Abstract
Legionella pneumophila requires the dot/icm genes to create an organelle inside eukaryotic host cells that will support bacterial replication. The dot/icm genes are predicted to encode a type IV-related secretion apparatus. However, no proteins have been identified that require the dot/icm genes for secretion. In this study we show that the DotA protein, which was previously found to be a polytopic membrane protein, is secreted by the Dot/Icm transporter into culture supernatants. Secreted DotA protein was purified and N-terminal sequencing of the purified protein revealed that a 19 amino acid leader peptide is removed from DotA prior to secretion. Extracellular DotA protein did not fractionate in membrane vesicles. Structures containing secreted DotA protein were visualized by electron microscopy and were shaped like hollow rings. These data indicate that the large poly topic membrane protein DotA is secreted from L.pneumophila by a unique process. This represents the first target secreted by the dot/icm-encoded apparatus and demonstrates that this transporter is competent for protein secretion.
Keywords: DNA conjugation/intracellular pathogen/phagosome biogenesis/protein secretion/type IV transporter
Introduction
Protein secretion is a remarkably complex biological process of fundamental importance to both free-living and pathogenic bacteria. Protein secretion by Gram-negative bacteria represents an interesting biological problem because two membrane barriers, the inner membrane and the bacterial envelope, must be traversed in order for proteins to be released into the extracellular environment. To accomplish this feat, these bacteria have evolved a variety of specialized multi-protein transporters that can deliver polypeptides across both membranes.
Currently, there are four general types of transporters used by Gram-negative bacteria for protein secretion (reviewed by Linton and Higgins, 1998; Christie, 2001; Plano et al., 2001; Sandkvist, 2001). These transporters can be classified by evolutionary relationships between components of the secretion apparatus and, to a certain extent, by shared functional properties. To drive protein transfer, all these systems have at least one integral inner membrane component that can be energized by ATP hydrolysis, which fuels the bioenergetically unfavorable secretion process. Proteins secreted by type II transporters are first translocated into the periplasm by a standard sec-dependent mechanism. The periplasmic intermediate is then engaged and delivered across the bacterial envelope by the type II transport apparatus. In contrast, type I and type III transporters secrete proteins directly from the bacterial cytosol without the factor being first exported into the periplasm by a sec-dependent process. For type IV systems, the subcellular compartments from which the protein substrates are secreted can vary. Some type IV transporters secrete proteins that are first translocated into the periplasm, and others secrete proteins directly from the cytosol.
Bacterial pathogens have taken protein secretion one step further by adapting secretion devices into syringe-like machines that will inject molecules directly into the cytoplasm of eukaryotic host cells (Kubori et al., 1998, 2000). Among the best studied of these micro-injection machines are the type III secretion systems found in a number of Gram-negative pathogens, which are used to translocate proteins into host cells that affect a variety of cellular processes (reviewed by Hueck, 1998; Galan and Collmer, 1999). Type IV secretion systems can also inject proteins directly into eukaryotic hosts. A type IV transfer apparatus in the phytopathogen Agrobacterium tumefaciens is used to deliver bacterial proteins into plant cells (reviewed by Christie, 1997). Inside the bacterium, one of these proteins will first be attached to the 5′-end of a plasmid-encoded DNA strand so that the nucleic acid molecule will also be co-injected into the plant cell along with the protein. Type IV transporters have been identified on many self-transmissible plasmids and it is now well appreciated that conjugal transfer of plasmid DNA by these machines is directed by a protein secretion process similar to that characterized in A.tumefaciens (Christie and Vogel, 2000; Christie, 2001).
Recently, it was discovered that the bacterial pathogen Legionella pneumophila requires a type IV transporter to create an organelle that permits replication inside phagocytic host cells such as macrophages and amoebae (Berger et al., 1994; Brand et al., 1994; Segal and Shuman, 1997; Andrews et al., 1998; Purcell and Shuman, 1998; Segal et al., 1998; Vogel et al., 1998). This transporter is encoded by 24 dot and icm genes, which are grouped on two regions of the bacterial chromosome. After phagocytosis, vacuoles containing wild-type L.pneumophila avoid immediate fusion with endosomes (Roy et al., 1998; Wiater et al., 1998; Clemens et al., 2000) and intimately associate with vesicles derived from the endoplasmic reticulum (Horwitz, 1983; Swanson and Isberg, 1995). However, L.pneumophila mutants that are defective in Dot/Icm transporter function cannot replicate intracellularly because the vacuoles in which they reside fuse rapidly with endosomes and lysosomes (Swanson and Isberg, 1995; Andrews et al., 1998; Roy et al., 1998; Vogel et al., 1998; Wiater et al., 1998; Zuckman et al., 1999; Coers et al., 2000; Matthews and Roy, 2000). These data indicate that the Dot/Icm transporter sends a signal to host cells that alters trafficking of the phagosome in which the bacterium resides. Based on these data, it is likely that some of the proteins secreted by the Dot/Icm transporter will be factors that have a direct effect on vesicle trafficking in eukaryotic cells. However, the actual proteins secreted by the Dot/Icm transporter have not been discovered.
The DotA protein was one of the first components of the Dot/Icm transporter to be investigated (Berger et al., 1994). This protein has a predicted mass of 113 kDa. Molecular, biochemical and genetic evidence indicates that the DotA protein is a polytopic inner membrane protein with eight hydrophobic transmembrane domains (Roy and Isberg, 1997). Significant regions of amino acid similarity are observed when the sequence of DotA is compared with that of TraY (Segal and Shuman, 1999; Komano et al., 2000; Wilkins and Thomas, 2000), which is a component of the type IV transporter required for conjugal transfer of the broad host range plasmids ColIb-P9 and R64. These data suggest that DotA could be playing a role that is conserved in other type IV systems, perhaps by functioning as a structural component of the membrane-bound transport complex. Consistent with this theory, bacteria lacking the dotA gene are defective in all virulence activities that require the Dot/Icm transporter (Berger and Isberg, 1993; Berger et al., 1994; Swanson and Isberg, 1995; Kirby et al., 1998; Coers et al., 2000).
In the course of identifying additional genes required for intracellular growth of L.pneumophila, newly isolated mutants had been screened by immunoblot analysis to determine whether they produce the DotA protein (Vogel et al., 1996). An interesting observation was made during this screening process. An overabundance of DotA protein was discovered in most of the mutants that were defective for other components of the Dot/Icm transporter. It was unclear why DotA protein levels would be higher in these mutants. In this study, we have investigated this phenomenon further and have discovered that the DotA protein is secreted into culture supernatants by the Dot/Icm transporter. Secretion of a large polytopic membrane protein represents a unique cellular process that has not been observed previously in either prokaryotic or eukaryotic organisms. The significance of this secretion process and a potential role for the secreted DotA protein during L.pneumophila infection of host cells are discussed.
Results
Mutations that disrupt Dot/Icm transporter function result in cellular accumulation of the DotA protein
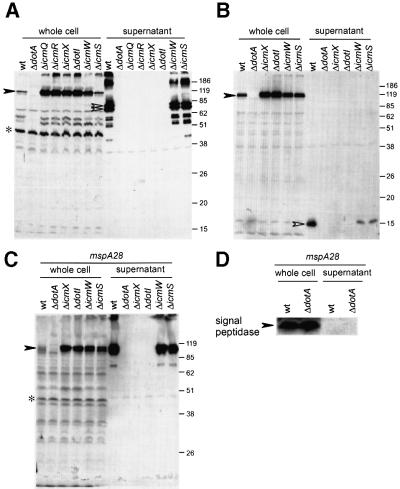
The cellular levels of DotA protein were determined for wild-type L.pneumophila and isogenic dot/icm mutant strains by immunoblot analysis. Legionella pneumophila cellular lysates were probed for DotA protein using the monoclonal antibody mAb2.29, which was generated against a 100 amino acid C-terminal fragment of the DotA protein (Matthews and Roy, 2000). An increase in the cellular concentration of DotA protein was observed in all dot/icm mutants examined (Figure 1A). In comparison with the icmS and icmW mutants, cellular levels of the DotA protein were higher in the icmX and dotI mutants; however, all mutants contained significantly more DotA protein than wild-type L.pneumophila. To determine whether transcriptional regulation may account for the observed cellular increases in DotA protein, gene expression was measured in wild-type and mutant bacteria. Quantitative slot-blot hybridization analysis of total cellular RNA indicates that there is no significant increase in the expression of dotA message by any dot or icm mutant compared with wild-type L.pneumophila (Figure 1B). In addition, we were unable to find any difference in translational regulation or in the stability of DotA protein that would account for the apparent increase in its cellular concentration observed in these dot/icm mutants (J.Kagan, M.Stern and C.R.Roy, unpublished results). Thus, a pool of DotA protein was being made by wild-type L.pneumophila for which we could not account.
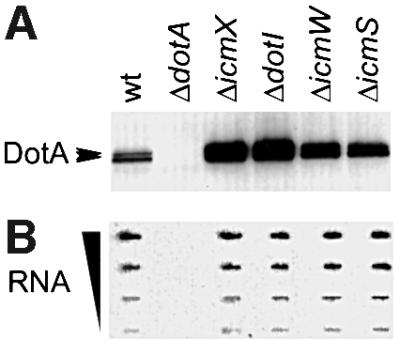
Fig. 1. DotA protein accumulates in a subset of L.pneumophila dot/icm mutants. Legionella pneumophila strains were grown in AYE broth, then whole-cell protein extracts and total cellular RNA were prepared in parallel. Strains used were CR39 (wt), CR58 (ΔdotA), CR416 (ΔicmX), CR419 (ΔdotI), CR157 (ΔicmW) and CR393 (ΔicmS). (A) DotA protein levels were determined by immunoblot analysis. Whole-cell lysates from parental strain CR39 (wt) and the indicated dot/icm null derivatives were separated by 12.5% SDS–PAGE and probed with mAb2.29, a monoclonal antibody generated against the 100 amino acid C-terminal region of the DotA protein. The DotA protein migrates as a doublet in the range of the 120 kDa molecular weight marker (arrowheads). Samples were not boiled prior to loading to prevent DotA protein aggregation. (B) Expression of the dotA gene was examined by RNA slot-blot hybridization. Dilutions of total RNA were blotted onto a nylon membrane and probed with a 32P-labeled probe consisting of the entire dotA gene. From top to bottom, the total amounts of RNA loaded were 6, 2, 0.67 and 0.22 µg, respectively.
The DotA protein is secreted by the Dot/Icm transporter during growth in liquid culture
These data led to speculation that wild-type L.pneumophila may secrete the DotA protein by a dot/icm-dependent mechanism, which would explain why the mutant bacteria contain more cellular DotA protein. To test this possibility, wild-type and mutant L.pneumophila were grown to stationary phase in N-(2-acetamido)-2-aminoethanesulfonic acid (ACES)-buffered yeast extract (AYE) broth. The amount of DotA protein in each bacterial cell pellet and corresponding culture supernatant was measured by immunoblot analysis using an affinity-purified polyclonal antibody generated against the DotA542–557 epitope (Roy and Isberg, 1997). According to membrane topology studies, the DotA542–557 epitope is located in a 509 amino acid periplasmic domain centered between the third and fourth transmembrane domains in the DotA protein (Roy and Isberg, 1997). Consistent with results obtained using mAb2.29, L.pneumophila cells with a defective Dot/Icm transporter contained abundant levels of DotA protein when probed with anti-DotA542–557; considerably less cell-associated DotA protein was found in wild-type, ΔicmS and ΔicmW bacteria (Figure 2A). When supernatants from these cultures were probed for DotA protein, the exact opposite pattern was observed. The anti-DotA542–557 antibody reacted strongly to polypeptides of between 70 and 80 kDa in culture supernatants from wild-type L. pneumophila, an icmS mutant and an icmW mutant (Figure 2A). No DotA542–557-immunoreactive products were found in supernatants isolated from L.pneumophila mutants with a defective Dot/Icm transporter. These results were verified using mAb2.29. Membrane topology studies indicate that the C-terminal DotA epitope recognized by mAb2.29 is cytoplasmic, making it distinct from the periplasmic DotA542–557 epitope. When the extracts from Figure 2A were probed with mAb2.29, a small peptide of ∼15 kDa was detected in supernatants from wild-type, ΔicmS and ΔicmW bacteria, but was absent in supernatants from all mutants with a defective Dot/Icm transporter (Figure 2B and data not shown). These data demonstrate that fragments of the DotA protein are secreted into culture supernatants by L.pneumophila and that this process requires a functional Dot/Icm transport complex.
Fig. 2. Legionella pneumophila secretes DotA protein into culture supernatants by a process requiring the Dot/Icm transport apparatus. Legionella pneumophila strains were grown in AYE broth as described in Materials and methods, and protein extracts were prepared from fractionated bacteria and culture supernatants. Equivalent amounts of whole-cell bacteria in the isolated cell pellet and their culture supernatant (4% of total) were loaded onto a 12% SDS–PAGE gel and separated proteins were detected by immunoblot analysis. Protein samples were not boiled prior to loading to prevent DotA aggregation. Closed arrowheads indicate full-length DotA protein. Open arrowheads indicate DotA-specific immunoreactive products. Positions of molecular weight markers are indicated on the right of each blot (kDa). (A) Immunoblot analysis using a polyclonal antibody specific for the DotA542–557 epitope, which is located in a central region of the DotA protein. Strains used were CR39 (wt), CR58 (ΔdotA), CR341 (ΔicmQ), CR343 (ΔicmR), CR416 (ΔicmX), CR419 (ΔdotI), CR157 (ΔicmW) and CR393 (ΔicmS). (B) Samples from (A) were probed with the monoclonal antibody mAb2.29, which reacts specifically with a C-terminal DotA epitope. (C) Samples were prepared from isogenic mspA28 mutant strains to examine whether the MspA protease is responsible for degradation of secreted DotA protein. The strains used were L.pneumophila CR214 (wt), which is an mpsA28 mutant with a fully functional Dot/Icm transporter, and strains CR206 (ΔdotA), CR823 (ΔicmX), CR821 (ΔdotI), 216 (ΔicmW) and CR388 (ΔicmS), which are mspA28 derivatives with the indicated dot/icm null mutation. (D) To determine whether secretion of DotA protein is specific, extracts from the mspA28 strains CR214 (wt) and CR206 (ΔdotA) were probed with an antibody specific for signal peptidase I, a conserved prokaryotic membrane protein.
The DotA fragments located in the supernatant of broth-grown L.pneumophila may represent digestion products generated during Dot/Icm transporter function that escape from the cell by a non-specific secretion event. Alternately, the intact DotA protein may be secreted by the Dot/Icm transporter and partially degraded after secretion. Legionella pneumophila secretes a potent zinc metalloprotease using a type II transporter that is unrelated to the Dot/Icm apparatus (Hales and Shuman, 1999). This zinc metalloprotease, encoded chromosomally by the mspA gene, is the most abundant protein found in L.pneumophila culture supernatants (Quinn et al., 1989; Szeto and Shuman, 1990). If the intact DotA protein were being secreted by wild-type L. pneumophila, it is likely that it would be subject to proteolysis by the MspA protein. To examine whether secreted DotA is degraded by the MspA protein, allelic exchange was used to introduce the mspA28 loss-of-function mutation onto the chromosome of wild-type L.pneumophila and several dot/icm mutants. Cell pellets and supernatants from the resulting mspA28 derivatives were collected after bacterial growth in liquid culture, and DotA protein levels were measured. Immunoblot analysis of mspA28 mutants indicates that a DotA product migrating slightly faster than the 119 kDa standard was present in secreted fractions isolated from bacteria with a functional Dot/Icm transporter (Figure 2C). As predicted, the mspA28 mutants that were defective for Dot/Icm transporter function had a higher cellular concentration of DotA protein and no detectable DotA product was found in their culture supernatants. The mass of the DotA immunoreactive protein identified in the supernatant of mspA28 bacteria with a functional Dot/Icm transporter closely matches the molecular weight (113 kDa) calculated for the intact dotA-encoded product. To ensure that the DotA protein was not being released from the inner membrane by a general phenomenon such as bacterial lysis, these mspA28 extracts were probed for protein signal peptidase I, which is a resident inner membrane protein (Paetzel et al., 2000). Signal peptidase I protein was present in equal amounts in the cellular fraction of each of the strains examined, but was undetectable in all secreted fractions (Figure 2D and data not shown). These data demonstrate that the intact DotA protein is secreted by L.pneumophila by a specific process that requires a functional Dot/Icm transporter.
Isolation of DotA protein from L.pneumophila culture supernatants
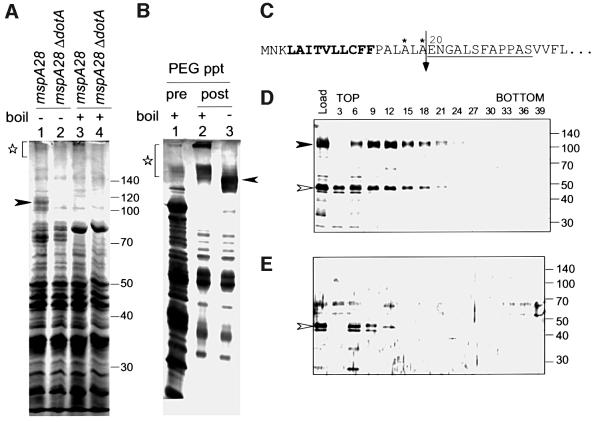
Proteins secreted into culture supernatants by the mspA28 mutant and the isogenic mspA28 ΔdotA derivative were separated by SDS–PAGE and stained with Coomassie Brilliant Blue (CBB), so that the relative abundance of secreted DotA protein could be examined. When the profiles of proteins secreted by these two strains were compared, a diffuse band that migrated just below the 120 kDa marker was identified in samples obtained from the mspA28 strain (Figure 3A, lane 1). This band was not present in the mspA28 ΔdotA supernatant (Figure 3A, lane 2). These data are consistent with this polypeptide being DotA protein that has been secreted by L.pneumophila. To determine whether this novel polypeptide observed in the mspA28-secreted fraction could be DotA protein, samples were boiled prior to being loaded on the gel. Like many other polytopic membrane proteins, the DotA protein will form high molecular weight aggregates that are difficult to resolve if samples are boiled in loading buffer prior to SDS–PAGE analysis. When the mspA28 sample was boiled prior to loading, the polypeptide observed below the 120 kDa marker was no longer detectable and several products with higher molecular weights appeared near the top of the gel (Figure 3A, lane 3). These high molecular weight products were not detectable in the supernatant isolated from mspA28 ΔdotA bacteria (Figure 3A, lane 4), suggesting that these bands represent aggregates of DotA protein. From these data, we conclude that DotA protein secretion into culture supernatants occurs at levels that allow easy detection by standard CBB gel staining.
Fig. 3. A complex containing DotA protein can be isolated from L.pneumophila culture supernatants. (A) Culture supernatants from CR214 (mspA28) and CR206 (mspA28 ΔdotA) were separated by 7.5% SDS–PAGE and proteins were visualized by CBB staining. Samples in lanes 3 and 4 were boiled for 3 min prior to loading. Boiling resulted in the loss of polypeptides with the same mobility as full-length DotA protein (lane 1, closed arrowhead) and the appearance of high molecular weight aggregates (lane 3, star), which were absent in samples prepared from the ΔdotA strain (lanes 2 and 4). (B) Partial purification by PEG precipitation. Culture supernatant from CR214 (lane 1) and PEG precipitants (lanes 2 and 3) were separated by 10% SDS–PAGE and proteins were detected by silver staining. Samples in lanes 1 and 2 were boiled for 3 min prior to loading. (C) The predicted N-terminal amino acid sequence of the full-length DotA protein is shown. Underlined is the peptide sequence obtained for the protein purified from L.pneumophila supernatants, which was identical to amino acid residues 20–31 of the DotA protein. Conserved motifs found for cleavable N-terminal signal peptides are shown. The hydrophobic domain of the leader peptide is in bold, amino acid residues recognized by signal peptidase are marked with an asterisk and the predicted leader peptide cleavage site is marked with an arrow. (D and E) PEG precipitants isolated from culture supernatants were fractionated by isopyncnic sucrose density gradient centrifugation. Every third fraction was separated by 10% SDS–PAGE and proteins were detected by silver staining. Proteins isolated from CR214 supernatants (D) were compared with those isolated from CR206 supernatants (E). The DotA protein (closed arrowheads) and the p46 protein (open arrowheads) were found to co-fractionate in CR214 supernatants.
To confirm that this secreted protein is DotA, supernatants were harvested from saturated liquid cultures of mspA28 bacteria and proteins were differentially extracted by polyethylene glycol (PEG) precipitation. This secreted protein was selectively precipitated from culture supernatants in 16% PEG (Figure 3B). Secreted proteins isolated by PEG precipitation were fractionated by SDS–PAGE and the protein that migrated just below the 120 kDa molecular weight standard was extracted from the gel. The N-terminal sequence of the extracted protein was determined. The N-terminal amino acid sequence obtained for this secreted protein was an exact match with amino acid residues 20–31 of the DotA protein (Figure 3C). Further analysis of DotA amino acid residues 1–19 reveals that this sequence has many of the standard features found for leader peptides cleaved by signal peptidase I (Figure 3C) (Pugsley, 1993; Fekkes and Driessen, 1999). From these data, we conclude that secreted DotA protein can be isolated from L.pneumophila mspA28 culture supernatants and that the N-terminal leader peptide is cleaved before secretion of DotA by the Dot/Icm apparatus.
To determine whether the secreted DotA protein is contained in a macromolecular complex, supernatant proteins precipitated by 16% PEG were further fractionated by sucrose density gradient centrifugation (see Materials and methods). Fractions were isolated starting from the top of the gradient and proteins in each fraction were identified on silver-stained gels. The majority of DotA protein was recovered in gradient fractions 6–24 (Figure 3D, closed arrowhead). A protein with an estimated mass of 46 kDa was also found in the fractions containing DotA. Interestingly, this 46 kDa protein appears to co-fractionate with DotA on the sucrose gradient, although the 46 kDa protein was also present in lighter fractions that had no detectable DotA protein. Supernatants from the mspA28 ΔdotA mutant were purified in parallel with the mspA28 supernatants to examine whether the 46 kDa protein fractionates similarly in the absence of DotA protein (Figure 3E). The 46 kDa protein was isolated from the mspA28 ΔdotA mutant supernatant under the conditions used to purify DotA protein, but the 46 kDa protein fractionated as a tight single peak when DotA was not present. These data indicate that secretion of the 46 kDa protein is DotA independent, but the observed fractionation pattern suggests that the 46 kDa protein and DotA could be part of a multiprotein complex.
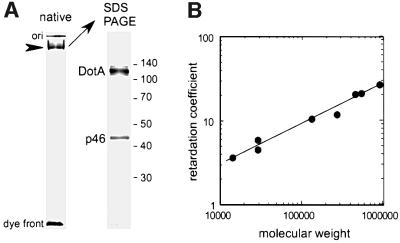
Native PAGE was used to estimate the size of the DotA complex and to determine whether p46 may be a component of this complex. When the enriched DotA complex purified on a sucrose gradient was analyzed by native PAGE, a single band was observed (Figure 4A, native). To determine the protein composition of this complex, the band was excised from the native gel and proteins were extracted by electroelution. When the electroeluted proteins were fractionated by SDS–PAGE, both the DotA and p46 proteins were identified (Figure 4A, SDS–PAGE). The molar ratio of DotA and p46 was estimated to be 1:1, based on the intensity of CBB staining of these two bands. A retardation coefficient was determined for the DotA complex by measuring its migration on 7, 6, 5.5 and 5% native gels. A Ferguson plot was constructed by measuring the migration of protein standards and calculating their retardation coefficients (Figure 4B). Based on a retardation coefficient of 25.9, the molecular weight of the secreted DotA complex was estimated to be 850 kDa. These data are consistent with the complex being either a pentameric or hexameric structure containing an equal molar ratio of DotA and p46; these would have predicted molecular weights of 795 and 954 kDa, respectively.
Fig. 4. Secreted DotA is contained in a high molecular weight complex. (A) A sucrose gradient fraction containing secreted DotA was separated by 6% native PAGE and proteins were visualized by CBB staining (native panel). A single high molecular weight complex was detected (arrowhead). Proteins in this complex were electroeluted from the gel and fractionated on a denaturing gel (SDS–PAGE), which revealed that the complex contained both the DotA protein and p46. (B) A Ferguson plot was generated by plotting retardation coefficients for several protein standards on the y-axis and their molecular weights on the x-axis. The retardation coefficient of the DotA–p46 complex was calculated to be 25.9, which correlates with an estimated molecular weight of 850 kDa.
A complex containing secreted DotA protein forms a ring-like structure
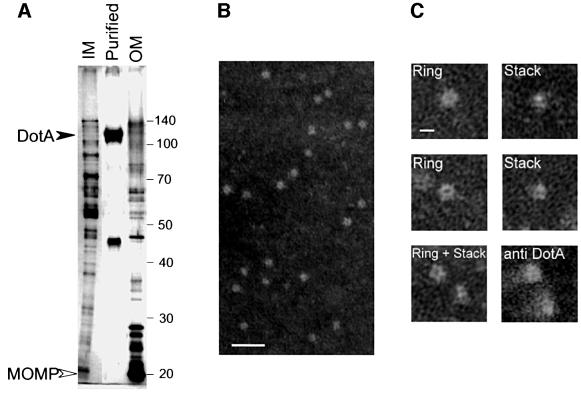
Horstman and Kuehn (2000) recently reported a novel mechanism for protein secretion that involves packaging of exported molecules in membrane vesicles derived from the bacterial surface. The possibility that DotA protein was being exported in membrane vesicles was explored since it was shown previously that cell-associated DotA protein is located in the inner membrane. We found that secreted DotA protein remained in suspension when culture supernatant was centrifuged at 100 000 g for 30 min—unlike DotA protein contained in the inner membrane, which will sediment quantitatively by centrifugation at 100 000 g for 30 min (data not shown). These results suggest that the secreted protein is no longer in a membrane vesicle. According to data by Horstman and Kuehn (2000), purified vesicles containing proteins that are secreted by the novel membrane-derived export pathway are highly enriched for bacterial proteins located in the outer membrane, such as porins. To determine whether the purified DotA complex is enriched for resident membrane proteins, both inner and outer membrane vesicles were purified from L.pneumophila mspA28 and their protein profiles were compared with fractions containing the secreted DotA protein. The sucrose gradient fractions containing inner and outer membrane vesicles from L.pneumophila had a very different protein profile compared with fractions containing the secreted DotA protein (Figure 5A). Proteins that were abundant in the inner and outer membrane vesicles were not detectable in the supernatant fraction containing DotA protein. These data strongly suggest that secreted DotA protein no longer resides in membrane vesicles.
Fig. 5. The complex containing secreted DotA forms a ring-like structure. (A) The protein profile of the purified DotA complex was compared with inner and outer membrane fractions isolated from strain CR214. Samples were separated by 10% SDS–PAGE and proteins were detected by silver staining. The closed arrowhead indicates the location of DotA protein. The open arrowhead indicates the location of the L.pneumophila major outer membrane protein (MOMP). (B) A sample containing DotA protein purified by isopyncnic sucrose density gradient centrifugation was negatively stained and examined by electron microscopy. Particles that were ∼10 nm in diameter were detected in these samples (bar, 50 nm). (C) High magnification electron micrographs of the 10 nm particles. Depending on the projection, the structures of the DotA-containing particles were shaped like a hollow ring (Ring) or a parallel stack (Stack). When samples were pre-incubated with the anti-DotA antibody mAb2.29, these structures aggregated and the center of the ring was occluded (anti DotA) (bar, 10 nm).
To examine further whether secreted DotA is found in membrane vesicles, fractions containing the purified DotA complex were negatively stained and visualized by transmission electron microscopy (TEM). Small particles with a uniform diameter of ∼10 nm were observed in these samples (Figure 5B). In contrast, proteins that are secreted in membrane vesicles were shown previously to be in particles that range in diameter from 50 to 200 nm (Horstman and Kuehn, 2000), indicating that the structures observed in the DotA samples were not similar organelles. Interesting structural features were observed upon close inspection of the 10 nm particles found in the DotA-containing samples. In general, the particles appeared either as hollow rings (Figure 5C, Ring) or as double-stacked disks (Figure 5C, Stack). These two distinct structures are probably different orientations of the same complex, with the rings representing a view from the top of the complex and the stacked disks being a side view of the complex. These 10 nm particles were observed only in the sucrose fractions purified from L.pneumophila mspA28 supernatant with detectable levels of DotA protein and were not seen in purified mspA28 ΔdotA supernatant fractions that contained the 46 kDa protein. Incubation of samples containing DotA protein with mAb2.29 prior to EM visualization resulted in particle aggregation. The single particles that were identified in mAb2.29-treated samples exhibited a change in morphology (Figure 5C, anti-DotA). The most striking difference was that the hollow center of the ring-like structure was no longer visible. Thus, these ring-shaped particles are DotA-containing structures. In conclusion, these data indicate that DotA protein is being secreted by a process that requires the Dot/Icm transporter, resulting in the formation of an extracellular protein complex that has the morphology of a hollow ring.
Discussion
Microbial pathogens use complex secretion systems to export virulence factors that modulate host cellular functions. Molecular analysis of these transporters and the factors that they export indicates that each system functions differently. When comparing the proteins that are secreted by these various systems, there are no common rules regarding where in the cell a protein must be located before engaging the secretion machinery or how recognition of the transport apparatus is mediated. Even systems that appear to be similar can be very different operationally. In other words, these secretion systems have all adapted to serve the particular needs of the organism in which they are expressed. For this reason, a number of novel biological processes, such as the direct injection of bacterial proteins into eukaryotic cells, have been uncovered while studying these secretion systems. In this study, we have shown that the DotA protein is secreted by the L.pneumophila Dot/Icm secretion apparatus. This is a novel finding as it is the first report of a large polytopic inner membrane protein being secreted by a bacterial transporter.
We have shown that there is an inverse correlation between the cellular concentration of DotA protein detected inside L.pneumophila and the amount of DotA protein present in culture supernatants. Analysis of L.pneumophila mutants indicates that the DotA protein is secreted by bacteria with a functional Dot/Icm transporter. Mutations that negatively affect Dot/Icm transporter function abolish secretion of the DotA protein and increase the amount of DotA protein inside the bacterial cell. There was no evidence indicating that dotA gene expression was altered in these dot/icm mutants, suggesting that cellular accumulation of DotA protein may simply be due to a lack of bacterial secretion. Interestingly, icmS and icmW mutants were able to secrete DotA protein. We have shown previously that these genes encode small cytoplasmic proteins that are not essential for Dot/Icm transporter function, even though they are required by L.pneumophila to create a vacuole that evades fusion with lysosomes (Zuckman et al., 1999; Coers et al., 2000). We have hypothesized that the IcmS and IcmW proteins are chaperones for a subset of effector proteins that are secreted by the Dot/Icm transporter. The observation that secretion of DotA protein by L.pneumophila occurs independently of IcmS or IcmW function indicates that these proteins are not required for the DotA export process. This places DotA secretion upstream of phagosome-trafficking events requiring IcmS and IcmW function.
Secretion of DotA protein from L.pneumophila is a specific export process. Previous studies have shown that the proteins IcmQ, IcmR, IcmS and IcmW are not membrane associated and are not found in culture supernatants, which suggests that they are located in the bacterial cytoplasm (Zuckman et al., 1999; Coers et al., 2000). If the DotA protein were released by a non-specific process such as enhanced autolysis of bacteria with a functional Dot/Icm transporter, we would expect that these cytoplasmic determinants should also be detected in culture supernatants and that cellular levels of these proteins would appear to be reduced, which does not occur. In this study, an unknown L.pneumophila antigen that cross-reacts with the polyclonal DotA542–557 antibody provided an internal control for equal loading of cellular proteins. The difference in DotA levels observed in Figure 2A could not have been due to a loading difference since nearly identical levels of this cross-reacting protein (marked by an asterisk) were observed in each whole-cell-extract lane. In addition, we have shown that the integral membrane protein, signal peptidase I, was not found in L.pneumophila culture supernatants (Figure 2D) and that secreted DotA protein was not located in vesicles containing resident proteins of the inner or outer membrane (Figure 5A). These results argue against the possibility that DotA protein secretion occurs by non-specific events such as membrane blebbing or bacterial lysis. From these data, we conclude that secretion of DotA protein is a specific export process that requires the Dot/Icm transporter. This is the first demonstration that the Dot/Icm transporter is capable of secreting proteins from L.pneumophila.
We found that the MspA protease can interfere with the detection of proteins that are secreted by L.pneumophila during growth in liquid culture. Using antibodies specific for two different domains in the DotA protein, we detected only proteolytic fragments of the DotA protein in culture supernatants from wild-type L.pneumophila (Figure 2A and B). When MspA protease activity was eliminated by mutating the mspA gene, the intact DotA product was identified in the culture supernatants (Figure 2C). These data indicate that extracellular DotA protein is subject to MspA degradation and explain why DotA was not detected in culture supernatants in previous studies using wild-type L.pneumophila to examine protein secretion. The number of secreted proteins detected on stained gels increased significantly when supernatants from the mspA28 mutant were compared with those from wild-type L.pneumophila. Identifying other proteins secreted by the Dot/Icm transporter will be facilitated greatly by comparing the secreted protein profile of an mspA28 mutant to profiles obtained for select icm mspA28 double mutants.
Topological analysis using translational fusions to alkaline phosphatase and β-galactosidase revealed that DotA is a polytopic inner membrane protein with eight transmembrane helices (Roy and Isberg, 1997). This brings up the question of how a large integral membrane protein could be extracted and secreted from the inner membrane. Secretion of a protein that first resides in the bacterial inner membrane is not without precedent. The major coat protein, pVIII, of Escherichia coli phage f1, fd and M13 is an inner membrane protein that is assembled into phage particles and secreted by a process with similarities to type II and type III transport (Kuhn, 1995; Russel et al., 1997). Packing of the transmembrane helices during oligomerization of pVIII is important for removal of the protein from the lipid membrane and secretion (Kuhn and Wickner, 1985; Williams et al., 1995). Interest ingly, the sequence similarities between DotA and the ColIb-P9 TraY protein are found almost exclusively in membrane-spanning domains and not the exposed periplasmic loops (Wilkins and Thomas, 2000). Furthermore, analysis of sequence variations found for dotA alleles encoded by other L.pneumophila serogroups (Thomas S.Whittam, DDBJ/EMBL/GenBank accession Nos. AF095231, AF095232, AF095233, AF095234, AF095235) indicates that the transmembrane helices are the most invariant regions of the protein. Thus, it is possible that the packing of transmembrane domains in DotA will also play an important role in an assembly process that is necessary for secretion.
It was hypothesized that DotA protein could serve as an inner membrane scaffold upon which other components of the Dot/Icm transporter are assembled (Roy and Isberg, 1997; Roy et al., 1998). The observation that DotA is secreted by L.pneumophila implies a role for this protein that is more intricate. Electron micrographs show that the secreted DotA protein is assembled into a ring-shaped structure with a central channel. Based on these findings, it is attractive to speculate that the DotA protein may be an essential component of a pore that is inserted into target membranes by the Dot/Icm apparatus upon host cell contact. Such a channel may be necessary for translocation of effector molecules into host cells after contact, which would explain why dotA mutant bacteria are defective for all virulence activities requiring transporter function. The observation that formation of pores in eukaryotic membranes and secretion of DotA protein are L.pneumophila virulence traits that do not require IcmS or IcmW function also supports this theory. This hypothesis predicts that DotA protein will interact directly with host membranes upon bacterial export. However, we have been unable to show that this protein can be delivered directly into host cells using a variety of assays that are based on immunodetection. Future efforts to understand the role of DotA protein in host cell pathogenesis will focus on whether the secreted product is a component of a pore-forming complex that can be delivered into eukaryotic host cells.
Materials and methods
Bacterial strains and media
Legionella pneumophila strains used in this study (Table I) were grown on charcoal-yeast extract (CYE) plates or in AYE broth as described previously (Feeley et al., 1979; Roy and Isberg, 1997).
Table I. Legionella pneumophila strains.
| Strain | Relevant genotype | Reference |
|---|---|---|
| CR39 | L.pneumophila serogroup 1 strain LP01 rpsL | Berger and Isberg (1993) |
| CR58 | LP01 ΔdotA | Zuckman et al. (1999) |
| CR341 | LP01 ΔicmQ | Coers et al. (2000) |
| CR343 | LP01 ΔicmR | Coers et al. (2000) |
| CR416 | LP01 ΔicmX (MM101) | Matthews and Roy (2000) |
| CR419 | LP01 ΔdotI (HL056) | Andrews et al. (1998) |
| CR157 | LP01 ΔicmW | Zuckman et al. (1999) |
| CR393 | LP01 ΔicmS | Coers et al. (2000) |
| CR214 | LP01 mspA28 | this study |
| CR206 | LP01 ΔdotA mspA28 | this study |
| CR216 | LP01 ΔicmW mspA28 | this study |
| CR388 | LP01 ΔicmS mspA28 | this study |
| CR821 | LP01 ΔdotI mspA28 | this study |
| CR823 | LP01 ΔicmX mspA28 | this study |
Construction of mspA28 protease mutants
To obtain isogenic L.pneumophila strains that lack the MspA protease, PCR mutagenesis was used to insert a stop codon after nucleotide position 84 in the mspA coding region. Forward primer CR16 (5′-GGGATCCGTCACACAAAAATTTCAGTTG-3′) and reverse primer CR17 (5′-GGATCCGAACAAAAGGCTTATTGGTTT-3′) were used to generate a DNA fragment encoding the C-terminal region of the MspA protein. Forward primer CR22 (5′-CCGAGCTCCAAACCTGGGCACTCCAGG-3′) and reverse primer CR23 (5′-CCGGATCCATTAGATCGGGTCGGCTGCCTTC-3′) were used to generate a DNA fragment encoding the N-terminal region of the MspA protein. The underlined sequence in primer CR23 identifies the position of the inserted TAA stop codon. After BamHI digestion, these two DNA fragments were ligated together in the gene replacement vector pSR47S to generate the mspA28 allele. Only the first 28 amino acid residues of the MspA protein will be produced by bacteria containing the mspA28 allele. Allelic exchange was used to introduce the mspA28 allele onto the chromosome of isogenic LP01 derivatives as described by Zuckman et al. (1999). Recombinant clones containing the mspA28 gene were identified by single-colony PCR.
Antibodies
The affinity-purified rabbit polyclonal antibody raised against the DotA542–557 epitope and the monoclonal antibody mAb2.29 generated against the C-terminal 100 amino acid residues of DotA were as described by Matthews and Roy (2000). A polyclonal antibody that recognizes the L.pneumophila signal peptidase protein was kindly provided by Dr R.Isberg (Tufts University). Antibodies were used at the following dilutions: rabbit polyclonal DotA antibody, 1:10 000; mAb 2.29, 1:100 for immunoblot and 1:10 for electron microscopy; anti-signal peptidase, 1:10 000.
Electrophoresis and immunoblot analysis
SDS–PAGE (7.5–12.5%) was employed throughout this study (Laemmli, 1970). For native PAGE analysis, SDS and reducing agent were omitted from the buffers. For immunoblot analysis, proteins were transferred onto Immobilon-P membrane (Millipore) and probed with antibodies as described by Matthews and Roy (2000).
Protein secretion assay
Legionella pneumophila strains were grown on CYE plates for 2 days at 37°C. Bacteria were recovered from plates and inoculated in 2.5 ml of AYE broth in 25 × 150 mm test tubes. The OD600 of the starting culture was 0.1. Bacteria were grown at 37°C for 20 h with shaking. The OD of the culture was determined and a volume equivalent to 2 OD600 units was removed (∼500 µl). Bacterial cells were pelleted for 15 min in a microfuge. A cleared supernatant fraction was obtained by passing the aqueous phase through a 0.22 µm filter (Millipore) to remove contaminating bacterial cells. The cell pellets were washed once with ice-cold water and suspended in 500 µl of ice-cold water. Each sample was precipitated with 10% (final) trichloroacetic acid (TCA) and dissolved in an appropriate volume of SDS–PAGE sample buffer by vortexing for >30 min at 4°C. Equal sample volumes were separated by SDS–PAGE and proteins were visualized either by gel staining or immunoblot analysis. Unless indicated otherwise, samples were not heat treated prior to loading to prevent aggregation of the DotA protein.
RNA slot-blot analysis
Legionella pneumophila strains were grown as described above. Total RNA from an estimated 4 × 109 bacteria was isolated using the RNeasy Mini Kit (Qiagen). Total RNAs (10 µg) were denatured by incubating at 65°C for 15 min in a total of 150 µl of 40 mM MOPS pH 7.0, 10 mM sodium acetate, 1 mM EDTA, 50% formamide and 6% formaldehyde, then quickly chilled in an ice–water bath and mixed with an equal volume of ice-cold 20× SSC. Samples (180 µl) containing the indicated amounts of total RNA were aspirated onto Immobilon-Ny+ membranes (Millipore) using a Hybri-slot manifold (BRL). Each slot was washed twice with 1 ml of 10× SSC. A probe containing the entire dotA coding region was labeled with 32P using the Rediprime II kit (Amersham Pharmacia Biotech). Hybridization was carried out in 50% formamide, 5× SSC, 5× Denhardt’s solution, 0.5% SDS and 0.1 mg/ml salmon sperm DNA at 42°C overnight. Stringent washes were carried out in 0.1× SSC, 0.1% SDS at 68°C.
Purification and analysis of the extracellular DotA complex
Legionella pneumophila strains were cultured as described above, but 100 tubes were employed so that a total of 250 ml of culture volume was obtained. After clearing bacteria by centrifugation at 7300 g for 15 min, phenylmethylsulfonyl fluoride (PMSF; final concentration 0.25 mM) and PEG 3350 [final concentration 16% (w/w)] were added to the fractionated supernatant and incubated at 4°C overnight. Precipitates were collected by centrifugation at 7300 g for 15 min at 4°C. The precipitate was extracted twice with 2.5 ml of HE buffer (10 mM HEPES pH 7.4, 1 mM EDTA) and the dissolved material was applied to a two-step sucrose gradient consisting of 0.8 ml of a 60% (w/w) sucrose cushion and 2.4 ml of 25% (w/w) sucrose in HE buffer. After ultracentrifugation for 17 h at 40 000 r.p.m. in a SW50.1 rotor at 4°C, complexes containing the DotA protein were isolated at the 25–60% sucrose interface. Material at this interface was extracted and the total sample volume was adjusted to 1 ml using HE buffer. The extracted material was loaded onto a sucrose step gradient consisting of a cushion containing 0.8 ml of 60% (w/w) sucrose followed by sequential layers consisting of 2.4 ml of 50, 45, 40% sucrose, 2.1 ml of 35% sucrose and 1.6 ml of 30% sucrose in HE buffer. Separation was carried out in a SW40Ti rotor at 36 000 r.p.m. for 17 h at 4°C. Fractions of ∼250 µl each were collected from the top of the tube using an AUTO DENSI-FLOW collector (Labconco). For native gel analysis, 150 µl of the peak fraction containing DotA were concentrated by centrifugation on microcon-100 (Millipore), then mixed with an equal volume of 2× native sample buffer. To estimate the molecular weight of the DotA complex, 10 µl of the concentrate protein solution were mixed with 2× native sample buffer, then analyzed on 7, 6, 5.5 and 5% native gels. The retardation coefficient was calculated by plotting 100log(100Rf) against the acrylamide concentration (where Rf is relative mobility) and determining the negative slope of this line. The retardation coefficients of apoferritin (monomer 450 kDa and dimer 900 kDa), urease (trimer 272 kDa and hexamer 545 kDa), bovine serum albumin (BSA; monomer 66 kDa and dimer 132 kDa), carbonic anhydrase (29 kDa) and α-lactalbumin (14 kDa) were determined in parallel to construct a Ferguson plot (Ferguson, 1964). This was used to estimate the molecular weight of the DotA complex. Following 6% native PAGE, the band containing the DotA complex was excised from the gel and proteins were electroeluted in buffer containing 50 mM NH4HCO3 and 0.1% SDS using a Model 422 Electro-eluter (Bio-Rad). Elution was carried out at 10 mA for 3 h at 4°C, followed by electrodialysis in elution buffer without SDS for 1 h. The eluate was lyophilized in a Speed-Vac concentrator (Savant), dissolved in sample buffer and the proteins identified by SDS–PAGE.
Electron microscopy
Protein samples obtained from sucrose density gradients (5 µl) were negatively stained with 2% phosphotungstic acid pH 7.4 on carbon-coated 200 mesh copper grids (CF200-Cu; Electron Microscopy Science) and observed under an EM410 electron microscope (Philips). Micrographs were taken at an accelerating voltage of 80 kV. Antibody labeling was carried out as described by Homma et al. (1984).
Legionella pneumophila membrane fractionation
Sucrose gradient fractionation of inner and outer membranes from L.pneumophila strain CR214 was carried out as described by Roy and Isberg (1997). The only modification being that the starting culture volume was reduced to 25 ml.
Acknowledgments
Acknowledgements
We thank Dr Ralph Isberg at Tufts University for kindly providing signal peptidase I antibody, Veronica Novik for constructing strains CR821 and CR823, and Jonathan Kagan for critical reading of the manuscript and helpful discussions. This work was supported by NIH Grant AI41699.
References
- Andrews H.L., Vogel,J.P. and Isberg,R.R. (1998) Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect. Immun., 66, 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K.H. and Isberg,R.R. (1993) Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol., 7, 7–19. [DOI] [PubMed] [Google Scholar]
- Berger K.H., Merriam,J.J. and Isberg,R.R. (1994) Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol. Microbiol., 14, 809–822. [DOI] [PubMed] [Google Scholar]
- Brand B.C., Sadosky,A.B. and Shuman,H.A. (1994) The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol. Microbiol., 14, 797–808. [DOI] [PubMed] [Google Scholar]
- Christie P.J. (1997) Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in Eubacteria. J. Bacteriol., 179, 3085–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P.J. (2001) Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol. Microbiol., 40, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P.J. and Vogel,J.P. (2000) Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol., 8, 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D.L., Lee,B.Y. and Horwitz,M.A. (2000) Deviant expression of rab5 on phagosomes containing the intracellular pathogens Myco bacterium tuberculosis and Legionella pneumophila is associated with altered phagosomal fate. Infect. Immun., 68, 2671–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coers J., Kagan,J.C., Matthews,M., Nagai,H., Zuckman,D.M. and Roy, C.R. (2000) Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol. Microbiol., 38, 719–736. [DOI] [PubMed] [Google Scholar]
- Feeley J.C., Gibson,R.J., Gorman,G.W., Langford,N.C., Rasheed,J.K., Mackel,D.C. and Blaine,W.B. (1979) Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila.J. Clin. Microbiol., 10, 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekkes P. and Driessen,A.J. (1999) Protein targeting to the bacterial cytoplasmic membrane. Microbiol. Mol. Biol. Rev., 63, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K.A. (1964) Starch-gel electrophoresis—application to the classification of pituitary proteins and polypeptides. Metabolism, 13, 985–1002. [DOI] [PubMed] [Google Scholar]
- Galan J.E. and Collmer,A. (1999) Type III secretion machines: bacterial devices for protein delivery into host cells. Science, 284, 1322–1328. [DOI] [PubMed] [Google Scholar]
- Hales L.M. and Shuman,H.A. (1999) Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect. Immun., 67, 3662–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Kutsukake,K., Iino,T. and Yamaguchi,S. (1984) Hook-associated proteins essential for flagellar filament formation in Salmonella typhimurium. J. Bacteriol., 157, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstman A.L. and Kuehn,M.J. (2000) Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem., 275, 12489–12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M.A. (1983) The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome lysosome fusion in human monocytes. J. Exp. Med., 158, 2108–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueck C.J. (1998) Type III protein secretion systems in bacterial patho gens of animals and plants. Microbiol. Mol. Biol. Rev., 62, 379–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J.E., Vogel,J.P., Andrews,H.L. and Isberg,R.R. (1998) Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol., 27, 323–336. [DOI] [PubMed] [Google Scholar]
- Komano T., Yoshida,T., Narahara,K. and Furuya,N. (2000) The transfer region of IncI1 plasmid R64: similarities between R64 tra and Legionella icm/dot genes. Mol. Microbiol., 35, 1348–1359. [DOI] [PubMed] [Google Scholar]
- Kubori T., Matsushima,Y., Nakamura,D., Uralil,J., Lara-Tejero,M., Sukhan,A., Galan,J.E. and Aizawa,S.I. (1998) Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science, 280, 602–605. [DOI] [PubMed] [Google Scholar]
- Kubori T., Sukhan,A., Aizawa,S.I. and Galan,J.E. (2000) Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc. Natl Acad. Sci. USA, 97, 10225–10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A. (1995) Major coat proteins of bacteriophage Pf3 and M13 as model systems for Sec-independent protein transport. FEMS Microbiol. Rev., 17, 185–190. [DOI] [PubMed] [Google Scholar]
- Kuhn A. and Wickner,W. (1985) Isolation of mutants in M13 coat protein that affect its synthesis, processing and assembly into phage. J. Biol. Chem., 260, 15907–15913. [PubMed] [Google Scholar]
- Laemmli E.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Linton K.J. and Higgins,C.F. (1998) The Escherichia coli ATP-binding cassette (ABC) proteins. Mol. Microbiol., 28, 5–13. [DOI] [PubMed] [Google Scholar]
- Matthews M. and Roy,C.R. (2000) Identification and subcellular localization of the Legionella pneumophila IcmX protein: a factor essential for establishment of a replicative organelle in eukaryotic host cells. Infect. Immun., 68, 3971–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetzel M., Dalbey,R.E. and Strynadka,N.C. (2000) The structure and mechanism of bacterial type I signal peptidases. A novel antibiotic target. Pharmacol. Ther., 87, 27–49. [DOI] [PubMed] [Google Scholar]
- Plano G.V., Day,J.B. and Ferracci,F. (2001) Type III export: new uses for an old pathway. Mol. Microbiol., 40, 284–293. [DOI] [PubMed] [Google Scholar]
- Pugsley A.P. (1993) The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev., 57, 50–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell M. and Shuman,H.A. (1998) The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect. Immun., 66, 2245–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn F.D., Keen,M.G. and Tompkins,L.S. (1989) Genetic, immuno logical and cytotoxic comparisons of Legionella proteolytic activities. Infect. Immun., 57, 2719–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C.R. and Isberg,R.I. (1997) Topology of Legionella pneumophila DotA: an inner membrane protein required for replication in macrophages. Infect. Immun., 65, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C.R., Berger,K. and Isberg,R.R. (1998) Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol., 28, 663–674. [DOI] [PubMed] [Google Scholar]
- Russel M., Linderoth,N.A. and Sali,A. (1997) Filamentous phage assembly: variation on a protein export theme. Gene, 192, 23–32. [DOI] [PubMed] [Google Scholar]
- Sandkvist M. (2001) Biology of type II secretion. Mol. Microbiol., 40, 271–283. [DOI] [PubMed] [Google Scholar]
- Segal G. and Shuman,H.A. (1997) Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect. Immun., 65, 5057–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal G. and Shuman,H.A. (1999) Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii. Mol. Microbiol., 33, 669–670. [DOI] [PubMed] [Google Scholar]
- Segal G., Purcell,M. and Shuman,H.A. (1998) Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl Acad. Sci. USA, 95, 1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M.S. and Isberg,R.R. (1995) Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun., 63, 3609–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto L. and Shuman,H.A. (1990) The Legionella pneumophila major secretory protein, a protease, is not required for intracellular growth or cell killing. Infect. Immun., 58, 2585–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.P., Roy,C. and Isberg,R.R. (1996) Use of salt to isolate Legionella pneumophila mutants unable to replicate in macrophages. Ann. N. Y. Acad. Sci., 797, 271–272. [DOI] [PubMed] [Google Scholar]
- Vogel J.P., Andrews,H.L., Wong,S.K. and Isberg,R.R. (1998) Conju gative transfer by the virulence system of Legionella pneumophila. Science, 279, 873–876. [DOI] [PubMed] [Google Scholar]
- Wiater L.A., Dunn,K., Maxfield,F.R. and Shuman,H.A. (1998) Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect. Immun., 66, 4450–4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B.M. and Thomas,A.T. (2000) DNA-independent transport of plasmid primase protein between bacteria by the I1 conjugation system. Mol. Microbiol., 38, 650–657. [DOI] [PubMed] [Google Scholar]
- Williams K.A., Glibowicka,M., Li,Z., Li,H., Khan,A.R., Chen,Y.M., Wang,J., Marvin,D.A. and Deber,C.M. (1995) Packing of coat protein amphipathic and transmembrane helices in filamentous bacteriophage M13: role of small residues in protein oligomerization. J. Mol. Biol., 252, 6–14. [DOI] [PubMed] [Google Scholar]
- Zuckman D.M., Hung,J.B. and Roy,C.R. (1999) Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol. Microbiol., 32, 990–1001. [DOI] [PubMed] [Google Scholar]