Methanol fixation was used for most stainings shown in the Supplementary data in order to reproduce the conditions used by Reinacher-Schick and Gumbiner (2001). The subcellular distribution of APC in all cell types used was essentially the same after the two different fixations (if anti-M-APC was used, see below). After three washes in PBS, fixed cells were permeabilized for 5 min with 0.1% Triton X-100 and blocked with bovine serum albumin for 30 min (or overnight, if followed by staining with GFP antibody), and subsequently incubated at room temperature with primary and secondary antibody for 60 and 30 min, respectively. In transfected polarized MDCK cells, GFP fusion proteins were visualized using a polyclonal antibody against GFP, allowing visualization of much lower levels of expressed protein.
The following primary and secondary antibodies were used: rabbit anti-M-APC (kindly provided by I.Näthke; 1:700 for immunofluorescence, 1:1000 for western blot analysis); rat anti-ZO-1 (Chemicon; 1:400); mouse anti-α-tubulin (Sigma T9026; 1:500); rabbit anti-GFP (kindly provided by R.Arkowitz; 1:5000 for immunofluorescence, 1:3000 for western blot analysis); mouse anti-E-cadherin (clone 36, Transduction Laboratories; 1:100); mouse anti-β-catenin (Transduction Laboratories; 1:500); mouse anti-vinculin (clone 11–5, Sigma; 1:50); Alexa IgG (Molecular Probes; 1:500) 594 goat anti-mouse, 594 goat anti-rat, 488 goat anti-rabbit. Alexa 594 phalloidin was used (1:50) to visualize filamentous actin. For the Supplementary data, N-15 raised against an N-terminal peptide of APC was used (rabbit polyclonal IgG; Santa Cruz Biotechnology, Inc.; 1:75), and Alexa IgG 568 goat anti-rabbit for Supplementary data, Figure 3. Images were collected at a BioRad MRC 1024 confocal microscope.
Supplementary data
Supplementary data is available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank I.Näthke and R.Arkowitz for antibody, T.Brabletz, I.Tomlinson and P.Digard for cell lines, A.Cliffe for help with image processing, S.Munro for discussions and comments on the manuscript, and J.P.Luzio for support. R.R.-A. is supported by a Wellcome travelling fellowship. G.I. was funded by Wellcome Trust project grant 057263.
References
- Ayscough K. (1998) Use of latrunculin-A, an actin monomer-binding drug. Methods Enzymol., 298, 18–25. [DOI] [PubMed] [Google Scholar]
- Barth A.I., Pollack,A.L., Altschuler,Y., Mostov,K.E. and Nelson,W.J. (1997) NH2-terminal deletion of β-catenin results in stable colocalisation of mutant β-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J. Cell Biol., 136, 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. (1999) APC: the plot thickens. Curr. Opin. Genet. Dev., 9, 595–603. [DOI] [PubMed] [Google Scholar]
- Bienz M. and Clevers,H. (2000) Linking colorectal cancer to Wnt signaling. Cell, 103, 311–320. [DOI] [PubMed] [Google Scholar]
- Decaens C., Rodriguez,P., Bouchaud,C. and Cassio,D. (1996) Establishment of hepatic cell polarity in the rat hepatoma-human fibroblast hybrid WIF-B9. A biphasic phenomenon going from a simple epithelial polarised phenotype to an hepatic polarised one. J. Cell Sci., 109, 1623–1635. [DOI] [PubMed] [Google Scholar]
- Fagotto F., Jho,E., Zeng,L., Kurth,T., Joos,T., Kaufmann,C. and Costantini,F. (1999) Domains of axin involved in protein–protein interactions, Wnt pathway inhibition, and intracellular localisation. J. Cell Biol., 145, 741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R. et al. (2001) Mutations in the APC tumour suppressor gene cause chromosomal instability. Nature Cell Biol., 3, 433–438. [DOI] [PubMed] [Google Scholar]
- Ihrke G., Neufeld,E.B., Meads,T., Shanks,M.R., Cassio,D., Laurent,M., Schroer,T.A., Pagano,R.E. and Hubbard,A.L. (1993) WIF-B cells: an in vitro model for studies of hepatocyte polarity. J. Cell Biol., 123, 1761–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K.B., Burds,A.A., Swedlow,J.R., Bekir,S.S., Sorger,P.K. and Näthke,I.S. (2001) A role for the Adenomatous polyposis coli protein in chromosome segregation. Nature Cell Biol., 3, 429–432. [DOI] [PubMed] [Google Scholar]
- Kinzler K.W. and Vogelstein,B. (1996) Lessons from hereditary colorectal cancer. Cell, 87, 159–170. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker,N., Morin,P.J., van Wichen,D., de Weger,R., Kinzler,K.W., Vogelstein,B. and Clevers,H. (1997) Constitutive transcriptional activation by a β-catenin–Tcf complex in APC–/– colon carcinoma. Science, 275, 1784–1787. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker,N., Moerer,P., van Donselaar,E., Huls,G., Peters,P.J. and Clevers,H. (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genet., 19, 379–383. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy,Y., Mostov,K. and Rodriguez-Boulan,E. (1990) Vectorial targeting of an endogenous apical membrane asialoglycoprotein and uvomorulin in MDCK cells. J. Cell Biol., 110, 1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney B.M., Dierick,H.A., Kirkpatrick,C., Moline,M.M., Baas,A., Peifer,M. and Bejsovec,A. (1999) Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J. Cell Biol., 146, 1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley C.A., White,S., Howitt,R., Save,V., Dunlop,M.G., Hall,P.A., Lane,D.P., Wyllie,A.H. and Bubb,V.J. (1997) APC expression in normal human tissues. J. Pathol., 181, 426–433. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., Shiina,N. and Tsukita,S. (2000) Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J. Cell Biol., 148, 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro I. et al. (1995) Subcellular localisation of the APC protein: immunoelectron microscopic study of the association of the APC protein with catenin. Oncogene, 11, 89–96. [PubMed] [Google Scholar]
- Morin P.J., Vogelstein,B. and Kinzler,K.W. (1996) Apoptosis and APC in colorectal tumorigenesis. Proc. Natl Acad. Sci. USA, 93, 7950–7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näthke I.S., Adams,C.L., Polakis,P., Sellin,J.H. and Nelson,W.J. (1996) The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol., 134, 165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima M., Oshima,H., Kitagawa,K., Kobayashi,M., Itakura,C. and Taketo,M. (1995) Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc. Natl Acad. Sci. USA, 92, 4482–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H., Oshima,M., Kobayashi,M., Tsutsumi,M. and Taketo,M.M. (1997) Morphological and molecular processes of polyp formation in ApcD716 knockout mice. Cancer Res., 57, 1644–1649. [PubMed] [Google Scholar]
- Polakis P. (2000) Wnt signaling and cancer. Genes Dev., 14, 1837–1851. [PubMed] [Google Scholar]
- Pollack A.L., Barth,A.I.M., Altschuler,Y., Nelson,W.J. and Mostov,K.E. (1997) Dynamics of β-catenin interactions with APC protein regulate epithelial tubulogenesis. J. Cell Biol., 137, 1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope B.J., Gonsior,S.M., Yeoh,S., McGough,A. and Weeds,A.G. (2000) Uncoupling actin filament fragmentation by cofilin from increased subunit turnover. J. Mol. Biol., 298, 649–661. [DOI] [PubMed] [Google Scholar]
- Reinacher-Schick A. and Gumbiner,B.M. (2001) Apical membrane localisation of the Adenomatous polyposis coli tumor suppressor protein and subcellular distribution of the β-catenin destruction complex in polarised epithelial cells. J. Cell Biol., 152, 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E. and Nelson,W.J. (1989) Morphogenesis of the polarised epithelial cell phenotype. Science, 245, 718–725. [DOI] [PubMed] [Google Scholar]
- Rosin-Arbesfeld R., Townsley,F. and Bienz,M. (2000) The APC tumour suppressor has a nuclear export function. Nature, 406, 1009–1012. [DOI] [PubMed] [Google Scholar]
- Rowan A.J., Lamlum,H., Ilyas,M., Wheeler,J., Straub,J., Papadopoulou,A., Bicknell,D., Bodmer,W.F. and Tomlinson,I.P. (2000) APC mutations in sporadic colorectal tumors: A mutational ‘hotspot’ and interdependence of the ‘two hits’. Proc. Natl Acad. Sci. USA, 97, 3352–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda T., Miyashiro,I., Matsumine,A., Baeg,G.H., Monden,T., Kobayashil,S., Monden,M., Toyoshima,K. and Akiyama,T. (1996) The tumor suppressor protein APC colocalizes with β-catenin in the colon epithelial cells. Biochem. Biophys. Res. Commun., 223, 329–334. [DOI] [PubMed] [Google Scholar]
- Shore E.M. and Nelson,W.J. (1991) Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin–Darby canine kidney epithelial cells. J. Biol. Chem., 266, 19672–19680. [PubMed] [Google Scholar]
- Simons K. and Fuller,S.D. (1985) Cell surface polarity in epithelia. Annu. Rev. Cell. Biol., 1, 243–288. [DOI] [PubMed] [Google Scholar]
- Smits R. et al. (1999) Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev., 13, 1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L.K., Kinzler,K.W., Vogelstein,B., Preisinger,A.C., Moser,A.R., Luongo,C., Gould,K.A. and Dove,W.F. (1992) Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science, 256, 668–670. [DOI] [PubMed] [Google Scholar]
- Takeichi M. (1991) Cadherin cell adhesion receptors as a morphogenetic regulator. Science, 251, 1451–1455. [DOI] [PubMed] [Google Scholar]
- Tepass U. (1997) Epithelial differentiation in Drosophila. BioEssays, 19, 673–682. [DOI] [PubMed] [Google Scholar]
- Townsley F. and Bienz,M. (2000) Actin-dependent membrane association of an epithelial APC and its effect on junctional Armadillo. Curr. Biol., 10, 1339–1348. [DOI] [PubMed] [Google Scholar]
- von Kries J.P., Winbeck,G., Asbrand,C., Schwarz-Romond,T., Sochnikova,N., Dell’Oro,A., Behrens,J. and Birchmeier,W. (2000) Hot spots in β-catenin for interactions with LEF-1, conductin and APC. Nature Struct. Biol., 7, 800–807. [DOI] [PubMed] [Google Scholar]
- Yu X. and Bienz,M. (1999) Ubiquitous expression of a Drosophila Adenomatous polyposis coli homolog and its localisation in cortical actin caps. Mech. Dev., 84, 69–73. [DOI] [PubMed] [Google Scholar]
- Yu X., Waltzer,L. and Bienz,M. (1999) A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nature Cell Biol., 3, 144–151. [DOI] [PubMed] [Google Scholar]
- Zeng L. et al. (1997) The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell, 90, 181–192. [DOI] [PubMed] [Google Scholar]