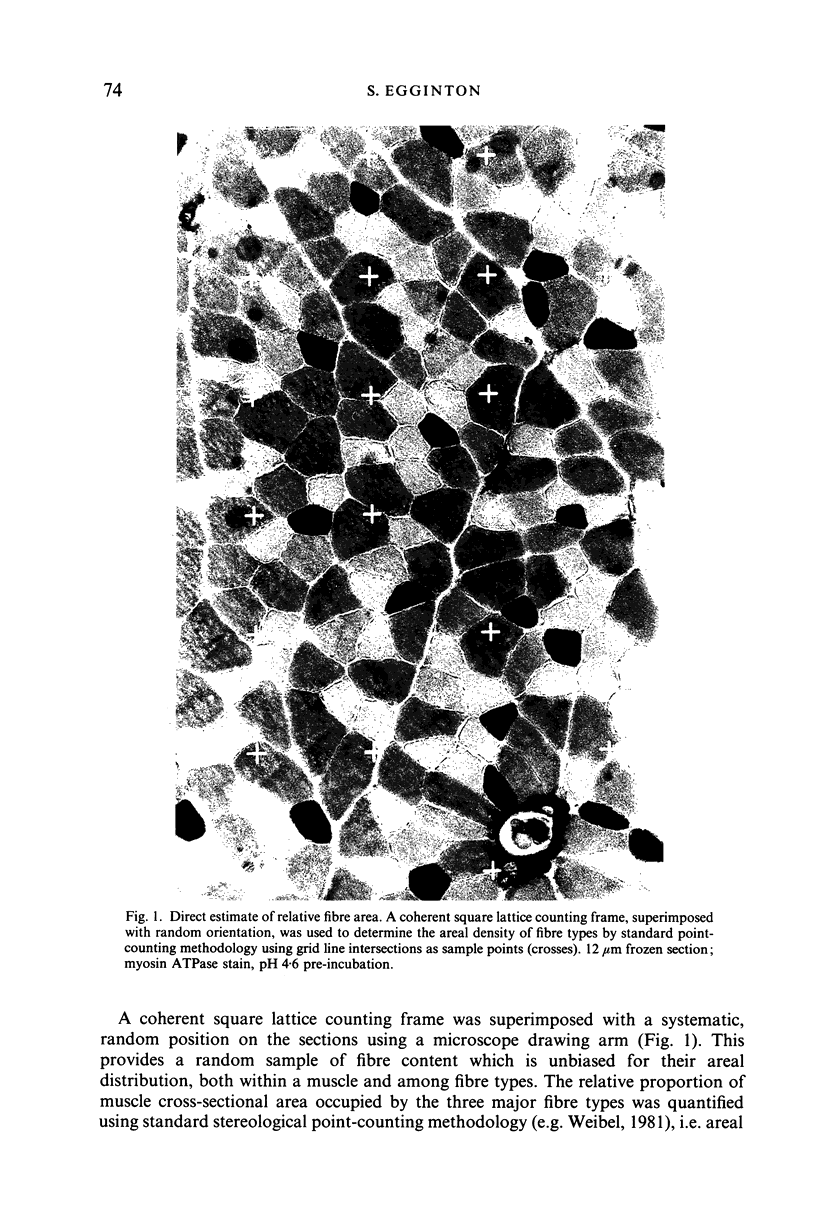
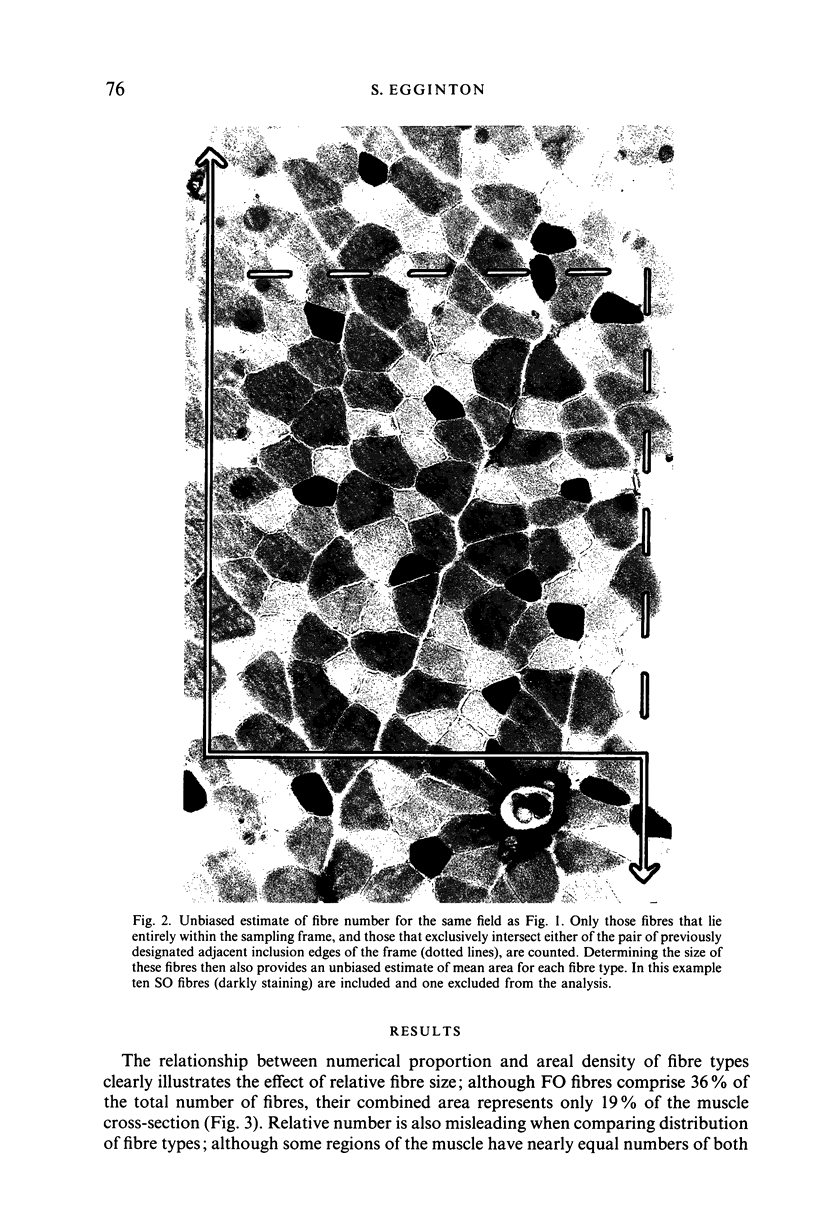
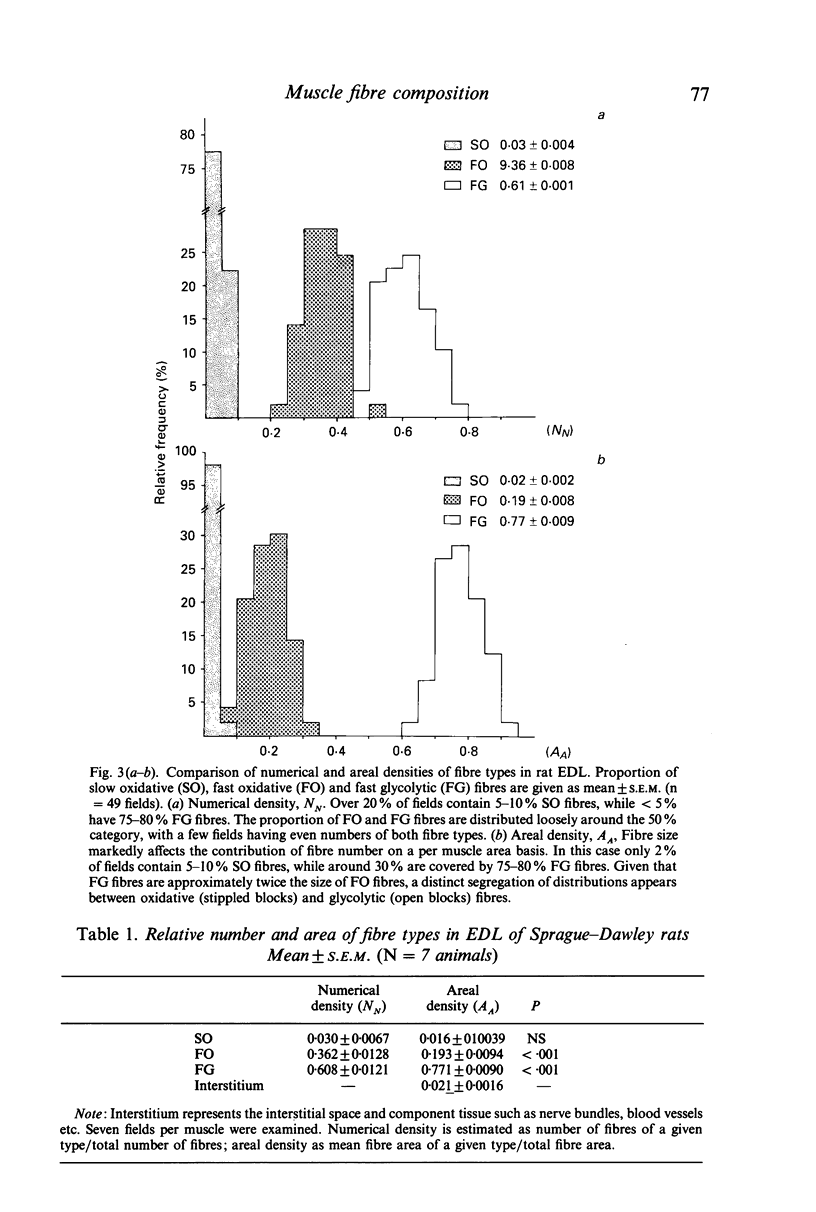
Abstract
The composition of a mixed fast skeletal muscle (rat extensor digitorum longus) was examined to quantify the difference between the relative number of the three major fibre types in a representative muscle and their relative contribution to muscle cross-section, i.e. numerical (NN) and areal (AA) densities, respectively. These two indices clearly differ in their physiological relevance. While the former may be useful in describing hyperplasia, the latter allows for differences in size among fibre types. When estimated as NN, over 20% of fields contained 5-10% SO fibres and less than 5% had 75-80% FG fibres. In contrast, only 2% of fields had an AA of 5-10% for SO fibres while around 30% contained 75-80% FG fibres. The importance of a direct method for estimating AA is emphasised, as an indirect approach may have an error of 20-30% when used for oxidative fibre types. The use of an unbiased sampling regime to minimise error in determining both numerical and areal densities of different fibre types is illustrated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. B., Laughlin M. H. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol. 1983 Nov;344:189–208. doi: 10.1113/jphysiol.1983.sp014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. B., Phelps R. O. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984 Nov;171(3):259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Trophic functions of the neuron. II. Denervation and regulation of muscle. The use and abuse of muscle histochemistry. Ann N Y Acad Sci. 1974 Mar 22;228(0):121–144. doi: 10.1111/j.1749-6632.1974.tb20506.x. [DOI] [PubMed] [Google Scholar]
- Egginton S. Effects of an anabolic hormone on aerobic capacity of rat striated muscle. Pflugers Arch. 1987 Nov;410(4-5):356–361. doi: 10.1007/BF00586511. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Parsons D., Oakley C. R. Differentiation of fiber types in skeletal muscle from the sequential inactivation of myofibrillar actomyosin ATPase during acid preincubation. Histochemistry. 1983;77(4):543–555. doi: 10.1007/BF00495808. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Spamer C., Pette D. Activity patterns of phosphofructokinase, glyceraldehydephosphate dehydrogenase, lactate dehydrogenase and malate dehydrogenase in microdissected fast and slow fibres from rabbit psoas and soleus muscle. Histochemistry. 1977 Jun 8;52(3):201–216. doi: 10.1007/BF00495857. [DOI] [PubMed] [Google Scholar]