Abstract
The protein brain-derived neurotrophic factor (BDNF) has been postulated to be a retrograde or paracrine synaptic messenger in long-term potentiation and other forms of activity-dependent synaptic plasticity. Although crucial for this concept, direct evidence for the activity-dependent synaptic release of BDNF is lacking. Here we investigate secretion of BDNF labelled with green fluorescent protein (BDNF–GFP) by monitoring the changes in fluorescence intensity of dendritic BDNF–GFP vesicles at glutamatergic synaptic junctions of living hippocampal neurons. We show that high-frequency activation of glutamatergic synapses triggers the release of BDNF–GFP from synaptically localized secretory granules. This release depends on activation of postsynaptic ionotropic glutamate receptors and on postsynaptic Ca2+ influx. Release of BDNF–GFP is also observed from extrasynaptic dendritic vesicle clusters, suggesting that a possible spatial restriction of BDNF release to specific synaptic sites can only occur if the postsynaptic depolarization remains local. These results support the concept of BDNF being a synaptic messenger of activity-dependent synaptic plasticity, which is released from postsynaptic neurons.
Keywords: BDNF–GFP/glutamatergic synapse/hippocampus/secretion/synaptic plasticity
Introduction
Long-term potentiation (LTP) is a widely accepted model of activity-dependent synaptic plasticity of excitatory synapses. It is believed to underlie memory formation and use-dependent reorganization of synaptic connections in the mammalian brain (Bliss and Collingridge, 1993). Induction of LTP at glutamatergic synapses in the hippocampus is typically induced by high-frequency presynaptic activation, which triggers a critical level of postsynaptic Ca2+ influx. For presynaptic LTP-induced changes to occur, the successful postsynaptic induction of LTP has to be communicated to the presynaptic neuron. Similarly, postsynaptic changes during LTP could result from autocrine/paracrine actions of such synaptic messengers. Among other molecules, brain-derived neurotrophic factor (BDNF) has been proposed to serve this role of an activity-dependent retrograde synaptic messenger in LTP and other forms of activity-dependent synaptic plasticity, which is secreted locally at frequently used glutamatergic synapses, thus inducing changes leading to enhanced synaptic efficacy (see for example Thoenen, 1995; Bonhoeffer, 1996; Katz and Shatz, 1996).
The protein family of neurotrophins [consisting of nerve growth factor (NGF), BDNF, NT-3, NT-4/5, NT-6 and NT-7] is known to regulate the survival and differentiation of peripheral nervous system (PNS) and central nervous system (CNS) neurons (Lewin and Barde, 1996). In recent years, evidence has accumulated that BDNF plays an additional important role in hippocampal synaptic plasticity (Lessmann et al., 1994; Kang and Schuman, 1995; Levine et al., 1995), by either facilitating transmitter release from presynaptic terminals (Figurov et al., 1996; Carmignoto et al., 1997; Lessmann and Heumann, 1998; Li et al., 1998) or enhancing postsynaptic N-methyl-d-aspartate (NMDA) receptor function (Levine et al., 1998). Since LTP is impaired in the hippocampus of BDNF knockout mice (Korte et al., 1995; Patterson et al., 1996), these data suggest that BDNF is among the candidate molecules that can mediate activity-dependent synaptic plasticity in the mammalian CNS.
However, this model of BDNF as a retrograde or paracrine messenger in synaptic plasticity requires activity-dependent postsynaptic release of this neurotrophin upon high-frequency synaptic stimulation. This fundamental hypothesis has never been proved directly. Neuronal release of BDNF and NGF has been investigated previously by western blot and enzyme-linked immunosorbent assay (ELISA) analysis of cell culture supernatants (Blöchl and Thoenen, 1995; Goodman et al., 1996; Canossa et al., 1997; Balkowiec and Katz, 2000), by immunocytochemical detection of endocytotic uptake of brain-derived neurotrophic factor tagged with green fluorescent protein (BDNF–GFP) (Kohara et al., 2001) and by detection of long-term changes in BDNF–GFP fluorescence in hippocampal neurons (Kojima et al., 2001). However, measurements of the real-time kinetics of BDNF secretion from synaptic sites have not been performed previously, and the necessary synaptic activity levels to elicit BDNF secretion and the role of Ca2+ influx in the synaptic release process of neurotrophins remained elusive.
Here we have expressed a BDNF–GFP fusion protein in microcultures of rat hippocampal neurons to monitor BDNF secretion in real-time as the decrease in fluorescence intensity of BDNF–GFP vesicle clusters at live glutamatergic synapses. Our results show that high-frequency synaptic stimulation elicits release of BDNF from postsynaptic structures of glutamatergic synapses. This release process is critically dependent on the postsynaptic influx of Ca2+ via ionotropic glutamate receptors or voltage-gated Ca2+ channels, thus validating the concept of BDNF being a possible retrograde or paracrine messenger in synaptic plasticity.
Results
Targeting of BDNF–GFP to dendrites and glutamatergic synapses
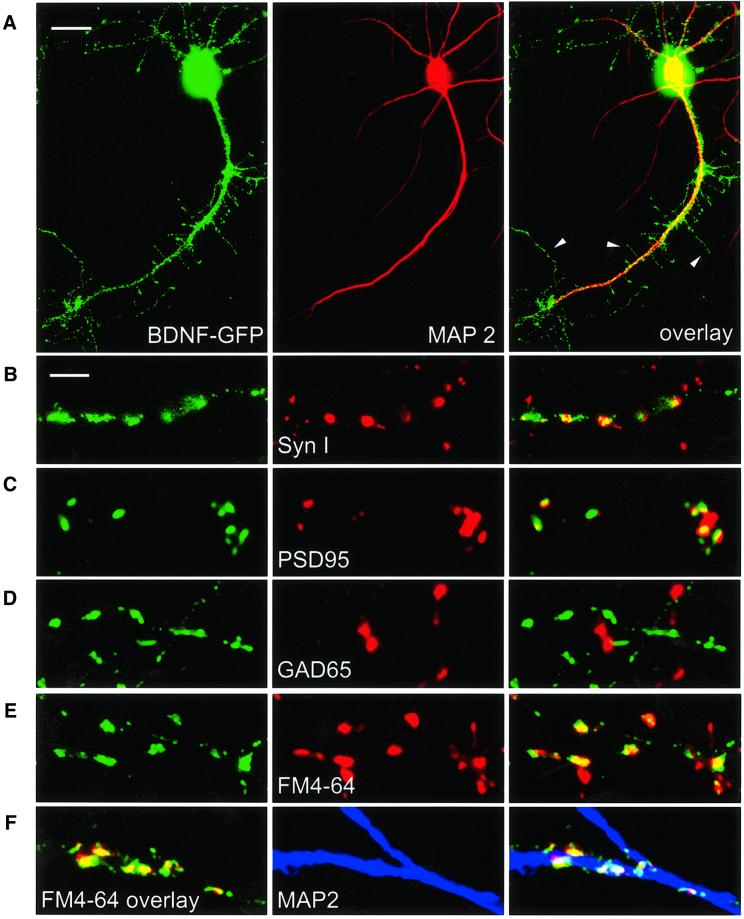
The expression of BDNF–GFP in cortical neurons yields a protein with biochemical properties that are indistinguishable from those of wild-type BDNF, including storage of the protein in secretory granules (Haubensak et al., 1998). Following transfection of primary hippocampal neurons with the BDNF–GFP construct, we observed prominent dendritic targeting (judged by co-localization with the dendritic marker protein MAP2; Figure 1A) of BDNF–GFP-containing secretory granules in all neurons investigated. The numerous thin, abruptly ending MAP2-negative processes sprouting from the primary dendrites (arrowheads in Figure 1A) are dendritic filopodia, which are prominently seen in differentiating hippocampal neurons (Kaech et al., 1997; Fiala et al., 1998). Quantification of MAP2 staining and of BDNF–GFP fluorescence, respectively, in the processes of individual cells (excluding the soma) revealed a dendritic origin of 97 ± 2% of the BDNF–GFP fluorescence in all neurons investigated (n = 23), suggesting that the vast majority of BDNF–GFP vesicles were present in dendrites.
Fig. 1. Synaptic targeting of BDNF–GFP. Immunofluorescent detection of the dendritic marker protein MAP2 (A), the general presynaptic marker protein Synapsin I (B), the marker protein PSD95 for glutamatergic synapses (C) and the marker protein GAD65 for GABAergic synapses (D), respectively. (E) Identification of presynaptic terminals in living neurons using activity-dependent labelling with FM 4-64. In (A–E), each panel shows dendrites of a BDNF–GFP-expressing hippocampal neuron (left), the fluorescence image for the respective marker (middle) and the overlay of both (right). (F) Left: live staining of FM 4-64-labelled synaptic terminals (red) of a BDNF–GFP (green)-expressing neuron shows synaptic localization of BDNF–GFP (yellow). Middle: posthoc labelling of dendrites using MAP2 antibody (blue). The overlay on the right shows co-localization of the three signals in white. Scale bars: 10 µm (A), 4 µm (B–F). The arrowheads in (A) depict dendritic filopodia.
Using immunocytochemistry with antibodies detecting a general presynaptic marker (Synapsin I, Figure 1B) and a marker of glutamatergic synapses (PSD95, Figure 1C), respectively, we show targeting of BDNF–GFP secretory vesicle clusters (i.e. fluorescent ellipsoids with long axis >2 µm) to glutamatergic synaptic junctions of hippocampal neurons. Immunocytochemical detection with a GAD65 antibody (GABAergic presynaptic marker) did not reveal targeting of BDNF–GFP vesicles to GABAergic synapses in our cultures (Figure 1D). To reveal synaptic localization of BDNF–GFP secretory granules in living cells, we labelled active presynaptic terminals with the styryl dye FM 4-64 (Betz et al., 1996). This method allows us to identify synaptic BDNF–GFP vesicle clusters via co-localization with FM 4-64 (Figure 1E). FM 4-64 staining followed by MAP2 immunocytochemistry of the same region of a BDNF–GFP-expressing cell (Figure 1F) suggests that the synaptic BDNF–GFP vesicle clusters are localized in dendrites.
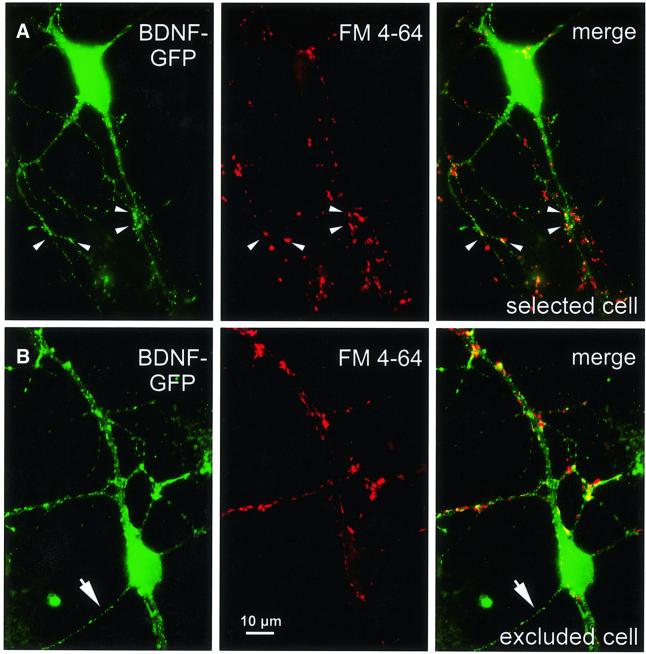
To investigate the release of BDNF–GFP from synapses, we selected these FM 4-64-labelled dendritic BDNF–GFP vesicle clusters. Microcultures with only one of the neurons being transfected were chosen to allow assignment of green processes to the transfected neuron under inspection. In addition, only those neurons that did not show BDNF–GFP fluorescence in a long soma-derived axon-like process were selected for release experiments (Figure 2A), whereas cells with a BDNF–GFP-containing axon (Figure 2B) were excluded. Thus, co-localization of BDNF–GFP and FM 4-64 signals resulting from presynaptic BDNF–GFP in the axon was highly unlikely.
Fig. 2. Cell selection for BDNF–GFP release experiments. (A) BDNF–GFP fluorescence (left), FM 4-64 labelling of presynaptic terminals (middle) and merged pictures (right) of a typical hippocampal neuron selected for release experiments. All four soma-derived processes are dendrites, as is evident from the large diameter of the proximal processes and from the FM 4-64-labelled terminals approaching them. Arrowheads depict synaptic BDNF–GFP clusters used for release measurements. For these clusters, postsynaptic localization is very likely because a BDNF–GFP-containing axon is not present. (B) Sequence of images as in (A), but from a neuron that was excluded from release experiments: the thin, soma-derived process (arrow), which is not contacted by FM 4-64-labelled terminals, is the presumed axon of the cell and contains BDNF–GFP fluorescence. Such cells were excluded because of possible additional axonal localization of BDNF–GFP. Scale bar: 10 µm.
Synaptic release of BDNF–GFP by K+-induced depolarization
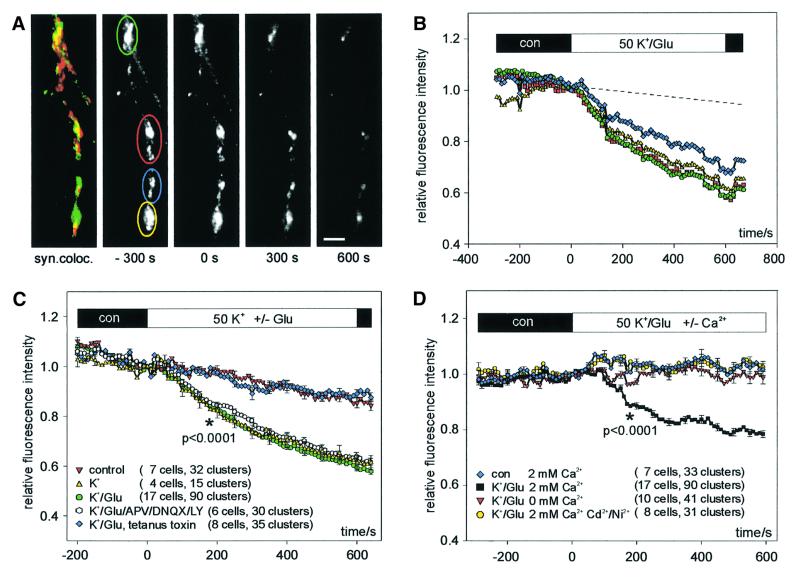
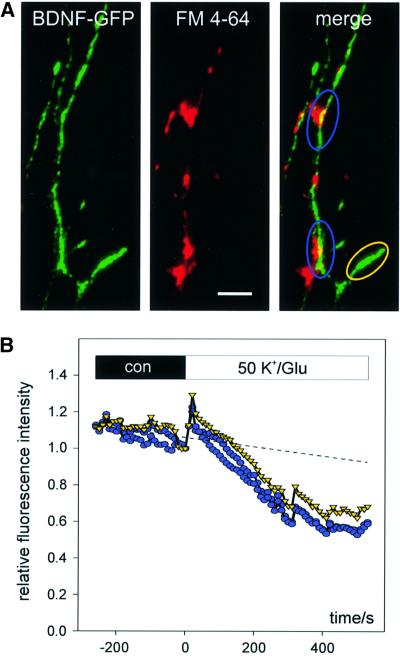
The basic properties of synaptic release of BDNF–GFP were investigated with high K+ (50 mM)- and l-glutamate (300 µM)-induced depolarization in physiological saline (2 mM Ca2+, 1 mM Mg2+) to provoke efficient Ca2+ influx together with activation of glutamate receptors. The cells were continuously superfused with a laminar jet of extracellular solution (Lessmann and Dietzel, 1995) and the average fluorescence intensity (relative to time of change of solution, t = 0) of synaptic BDNF–GFP vesicle clusters (Figure 3A) was plotted versus time. Application of K+/glutamate (K+/Glu) solution (Figure 3B) induced a graded decrease in fluorescence intensity that became apparent within the first 2 min after solution exchange. The best fit of the time course of fluorescence decrease was obtained with a monoexponential function using a time constant of 300 ± 24 s. On average (Figure 3C, 90 clusters of 17 cells), 10 min after K+/Glu application, fluorescence intensities had decreased to 59 ± 2% relative to values just prior to drug application (t = 0). This value was significantly different (p <0.0001) from the 83 ± 2% remaining fluorescence intensity in unstimulated control cells (33 clusters of seven cells).
Fig. 3. Monitoring depolarization-induced synaptic secretion of BDNF–GFP as a decrease in fluorescence intensity. (A) Synaptic co-localization (yellow) of BDNF–GFP (green) with FM 4-64 (red). Monochrome images show original BDNF–GFP fluorescence at the time points indicated. Scale bar: 5 µm. (B) Relative change (standardized to t = 0, start of stimulation) of averaged BDNF–GFP fluorescence intensities of colour-coded regions of interest marked in (A) upon application of 50 mM K+ and 300 µM l-glutamate in physiological saline (2 mM Ca2+, 1 mM Mg2+). The dashed line is extrapolated from photobleaching observed during 5 min of control superfusion (see Materials and methods). (C) Time course of the average fluorescence decrease of BDNF–GFP clusters using the different stimulation solutions as indicated. All solutions contained 2 mM Ca2+ and 1 mM Mg2+. dl-APV (200 µM), DNQX (20 µM) and LY 341495 (100 µM) were added to one group of cells to inhibit ionotropic and metabotropic glutamate receptors. Tetanus toxin: BDNF–GFP-expressing neurons were pre-incubated with tetanus toxin (1 nM, 20 h) prior to recording. The asterisk represents the starting point of significant difference between ‘control’ and ‘K+/glu’ at the p-level indicated. (D) Release of BDNF–GFP was blocked in the absence of extracellular Ca2+ and by inhibiting VGCC with Ni2+ (100 µM) and Cd2+ (100 µM). Average change in fluorescence intensity of BDNF–GFP clusters from different groups of cells as indicated. Mg2+ concentration was 1 mM, except for ‘K+/Glu/0 Ca2+’ (3 mM Mg2+). The asterisk represents the starting point of significant difference between K+/Glu ± 2 mM Ca2+ at the p-level indicated. All traces were corrected for photobleaching by subtraction of the fluorescence decrease in negative controls (0 mM Ca2+, 3 mM Mg2+, 5 mM EGTA).
To correlate the fluorescence decrease directly with the release of BDNF–GFP from secretory vesicles, we pre-incubated cells with tetanus toxin, which specifically blocks fusion of transmitter vesicles and secretory granules with the plasma membrane (see for example Ahnert-Hilger and Bigalke, 1995). Pretreatment with tetanus toxin (1 nM) completely abolished the K+/Glu-induced fluorescence decrease (Figure 3C; remaining fluorescence intensity 87 ± 2%, n = 8 cells), strongly suggesting that the observed fluorescence decrease in the absence of tetanus toxin reflects release of BDNF–GFP from secretory vesicles into the extracellular medium. This interpretation is corroborated by the detection of BDNF–GFP immunoreactivity in the supernatant of our stimulated cultures (50 mM KCl, 40 min): the level of BDNF–GFP was 5.8 ± 0.8 pg/ml in the presence of Ca2+, compared with 3.3 ± 1.3 pg/ml in the absence of Ca2+ (significantly different, p <0.05, n = 5 independent experiments). Taken together, these results suggest that the observed stimulus-induced fluorescence decrease indeed reflects release of BDNF–GFP from secretory vesicles.
To provide additional evidence that the loss of BDNF–GFP fluorescence does not result from excitotoxic damage of the plasma membrane, cell viability was determined using a propidium iodide exclusion assay (see Materials and methods). Following 10 min of K+/Glu stimulation, 99 ± 6% of the neurons excluded the dye, compared with 93 ± 4% in buffer-treated controls (not significantly different with p >0.15). Likewise, even 24 h after 10 min of depolarization, neuron survival was unaffected (not shown). These results exclude the possibility that the decline in BDNF–GFP fluorescence was due to loss of membrane integrity.
To characterize the minimal requirements to elicit release, we depolarized the neurons by high K+ alone (50 mM, without added glutamate). This paradigm was as effective as K+/Glu stimulation in releasing BDNF–GFP (Figure 3C, fluorescence decrease to 61 ± 3%, n = 15 clusters in four cells). To exclude the possibility that additional glutamate receptor activation (which could still occur by endogenously released glutamate upon high K+ application) contributes to elicit release of BDNF–GFP, we stimulated release with K+/Glu in the presence of antagonists of NMDA receptors [200 µM d,l-2-aminophosphonovaleric acid (APV)], α-amino-3-hydroxy-5-methyl-4-isoazolepropionate (AMPA) receptors [20 µM 6,7-dinitroquinoxaline-2,3-dione (DNQX)], and metabotropic glutamate receptors type I–III (100 µM LY 341495; Bortolotto et al., 1999). This treatment changed neither the kinetics nor the extent of BDNF–GFP secretion (fluorescence decrease to 64 ± 2%, six cells, 30 clusters; Figure 3C), indicating that, upon general K+-induced depolarization, additional activation of glutamate receptors (e.g. as a cofactor) is not necessary to release BDNF–GFP.
Depolarization-induced vesicular release is generally believed to depend on the entry of Ca2+ from the extracellular space (Lim et al., 1990; Thomas et al., 1990), whereas the role of extracellular Ca2+ in the release of neurotrophins is still unclear (see for example Blöchl and Thoenen, 1995; Goodmann et al., 1996). Thus, we investigated whether release of BDNF–GFP was inhibited in the absence of extracellular Ca2+ (0 mM Ca2+, 3 mM Mg2+, 5 mM EGTA; n = 10 cells) or in the presence of inhibitors of voltage-gated Ca2+ channels (VGCC; 100 µM Cd2+, 100 µM Ni2+ in 2 mM Ca2+, 1 mM Mg2+; n = 8 cells). Both treatments completely blocked the release of BDNF–GFP, and the remaining fluorescence levels after 10 min of stimulation were indistinguishable from those of unstimulated controls (Figure 3D). This dependence on extracellular Ca2+ was further supported in a set of experiments (n = 3 cells) where successive stimulations of individual cells in the absence and presence of extracellular Ca2+, respectively, yielded release of BDNF–GFP only when Ca2+ was available (data not shown). This Ca2+ dependence is not an indirect effect simply reflecting the need for Ca2+-dependent glutamate release (which subsequently initiates BDNF secretion), since K+/Glu-induced release of BDNF–GFP does not rely on glutamate receptor stimulation (see above). Thus, Ca2+ influx is directly involved in the BDNF release process itself (see also Figure 6). Because of this strict dependence on extracellular Ca2+, we did not investigate further possible additional contributions of Ca2+ release from intracellular stores.
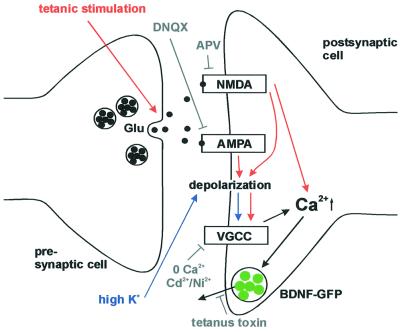
Fig. 6. Synaptic secretion of BDNF–GFP by different routes of postsynaptic depolarization. Tetanic presynaptic stimulation leads, via intermediate repetitive glutamate release, to postsynaptic depolarization and Ca2+ influx (red route). This depolarization can be blocked by ionotropic glutamate receptor antagonists (see Figure 5). In contrast, high K+ solution directly depolarizes the postsynaptic membrane (blue route), activates VGCCs, and can thus bypass intermediate glutamate release and activation of postsynaptic glutamate receptors (see Figure 3). The observed inhibition of BDNF release by blocking Ca2+ influx (0 Ca2+; Ni2+/Cd2+) and the independence of BDNF release from glutamate receptors (both during stimulation with high K+) demonstrate the direct dependence of BDNF secretion on postsynaptic Ca2+ influx. Likewise, since K+-induced postsynaptic depolarization is independent of glutamate release, the inhibition of high K+-induced BDNF secretion by tetanus toxin indicates that BDNF secretion depends on the fusion of secretory vesicles.
To examine whether release of BDNF–GFP is restricted to synaptic sites, we performed a series of experiments where release of BDNF–GFP from synaptic and extrasynaptic clusters (lacking co-localization with FM 4-64), respectively, was compared in the same cells. As depicted in Figure 4, depolarization with K+/Glu yielded efficient release of BDNF–GFP also from extrasynaptic sites in all the cells investigated (n = 6). Since vesicular axonal release is restricted to active (and thus FM 4-64-labelled) presynaptic terminals, which obviously lack these extrasynaptic BDNF–GFP clusters (see the yellow marked region in Figure 4A), this extrasynaptic release provides additional evidence that our observed secretion of BDNF–GFP takes place from dendrites.
Fig. 4. Simultaneous release of BDNF–GFP from postsynaptic and extrasynaptic dendritic locations. (A) BDNF–GFP fluorescence (left), FM 4-64 labelling of presynaptic terminals (middle) and merged images (right) obtained from a dendritic branch of a transfected neuron. Synaptic (blue) and extrasynaptic (yellow) BDNF–GFP vesicle clusters are marked. Scale bar: 5 µm. (B) The time course of fluorescence decrease upon stimulation with K+/Glu for colour-coded regions shown in (A) reveals a comparable degree of BDNF–GFP release from synaptic and extrasynaptic clusters. Since vesicular axonal release is restricted to presynaptic terminals, which are absent from extrasynaptic BDNF–GFP clusters (marked in yellow), the release must have occurred from dendrites.
Postsynaptic release of BDNF–GFP after tetanic presynaptic stimulation
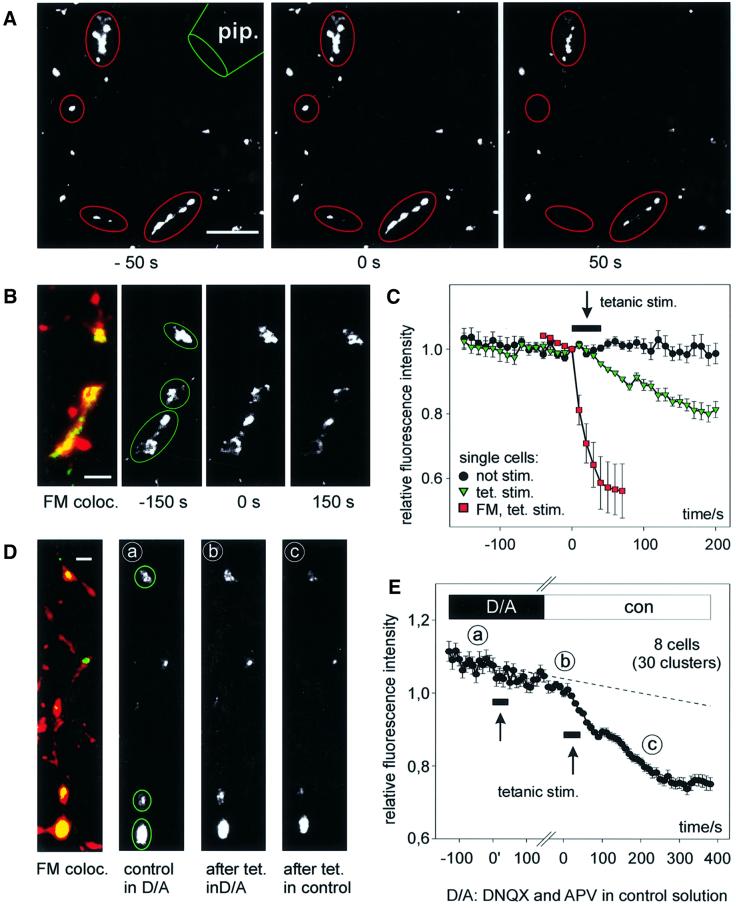
To investigate synaptic secretion of BDNF–GFP after high-frequency stimulation, synaptic activity was induced by extracellular electrical stimulation via a nearby glass pipette. This paradigm reliably induced destaining of FM 4-64-labelled presynaptic terminals, indicating physiological levels of transmitter release (Figure 5A and C). The same high-frequency stimulation paradigm (16 bursts of 50 pulses at 50 Hz within 40 s) induced release of BDNF–GFP from synaptically localized dendritic vesicle clusters (Figure 5B and C). This paradigm was successful in eight out of the 16 cells investigated. On average, the mean remaining fluorescence intensity 5 min after tetanic stimulation was 79 ± 1%, compared with 97 ± 2% in unstimulated controls (significantly different with p <0.001). The average time constant of fluorescence decrease was 238 ± 49 s and thus comparable to the time course observed with K+/Glu stimulation. In all experiments, successful presynaptic stimulation was evident from destaining of FM 4-64-labelled terminals at the end of a recording (data not shown).
Fig. 5. Synaptic release of BDNF–GFP upon high-frequency synaptic stimulation. (A) Monochrome images (at the time points indicated) from a representative destaining experiment of FM 4-64-labelled presynaptic terminals using extracellular tetanic stimulation [800 pulses in 16 × 1 s bursts (2.5 s intervals) at 50 Hz]. pip., stimulation pipette. (B) Synaptic co-localization of BDNF–GFP clusters with FM 4-64 (left) and monochrome images of BDNF–GFP fluorescence before and after tetanic stimulation [same paradigm as in (A)]. Images in (A) and (B) are from different cells. (C) Average change in fluorescence intensity of the postsynaptic BDNF–GFP clusters marked in (B) (green triangles) versus results of a non-stimulated cell under identical conditions (black circles). Red squares show averaged release of FM 4–64 from the presynaptic terminals marked in (A). The arrow pointing to a black bar indicates the duration of stimulation. (D) Synaptic co-localization (yellow) of BDNF–GFP clusters (green) with FM 4–64 (red) of a cell subjected to two rounds of tetanic stimulation. Monochrome images show BDNF–GFP fluorescence prior to first tetanus in DNQX/APV (a), 250 s after first tetanus in D/A and immediately prior to the second tetanus (b), and 250 s after second tetanus in control (c). Note the stimulus-induced loss of fluorescence only between b and c. (E) Averaged data from eight cells as in (D). The labels a–c indicate time points of images shown in (D). Data are aligned with respect to the two tetani (at 0′ and 0; duration of tetani indicated by arrows pointing to black bars). The gap on the time axis accounts for slightly different intervals between the two tetani in individual cells (210–380 s). The dashed line is extrapolated from photobleaching during 5 min prior to first tetanus. The reversible block of BDNF–GFP release by APV and DNQX reveals that synaptic secretion of BDNF–GFP upon presynaptic tetanic stimulation depends on depolarization via postsynaptic glutamate receptors. Scale bars: 10 µm (A), 3 µm (B and D).
As in the case of K+-induced secretion, the release of BDNF–GFP after high-frequency stimulation was not restricted to synaptic sites, and in five out of the eight cells investigated we observed additional release of BDNF–GFP from extrasynaptic dendritic BDNF–GFP clusters (compare with extrasynaptic release after K+-induced secretion as shown in Figure 4), indicating spread of the postsynaptic depolarization in the dendritic tree. This extrasynaptic release was slightly less efficient and amounted to ∼60% of the fluorescence decrease observed at synaptic BDNF–GFP clusters of the same cell (data not shown).
The synaptic release of dendritic BDNF–GFP upon electrical stimulation could result either from direct depolarization of the postsynaptic plasma membrane leading to activation of VGCC or from glutamate receptor-induced postsynaptic depolarization, subsequently eliciting Ca2+ influx. To distinguish between these two possibilities, we performed experiments with successive tetanic stimulations of the same cell: first, in the presence of ionotropic glutamate receptor antagonists (DNQX and APV), followed by identical stimulation in the absence of these antagonists (Figure 5D and C). Release of BDNF–GFP in DNQX/APV was sensitive to the strength of extracellular stimulation (between –40 and –80 V; Materials and methods). Thus, in seven out of 15 experiments, release of BDNF–GFP was elicited by the first tetanus stimulation due to direct postsynaptic depolarization (Lessmann and Heumann, 1998); these cells were excluded from the analysis. In the remaining eight cells in which electrical stimulation in the presence of DNQX/APV failed to induce secretion, subsequent stimulation of the same synapses in the absence of DNQX/APV was successful in releasing BDNF–GFP; the residual average fluorescence intensity was 75 ± 2%, 5 min after the second stimulation (Figure 5E). As expected, tetanus-induced destaining of FM 4-64-labelled presynaptic terminals was not affected by the DNQX/APV treatment (data not shown), indicating that presynaptic transmitter release takes place during both rounds of tetanic stimulation. Thus, in contrast to the results obtained after direct postsynaptic depolarization with high K+ (Figure 3), the postsynaptic depolarization after tetanic presynaptic stimulation depends on postsynaptic glutamate receptors (Figure 6). Most importantly, this dependence of the release of BDNF–GFP after tetanic presynaptic stimulation on activation of ionotropic glutamate receptors provides direct physiological evidence that release of BDNF–GFP occurs from postsynaptic structures: although presynaptic elements were depolarized in both rounds of tetanic stimulation, release of BDNF–GFP only occurred when postsynaptic depolarization was possible (i.e. in the absence of DNQX/APV).
Taken together, these results suggest release of BDNF from postsynaptic structures of frequently used excitatory synapses.
Discussion
Using time-lapse video microscopy of BDNF–GFP fluorescence, our results directly demonstrate vesicular release of BDNF–GFP from postsynaptic sites of glutamatergic synapses in hippocampal neurons. This release process depends on postsynaptic influx of Ca2+, either via activation of ionotropic glutamate receptors after tetanic synaptic stimulation or via direct postsynaptic gating of VGCC (e.g. upon stimulation with high K+). Since activity-dependent postsynaptic Ca2+ influx triggers LTP and long-term depression (LTD) at many excitatory synapses, our data are consistent with the view of BDNF being a retrograde or paracrine synaptic messenger in synaptic plasticity, as has been postulated for a number of neuronal circuits (Thoenen, 1995; Berninger and Poo, 1996; Bonhoeffer, 1996; Katz and Shatz, 1996; Schuman, 1999). Although we did not directly correlate release of BDNF–GFP with electrophysiological recordings, the wealth of data showing BDNF-induced enhancement of excitatory synaptic transmission favours BDNF-mediated LTP rather than LTD.
Despite the fact that activity-dependent neuronal release of BDNF has been investigated previously by western blot and ELISA analysis of cell culture supernatants (Goodman et al., 1996; Canossa et al., 1997; Balkowiec and Katz, 2000), neither the sites of neuronal BDNF secretion nor the required synaptic activity levels to initiate such a release process could be determined. Using the presynaptic marker Synapsin I, we have shown previously synaptic targeting of BDNF–GFP secretory granules in cortical neurons (Haubensak et al., 1998). In addition, Kojima et al. (2001) have recently shown co-localization of BDNF–GFP with the postsynaptic marker PSD95 and high K+-induced release of BDNF–GFP from cortical neurons. However, real-time measurement and characterization of synaptic secretion of neurotrophins have not been performed previously. In addition, the role of extracellular Ca2+ in neuronal release of neurotrophins has been a matter of debate, with most of the previous studies favouring a mechanism independent of extracellular Ca2+ (see for example Blöchl et al., 1995; Canossa et al., 1997; Griesbeck et al., 1999). The strict dependence of release of BDNF–GFP on extracellular Ca2+, as observed here, is at variance with these previous results. However, since our approach enables us to focus on synaptic release of BDNF in single cells and investigates release on a faster time scale than previous studies, it is possible that the properties of synaptic release of BDNF differ from the characteristics of neurotrophin secretion observed from other release sites.
In a recent study, presynaptic transfer of BDNF–GFP from transfected neurons was determined in fixed preparations by quantifying accumulated BDNF–GFP in neighbouring untransfected neuronal somata by means of anti–GFP immunocytochemistry (Kohara et al., 2001). These results are not in conflict with the data presented here. However, due to the very predominant (i.e. 97%) dendritic localization of BDNF–GFP and to the selection procedure of cells in our release experiments (i.e. excluding cells showing axonal localization; see Figure 2), our focus was on postsynaptic release of BDNF–GFP. Also, the concomitant dendritic release of BDNF–GFP at extrasynaptic locations (see Figure 4) and the dependence of BDNF–GFP secretion on activation of postsynaptic glutamate receptors upon presynaptic tetanic stimulation (Figure 5) clearly indicate release of BDNF from postsynaptic structures in our experiments. However, future studies will be needed to explore whether pre- and postsynaptic release of BDNF–GFP can co-exist in a given cell.
The block of BDNF–GFP release by tetanus toxin strongly suggests that BDNF secretion results from a vesicular fusion event. The dependence of the release of BDNF–GFP on Ca2+ influx indicates that the BDNF vesicles are secretory granules of the regulated pathway of secretion, rather than constitutively released vesicles, which are released independently of Ca2+ signalling (Halban and Irminger, 1994). This is in line with the co-localization of GFP- and myc-tagged BDNF with marker proteins of secretory granules (Haubensak et al., 1998; Möller et al., 1998).
The slow onset of release of BDNF–GFP as compared with transmitter release (Figure 5) is in line with previous studies of peptide secretion from secretory granules of neuroendocrine PC12 cells (Burke et al., 1997; Lang et al., 1997, 2001) and of other model systems of peptide secretion (Kasai, 1999). This delayed onset of secretion is believed to reflect diffusion of fusion competent-secretory granules to the release sites at the plasma membrane (Burke et al., 1997; Mansvelder and Kits, 2000).
In recent years, evidence has accumulated that regulated exocytosis can also occur in the somatodendritic compartment of neurons, with properties reminiscent of peptide secretion (Huang and Neher, 1996; Lledo et al., 1998; Maletic-Savetic, 1998). These data are compatible with the regulated postsynaptic secretion of BDNF–GFP from glutamatergic synapses reported here. Interestingly, the intracellular Ca2+ levels (<1 µM) that trigger exocytosis of secretory granules (see for example Lim et al., 1990; Verhage et al., 1991; Huang and Neher, 1996) are an order of magnitude below those necessary for transmitter secretion from nerve terminals (>50 µM) (see for example Heidelberger et al., 1994). Thus, the typical slow offset kinetics of peptide secretion after the end of depolarization—as also observed here (see for example Figure 5)—could be explained by the slow decline of residual Ca2+ levels, maintaining exocytosis after Ca2+ influx has terminated (Kasai, 1999). Alternatively, the slow decay kinetics could result either from constitutively activated CaM kinase II, which has been suggested to mediate exocytosis also at resting intracellular Ca2+ levels (Schweitzer et al., 1995; Maletic-Savetic et al., 1998), or from delayed dissolution of peptide dense cores following the actual secretion event (Angleson et al., 1999).
In contrast to the high K+-induced secretion, the release of BDNF–GFP upon high-frequency presynaptic stimulation was dependent on the activity of postsynaptic ionotropic glutamate receptors (compare Figures 3 and 5). Although at first glance this appears contradictory, this different dependence on ionotropic glutamate receptors was an expected finding and reflects the different routes to postsynaptic depolarization that are used in the experiments. In the case of a localized presynaptic stimulation, BDNF release depends on the glutamate receptor-mediated postsynaptic depolarization to trigger Ca2+ influx, whereas general depolarization of the neuronal membrane by high K+ can directly gate postsynaptic VGCCs to trigger Ca2+ influx independent of glutamate receptor activation (Figure 6). This suggests that a possible spatial restriction of synaptic BDNF–GFP release can only occur if Ca2+ influx remains local.
The electrical stimulation pattern that we used to elicit synaptic release of BDNF–GFP is comparable to electrophysiological LTP-inducing paradigms (see for example Bliss and Lomo, 1973; Kang et al., 1997). Likewise, although BDNF is certainly not the only mediator of LTP, reduced endogenous levels of BDNF inhibit the expression of tetanus-induced LTP in the rodent hippocampus (Korte et al., 1995; Kang et al., 1997); a deficit that can be overcome by prolonged supply of exogenous BDNF (Figurov et al., 1996; Korte et al., 1996; Patterson et al., 1996). However, it remained unresolved whether these effects reflect a permissive role of BDNF in LTP, just favouring the induction process, or whether acute release of BDNF itself can directly trigger the induction of LTP. Our observation of activity-dependent postsynaptic secretion of BDNF–GFP within the first 2 min after tetanic stimulation, together with the known effects of BDNF to enhance the efficacy of glutamatergic synaptic transmission on a short time scale (Lessmann, 1998; Schuman, 1999), support the notion that BDNF could in fact mediate the induction of some types of activity-dependent synaptic plasticity in the hippocampus. However, it remains to be determined whether dendritic secretion of BDNF after high-frequency stimulation can occur with sufficient spatial restriction to account for input specificity of synaptic plasticity.
Materials and methods
Hippocampal microcultures
Dissociated postnatal rat hippocampal microcultures were prepared as described by Lessmann and Heumann (1998) with minor modifications. Primary postnatal (P0–P2) neocortical astrocytes were isolated and cultured on glass cover slips at low density in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS) to yield astrocyte islands of 100–300 µm in diameter after 7–14 days in vitro (DIV). Postnatal rat hippocampal neurons were plated at low density (1–5 neurons per astrocyte island) onto the astrocyte cover slips and the neurons were cultured in serum-free medium (Neurobasal with 2% B27; Life Technologies). After 4 days, 4 µM cytosine arabinofuranoside (AraC) was added to inhibit glial proliferation.
Transfection
Hippocampal microcultures were transfected with the rat pre-pro-BDNF–GFP expression plasmid at 7–9 DIV, using the Ca2+ phosphate precipitation method described by Haubensak et al. (1998). Cells were used for experiments 3–5 days after transfection (11–14 DIV), when mature glutamatergic synapses have formed (Lessmann and Heumann, 1998). To check for vesicular release of BDNF–GFP, transfected cells were incubated for 20 h with 1 nM tetanus toxin (kind gift of G.Ahnert-Hilger) in culture medium prior to recording. Successful block of vesicular release was judged by lack of FM 4-64 staining of presynaptic terminals.
Fluorescence imaging
Cover slips with transfected cells were transferred into Petriperm dishes (Heraeus) with a folio bottom. Cells were inspected through high-aperture oil immersion objectives (40×, n.a. 1.0; 100×, n.a. 1.35) of an inverted epifluorescence microscope (Olympus IX 70). GFP fluorescence was detected with narrow excitation (450–490 nm) and emission (dichroic mirror: 495 nm; 500–550 nm) band pass filters. The red Cy3, FM 4-64 and propidium iodide fluorescence, respectively, was detected with a custom-built filter set (excitation: 530–550 nm; dichroic mirror: 570 nm; emission: 590–650 nm). Illumination of the probe was restricted to image capture and controlled with an electronic shutter device (Uni-Blitz). Pictures were captured with a digital CCD camera (Sensys 1401E; Photometrics). Time-lapse images were acquired at 10 s intervals. Data analysis was performed with MetaView software (Universal Imaging). In time-lapse recordings, fluorescence intensities (minus background) were analysed in manually adjusted regions of interest and the corresponding average fluorescence intensities were standardized to the start of a stimulation (t = 0 in graphs). Due to differences in the level of BDNF–GFP expression between individual cells, the illumination strength and exposure times changed from cell to cell, resulting in different levels of photobleaching during control periods. A trend line extrapolating the fluorescence change during 5 min of control superfusion at the beginning of each recording was inserted in the graphs and gives an estimate of the fluorescence decrease due to photobleaching. Time-lapse data from cells with >20% photobleaching during these 5 min of control superfusion were discarded. All experiments were performed at room temperature (22°C). Errors represent either the SEM (pooled data from different cells) or SD (data from the same cell). The significance of differences was tested using the unpaired Student’s t-test.
Superfusion system and FM staining
During release experiments, the cells were continuously superfused by a gravity-driven laminar local superfusion system (Lessmann and Dietzel, 1995), allowing complete exchange of successively applied solutions within 10 s. The standard extracellular solution (ECS) contained 100 mM NaCl, 4 mM KCl, 20 mM HEPES, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 0.01 mM glycine pH 7.3. For high K+-induced depolarization, 46 mM KCl was substituted for an equal amount of NaCl. In some experiments, the concentration of divalent cations was changed to 0 mM Ca2+, 3 mM Mg2+ (including 5 mM EGTA) to minimize Ca2+ influx. Where indicated, 20 µM DNQX, 200 µM APV or 100 µM LY 341495 was added to ECS to inhibit AMPA, NMDA and metabotropic glutamate receptors (class I–III), respectively. To inhibit voltage-gated Ca2+ channels, 100 µM Ni2+ and 100 µM Cd2+ were added to ECS in some experiments. Live presynaptic terminals were labelled by a 40 s challenge of cells with 10 µM FM 4-64 in high K+ (50 mM) ECS, followed by extensive washing in 0 Ca2+ ECS.
Electrical stimulation
For release of BDNF–GFP and FM 4-64, electrical synaptic stimulation was performed using a large diameter (10 µm) glass pipette filled with ECS and connected to an extracellular stimulator (Lessmann and Heumann, 1998). One-second bursts of square pulses (–40 to –80 V, 2 ms in duration) were applied at a frequency of 50 Hz. This paradigm reliably induced action potentials in nearby cells, as was evident from destaining of FM 4-64-labelled presynaptic terminals, which was blocked in the presence of 1 µM tetrodotoxin (TTX). Using this approach, successful presynaptic stimulation was checked immediately prior to all tetanus-induced BDNF–GFP release experiments.
Cell viability
Transfected neurons were stimulated for 10 min with K+/Glu solution and incubated (30 min, 37°C) with 2.5 µg/ml propidium iodide (Molecular Probes) in ECS. Viable cells exclude the DNA dye, whereas necrotic and apoptotic cells reveal red-fluorescing nuclei (Ankarcrona et al., 1995). The number of viable neurons was determined for n = 30 microculture islands (5–20 neurons/island) in three independent experiments, and compared with the results in buffer-treated controls (10 min ECS without K+/Glu) and untreated controls (left in culture medium until staining), respectively. Cell viability is given as percentage survival relative to untreated controls.
Immunocytochemistry
Cover slips of BDNF–GFP-transfected hippocampal neurons were fixed (4% formaldehyde in phosphate-buffered saline) and permeabilized (0.1% Triton X-100) using standard procedures. Monoclonal Synapsin I antibody (1:400; kindly provided by L.DeGennaro), PSD95 antibody (1:200; Calbiochem), MAP2 antibody (1:200; Sigma) and GAD65 antibody (1:2000; BioTrend) were incubated for 1 h at room temperature (RT) followed by incubation (RT, 1 h) with Cy3-coupled anti-mouse secondary antibody (1:1000; Sigma). To relocate cells for successive FM 4-64 and MAP2 staining, neurons were cultured on Cellocate cover slips (Eppendorf). The percentage of BDNF–GFP fluorescence in dendrites was calculated from the overlay of BDNF–GFP fluorescence (excluding the soma) and the respective MAP2 staining of a cell. Dendritic filopodia were judged by their regular emergence from primary dendrites and their abrupt termination after <50 µm (see Figure 1A).
BDNF ELISA
BDNF–GFP-transfected postnatal rat cortical mass cultures (see Haubensak et al., 1998; 800 000 cells/3.5 cm dish, 13–15 DIV, 5–6 days after transfection) were incubated for 45 min with 400 µl of high (50 mM) K+-ECS in the presence (2 mM) or absence of extracellular Ca2+. The cultures were subsequently processed for immunoadsorbent detection of BDNF using a commercial kit (BDNF-ELISA E-max; Promega). BDNF immunoreactivity in this assay was below the limits of detection in the supernatant of equally treated untransfected cultures.
Acknowledgments
Acknowledgements
The authors wish to thank T.Bonhoeffer and K.Gottmann for valuable suggestions; I.Dietzel, K.Erdmann, U.Eysel, T.Mittmann and M.Volgushev for critical reading of the manuscript; G.Ahnert-Hilger for kindly providing tetanus toxin; and S.Hoppe for excellent technical assistance. Supported by grant SFB 509/C9 from the DFG.
References
- Ahnert-Hilger G. and Bigalke,H. (1995) Molecular aspects of tetanus and botulinum neurotoxin poisoning. Prog. Neurobiol., 46, 83–96. [DOI] [PubMed] [Google Scholar]
- Angleson J.K., Cochilla,A.J., Kilic,G., Nussinovitch,I. and Betz,W.J. (1999) Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytotic events. Nature Neurosci., 2, 440–446. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M., Dypbukt,J.M., Bonfoco,E., Zhivotovsky,B., Orrenius,S., Lipton,S.A. and Nicotera,P. (1995) Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron, 15, 961–973. [DOI] [PubMed] [Google Scholar]
- Balkowiec A. and Katz,D.M. (2000) Activity dependent release of endogenous BDNF from primary sensory neurons detected with ELISA in situ. J. Neurosci., 20, 7417–7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger B. and Poo,M. (1996) Fast actions of neurotrophic factors. Curr. Opin. Neurobiol., 6, 324–330. [DOI] [PubMed] [Google Scholar]
- Betz W.B., Mao,F. and Smith,C.B. (1996) Imaging exocytosis and endocytosis. Curr. Opin. Neurobiol., 6, 365–371. [DOI] [PubMed] [Google Scholar]
- Bliss T.V.P. and Collingridge,G.L. (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature, 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Bliss T.V.P. and Lomo,T. (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol., 232, 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blöchl A. and Thoenen,H. (1995) Characterization of nerve growth factor (NGF) release from hippocampal neurons: evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur. J. Neurosci., 7, 1220–1228. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T. (1996) Neurotrophins and activity-dependent development of the neocortex. Curr. Opin. Neurobiol., 6, 119–126. [DOI] [PubMed] [Google Scholar]
- Bortolotto Z.A., Fitzjohn,S.M. and Collingridge,G.L. (1999) Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr. Opin. Neurobiol., 9, 299–304. [DOI] [PubMed] [Google Scholar]
- Burke N.V., Han,W., Li,D., Takimoto,K., Watkins,S.C. and Levitan,E.S. (1997) Neuronal peptide release is limited by secretory granule mobility. Neuron, 19, 1095–1102. [DOI] [PubMed] [Google Scholar]
- Canossa M., Griesbeck,O., Berninger,B., Campana,G., Kolbeck,R. and Thoenen,H. (1997) Neurotrophin release by neurotrophins: implications for activity-dependent neuronal plasticity. Proc. Natl Acad. Sci. USA, 94, 13279–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G., Pizzorusso,T., Tia,S. and Vicini,S. (1997) BDNF and NGF potentiate excitatory synaptic transmission in the rat visual cortex. J. Physiol., 498, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala J.C., Feinberg,M., Popov,V. and Harris,K.M. (1998) Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J. Neurosci., 18, 8900–8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A., Pozzo-Miller,L.D., Olafsson,P., Wang,T. and Lu,B. (1996) Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature, 381, 706–709. [DOI] [PubMed] [Google Scholar]
- Goodman L.J., Valverde,J., Lim,F., Geschwind,M.D., Federoff,H.J., Geller,A.I. and Hefti,F. (1996) Regulated release and polarized localization of BDNF in hippocampal neurons. Mol. Cell. Neurosci., 7, 222–238. [DOI] [PubMed] [Google Scholar]
- Griesbeck O., Canossa,M., Campana,G., Gärtner,A., Hoener,M., Nawa,H., Kolbeck,R. and Thoenen,H. (1999) Are there differences between the secretion characteristics of NGF and BDNF? Implications for the modulatory role of neurotrophins in activity-dependent neuronal plasticity. Microsc. Res. Tech., 45, 262–275. [DOI] [PubMed] [Google Scholar]
- Halban P.A. and Irminger,J.-C. (1994) Sorting and processing of secretory proteins. Biochem. J., 299, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W., Narz,F., Heumann,R. and Lessmann,V. (1998) BDNF–GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J. Cell Sci., 111, 1483–1493. [DOI] [PubMed] [Google Scholar]
- Heidelberger R., Heinemann,C., Neher,E. and Matthews,G. (1994) Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature, 371, 513–515. [DOI] [PubMed] [Google Scholar]
- Huang L.Y.M. and Neher,E. (1996) Ca2+-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron, 17, 135–145. [DOI] [PubMed] [Google Scholar]
- Kaech S., Fischer,M., Doll,T. and Matus,A. (1997) Isoform specificity in the relationship of actin to dendritic spines. J. Neurosci., 17, 9565–9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. and Schuman,E.M. (1995) Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science, 267, 1658–1662. [DOI] [PubMed] [Google Scholar]
- Kang H., Welcher,A., Shelton,D. and Schuman,E.M. (1997) Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron, 19, 653–664. [DOI] [PubMed] [Google Scholar]
- Kasai H. (1999) Comparative biology of Ca2+ dependent exocytosis: implications of kinetic diversity for secretory function. Trends Neurosci., 22, 88–93. [DOI] [PubMed] [Google Scholar]
- Katz L.C. and Shatz,C.J. (1996) Synaptic activity and the construction of cortical circuits. Science, 274, 1133–1138. [DOI] [PubMed] [Google Scholar]
- Kohara K., Kitamura,A., Morishima,M. and Tsumoto,T. (2001) Activity-dependent transfer of BDNF to postsynaptic neurons. Science, 291, 2419–2423. [DOI] [PubMed] [Google Scholar]
- Kojima M., Takei,N., Numakawa,T., Ishikawa,Y., Suzuki,S., Matsumoto,T., Katoh-Semba,R., Nawa,H. and Hatanaka,H. (2001) Biological characterization and optical imaging of BDNF–GFP suggest an activity-dependent local release of BDNF in neurites of cultured hippocampal neurons. J. Neurosci. Res., 64, 1–10. [DOI] [PubMed] [Google Scholar]
- Korte M., Carroll,P., Wolf,E., Brem,G., Thoenen,H. and Bonhoeffer,T. (1995) Hippocampal long-term potentiation is impaired in mice lacking BDNF. Proc. Natl Acad. Sci. USA, 92, 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M., Griesbeck,O., Gravel,C., Carroll,P., Staiger,V., Thoenen,H. and Bonhoeffer,T. (1996) Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in BDNF mutant mice. Proc. Natl Acad. Sci. USA, 93, 12547–12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T., Wacker,I., Steyer,J., Kaether,C., Wunderlich,I., Soldati,T., Gerdes,H.-H. and Almers,W. (1997) Ca2+ triggered peptide secretion in single cells imaged with green fluorescent protein and evanescent-wave microscopy. Neuron, 18, 857–863. [DOI] [PubMed] [Google Scholar]
- Lang T., Bruns,D., Wenzel,D., Riedel,D., Holroyd,P., Thiele,C. and Jahn,R. (2001) SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J., 20, 2202–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V. (1998) Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen. Pharmacol., 31, 667–674. [DOI] [PubMed] [Google Scholar]
- Lessmann V. and Dietzel,I.D. (1995) Two kinetically distinct 5-hydroxytryptamine activated Cl– conductances at Retzius-P cell synapses of the medicinal leech. J. Neurosci., 15, 1496–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V. and Heumann,R. (1998) Modulation of unitary glutamatergic synapses by NT-4/5 or BDNF in hippocampal microcultures: presynaptic enhancement depends on pre-established paired-pulse facilitation. Neuroscience, 86, 399–413. [DOI] [PubMed] [Google Scholar]
- Lessmann V., Gottmann,K. and Heumann,R. (1994) BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurons. Neuroreport, 6, 21–25. [DOI] [PubMed] [Google Scholar]
- Levine E., Dreyfus,C.F., Black,I.B. and Plummer,M.P. (1995) BDNF rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc. Natl Acad. Sci. USA, 92, 8074–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine E.S., Crozier,R.A., Black,I.B. and Plummer,M. (1998) BDNF modulates hippocampal synaptic transmission by increasing N-methyl-d-aspartic acid receptor activity. Proc. Natl Acad. Sci. USA, 95, 10235–10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin G.R. and Barde,Y.A. (1996) Physiology of the neurotrophins. Annu. Rev. Neurosci., 19, 289–317. [DOI] [PubMed] [Google Scholar]
- Li Y., Xu,Y.F., Ju,D.S., Lester,H.A., Davidson,N. and Schuman,E.M. (1998) Expression of a dominant negative TrkB receptor T1, reveals a requirement for presynaptic signalling in BDNF-induced synaptic potentiation in hippocampal neurons. Proc. Natl Acad. Sci. USA, 95, 10884–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim N.F., Nowycky,M.C. and Bookman,R.J. (1990) Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature, 344, 449–451. [DOI] [PubMed] [Google Scholar]
- Lledo P.M., Zhang,X., Südhof,T.C., Malenka,R.C. and Nicoll,R.A. (1998) Postsynaptic membrane fusion and long-term potentiation. Science, 279, 399–403. [DOI] [PubMed] [Google Scholar]
- Maletic-Savetic M., Koothan,T. and Malinow,R. (1998) Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part II: mediation by calcium/calmodulin-dependent protein kinase II. J. Neurosci., 18, 6814–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder H. and Kits,K. (2000) Calcium channels and the release of large dense core vesicles from neuroendocrine cells: spatial organization and functional coupling. Prog. Neurobiol., 62, 427–441. [DOI] [PubMed] [Google Scholar]
- Möller J.C., Krüttgen,A., Heymach,J.V., Ghori,N. and Shooter,E.M. (1998) Subcellular localization of epitope-tagged neurotrophins in neuroendocrine cells. J. Neurosci. Res., 51, 463–472. [DOI] [PubMed] [Google Scholar]
- Patterson S.L., Abel,T., Deuel,T.A.S., Martin,K.C., Rose,J.C. and Kandel,E.R. (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron, 16, 1137–1145. [DOI] [PubMed] [Google Scholar]
- Schuman E.M. (1999) Neurotrophin regulation of synaptic transmission. Curr. Opin. Neurobiol., 9, 105–109. [DOI] [PubMed] [Google Scholar]
- Schweitzer E.S., Sanderson,M.J. and Wasterlain,C.G. (1995) Inhibition of regulated catecholamine secretion from PC12 cells by the Ca2+/calmodulin kinase II inhibitor KN-62. J. Cell Sci., 108, 2619–2628. [DOI] [PubMed] [Google Scholar]
- Thoenen H. (1995) Neurotrophins and neuronal plasticity. Science, 270, 593–598. [DOI] [PubMed] [Google Scholar]
- Thomas P., Surprenant,A. and Almers,W. (1990) Cytosolic Ca2+, exocytosis and endocytosis in single melanotrophs of the rat pituitary. Neuron, 5, 723–733. [DOI] [PubMed] [Google Scholar]
- Verhage M., McMahon,H.T., Ghijsen,W.E., Boomsma,F., Scholten,G., Wiegant,V.M. and Nicholls,D.G. (1991) Differential release of amino acids, neuropeptides and catecholamines from isolated nerve terminals. Neuron, 6, 517–524. [DOI] [PubMed] [Google Scholar]