Abstract
Recent studies have highlighted the potential of various mushroom or fungal species in promoting health and combating cancer through their bioactive compounds. This growing body of evidence encourages further exploration into their mechanisms of action and possible applications in cancer prevention and treatment. Fruit bodies of wild fungus Daldinia eschscholtzii were extracted in ethyl acetate solvent (purity: 99.9%), then lyophilized to powder known as Daldinia ethyl acetate extract (DEAE). LC-MS (liquid chromatography mass spectrum) was used to detect the bioactive compounds present in DEAE. Antioxidant contents and activity of DEAE were determined by standard methods. The anticancer activity of this extract was evaluated against the A549 cell line (lung cancer) by MTT assay, apoptosis (flow cytometry), and expression of pro- and anti-apoptotic genes; anti-migration was determined by qPCR. The drug likeness of DEAE was evaluated by WebPredictor (SwissADME). Mycochemistry analysis by LC-MS demonstrated that DEAE was a mixture of a total of 28 compounds, among them 8 were flavonoids, 3 polyphenols, and 8 alkaloids. The TPC (total phenolic content), TFC (total flavonoid content), and TTC (total tannin content) of DEAE were 56.7 ± 1.3 mg GAE/g dry weight, 24.0 ± 0.28 mg QE/g dry weight, and 10.39 ± 0.24 mg TAE/g dry weight of the extract, respectively. The total antioxidant capacity (TAC) was 110.2 ± 1.3 mg AAE/g dry weight of the DEAE. The EC50 value for DPPH scavenging was 3.55 ± 0.02 mg/mL. Cytotoxicity assay exhibited anti-proliferation activity with IC50 values of 149.80 ± 0.76, 104.60 ± 1.43, and 87.86 ± 2.29 µg/mL against the A549 lung cancer cell line at 24, 48, and 72 h, respectively. The mechanism of the anticancer effect of DEAE on the cancer cell line (A549) included induction of apoptosis and a change of gene expression levels of Caspase 3, Caspase 9, p53, and Bcl 2 of the cell line. The qPCR study showed that Caspase 3, 9, BAX, and p53 were up-regulated, whereas Bcl 2 was down-regulated after the treatment with DEAE (50, 150, and 200 µg/mL) for 24 h. It had an anti-migration activity, which was justified by the downregulation of MMP 2 & 9 genes. The physicochemical, pharmacokinetic, and medicinal properties of ten compounds in DEAE were screened by web predictors (SwissADME), and among them, skimmianine, lycorine-diacetate, isocorydine, glycyrrhetic acid Me ester, kaempferol-3-O-rhamnoside, and apigenin compounds followed the rules of 5 (Lipinski filter). Skimmianin, glycyrrhetic acid Me ester, and apigenin were found to inhibit some members of the cytochrome family, like CYP1A2, CYP2D6, CYP3A4, CYP2C19, and CYP2C9, indicating their anti-drug-resistant properties. These findings suggest that DEAE may serve as a promising natural therapeutic as an antioxidant and also as an anticancer and anti-metastasis candidate against lung cancer.
Keywords: Daldinia eschscholtzii, Ethyl acetate extract, LC-MS, Antioxidant content, Antioxidant activity, Anticancer activity, Drug likeness
Subject terms: Biochemistry, Cancer, Chemical biology, Drug discovery
Introduction
Plants are the source of many anticancer medications; however, plant-derived natural chemicals have drawbacks, such as extremely low quantities, bioactive substances that depend on the environment, laborious and time-consuming isolation procedures, etc. Researchers have looked into fungi, or mushrooms, as a source of anticancer medicines or medications to address these issues. Using fungi for this purpose has several benefits1. At the same time, the chemotherapeutic agents that are available in the market have different modes of action, like anti-tubulin (e.g., vinblastine), DNA-interactive agents (e.g., cisplatin, doxorubicin), antimetabolites (e.g., methotrexate), hormones, and molecular targeting agents2,3. However, using these medications resulted in a number of negative side effects, such as drug resistance, toxic effects on non-targeted organs, and a decline in the patient’s quality of life3. As a result, in order to address the issues with current anti-cancer therapy, efforts are being made to find new, promising anticancer medicines from natural sources that are more effective and have less side effects. Fungi are now ranked as the top candidate. Fungal fruit bodies, or mushrooms, are “mini-factories” of bioactive compounds that have valuable health effects without any known negative side effects and can be used as significant therapeutic products against cancer4–6. Globally numbers of fungal species were estimated as 2–11 million7,8, while described species were 1,50,6009 (http://www.speciesfungorum.org/; December 2021). The Ascomycota of the fungal kingdom is the largest phylum, with ~ 110,000 species10, ~ 65,000 described species11, and the phylum Ascomycota exhibits high species diversity12. Antioxidant and anti-inflammatory qualities, as well as anti-cancer activity against leukemia, breast, lung, colon, or liver cancer, have been reported for some of the genera, such as Aspergillus, Penicillium, Fusarium, or Cordyceps13–17. In their review on the anticancer activity of Ascomycota fungi, Lucue et al.18 demonstrated that certain species of Ascomycota and their extracts or compounds were effective against colorectal cancer (CRC) cells. Many endophytic Ascomycota fungi are reported as anticancer, like Phomopsis, Pezicula, Penicillium sp., and Aspergillus sp.6. Very recently, Wong Chin et al.19 compiled the anticancer properties of metabolites from marine species of Aspergillus, and a total of 208 most active cytotoxic molecules were recorded from Aspergillus. Cordycepin (a.k.a 3’-deoxyadenosine) isolated from Cordyceps sinensis (Ascomycota) has been used as an anticancer agent, but an anti-cancer drug (NUC-7738) modification of Cordycepin developed by NuCana, has been reported to be 40 times more active against cancer cells without toxic side effects (https://www.ox.ac.uk/news/2021-10-08-anti-cancer-drug-derived-fungus-shows-promise-clinical-trials). Daldinia eschscholtzii, belonging to the phylum Ascomycota, class Sordariomycetes, order Xylariales, and family Xylariaceae, is known as a saprophytic or endophytic fungus. On a global scale, there has been inadequate investigation into the pharmacological properties of Daldinia eschscholtzii20. It was found that Daldinia concentrica extract showed some estrogen-like activities in vivo21. Chutulo & Chalannavar22 reported that the solvent extract of Daldinia eschscholtzii showed antioxidant and cytotoxic activities. Similarly, in India, relatively few works have been done on this fungus22,23, most probably since it is a purely wild fungus or mushroom with no culinary importance and is not readily available everywhere at all times. A proper mechanistic study was lacking to determine the anticancer activity of Daldinia eschscholtzii, and no one had previously used it against the lung cancer cell line (A549), despite the fact that Mishra et al.23 reported the dose-dependent anticancer effect of the crude extract of Daldinia eschscholtzii against the MCF-7 (breast cancer) and HeLa cell line (cervical cancer). Therefore, there was relatively little appropriate research done on a medically significant application. There is a lack of knowledge. We investigated the anticancer activity of Daldinia eschscholtzii (DEAE) against the lung cancer cell line (A549) and the relevant molecular mechanisms that underlie the extract’s anticancer activity, such as apoptosis as assessed by flow cytometry, apoptotic (Caspase 3 & 9, BAX, and p53) and anti-apoptotic (Bcl 2) gene expression, as well as migration gene expression (MMP 2 & 9) by qPCR. Furthermore, we have performed drug-likeness analysis on the DEAE compounds. This closes this knowledge gap and is novelty of our current work. Therefore, the main objectives of this work included the extraction of Daldinia eschscholtzii fruit body by ethyl acetate solvent, identification of its bioactive compounds using LC-MS, evaluation of its antioxidant and anticancer potential against lung cancer cell line (A549), and assessment of drug-likeness of the selected compounds by web predictor.
Result
Yield and LC-MS of DEAE
The extraction yield of DEAE was 2.84 ± 0.03%. The identification of secondary metabolites in DEAE was based on the retention times obtained from liquid chromatography and analysis of mass spectra (Fig. 1). The identification of metabolites was facilitated by a mass spectra database (MassBank Europe Mass Spectral Data Base) and supported by literature references (Table 1). The identified peaks are tentatively mentioned here because the natural products are present also in isomeric form, like isomerization of aglycones (e.g., isoflavones and flavones) or as isobaric compounds with distinct element composition but the identical molecular weight. Acylating groups may also coexist in different configurations with the sugar moieties. Total 28 compounds in DEAE were detected and identified from LC-MS chromatogram, among them 8 flavonoids (myricetin-3-O-xyloside, naringenin-7-O-glucoside, flavanomarein, apigenin; quercetin-3-glucuronide, dihydroquercetin, gossypin and puerarin), 3 polyphenols (rosmarinic acid, kaempferol-3-O-rhamnosid, and gallic acid hexoside), 8 alkaloids (lycoctonine, skimmianine, lycorine-diacetate, germine, dubinidine, isocorydine, ethylrhoeagenine and (-)-β-hydrastine), 1 cumarin (capensine), 1 alkylglucosinolates ((2R)−2-Hydroxy-2-phenethylglucosinolate), 1 isoflavones (genistein − 7-O-Glc-Xyl, acetate), 1 glucosinolate (Glucoraphanin), 1 triterpene glycoside (Glycyrrhetic acid, methyl ester) and 1 benzoxazinoid (DIMBOA + O-Hex-Hex) compounds were identified. Isoflavone base + 1O, 2MeO, O-Hex + C7H12NO; flavone base + 3O, 2MeO, O-HexA and flavonol base + 4O, O-Hex, 1MeO were also identified compounds.
Fig. 1.
Total ion chromatogram (TIC) of DEAE separated by LC-MS.
Table 1.
Identification of bioactive compounds with their bioactivity present in DEAE by LC-MS.
| No of peak | Experimental m/z | Bioactive compound | Retention time | Bioactivity | References |
|---|---|---|---|---|---|
| 1. |
467.28 (Fragments: 275.96, 1367.95, 189.95, 630.45, 760.33, 970.41, 1076.07, 1537.91, 1832.18) |
Lycoctonine | 0.98 | NA | - |
| 2. |
259.11 (Fragments: 328.04, 371.36, 1619.97, 388.18, 406.33, 583.23, 752.96, 1016.22, 894.01, 1172.69, 1460.17, 1833.51) |
Skimmianine | 1.38 | Anticancer | 24 |
| 3. |
371.25 (Fragments: 268.06, 605.50, 764.47, 781.86, 983.07, 1133.45, 1417.95, 1560.59, 1851.94) |
Lycorine-diacetate | 8.13 | Anticancer | 25 |
| 4. |
509.47 (Fragments: 276.01, 371.18, 912.04, 531.52, 189.98, 547.39, 864.41, 981.85, 1136.86, 1340.11, 1601.42, 1892.04) |
Germine | 8.65 | NA | - |
| 5. |
276.01 (Fragments: 467.32, 1353.19, 327.07, 189.93, 579.81, 887.13, 1115.07, 1494.90, 1731.48, 1972.37) |
Capensine | 8.65 | NA | - |
| 6. |
585.48 (Fragments: 275.84, 341.23, 189.76, 1634.88, 1188.63, 961.14, 733.50, 1304.76, 1951.47) |
Isoflavone base + 1O, 2MeO, O-Hex + C7H12NO | 8.94 | NA | - |
| 7. |
535.33 (Fragments: 200.10, 275.90, 359.27, 433.50, 535.33, 579.39, 601.20, 897.30, 1101.94, 1339.85, 1463.16, 1677.00, 1807.50, 1846.70) |
DIMBOA + O-Hex-Hex | 9.74 | Anticancer | 26 |
| 8. |
275.89 (Fragments: 371.25, 189.91, 453.39, 475.51, 1546.30, 1902.91, 683.21, 1017.77, 1209.27, 1638.13) |
Dubinidine | 9.74 | NA | - |
| 9. |
451.41 (Fragments: 468.37, 433.46, 330.76, 1101.54, 540.46, 790.21, 918.12, 1246.56, 1424.32, 1761.41, 1969.52) |
Myricetin-3-O-xyloside | 10.10 | NA | - |
| 10. |
359.26 (Fragments: 341.37, 275.93, 450.34, 1333.61, 1034.75, 605.56, 251.04, 1937.20, 1497.69, 726.28, 897.36, 1074.96, 1722.27, 1662.06, 1859.39) |
Rosmarinic acid | 10.33 |
Anticancer and Antioxidant |
27–29 |
| 11. |
341.37 (Fragments: 432.41, 769.59, 450.36, 979.70, 300.36, 504.43, 1232.17, 1555.86, 1687.48, 1899.66) |
Isocorydine | 10.33 | Anticancer | 30 |
| 12. |
484.34 (Fragments: 506.39, 450.42, 1176.49, 1418.55, 1018.21, 1457.95, 430.32, 863.82, 1644.50, 359.42, 1949.24, 584.35, 745.25, 332.11) |
Glycyrrhetic acid, Me ester | 11.06 | Anticancer | 31 |
| 13. |
506.39 (Fragments: 482.31, 451.42, 381.52, 573.50, 985.45, 275.79, 1054.66, 263.25, 674.99, 933.40, 1213.82, 1377.65, 1638.49, 1791.31) |
Flavone base + 3O, 2MeO, O-HexA | 11.06 | NA | - |
| 14. |
434.40 (Fragments: 452.20, 903.47, 474.32, 416.37, 510.29, 961.55, 557.38, 696.94, 1268.09, 1454.73, 306.87, 1591.54, 1055.62, 1833.42) |
Naringenin-7-O-glucoside | 11.66 | Anticancer | 32 |
| 15. |
432.41 (Fragments: 305.91, 414.37, 450.30, 472.45, 643.35, 694.15, 863.67, 899.48, 921.60, 943.52, 1141.47, 1370.24, 1500.66, 1643.42, 1886.09) |
Kaempferol-3-O-rhamnoside | 11.78 | Anticancer | 33 |
| 16. |
899.55 (Fragments: 432.36, 450.33, 472.37, 921.41, 1371.54, 414.40, 694.69, 937.36, 1251.60, 267.23, 1501.53, 1725.24, 1850.71) |
Genistein − 7-O-Glc-Xyl, Acetate | 11.78 | NA | - |
| 17. |
450.18 (Fragments: 532.46, 432.44, 933.44, 1041.62, 1255.10, 1376.73, 315.16, 720.31, 1594.63, 200.17, 763.94, 1085.58, 1813.88) |
Flavanomarein | 12.52 | Antioxidant | 34 |
| 18. |
270.32 (Fragments: 439.39, 467.45, 383.32, 1166.25, 1796.49, 1265.32, 922.66, 1611.54, 621.59, 789.53, 1116.48, 1828.64, 200.18, 645.69, 1987.86, 1447.54) |
Apigenin | 12.79 |
Anticancer and Antioxidant |
35–38 |
| 19. |
439.39 (Fragments: 1034.79, 220.24, 537.49, 275.42, 642.33, 801.04, 1065.61, 1433.64, 1760.94, 1893.64) |
(2R)−2-Hydroxy-2-phenethylglucosinolate | 12.79 | NA | - |
| 20. |
478.36 (Fragments: 253.08, 270.32, 414.41, 432.43, 518.36, 648.55, 729.44, 762.94, 991.45, 1148.77, 1417.14, 1694.89, 1965.04) |
Quercetin-3-glucuronide | 13.21 |
Antioxidant and Anticancer |
39,40 |
| 21. |
304.30 (Fragments: 434.40, 467.25, 1312.36, 212.22, 1969.24, 1589.78, 1096.67, 679.56, 934.57) |
Dihydroquercetin | 13.68 |
Antioxidant, Anti-inflammatory and Antitumor |
41,42 |
| 22. |
494.39 (Fragments: 439.45, 416.35, 516.56, 383.46, 987.23, 743.46, 1357.04, 1090.00, 588.48, 1722.97, 337.28, 1898.30) |
Flavonol base + 4O, O-Hex, 1MeO | 14.52 | NA | - |
| 23. |
437.46 (Fragments: 479.53, 397.29, 708.74, 917.40, 1302.31, 1435.63, 383.36, 1228.70, 284.46, 1485.09, 1705.98, 1843.80) |
Glucoraphanin | 15.03 | Anticancer | 43 |
| 24. |
479.53 (Fragments: 423.46, 498.42, 957.82, 702.47, 1259.76, 935.63, 375.42, 732.84, 794.60, 1503.88, 1086.80, 1715.89, 1932.38) |
Gossypin | 15.03 | Antioxidant, Anti-inflammatory, Anticancer and Antitumor | 44 |
| 25. |
332.44 (Fragments: 437.44, 479.53, 284.39, 507.36, 795.77, 1412.28, 1073.68, 1678.69, 1939.11) |
Gallic acid hexoside | 15.37 |
Anticancer and Antioxidant |
45–47 |
| 26. |
416.35 (Fragments: 434.30, 867.51, 456.27, 889.51, 1322.35, 398.32, 670.42, 1498.99, 1139.89, 915.69, 624.00, 335.37, 479.63, 1649.57, 1888.83) |
Puerarin | 15.74 | Anticancer | 48,49 |
| 27. |
397.43 (Fragments: 478.12, 1159.55, 357.44, 757.55, 929.67, 271.16, 495.58, 1528.10, 253.47, 1063.97, 1312.85, 1667.03, 1973.76) |
Ethylrhoeagenine | 15.90 | NA | - |
NA: not available; (-): no reference.
Determination of total phenolic content (TPC), total flavonoid content (TFC), total tannin content (TTC), and total antioxidant capacity (TAC) of DEAE
TPC, TFC, and TTC of DEAE were 56.7 ± 1.3 mg GAE/g dry weight, 24.0 ± 0.28 mg QE/g dry weight, and 10.39 ± 0.24 mg TAE/g dry weight of the extract, respectively. The total antioxidant capacity (TAC) of DEAE was measured by the phospho-molybdenum assay using spectrophotometry, and it was found to be 110.2 ± 1.3 mg AAE/g dry weight of the DEAE.
Determination of antioxidant activity of DEAE in vitro
DPPH free radical scavenging activity
The result showed that, the extract was able to scavenge DPPH radical in the concentration range of 0.1–5.0 mg/mL and the scavenging percentages in this range were 3.9–54.6% respectively (Fig. 2A). The Pearson correlation coefficient test result showed that the relationship between the concentration of DEAE and DPPH free radical scavenging percentage was statistically significant (p < 0.05). The EC50 value was 3.55 ± 0.02 mg/mL (Table 2). Butylated hydroxy anisole (BHA), the commercial synthetic antioxidant, was used as a standard, and at 0.05 mg/mL, the scavenging percentage was 65.9 ± 0.44%. The EC50 value of the standard was 0.0364 ± 0.0002 mg/mL.
Fig. 2.
DPPH (A), OH radical scavenging (B), lipid peroxidation inhibition (C), and reducing power activity (D) of DEAE.
Table 2.
EC50 values and FRAP activity of DEAE for antioxidant property.
| Sample name | DPPH (mg/mL) | FRAP (mM Fe2+/mg extract) | OH free radical (mg/mL) | Reducing power (mg/mL) | Lipid peroxidation inhibition (mg/mL) |
|---|---|---|---|---|---|
| DEAE | 3.55 ± 0.02 | 0.990 ± 0.017 | 1.75 ± 0.04 | 3.67 ± 0.05 | 3.18 ± 0.03 |
Values are expressed as mean ± SD (n = 3).
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging capacity of DEAE was evaluated by the ability of the extract to compete with salicylic acid for hydroxyl radical in the hydroxyl radical generating/detecting system. DEAE was shown to scavenge the ·OH in the concentration range between 0.1-2 mg/mL, and the corresponding inhibition percentages were 4.25 ± 0.23, 13.4 ± 0.68, 27.51 ± 1.01 and 57.66 ± 1.24% respectively (Fig. 2B). The Pearson correlation coefficient test result showed that the concentrations of DEAE were statistically significant with the hydroxyl radical scavenging percentages (p < 0.05). The EC50 value was 1.75 ± 0.04 mg/mL (Table 2). BHA was used as a standard, and its EC50 value was 0.296 ± 0.002 mg/mL.
Lipid peroxidation Inhibition activity
In our study, it was found that, in a concentration-dependent manner, DEAE was capable of inhibiting MDA formation. In a concentration range of 0.1–5.0 mg/mL, the inhibition percentages were 5.27 ± 1.79, 14.82 ± 1.13, 28.3 ± 1.32, 40.79 ± 0.66, 48.93 ± 1.04, 55.61 ± 0.09, 62.42 ± 0.28 respectively (Fig. 2C). The Pearson correlation coefficient test result showed that the concentrations of DEAE were statistically significant with the percentages of lipid peroxidation inhibition (p < 0.05). The EC50 value was 3.18 ± 0.03 mg/mL for DEAE, and for the BHA standard, the EC50 value was 0.093 ± 0.0004 mg/mL (Table 2).
Reducing power activity
It was found that the reducing power was increased when the DEAE concentration was increased. At 0.1–5.0 mg/mL, the reducing power was 0.009 ± 0.001, 0.047 ± 0.004, 0.115 ± 0.008, 0.266 ± 0.006, 0.413 ± 0.012, 0.562 ± 0.011, and 0.674 ± 0.003, respectively (Fig. 2D). The Pearson correlation coefficient test result showed that the relationship between the concentration of DEAE and reducing power was statistically significant (p < 0.05). The EC50 value of DEAE was 3.67 ± 0.05 mg/mL, and in the case of standard (BHA), the EC50 value was 0.039 ± 0.0003 mg/mL (Table 2).
Ferric reducing antioxidant potential (FRAP)activity
The ferric reducing antioxidant potential of DEAE was 0.990 ± 0.017 mM Fe2+/mg of the extract (Table 2).
Spearman rank correlation test
The Spearman rank correlation was done to correlate the antioxidant content (TPC, TFC, TTC, and TAC) with the EC50 value of DPPH free radical scavenging, OH radical scavenging, lipid peroxidation inhibition reducing power, and the FRAP activity of DEAE (Fig. 3). A positive correlation was also found between TPC with EC50 of DPPH radical scavenging, EC50 of OH radical scavenging and EC50 of lipid peroxidation inhibition; TFC with EC50 of DPPH radical scavenging, EC50 of OH radical scavenging and EC50 of lipid peroxidation inhibition; TTC with EC50 of DPPH radical scavenging, EC50 of OH radical scavenging and EC50 of lipid peroxidation inhibition; TAC with EC50 of reducing power and FRAP. Positive correlation was also found between EC50 of DPPH radical scavenging and EC50 of OH radical scavenging; EC50 of lipid peroxidation inhibition and EC50 of OH radical scavenging; EC50 of reducing power and FRAP. TPC with TFC and TTC; TFC with TPC and TTC; TTC with TPC and TFC were also positively correlated. Negative correlations were also noticed in this correlation study. The relationship was found negative between TPC, TFC, and TTC with EC50 of reducing power and FRAP.
Fig. 3.
Spearman rank correlation test between antioxidant content (TPC, TFC, TTC, and TAC) and EC50 of DPPH, OH radical scavenging activity, lipid peroxidation inhibition, reducing power, and FRAP of DEAE.
Anticancer activity of DEAE
Cytotoxicity/antiproliferative assay/MTT assay
The result showed that, proliferation of the A549 cell line was gradually reduced when DEAE dosage was gradually increased from 10 to 200 µg/mL at 24, 48, and 72 h, respectively. At a 10–200 µg/mL concentration range, the cell growth inhibition percentages at 24 h were 19.5 ± 0.92, 27.8 ± 0.17, 42.3 ± 0.10, and 59.5 ± 1.09%, respectively. At 48 h, the inhibition percentages were 25.97 ± 0.27, 34.84 ± 0.72, 49.29 ± 0.54, and 69.34 ± 1.20%, respectively, and at 72 h, the inhibition percentages were 27.68 ± 0.16, 41.63 ± 0.46, 52.52 ± 0.22, and 70.03 ± 1.20%, respectively. The positive control (PC), adriamycin, exhibited 75.36 ± 3.87% inhibition against A549 cells at 5 µg/mL after 24 h. DMSO (0.1%) was used as a vehicle control to detect the toxicity of it, and it showed no inhibition against the cancer cell lines (result not shown). The study also investigated the toxicity of DEAE towards the normal cell line, HEK 293T. It was found that DEAE showed a very negligible percentage of inhibition towards HEK 293 T at 500 µg/mL for 24, 48, and 72 h (Fig. 4.). One sample t test was used to check the relationship between the concentrations of DEAE and the percentages of inhibition at 24, 48 and 72 h, and the result was statistically significant [p < 0.05 (*)]. The IC50 values of DEAE at 24, 48, and 72 h were 149.80 ± 0.76, 104.60 ± 1.43, and 87.86 ± 2.29 µg/mL, respectively. Tukey’s multiple comparisons test showed that the differences in IC50 values between time points [24 vs. 48 h (***), 24 vs. 72 h (****), and 48 vs. 72 h (**)] were statistically significant (p < 0.05) (Fig. 5).
Fig. 4.
Cytotoxicity activity of DEAE against A549 cells and HEK 293 T cells at 24, 48, and 72 h [p < 0.05 (*)].
Fig. 5.

Tukey’s multiple comparison test between IC50 values of DEAE at different hours (24, 48, and 72 h). Differences in IC50 values between time points [24 vs. 48 h (***), 24 vs. 72 h (****), and 48 vs. 72 h (**)] were statistically significant (p < 0.05).
Study of cell morphology changes by inverted fluorescence microscope under bright field
In this study, after treatment with different doses of DEAE (50–200 µg/mL), the changes in A549 cell morphology from normal, elongated to irregular or round shapes were seen under bright field microscopy, and cell confluence levels were also reduced. The result showed that untreated control (negative control) cells exhibited a normal elongated shape (Fig. 6A), and confluence reached 90% after 24 h of culture. Cell confluence decreased and cells lost their attachment when treated with 50 µg/mL (the lowest dosage) (Fig. 6B). At 150 µg/mL (IC50 dose), cells became smaller in size and lost their shape, and some cells floated (Fig. 6C). At 200 µg/mL (highest dose), cells became round or irregularly shaped, shrunken, the plasma membrane blebbed, cell confluence was noticeably lower, and cell debris and floating dead cells were seen (Fig. 6D).
Fig. 6.
Effect of DEAE on cell morphology of A549 cells at 24 h under a bright field microscope (A) Negative control showed the normal cells of A549 (B) Cell confluences decreased when treated with 50 µg/mL of DEAE. (C, D) Cells became round-shaped, plasma membrane blebbed when treated with 150 and 200 µg/mL of DEAE, respectively (at 10×magnification; by inverted fluorescence microscope under bright field, Olympus Corporation, Tokyo, Japan).
Study of nuclear morphology changes under flurescence microscopy
Results showed that the negative control showed normal nuclei of A549 (Fig. 7A with inset zoom of one nucleus), while cells with distorted nuclear morphology/apoptotic nuclei (chromatin condensed, fragmented nuclei) were observed after treatment with 50, 150, and 200 µg/mL of DEAE, respectively (Fig. 7B, C, and D with inset zoom of one nucleus) at 24 h. It might result from DEAE’s ability to disrupt membrane on nuclear envelope. As a result, during DEAE treatment, the changes in the structure of the nucleus trigger the apoptosis progression in A549 lung cancer cells.
Fig. 7.
Effect of DEAE on cell nuclear morphology of A549 cells at 24 h after DAPI staining under fluorescence microscope (A) Negative control showed normal nuclei of A549 (inset zoom of one nucleus) (B, C, and D) Clear disorganization of nuclear morphology/apoptotic nuclei (chromatin condensed, fragmented nuclei) found (inset zoom of one nucleus) after treatment with 50, 150, and 200 µg/mL of DEAE, respectively (at 10×magnification; inverted fluorescence microscope, Olympus Corporation, Tokyo, Japan).
Determination of apoptosis through flow cytometer
The results showed that apoptosis was remarkably induced after treatment with DEAE for 24 h (Fig. 8). The percentage of apoptotic cells in the control group (negative control) was 4.6% (Fig. 8A). Notably, the percentage of late apoptotic cells increased significantly with DEAE treatment. The percentages of late apoptotic cells at concentrations of 50, 150, and 200 µg/mL were 10.9, 34.9, and 35.9%, respectively (Fig. 8B, C, and D). These data suggested that, for the growth inhibition of A549 cells, at least in part, induction of apoptosis was involved. Tukey’s multiple comparisons test showed that, except for the comparison between 150 and 200 µg/mL [p = 0.0609 (ns)], the differences in late apoptotic cell percentages among the DEAE concentrations [Control (0.0), 50, 150, and 200 µg/mL] were statistically significant [p < 0.0001 (****)] (Table 3). Šídák’s multiple comparisons test was performed to check the statistically significant variation between live cells and apoptotic cells (early + late apoptotic cells) for control and DEAE-treated (50, 150, and 200 µg/mL, respectively) cells at 24 h. The results indicated that the percentages of live cells vs. apoptotic cells at 24 h were statistically significant for the control set and the 50 and 150 µg/mL DEAE-treated set. The p-value for the control set and the 50 µg/mL of DEAE-treated set was < 0.0001 (****). In the case of 150 µg/mL of DEAE-treated set, the p-value was 0.0030 (**). Whereas for the 200 µg/mL treatment set at 24 h, the result was not statistically significant [p = 0.9900 (ns)] (Table 4).
Fig. 8.

Detection of apoptosis by annexin V-FITC assay induced by DEAE (50–200 µg/mL) in A549 cells by Flow cytometer (BD Accuri C6 plus) (A) Negative control. (B, C, and D) Cells were treated with 50, 150, and 200 µg/mL of DEAE, respectively. Quadrants: lower left: live cells; lower right: early apoptotic cells; upper right: late apoptotic cells; and upper left: necrotic cells.
Table 3.
Multiple comparison test for statistical significance between different doses of DEAE for late apoptosis.
| Tukey’s multiple comparisons test | Below threshold? | Summary | Adjusted P Value |
|---|---|---|---|
| Control vs. 50 µg/mL | Yes | **** | < 0.0001 |
| Control vs. 150 µg/mL | Yes | **** | < 0.0001 |
| Control vs. 200 µg/mL | Yes | **** | < 0.0001 |
| 50 µg/mL vs. 150 µg/mL | Yes | **** | < 0.0001 |
| 50 µg/mL vs. 200 µg/mL | Yes | **** | < 0.0001 |
| 150 µg/mL vs. 200 µg/mL | No | ns | 0.0609 |
Note: ns means not significant, **** means significant.
Table 4.
Multiple comparison test for statistical significance between different doses of DEAE for live cells vs. apoptotic cells (early + late).
| Concentration (µg/mL) of DEAE | Live cells vs. apoptotic cells (early + late) (%) | Adjusted P Value |
|---|---|---|
| 0 (Control) | 90.3 vs. 9.4 | < 0.0001 (****) |
| 50 | 82.1 vs. 16.6 | < 0.0001 (****) |
| 150 | 54.1 vs. 44.1 | 0.0030 (**) |
| 200 | 48.7 vs. 47.9 | 0.9900 (ns) |
Note: ns = not significant.
Study of gene expression related to apoptosis by qPCR
The gene expression levels were reported as fold change relative to control using the 2−ΔΔCt method. The qPCR results showed statistically significant differences in mRNA expression fold changes of Caspase 3, Caspase 9, BAX, p53, and Bcl 2 in DEAE-treated A549 cells compared to untreated cells (p < 0.05). The result showed that, when cells were treated with different concentrations (50, 150, and 200 µg/mL) of DEAE for 24 h, up-regulated expressions were found for all the pro-apoptotic genes, whereas for the anti-apoptotic gene, down-regulated expressions were found in comparison to the control. These findings strongly supported that DEAE induced apoptosis in A549 lung cancer cells by up-regulating Caspase 9, Caspase 3, BAX, and p53 (Fig. 9A), while down-regulating Bcl 2 (Fig. 9B). All results were statistically significant (p < 0.05). Interestingly, our flow cytometric study for apoptosis confirmed that DEAE was able to induce apoptosis, which aligns with the qPCR results.
Fig. 9.
Relative normalized expression studies of (A) pro-apoptotic genes (Caspase 9, Caspase 3, BAX, and p53) and (B) anti-apoptotic gene (Bcl 2) in A549 cells using qPCR after treatment with DEAE (50, 150, and 200 µg/mL) at 24 h (p < 0.05).
Study of gene expression related to anti-migration by qPCR
To study the antimigration activity of DEAE and the mechanism of antimigration, we determined the gene expression of MMP 2 and MMP 9 (matrix metallopeptidase 2 and 9) of A549 cells. The result showed that, after the treatment with different concentrations of DEAE (50, 150, and 200 µg/mL) at 24 h, the gene expressions of MMP 2 and 9 were significantly downregulated. The expression levels of MMP 2 and 9 were significantly decreased in a concentration-dependent manner (Fig. 10A, B). For MMP 2, the relative normalized expressions were 0.0980, 0.0037, and 0.0006-fold, respectively (p < 0.05) (Fig. 10A), whereas in the case of MMP 9, the relative normalized expressions were 0.0451, 0.0062, and 0.0006-fold, respectively, at 50, 150, and 200 µg/mL concentrations of DEAE comparing control (p < 0.05) (Fig. 10B). So, the significant reduction of MMP 2 and MMP 9 gene expression levels after the treatment with DEAE indicating that DEAE able to suppress the lung cancer migration.
Fig. 10.
Relative normalized expression studies of migratory genes (A) MMP 2 and (B) MMP 9 in A549 cells using qPCR after treatment with DEAE (50, 150, and 200 µg/mL) at 24 h (p < 0.05).
Physicochemical, pharmacokinetics, drug likeness, and medicinal chemistry properties of compounds of DEE by the SwissADME predictor
Based on SwissADME, prediction web servers, the physicochemical, lipophilicity, water solubility, pharmacokinetics, drug likeness, and medicinal chemistry properties of some compounds or ligands were studied (Table 5). Web predictor like SwissADME50, prediction was used to screen ten ligands (Skimmianine, lycorine-diacetate, DIMBOA + O-Hex-Hex, rosmarinic acid, isocorydine, glycyrrhetic acid, Me ester, kaempferol-3-O-rhamnoside, apigenin, quercetin-3-glucuronide, and glucoraphanin) identified by the LC-MS study of DEAE. The dataset (Table 5) provided an in-depth analysis of 10 natural compounds, evaluating their physicochemical, pharmacokinetic, drug-likeness, and medicinal chemistry properties. Out of ten compounds, 7 compounds like skimmianine, lycorine-diacetate, isocorydine, glycyrrhetic acid, Me ester, kaempferol-3-O-rhamnoside, apigenin, and glucoraphanin) were passed by Lipinski’s rule and Ghose filter. Among them, skimmianine, lycorine-diacetate, isocorydine, and glycyrrhetic acid methyl ester demonstrated the most favorable drug-like characteristics. Skimmianine was a low molecular weight compound (259.26 g/mol), with appropriate lipophilicity (Consensus LogP 2.52), and moderate topological polar surface area (TPSA 53.72 Ų). It also exhibited high gastrointestinal (GI) absorption and blood–brain barrier (BBB) permeability. It fulfilled all the filters of drug-likeness rules (Lipinski filter, Ghose filter, Veber filter, Egan filter, and Muegge filter)51–55. It had a bioavailability score of 0.55 and showed no structural alerts. Lycorine-diacetate was also a low molecular weight compound (MW 371.38) and exhibited high GI absorption. It satisfied all drug-likeness criteria. On the other hand, DIMBOA + O-Hex-Hex, rosmarinic acid, and quercetin-3-glucuronide were high molecular weights (> 500). The TPSA values (> 230 Ų) were also found to be high. These factors resulted in low GI absorption, poor BBB permeability, and violations of several drug-likeness rules (Lipinski, Veber, Egan, and Muegge). These compounds were highly water-soluble, and they had low predicted oral bioavailability scores (0.17 or 0.11). Isocorydine and glycyrrhetic acid Me ester exhibited balanced properties. The molecular weight of these compounds was ~ 360 and 341 g/mol, respectively. These compounds were moderately to highly lipophilicity, and complied with major drug-likeness filters. Notably, glycyrrhetic acid Me ester showed BBB permeability, indicating its potential for central nervous system (CNS) applications. Out of ten compounds, skimmianine was found to inhibit the cytochrome family like CYP1A2 (over-expressed in tumor cells than the normal cells), CYP2C19, CYP2C9, CYP2D6, and CYP3A4. The other two compounds, like glycyrrhetic acid, Me ester, and apigenin, were found to inhibit CYP1A2, CYP2D6, and CYP3A4, indicating their anti-drug-resistant property. Apigenin and kaempferol-3-O-rhamnoside fell into an intermediate category. Most of these compounds showed one or two violations of drug-likeness criteria and had moderate to low GI absorption(Table 5).
Table 5.
Prediction of chemical properties of some important bioactive compounds of DEAE by SwissADME predictor50.
| Compounds serial no | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Properties | Physicochemical properties | |||||||||
| Molecular weight (g/mol) | 259.26 | 371.38 | 535.45 | 535.45 | 360.31 | 341.40 | 432.38 | 270.24 | 478.36 | 437.51 |
| Num. heavy atoms | 19 | 27 | 37 | 37 | 26 | 25 | 31 | 20 | 34 | 26 |
| Num. arom. heavy atoms | 13 | 6 | 6 | 6 | 12 | 12 | 16 | 16 | 16 | 0 |
| Num. rotatable bonds | 3 | 4 | 7 | 7 | 7 | 3 | 3 | 1 | 4 | 10 |
| Num. H-bond acceptors | 5 | 7 | 15 | 15 | 8 | 5 | 10 | 5 | 13 | 11 |
| Num. H-bond donors | 0 | 0 | 8 | 8 | 5 | 1 | 6 | 3 | 8 | 5 |
| Molar Refractivity | 70.99 | 97.88 | 116.51 | 116.51 | 91.4 | 100.47 | 106.97 | 73.99 | 110.77 | 94.59 |
| TPSA | 53.72 Ų | 74.30 Ų | 237.53 Ų | 237.53 Ų | 144.52 Ų | 51.16 Ų | 170.05 Ų | 90.90 Ų | 227.58 Ų | 236.07 Ų |
| Lipophilicity | ||||||||||
| Log Po/w (iLOGP) | 2.78 | 3.12 | 1.88 | 1.88 | 1.48 | 3.34 | 1.84 | 1.89 | 0.75 | 1.01 |
| Log Po/w (XLOGP3) | 2.84 | 1.13 | −3.2 | −3.2 | 2.36 | 2.57 | 1.22 | 3.02 | 0.61 | −2.07 |
| Log Po/w (WLOGP) | 3.01 | 1.36 | −4.6 | −4.6 | 1.65 | 2.46 | 0.78 | 2.58 | −0.45 | 0.15 |
| Log Po/w (MLOGP) | 1.09 | 1.86 | −3.89 | −3.89 | 0.9 | 1.98 | −1.34 | 0.52 | −2.6 | −2.77 |
| Log Po/w (SILICOS-IT) | 2.90 | 1.73 | −4.75 | −4.75 | 1.5 | 3.49 | 0.48 | 2.52 | −1.04 | −2.77 |
| Consensus Log Po/w | 2.52 | 1.84 | −2.91 | −2.91 | 1.58 | 2.77 | 0.6 | 2.11 | −0.55 | −1.29 |
| Water solubility | ||||||||||
| Log S (ESOL) | Soluble | Soluble | Very soluble | Very soluble | Soluble | Soluble | Soluble | Soluble | Soluble | Very soluble |
| Log S (Ali) | Soluble | Soluble | Very soluble | Very soluble | Moderately Soluble | Soluble | Moderately Soluble | Moderately Soluble | Moderately Soluble | Soluble |
| Log S (SILICOS-IT) | Moderately soluble | Soluble | Soluble | Soluble | Soluble | Moderately Soluble | Soluble | Moderately Soluble | Soluble | Soluble |
| Pharmacokinetics | ||||||||||
| Gastrointestinal (GI) absorption | High | High | Low | Low | Low | High | Low | High | Low | Low |
| BBB (Blood Brain Barrier) permeant | Yes | No | No | No | No | Yes | No | No | No | No |
| CYP1A2 inhibitor | Yes | No | No | No | No | Yes | No | Yes | No | No |
| CYP2C19 inhibitor | Yes | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | Yes | No | No | No | No | No | No | No | No | No |
| CYP2D6 inhibitor | Yes | No | No | No | No | Yes | No | Yes | No | No |
| CYP3A4 inhibitor | Yes | Yes | No | No | No | Yes | No | Yes | No | No |
| Log Kp (skin permeation) | −5.87 cm/s | −7.76 cm/s | −11.84 cm/s | −11.84 cm/s | −6.82 cm/s | −6.56 cm/s | −8.07 cm/s | −5.80 cm/s | −8.78 cm/s | −10.44 cm/s |
| Druglikeness | ||||||||||
| Lipinski51 | Yes; 0 violation | Yes; 0 violation | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 | Yes; 0 violation | Yes; 0 violation | Yes; 1 violation: NHorOH > 5 | Yes; 0 violation | No; 2 violations: NorO > 10, NHorOH > 5 | Yes; 1 violation: NorO > 10 |
| Ghose52 | Yes | Yes | No; 2 violations: MW > 480, WLOGP<−0.4 | No; 2 violations: MW > 480, WLOGP<−0.4 | Yes | Yes | Yes | Yes | No; 1 violation: WLOGP<−0.4 | Yes |
| Veber53 | Yes | Yes | No; 1 violation: TPSA > 140 | No; 1 violation: TPSA > 140 | No; 1 violation: TPSA > 140 | Yes | No; 1 violation: TPSA > 140 | Yes | No; 1 violation: TPSA > 140 | No; 1 violation: TPSA > 140 |
| Egan54 | Yes | Yes | No; 1 violation: TPSA > 131.6 | No; 1 violation: TPSA > 131.6 | No; 1 violation: TPSA > 131.6 | Yes | No; 1 violation: TPSA > 131.6 | Yes | No; 1 violation: TPSA > 131.6 | No; 1 violation: TPSA > 131.6 |
| Muegge55 | Yes | Yes | No; 4 violations: XLOGP3<−2, TPSA > 150, H-acc > 10, H-don > 5 | No; 4 violations: XLOGP3<−2, TPSA > 150, H-acc > 10, H-don > 5 | Yes | Yes | No; 2 violations: TPSA > 150, H-don > 5 | Yes | No; 3 violations: TPSA > 150, H-acc > 10, H-don > 5 | No; 3 violations: XLOGP3<−2, TPSA > 150, H-acc > 10 |
| Bioavailability Score | 0.55 | 0.55 | 0.17 | 0.17 | 0.56 | 0.55 | 0.55 | 0.55 | 0.11 | 0.11 |
| Medicinal chemistry | ||||||||||
| PAINS | 0 alert | 2 alerts: isolated_alkene | 0 alert | 0 alert | 1 alert: catechol_A | 0 alert | 0 alert | 0 alert | 1 alert: catechol_A | 0 alert |
| Brenk | 0 alert | 2 alerts: isolated_alkene | 1 alert: hydroxamic_acid | 1 alert: hydroxamic_acid | 2 alerts: catechol, michael_acceptor_1 | 0 alert | 0 alert | 0 alert | 1 alert: catechol | 4 alerts: imine_1, imine_2, oxygen-nitrogen_single_bond, sulfonic_acid_2 |
| Leadlikeness | Yes | No; 1 violation: MW > 350 | No; 1 violation: MW > 350 | No; 1 violation: MW > 350 | No; 1 violation: MW > 350 | Yes | No; 1 violation: MW > 350 | Yes | No; 1 violation: MW > 350 | No; 2 violations: MW > 350, Rotors > 7 |
| Synthetic accessibility | 2.93 | 4.72 | 5.97 | 5.97 | 3.38 | 3.8 | 5.25 | 2.96 | 5.26 | 5.87 |
1: Skimmianine, 2: Lycorine-diacetate, 3: DIMBOA + O-Hex-Hex, 4: Rosmarinic acid, 5: Isocorydine, 6: Glycyrrhetic acid, Me ester, 7: Kaempferol-3-O-rhamnoside, 8: Apigenin, 9: Quercetin-3-glucuronide, 10: Glucoraphanin.
From a medicinal chemistry perspective, skimmianine stood out with the fewest alerts, the lowest synthetic complexity (score 2.93), and a favorable pharmacokinetic profile. In contrast, compounds like DIMBOA + O-Hex-Hex and rosmarinic acid presented challenges in terms of synthetic accessibility and drug-likeness due to their structural complexity, high polarity, and poor absorption. In summary, while several compounds demonstrated pharmacological potential, only a few, such as skimmianine and glycyrrhetic acid Me ester, emerged as promising leads for oral drug development due to their favorable balance of physicochemical, pharmacokinetic, and medicinal chemistry properties. Others might have required structural modification or alternative routes of administration.
Discussion
Searching for natural compounds from fungi or mushrooms is an emerging field, and these compounds are used both for cancer prevention and for cancer management. In this study, one fungus or mushroom (Daldinia eschscholtzii) was taken, and its ethyl acetate extract, namely DEAE, was used for antioxidant and anticancer activity. An LC-MS study of DEAE revealed 28 compounds were present in it, and many of them (Isocorydine, naringenin-7-O-glucoside, skimmianine, lycorine diacetate, rosmarinic acid, apigenin, dihydroquercetin, gossypin, gallic acid hexoside) showed antioxidant and anticancer activity24,25,28,29,32,35,42,44,45. In the previous research, TPC of Daldinia eschscholtzii ethanolic extract (DEE) was reported, and it was 74.55 ± 1.44 mg GAE/g dry weight of DEE56. According to Mishra et al.23, the TPC of the ethyl acetate extract of the endophytic fungus Daldinia eschscholtzii was 87.32 ± 8.96 µg GAE/mL. Chutulo & Chalannavar22 also reported that the TPC of the mixture of ethyl acetate and acetone solvent extract of Daldinia eschscholtzii was 43.853056 ± 0.05894 GAE/g of the extract. In this study, we found that our DEAE contained more TPC. It might be since Chutulo & Chalannavar22 conducted their work on the culture extract of Daldinia eschscholtzii, whereas our experiment used the fruiting bodies of the fungus. This could be a probable cause for the difference in the same species. The antioxidant ability of phenolic compounds is positively associated with the hydroxyl content present in them. The TFC and TTC of Daldinia ethanolic extract were recorded22,56. The total tannin content (TTC) found in DEE was 15.83 ± 0.47 mg TAE/g dry weight of extract56. Tannin contents in QUENCHER extracts and water extract of Terfezia boudieri (Ascomycota) were 8.12 mg TAE/g DW and 4.34 mg TAE/g DW, respectively57. Bera et al.56 reported that the total antioxidant capacity of the DEE was 135.9 ± 1.09 mg AAE/g dry weight of the extract.
DPPH radical-scavenging activity of any compounds or extract indicated its antioxidant potential; it was concentration-dependent, and the EC50 value (DPPH) of DEE was 3.13 ± 0.14 mg/mL56. Similarly, Chutulo & Chalannavar22 demonstrated that the DPPH radical scavenging percentages of the crude extract of Daldinia eschscholtzii were concentration-dependent, and at the highest concentration, the scavenging percentage was 80.298 ± 0.0608%. The antioxidant potential of all ethyl acetate extracts from ten isolates of Xylaria species was estimated using the DPPH and ABTS radical scavenging assays, and data exhibited that all extracts had antioxidant activities, but out of them, X. vinacea SWUF18-2.3 had the lowest EC50 values (0.020 ± 0.004 to 0.194 ± 0.031 mg/mL)58.
In our previous work, we found that DEE was shown to scavenge hydroxyl radicals by 8.13–66.35% within a concentration range of 0.1–2.0 mg/mL. The EC50 was 1.57 ± 0.06 mg/mL56. The hydroxyl radical scavenging activity of the ethyl acetate extract of Xylaria sp. was recorded by Pham et al.59, and it was 39.7 ± 2.6%. Bera et al.56 reported that the DEE was shown to inhibit lipid peroxidation from 9.93 to 63.02%. The EC50 was 2.71 ± 0.09 mg/mL. Murcia et al.60 showed the inhibition of lipid peroxidation of two truffles (Terfezia sp. and Picoa sp.) (Ascomycota), like 87.8% and 94.3%, respectively. Bera et al.56 found that the reducing power of DEE ranged from 0.011 to 0.983, and the EC50 value was 2.47 ± 0.05 mg/mL. Nath et al.61 determined the significant reducing power of the ethanolic extract of four ascomycetes (Phomopsis sp., Xylaria sp., Diaporthe sp., and Epacris sp.). The FRAP of DEE was 0.921 ± 0.005 mM Fe2+/mg of the extract56. In this study, Daldinia ethyl acetate extract has better hydroxyl radical scavenging, inhibition of lipid peroxidation, and ferric reducing activity than previous reports.
The cytotoxicity of ethyl acetate extract of D. concentrica against carcinoma vulvar squamous CA 431 cells was found, and the IC50 value of this extract was 0.46 mg/mL at 24 h62, which was higher than our result, which indicated our DEAE had more potential. The dose-dependent anticancer effect of the crude extract of Daldinia eschscholtzii against the MCF-7 (breast cancer) and HeLa cell line (cervical cancer) was demonstrated by a few workers23. As we found that DEAE contained skimmianine, naringenin-7-O-glucoside, rosmarinic acid (RA), isocorydine, etc., literature showed that these compounds were anticancer. For example, skimmianine showed potential for breast cancer therapy by simultaneously targeting tumor growth and immune regulation24, and naringenin-7-O-glucoside was found to induce apoptosis in triple-negative breast cancer (MDA-MB-231)32. Similarly, rosmarinic acid (RA) exhibited antioxidant activity, and it was found to suppress the activity and the expression of MMP 2 & 9 (matrix metalloproteinase 2 & 9) in the cancer cells. The impact of RA on tumor metastasis in the human colon cancer cell line (Ls174-T) was revealed through alteration of the ERK signaling pathway27. Isocorydine was found to induce mitochondrial dysfunction and energy metabolism disorder of oral squamous cell carcinoma (OSCC) cancer cells, and it was also responsible for accelerating the cell apoptosis of OSCC30. In another study, Ghosh et al.63 reported that F12 of AAEAE (ethyl acetate extract of Astraeus asiaticus) showed anticancer activity against HeLa, MCF-7, and A549 cell lines at 24 h. IC50 values were 701.00 ± 11.54, 728.71 ± 10.53, and 806.88 ± 11.52 µg/mL, respectively, for HeLa, MCF-7, and A549. James et al.32 determined the IC50 of naringenin-7-O-glucoside against the triple-negative breast cancer cell line (MDA-MB-231) by MTT (3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide) assay, and it was found to be 233.56 µg/µL.
After exposure to any chemical, the changes in cancer cell morphology were a major indication of the anticancer properties of the chemical, and the anticancer drugs primarily inhibited cancer cell proliferation by triggering apoptosis64. The nuclear morphology of A549, MCF-7, and HeLa became condensed and irregular, and also became fragmented, and apoptosis was noted in those cells when cells were treated with F12 of ethyl acetate extract of A. asiaticus63. To determine if the growth-inhibitory impact of DEAE was connected to the activation of apoptosis, A549 cells were treated with different doses of DEAE for 24 h, and results showed DEAE’s antiproliferative nature via apoptosis. As previous nuclear morphology changes results showed the quantity of condensing point due to chromatin condensation, which represents DNA damage after treatment with different doses of DEAE at 24 h, was increased compared to the control, we have performed an Annexin V-FITC/PI assay by flow cytometer to confirm apoptosis induced by DEAE, and the flow cytometer was used to measure the intensity of fluorescence. Ghosh et al.63 reported that F12 of AAEAE was able to induce apoptosis in A549, and the percentages of apoptosis were found to be 20.7, 25.1, and 31.2% for concentrations of 250, 500, and 750 µg/mL, respectively, in comparison to the control.
The mitochondrial pathway is a key modulator of apoptosis. In this study, the effect of DEAE on the intrinsic pathway was observed. In this study, qPCR was conducted to analyse the expression of mRNAs (pro-apoptotic Caspase 9, Caspase 3, BAX, and p53, and anti-apoptotic Bcl 2) that revealed more expression of Caspase 3 & 9, BAX, p53, and lesser expression of Bcl 2 gene, and this phenomenon was related to the apoptosis of A549 cells. In the intrinsic pathway of apoptosis, when apoptosis is initiated, initiator caspase (Caspase 9) is activated, which then activates executioner caspase (Caspase 3) and develops a caspase cascade signaling pathway. Similarly, the crude extract of Ganoderma applanatum was found to enhance the expression of Caspase 3, 9, and BAX genes significantly through RT-qPCR study65.
Another important property of our DEAE was the inhibition of migration of A549 cancer cells by down-regulating the expression of MMP2 and 9 (matrix metallopeptidase2 & 9) genes, which were responsible for the degradation of basement membrane materials like interstitial collagens I and III, and collagen IV66–68 and more distant migration of cancer cells or metastasis69. Our experimental results were supported by Haque et al.70, who reported that the mRNA expression of MMP9 was significantly reduced by the purified PEF-III fraction of Pleurotus highking.
Some properties of compounds were extremely linked with intestinal permeability and solubility in the initial step of oral bioavailability. Lipinski51 framed and introduced the “Rule of 5“(Ro5) (Lipinski’s rule) as the parameter cut-off values all contained 5’s. This rule is used to determine the drug-likeness51,71. To be potentially used as a drug, the bioactive compounds must not violate more than one parameter of Ro5 for oral administration. The absorption or permeation of a drug will be poor if molecular weight (MW) > 500 g/mol, number of H-bond acceptors > 10, number of H-bond donors > 5, MlogP > 4.1551. The drug score is used to select the bioactive compounds. The greater drug score value of the bioactive compounds denotes better drug candidates72. Furthermore, Ghose filter52, Veber filter53, Egan filter54, and Muegge filter55 were applied for validation of drug-likeness of any compound. In this study, 7 compounds of DEAE passed Ro5 (Lipinski’s rule) and Ghose filter. But in our previous work, eight compounds of Daldinia ethanol extract (DEE) (identified from LC-MS study) like phloretin, myricetin, phloridzin, quercetin-3-glucuronide, luteolin-7-O-glucuronide, myricetin-3-o-galactoside, quercetin-3-(6’’-malonyl)-glucoside and amentoflavone were characterized by SwissADME and it was found that only the phloretin compound fulfilled all the criteria of different filters (Lipinski filter, Ghose filter, Veber filter, Egan filter and Muegge filter)56. Cytochrome P450 (CYP) genes, have a function in metabolism of many anti-cancer drugs and also the development of anti-cancer-drug- resistance. For example, CYP3A4 was able metabolize many anticancer- drugs. Paclitaxel and Docetaxel cancer drug, were metabolized by CYP2C8 and CYP3A4/5, respectively73,74. In this study we found that some compounds of DEAE were found to inhibit Cytochrome 450 family which indicated DEAE might act as anti- drug-resistant.
Every research project has some restrictions. Gelatin zymography, which directly evaluates the proteolytic activity of secreted MMP 2 and MMP 9, is one of the limitations of our work. Others are the absence of protein-level validation (e.g., Western blot) of gene expression, the limited use of positive controls in mechanistic assays, and the lack of in vivo (mouse model) research to determine its toxicity and anticancer activity. Our present study provides a strong basis for further research.
After foregoing in-depth discussion, we came to conclude that the ethyl acetate extract of the fruit body of Daldinia eschscholtzii, containing many bioactive compounds, showed good antioxidant content and antioxidant activity as per several assays like DPPH, FRAP, lipid peroxidation, etc. This extract demonstrated excellent anti-proliferative or cytotoxic activity against the A549 lung cancer cell line via the apoptosis mechanism, which was operated by upregulation of apoptotic genes like Caspase 3 & 9, p53, and BAX, and by downregulation of the anti-apoptotic gene Bcl 2. This extract also exhibited anti-metastasis property by downregulating MMP 2 & 9 genes. LC-MS analysis revealed that this extract contained 28 compounds. The web predictor demonstrated that seven compounds were very suitable for oral drug preparation, as they passed both Lipinski’s rules and the Ghose filter. Therefore, this fungal extract is an excellent source for future drug development against lung cancer or other cancers after animal and human trials.
Materials and methods
Chemicals
Folin-Ciocalteau’s (FC) reagent, aluminium chloride (AlCl3), ammonium molybdate, ascorbic acid, ethylenediaminetetraacetic acid (EDTA), ferric chloride (FeCl3), ferrous sulphate (FeSO4.7H2O), potassium acetate, and potassium ferricyanide were obtained from Merck, India. The 2,2′- diphenyl-1-picrylhydrazyl (DPPH), gallic acid, quercetin, tannic acid, and 2, 4, 6-tripyridyl-s-triazine (TPTZ), butylated hydroxy anisole (BHA), and thiobarbituric acid (TBA) were purchased from Sigma–Aldrich (St. Louis, MO, USA). The DAPI (4’,6-diamidino-2-phenylindole), MTT (3-[4,5-dimethylthiazol-2-yl]−2,5 diphenyl tetrazolium bromide), and DMEM (Dulbecco’s Modified Eagle Medium) were purchased from Hi-Media Laboratories Pvt. Ltd., Mumbai, India. All other reagents were of analytical grade.
Fungal fruit body
Previously, the fruit bodies of this fungus were harvested from old fallen logs of trees at Doperia village (Bandipur), Khardaha, North 24 Parganas (N), W.B., India in the month of September, 2020, carried to our laboratory, cleaned, characterized, and identified by phenotypically (morpho-anatomically) and molecularly (ITSs marker), and the nucleotide sequence of ITSs of RNA was submitted to NCBI GenBank for publication and published with the accession number (PP024880.1) as Daldinia eschscholtzii strain SKGTBON1956. This fruitbody of Daldinia eschscholtzii was taken here for further work.
Solvent extraction of fungal fruit bodies
Fruit bodies of D. eschscholtzii were cleaned with distilled water and air dried at room temperature (30 ± 2 °C) for 72 h, and later on they were chopped into small pieces and finally ground into powder by a mixer grinder. For the extraction procedure, we used 65 g of fungal powder and have followed the method of Bera et al.56 except that here we used pure ethyl acetate (purity: 99.9%), and this solvent was selected from our previous experience63,64, which indicated that this solvent extract gave the majority of bioactive compounds. The extract was condensed into powder by a lyophilizer. The final yield of extract was determined by weighing the obtained extract powder. The lyophilized extract powder was placed in an airtight container in a 4 °C chamber for further use and named as Daldinia ethyl acetate extract (DEAE).
LC-MS (Liquid Chromatography-Mass Spectrometry) study for the characterization of the secondary metabolite
The LC-MS analysis of DEAE was conducted following Hajji et al.75 using a Thermo Scientific a3000 LTQXL system equipped with a binary pump, degasser, autosampler, and column heater. Chromatographic separation was achieved using a C18 column coupled to a photodiode array (PDA) detector. The mobile phase comprised solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile) at a flow rate of 0.5 mL/min. The gradient program began with 95% A and 5% B for 1 min, followed by an 11 min linear increase from 5% B to 100% B. This composition was held for 4 min before returning to the initial conditions (5% B) over 2 min. The flow rate was maintained at 0.5 mL/min, with an injection volume of 5 µL, giving a total run time of 18 min. Mass spectrometry was performed in positive electrospray ionization mode (e.g., ESI+) with a capillary voltage of 3.5 kV, drying temperature of 350 °C, nebulizer pressure of 40 psi, and drying gas flow of 10 L/min. UV–Vis spectra were used for chemical characterization, and compounds were identified by matching mass spectra with the MassBank database.
Determination of antioxidant content
Total phenolic content (TPC) determination
The total phenolic content of the DEAE was determined by the Folin-Ciocalteau (FC) method56,76. The absorbance was taken by a UV-Vis spectrophotometer at 765 nm (UV-1800, SHIMADZU, UV SPECTROPHOTOMETER). A standard curve was prepared utilizing a solution of gallic acid standard (10–80 µg/mL). From the standard curve, the TPC of the DEAE was estimated, and the data were expressed as mg gallic acid equivalent (GAE) per g of the DEAE. The experiment was run three times, and the mean ± standard deviation (SD) data values were taken.
Total flavonoid content (TFC) determination
TFC of the DEAE was measured as per the Dowd method77. In brief, 1 mL DEAE (from 1 mg/mL stock) was taken in a glass test tube. 0.2 mL AlCl3 solution (10%, w/v) in methanol, 0.2 mL potassium acetate (1 M), and 5.6 mL distilled water were added to the test tube and mixed thoroughly. Later on, the mixture was incubated a dark room for 30 min at room temperature (27 ± 2 °C). After incubation, absorbance was taken at 415 nm wavelength by a UV-Vis spectrophotometer against a reagent blank. The TFC of the DEAE was estimated against a standard curve of quercetin (10–400 µg/mL). The data (total flavonoid content) was expressed as mg quercetin equivalent (QE) per g of the DEAE powder. The experiment was repeated three times, and the mean ± SD was presented in the table.
Total tannin content (TTC) determination
TTC was estimated as per the method described by Price & Butler78 and Bera et al.56. In short, 0.5 mL DEAE (1 mg/mL stock) was taken in a clean glass tube. Then 0.5 mL FeCl3 (0.1 M), 0.5 mL potassium ferricyanide (8 mM), and 8 mL distilled water were added into the tube and mixed nicely. Later on, the mixture was kept at room temperature (30 ± 2 °C) for 10 min, and the optical density (OD) was taken at 720 nm wavelength against a reagent blank by a spectrophotometer. The TTC was measured from the standard curve prepared with different concentrations of tannic acid (5–25 µg/mL). The TTC was expressed as mg tannic acid equivalent (TAE) per g of dry weight of the DEAE. The experiment was repeated three times, and the mean ± SD values were presented.
Total antioxidant capacity (TAC) determination
TAC of the DEAE was measured by phospho-molybdenum, the method described by Prieto et al.79 and Bera et al.56. In short, in a clean test tube, 0.5 mL DEAE (1 mg/mL stock) was taken. Later on, 3 mL reagent solution (28 mM sodium phosphate, 4 mM ammonium molybdate, and 0.6 M sulfuric acid) and 0.5 mL distilled water were poured into the test tube and mixed thoroughly. Then, the tube was capped and placed in a water bath at 90 ± 1 °C for 90 min. After that, the tube was taken out water bath and cooled down to room temperature (30 ± 2 °C). The OD of the reaction mixture was measured by a spectrophotometer at 695 nm wavelength against a reagent blank. The amount of TAC of DEAE powder was estimated from a standard curve of ascorbic acid prepared with various concentrations (10–140 µg/mL), and TAC was expressed as mg ascorbic acid equivalent (AAE) per g of the DEAE. The experiment was run three times, and the data were presented as mean ± SD.
Determination of antioxidant activity
DPPH free radical scavenging activity assay
The antioxidant activity of DEAE against free radicals was measured by the DPPH (2,2′- diphenyl-1-picrylhydrazyl) assay as described by Brand-Williams et al.80 and Ghosh et al.63. In our previous paper56, we had taken the concentrations of DEE (Daldinia ethanol extract) in the range of 0.1 to 5.0 mg/mL, and it showed good results for antioxidant activity. So, in this study, we had taken the same concentration range for DEAE. In short, DEAE powder was dissolved in methanol solution at different concentrations (0.1 to 5.0 mg/mL), and 50 µL from each concentration was mixed separately with 1.2 mL of DPPH solution (6 × 10−5 M), and the mixture was properly vortexed. Then, the resultant mixtures were placed for 15 min in the dark at room temperature (30 ± 2 °C). After that, the OD was measured using a spectrophotometer at 517 nm wavelength against a blank (methanol). The antioxidant activity of the DEAE was expressed as the percentage of DPPH• radical scavenged, and it was estimated according to the following equation:
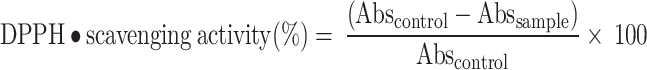 |
Where 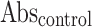 means Absorbance of the control,
means Absorbance of the control, 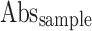 means Absorbance of the test sample.
means Absorbance of the test sample.
The EC50 of the DPPH• radical was measured from a graphical plot where the scavenging percentages were plotted against different concentrations of an extract. BHA (butylated hydroxy anisole) was used as a standard. Three replicas were run to collect data, and the mean ± SD of the data was presented.
Hydroxyl radical scavenging assay
It was estimated using the method described by Smirnoff & Cumbes81, and Bera et al.56 The ODs of the hydroxylated salicylate complex were taken at 562 nm wavelength by a UV-Vis spectrometer. The hydroxyl radical scavenging activity was calculated as per the formula:
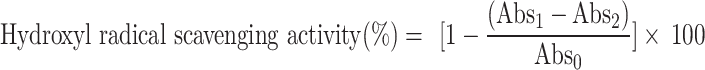 |
where, 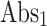 means Absorbance presence of the DEAE solution, Abs2 means Absorbance without sodium salicylate, and Abs0 means Absorbance of the control.
means Absorbance presence of the DEAE solution, Abs2 means Absorbance without sodium salicylate, and Abs0 means Absorbance of the control.
The EC50 of the hydroxyl radical scavenging was measured from a graphical plot where the scavenging percentages were plotted against different concentrations of DEAE. BHA (butylated hydroxy anisole) was taken as a standard. Three replicas were run to collect data, and the mean ± SD of the data was presented.
Lipid peroxidation Inhibition assay
This assay was done according to the method described by Damien Dorman et al.82 and Bera et al.56. A lipid-rich medium (Egg yolk homogenate) was utilized to estimate the lipid peroxide formed. In brief, egg yolk homogenate (10% v/v) was made in 1.15% (w/v) KCl in a test tube. 0.1 mL of each concentration (0.1 to 5 mg/mL) of the test DEAE was taken in a test tube, and 0.5 mL homogenate was added to each test tube and mixed. Later on, the volume of the mixture was made up to 1 mL by adding double-distilled water. After that, 0.05 mL of 0.07 M FeSO4 was added to each test tube to induce lipid peroxidation, and the mixture was kept for incubation at room temperature (30 ± 2 °C) for 30 min. Later on, 1.5 mL of TCA (tri-chloro acetic acid) followed by 1.5 mL of thiobarbituric acid (TBA) (0.06 M) in 0.04 M sodium dodecyl sulphate was poured into each tube. The mixture was mixed nicely, vortexed, and warmed for 1 h at 95 °C. Then, tubes were cooled down, and 5 mL butanol was poured into the mixture of each tube and subjected to centrifugation for 10 min at 3000 rpm. The color intensity of the mixture was estimated by OD measurement by a spectrophotometer at 532 nm wavelength. Side by side, we run a control experiment where 0.1 mL of SDS was taken instead of DEAE. The lipid peroxidation inhibition potential of the DEAE was determined as per the equation:
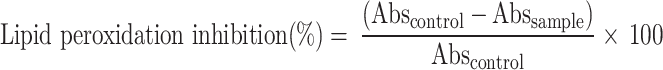 |
Where 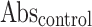 = Absorbance of the control,
= Absorbance of the control, 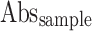 = Absorbance of the test sample.
= Absorbance of the test sample.
The EC50 of the lipid peroxidation inhibition was measured from a graphical plot where the inhibition percentages were plotted against different concentrations of DEAE. BHA (butylated hydroxy anisole) was taken as a standard. Three replicas were run to collect data, and the mean ± SD of the data was presented.
Reducing power capacity determination
The reducing power of the DEAE was tested as per the method of Oyaizu83 and Bera et al.56. In a 10 mL test tube, 2.5 mL DEAE solution of each concentration was taken separately in 10 mL test tube and later on, 200 mM/L (2.5 mL) sodium phosphate buffer (pH 6.6) and 1% potassium ferricyanide (2.5 mL) were added to each tube and mixed nicely by vortex and placed at 50 °C temperature for 20 min. After that, 2.5 mL (10%) tri-chloro acetic acid (TCA) was added to each tube, and the mixture was subjected to centrifugation for 10 min at 650 rpm. Carefully, 5 mL upper layer from each tube was taken in a separate tube, and 5 mL of deionised water and 0.1% ferric chloride (0.1%) were added separately to each. Then, OD of each mixture was taken at 700 nm wavelength by a spectrophotometer. The experiment was run three times. EC50 of DEAE was determined from the graph of OD of different DEAE concentrations at 700 nm. BHA (butylated hydroxy anisole) was taken as a standard. Three replicas were run to collect data, and the mean ± SD of the data was presented.
Ferric reducing antioxidant potential (FRAP) assay
For this purpose, we have followed the method of Benzie & Strain84 and Bera et al.56. Here, at low pH, the electron-donating antioxidant substance reduced the colourless ferric complex (Fe3+) to a blue-coloured ferrous complex (Fe2+). The FRAP reagent was freshly prepared, and it consisted of 1 volume of 20 mM FeCl3 and 1 volume of 10 mM TPTZ in 40 mM hydrochloric acid, and 10 volumes of 300 mM sodium acetate buffer (pH 3.6). It was kept at 37 °C before use. In short, 300 µL of DEAE (from 1 mg/mL stock) was added to 2.7 mL of FRAP reagent in a test tube and then again kept at 37 °C for 5 min. Then, OD was taken at the 593 nm wavelength. The FRAP value was determined from the standard curve prepared from ODs of different concentrations of ferrous sulphate, and the values were expressed as mM Fe2+/mg of sample. Three replicas were run to collect data, and the mean ± SD of the data was presented.
Anticancer activity
Cell culture
The cell culture and other anticancer bioassays including mechanistic experiments were conducted in the Cancer Research Unit of RKMVC College, Rahara, West Bengal, India. The cell culture was performed as per the method described by Freshney85. The human lung cancer cell line (A549) was procured from the National Centre for Cell Science (NCSS), Pune, India, and cultured in fresh medium (DMEM) amended with 1% antibiotic (streptomycin/penicillin) and 10% (v/v) fetal bovine serum in 25-mm tissue culture flasks at 37 °C temperature with 5% CO2 until the cells reached 90% confluence in a CO2 incubator. The cells were passaged once time in a week in the fresh culture medium.
Anticancer activity of DEAE
Cytotoxicity assay/MTT assay
MTT (3-[4,5-dimethylthiazol-2yl]−2.5-diphenyl tetrazolium bromide) (Hi-Media Laboratories Pvt. Ltd., Mumbai, India) assay, as described by Mosmann86 and Ghosh et al.63, was conducted to evaluate the anti-proliferative effect of DEAE. In cytotoxic assay of DEAE, 10, 50, 100, and 200 µg/mL concentrations of DEAE were used to treat A549 cells at 24, 48, and 72 h and for the determination of IC50. Adriamycin was used as a positive control (5 µg/mL). DMSO (0.1%) as vehicle control was utilized here to detect any toxicity of it. DMEM was used as a negative control here. Normal human cell line, HEK 293 T (Human embryonic kidney cell line) was also subjected to MTT assay at the highest tested concentration of DEAE (500 µg/mL) to check the cytotoxicity effect of this extract toward a normal human cell line. The percentage of cell cytotoxicity or the percentage of inhibition of cell growth was calculated by the formula:
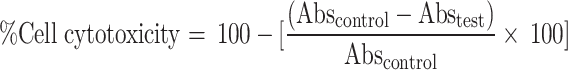 |
Where  means Absorbance of the control,
means Absorbance of the control, 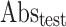 means Absorbance of the test sample.
means Absorbance of the test sample.
The experiments were run three times, and the data were represented as the mean ± SD.
The IC50 was measured by drawing a dose-response graph of the cytotoxicity data.
Cell morphology by inverted fluorescence microscope under bright field
Cell morphology, after exposure to DEAE at different concentrations [lower dose of IC50 (50 µg/mL), near dose of IC50 (150 µg/mL) and higher dose of IC50 (200 µg/mL) (doses were selected on the basis of IC50 determination, as IC50 was calculated from the above MTT assay)] for 24 h, was observed and recorded by inverted fluorescence microscope under bright field63 and, the cell images were captured by Canon camera fitted in microscope (at 10×magnification; bright field of inverted fluorescence microscope, Olympus Corporation, Tokyo, Japan).
Nuclear morphology by DAPI staining under an inverted fluorescence microscope
The nuclear morphology changes of the A549 cells under the exposure of DEAE (50, 150 and 200 µg/mL) at 24 h were recorded by DAPI (4′, 6-Diamidino-2-phenylindole) (Thermo Fisher Scientific, Inc.) staining according to the method described by64,87 and, the cell images were captured by Canon camera fitted in microscope (at 10×magnification; inverted fluorescence microscope, Olympus Corporation, Tokyo, Japan).
Determination of apoptosis by flow cytometer
The apoptosis of A549 cells under the exposure of DEAE (50, 150, and 200 µg/mL) at 24 h was determined through a flow cytometer (BD Accuri C6 plus) by AnnexinV-FITC (Fluorescein isothiocyanate)/PI (propidium iodide) apoptosis detection kit (Santacruze Biotechnology, USA) as per the manufacturer’s protocol and the method previously described by Wlodkowic et al.88 and Ghosh et al.63. These doses were selected on the basis of IC50 determination.
Gene expression study
Gene expression analysis of Caspase 3 & 9, BAX, p53, Bcl 2, MMP 2 & 9 was conducted by real-time qPCR. TRIzol (Thermo Fisher Scientific) was used to extract total RNA from the treated (with 50, 150, and 200 µg/mL of DEAE) and non-treated (Control) A549 cells (doses were selected on the basis of IC50 determination), and reverse transcription of RNA into cDNA by the cDNA synthesis kit (Verso, Thermo Fisher Scientific) was performed. The quantitative real-time PCR (qPCR) was done on Bio-Rad CFX 96 manager (Bio-Rad). The condition of amplification in qPCR was: initial denaturation at 95 °C for 3 min, followed by 39 cycles of denaturation for 10 s at 95 °C, then annealing at 55 °C for 60 s. β-actin (housekeeping gene) was utilized as an internal control for normalization. The 2-ΔΔCt method was used to determine the gene expression levels as fold change relative to the control after treatment. Three replicates were used for each experiment of gene expression. The primers list provided in Table 6.
Table 6.
List of primers for qPCR study.
| Caspase 3 | Forward | 5′-CTGGGTGAGAAAGCTGGTAA-3 |
| Reverse | 5′-AGCCTTCCTGGATGATGTTGG-3′ | |
| Caspase 9 | Forward | 5′-CATCTTCAGTTACCGACAGCTCAG-3′ |
| Reverse | 5′-TGGTCGAGAATTGTAAGGCGTAT-3′ | |
| p53 | Forward | 5′-GTTGTGTGTGTCCGACCGT-3′ |
| Reverse | 5′-GTCAGAAACAACCACCACCATGC-3′ | |
| BAX | Forward | 5′-CCAAAGAAGGACACGACAGAATC-3′ |
| Reverse | 5′-GGCAGGCTATTGCTCATCACA-3′ | |
| Bcl 2 | Forward | 5′CCAAAGAAGGACACGACAGAATC-3′ |
| Reverse | 5′-GGCAGGCTATTGCTCATCACA-3′ | |
| MMP 2 | Forward | 5′-GGAATGCCATCCCCGATAAC-3′ |
| Reverse | 5′-CAGCCTAGCCAGCCAGTCGGATTT-3′ | |
| MMP 9 | Forward | 5′-ATCCAGTTTGGTGTCGCGGAGC-3′ |
| Reverse | 5′-GAAGGGGAAGACGCACAGCT-3′ | |
| β-actin | Forward | 5′-AAATCTGGCACCACACCTTC-3′ |
| Reverse | 5′-GGGGTGTTGAAGGTCTCAAA-3′ |
Prediction of physicochemical properties, lipophilicity, water solubility, pharmacokinetics, drug-likeness, and medicinal chemistry of the compounds
In our study, 10 bioactive compounds (skimmianine, lycorine-diacetate, DIMBOA + O-Hex-Hex, rosmarinic acid, isocorydine, glycyrrhetic acid, Me ester, kaempferol-3-O-rhamnoside, apigenin, quercetin-3-glucuronide, and glucoraphanin) from the LC-MS result of DEAE were selected because they have antioxidant and anticancer properties as reported in previous studies24–31,33,35–40,43. For the screening of these properties of the bioactive compounds, the SwissADME predictor50 was used. It gave details about the physicochemical properties, lipophilicity, water solubility, pharmacokinetics, drug-likeness, and medicinal chemistry of the bioactive compounds. Lipinski’s Rule of 5 and other filters of different researchers51–55 were used to screen the analyzed compounds.
Statistical analysis
Data were presented as mean ± standard deviation (SD). The Pearson correlation coefficient test was used to determine the relationship between the concentrations of DEAE and radical scavenging percentage, reducing power, and lipid peroxidation inhibition percentage (p < 0.05). The Spearman rank correlation test was executed to understand the probable relationship between the following parameters: TFC, TPC, TTC, TAC, EC50 of OH radical scavenging, EC50 of DPPH radical scavenging, EC50 of reducing power, EC50 of lipid peroxidation inhibition, and FRAP. One-sample t-test was also used to check the relationship between concentrations of DEAE and the percentage of inhibition of A549 cells. A statistically significant difference was analyzed at the p < 0.05 level. Tukey’s multiple comparisons test was performed to understand the statistically significant variation (p < 0.05) between the IC50 values of DEAE at 24, 48, and 72 h. Again, Tukey’s multiple comparisons test was performed to check the statistically significant variation between the late apoptotic cell percentages after 24 h treatment between the different concentrations of DEAE (50, 150, and 200 µg/mL, respectively) and control treatment. Šídák’s multiple comparisons test was performed to check the statistically significant variation between live cells and apoptotic cells (early + late apoptotic cells) for control and DEAE-treated (50, 150, and 200 µg/mL, respectively) cells at 24 h. All developed graphs and the statistical analysis were done using GraphPad Prism 9 software.
Acknowledgements
Authors are grateful to the principal for providing Lab. facilities and also FIST, GoI for providing qPCR to conduct the research work.
Author contributions
S.K.G.: design, methodology and investigation of this work, collection of literatures, data analysis, writing, reviewing and editing of the Ms, fund acquisition. T. B.: experimental works, data collection and drawing of some Figs. M.G.: some experimental work, data collection, literature collection, drawing of some Figs. P.K.S.: writing, reviewing and editing of the Ms All authors reviewed and approved the article for submission.
Funding
This research work was funded by Department of Science and technology and biotechnology, Government of West Bengal, India. Grant No 820(Sanc)/ST/P/S&T/1G/2014.
Data availability
The nucleotide sequence of fungal ITSs zone has been deposited to the NCBI GenBank Nucleotide database (Baltimore, USA) and published with the Accession number PP024880.1.
Declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors gave consent to publish this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.How, C. W. et al. How Far have we explored fungi to fight cancer? Seminars Cancer Biol.86, 976–989 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Martino, E. et al. Vinca alkaloids and analogues as anti-cancer agents: looking back, peering ahead. Bioorg. Med. Chem. Lett.28 (17), 2816–2826 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Nussbaumer, S., Bonnabry, P., Veuthey, J. L. & Fleury-Souverain, S. Analysis of anticancer drugs: a review. Talanta85 (5), 2265–2289 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Zade, S. et al. Mushroom-derived bioactive compounds Pharmacological properties and cancer targeting: a holistic assessment. Discover Oncol.16 (1), 654. 10.1007/s12672-025-02371-z (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fogarasi, M. et al. Bioactive secondary metabolites in mushrooms: A focus on polyphenols, their health benefits and applications. Food Biosci.62, 105166. 10.1016/j.fbio.2024.105166 (2024). [Google Scholar]
- 6.Kousar, R. et al. Exploring the anticancer activities of novel bioactive compounds derived from endophytic fungi: mechanisms of action, current challenges and future perspectives. Am. J. Cancer Res.12 (7), 2897–2919 (2022). [PMC free article] [PubMed] [Google Scholar]
- 7.Lücking, R. et al. Fungal taxonomy and sequence-based nomenclature. Nat. Microbiol.6 (5), 540–548 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldrian, P., Větrovský, T., Lepinay, C. & Kohout, P. High-throughput sequencing view on the magnitude of global fungal diversity. Fungal Diver. 114 (1), 539–547 (2022). [Google Scholar]
- 9.Phukhamsakda, C. et al. The numbers of fungi: contributions from traditional taxonomic studies and challenges of metabarcoding. Fungal Diver. 114 (1), 327–386 (2022). [Google Scholar]
- 10.Wijayawardene, N. N., Hyde, K. D. & Dai, D. Q. Outline of Ascomycota in Encyclopedia of Mycology (eds Óscar, Z. & Arturo, C.) 246–254 (Elsevier, (2021).
- 11.Halbwachs, H., Harper, C. J. & Krings, M. Fossil Ascomycota and Basidiomycota, with notes on fossil lichens and nematophytes in Encyclopedia of Mycology (eds Óscar, Z. & Arturo) C.) 378–395 (Elsevier, 2021).
- 12.Tedersoo, L. et al. High-level classification of the fungi and a tool for evolutionary ecological analyses. Fungal Diver. 90, 135–159 (2018). [Google Scholar]
- 13.El-Bondkly, E. A. M., El-Bondkly, A. A. M. & El-Bondkly, A. A. M. Marine endophytic fungal metabolites: A whole new world of pharmaceutical therapy exploration. Heliyon7 (3), e06362. 10.1016/j.heliyon.2021.e06362 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rupa, E. J. et al. Cordyceps militaris fungus extracts-mediated nanoemulsion for improvement antioxidant, antimicrobial, and anti-inflammatory activities. Molecules (Basel Switzerland). 25 (23), 5733. 10.3390/molecules25235733 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ukwatta, K. M., Lawrence, J. L. & Wijayarathne, C. D. Antimicrobial, anti-cancer, anti-filarial and anti-inflammatory activities of cowabenzophenone A extracted from the endophytic fungus Aspergillus terreus isolated from a Mangrove plant Bruguiera gymnorrhyza. Mycology11 (4), 297–305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Gendy, M. M. A. A., Awad, M. F., El-Shenawy, F. S. & El-Bondkly, A. M. A. Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J. Biol. Sci.28 (4), 2540–2548 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi, W. et al. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr. Polym.237, 116113. 10.1016/j.carbpol.2020.116113 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Luque, C. et al. In vitro efficacy of extracts and isolated bioactive compounds from Ascomycota fungi in the treatment of colorectal cancer: a systematic review. Pharmaceuticals (Basel Switz.16 (1), 22. 10.3390/ph16010022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong Chin, J. M. et al. Marine-derived fungi from thegenus Aspergillus (Ascomycota) and their anticancer properties. Mycology16 (2), 545–592 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu, Z. et al. New Naphthalene Derivatives from the Mangrove Endophytic Fungus Daldinia eschscholzii MCZ-18. Marine Drugs. 22 (6), 242; (2024). 10.3390/md22060242 [DOI] [PMC free article] [PubMed]
- 21.Kouakou, K. & Benie, T. Effect antifertilisant de Daldinia concentrica et Psathyrella efflorescens. Recherche des. Effets oestrogéniques Ethnopharmacologia. 31, 45–57 (2003). [Google Scholar]
- 22.Chutulo, E. C. & Chalannavar, R. K. Daldinia eschscholtzii: an endophytic fungus isolated from Psidium Guajava as an alternative source of bioactive secondary metabolites. Asian J. Mycol.3 (1), 376–398 (2020). [Google Scholar]
- 23.Mishra, R., Kushveer, J. S., Khan, M. K. & Sarma, V. V. Evaluation of antioxidant potential, DNA damage protection and anti-cancer activities of three endophytic fungi associated with selected medicinal plants. Int. J. Pharm. Biol. Sci.9, 1174–1184 (2019). [Google Scholar]
- 24.Korak, T., Ayaz, H. & Aşır, F. Skimmianine modulates tumor proliferation and immune dynamics in breast cancer by targeting PCNA and TNF-α. Pharmaceuticals (Basel Switz.18 (5), 756. 10.3390/ph18050756 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, P. Lycorine inhibits cell proliferation, migration and invasion, and primarily exerts in vitro cytostatic effects in human colorectal cancer via activating the ROS/p38 and AKT signaling pathways. Oncol. Rep.45 (4), 1–1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adhikari, K. B. et al. Benzoxazinoids: cereal phytochemicals with putative therapeutic and health-protecting properties. Mol. Nutri Food Res.59 (7), 1324–1338 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Ijaz, S. et al. Rosmarinic acid and its derivatives: current insights on anticancer potential and other biomedical applications. Biomed. Pharmacother. 162, 114687. 10.1016/j.biopha.2023.114687 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Jin, B. et al. Detailed studies on the anticancer action of Rosmarinic acid in human Hep-G2 liver carcinoma cells: evaluating its effects on cellular apoptosis, caspase activation and suppression of cell migration and invasion. J. BUON. 25 (3), 1383–1389 (2020). [PubMed] [Google Scholar]
- 29.Aldoghachi, F. E. H., Al-Mousawi, N., Shari, F. H. & U. M. & Antioxidant activity of Rosmarinic acid extracted and purified from Mentha Piperita. Arch. Razi Inst.76 (5), 1279–1287 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou, Q. et al. Isocorydine exerts anticancer activity by disrupting the energy metabolism and filamentous actin structures of oral squamous carcinoma cells. Curr. Issues Mol. Biol.46 (1), 650–662 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song, J. et al. Inhibition of protein kinase C α/βII and activation of c-Jun NH2-terminal kinase mediate glycyrrhetinic acid induced apoptosis in non-small cell lung cancer NCI-H460 cells. Bioorg. Med. Chem. Lett.24 (4), 1188–1191 (2014). [DOI] [PubMed] [Google Scholar]
- 32.James, A. R., Jayaprakash, S. & Sundeep, L. M. In-vitro cytotoxicity, apoptotic property, and gene expression changes induced by naringenin-7-o-glucoside in triple-negative breast cancer. Cureus16 (4), e58634. 10.7759/cureus.58634 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barliana, M. I., Diantini, A., Subarnas, A. & Abdulah, R. Kaempferol-3-O-Rhamnoside inhibits the proliferation of Jurkat cells through Jun amino-terminal kinase signaling. Nat. Prod. J.12 (4), 57–63 (2022). [Google Scholar]
- 34.Chen, L. X. et al. Comparison of antioxidant activities of different parts from snow chrysanthemum (Coreopsis tinctoria Nutt.) and identification of their natural antioxidants using high performance liquid chromatography coupled with diode array detection and mass spectrometry and 2,2’-azinobis (3-ethylbenzthiazoline-sulfonic acid) diammonium salt-based assay. J. Chromatogr. A. 1428, 134–142 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Yang, J., Pi, C. & Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 103, 699–707 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Ittiudomrak, T., Puthong, S., Roytrakul, S. & Chanchao, C. α-Mangostin and apigenin induced cell cycle arrest and programmed cell death in SKOV-3 ovarian cancer cells. Toxicol. Res.35 (2), 167–179 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan, X., Qi, M., Li, P., Zhan, Y. & Shao, H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell. Biosci.7, 50. 10.1186/s13578-017-0179-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pápay, Z. E. et al. Study on the pulmonary delivery system of apigenin-loaded albumin nanocarriers with antioxidant activity. J. Aerosol Med. Pulm Drug Deliv. 30 (4), 274–288 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Shirai, M., Moon, J. H., Tsushida, T. & Terao, J. Inhibitory effect of a Quercetin metabolite, Quercetin 3-O-beta-D-glucuronide, on lipid peroxidation in liposomal membranes. J. Agri Food Chem.49 (11), 5602–5608 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Wu, Q. et al. Different antitumor effects of quercetin, quercetin-3’-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Func. 9 (3), 1736–1746 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Sunil, C. & Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochem166, 112066. 10.1016/j.phytochem.2019.112066 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Wang, R. et al. The anti-tumor effect of taxifolin on lung cancer via suppressing stemness and epithelial-mesenchymal transition in vitro and oncogenesis in nude mice. Ann. Transl Med.8 (9), 590. 10.21037/atm-20-3329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baenas, N. et al. Antiproliferative effects and metabolism of sulforaphane and glucoraphanin from broccoli sprouts in human colon and liver cancer cells in II International Congress, Food Technology, Quality and Safety. Proceedings. 28–30 (2014).
- 44.Song, B. et al. Gossypin: A flavonoid with diverse Pharmacological effects. Chem. Biol. Drug Des.101 (1), 131–137 (2023). [DOI] [PubMed] [Google Scholar]
- 45.Yang, J. T. et al. Gallic acid enhances the anti-cancer effect of Temozolomide in human glioma cell line via Inhibition of Akt and p38‐MAPK pathway. Processes10, 448. 10.3390/pr10030448 (2022). [Google Scholar]
- 46.Moghtaderi, H., Sepehri, H., Delphi, L. & Attari, F. Gallic acid and Curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231. BioImpacts: BI. 8 (3), 185–194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badhani, B., Sharma, N. & Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. Rsc Advan. 5 (35), 27540–27557 (2015). [Google Scholar]
- 48.Ahmad, B. et al. Molecular mechanisms of anticancer activities of puerarin. Cancer Manag Res.12, 79–90 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bisol, Â., de Campos, P. S. & Lamers, M. L. Flavonoids as anticancer therapies: A systematic review of clinical trials. Phytother Res.34 (3), 568–582 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Daina, A., Michielin, O. & Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep.7, 42717. 10.1038/srep42717 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv Rev.23, 3–25 (1997). [DOI] [PubMed] [Google Scholar]
- 52.Ghose, A. K., Viswanadhan, V. N. & Wendoloski, J. J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem.1 (1), 55–68 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Veber, D. F. et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Medi Chem.45 (12), 2615–2623 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Egan, W. J., Merz, K. M. Jr. & Baldwin, J. J. Prediction of drug absorption using multivariate statistics. J. Med. Chem.43 (21), 3867–3877 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Muegge, I., Heald, S. L. & Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem.44 (12), 1841–1846 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Bera, T., Ghosh, S. K. & Chakrabarty, R. Daldinia eschscholtzii (Ehrenb.: Fr) Rehm, a new record in West Bengal and its mycochemistry, antioxidant contents and activity, Lipoxygenase inhibitory activity and molecular Docking of its prevalent compound, phloretin, with reactive oxygen species (ROS) producing enzymes. Asian J. Mycol.8 (1), 57–85 (2025). [Google Scholar]
- 57.Hammami, R. et al. HPLC analysis, mycochemical contents and biological activities of two edible hypogeous ascomycetes: Tirmania nivea and Terfezia boudieri. Heliyon 9(3), e14331; (2023). 10.1016/j.heliyon.2023.e14331 [DOI] [PMC free article] [PubMed]
- 58.Wangsawat, N. et al. Antioxidant activity and cytotoxicity against cancer cell lines of the extracts from novel xylaria species associated with termite nests and LC-MS analysis. Antioxid. (Basel Switzerland). 10 (10), 1557. 10.3390/antiox10101557 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pham, N. S. et al. The cytotoxicity and antioxidant potentials of the endophytic fungus Xylaria sp. KET18 associated with keteleeria Evelyniana mast. Appl. Sci.14 (23), 11070. 10.3390/app142311070 (2024). [Google Scholar]
- 60.Murcia, M. A. et al. Antioxidant activity of edible fungi (truffles and mushrooms): losses during industrial processing. J. Food Protec. 65 (10), 1614–1622 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Nath, A., Raghunatha, P. & Joshi, S. R. Diversity and biological activities of endophytic fungi of Emblica officinalis, an ethnomedicinal plant of India. Mycobiol40 (1), 8–13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boua, B. et al. Chemical composition and anticancer activity of Daldinia concentrica (Xylariaceae). World J. Pharma Res.8 (1), 257–264 (2019). [Google Scholar]
- 63.Ghosh, S. K., Pandey, K., Ghosh, M. & Sur, P. K. Mycochemistry, antioxidant, anticancer activity, and molecular Docking of compounds of F12 of Ethyl acetate extract of Astraeus Asiaticus with BcL2 and caspase 3. Sci. Rep.15 (1), 4313. 10.1038/s41598-025-87775-1 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh, S. K., Bera, T. & Pal, S. Antiproliferative, apoptotic, and antimigration property of Ethyl acetate extract of Calocybe indica against HeLa and CaSki cell lines of cervical cancer, and its antioxidant and mycochemistry analysis. Middle East. J. Cancer. 11 (4), 454–468 (2020). [Google Scholar]
- 65.Kiddane, A. T. et al. Anticancer and apoptotic activity in cervical adenocarcinoma HeLa using crude extract of Ganoderma applanatum. Curr. Issues Mol. Biol.44 (3), 1012–1026 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chetty, C., Bhoopathi, P., Rao, J. S. & Lakka, S. S. Inhibition of matrix metalloproteinase-2 enhances radiosensitivity by abrogating radiation-induced FoxM1-mediated G2/M arrest in A549 lung cancer cells. Int. J. Cancer. 124 (10), 2468–2477 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin, U. H. et al. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J. Cell. Biochem.104 (1), 15–26 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Kato, Y., Yamashita, T. & Ishikawa, M. Relationship between expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 and invasion ability of cervical cancer cells. Oncol. Rep.9 (3), 565–569 (2002). [PubMed] [Google Scholar]
- 69.Vizoso, F. J. et al. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br. J. Cancer. 96 (6), 903–911 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haque, M. A., Reza, A. S. M. A., Nasrin, M. S. & Rahman, M. A. Pleurotus highking mushrooms potentiate antiproliferative and antimigratory activity against triple-negative breast cancer cells by suppressing Akt signaling. Integr. Cancer Ther.19, 1534735420969809. 10.1177/1534735420969809 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lipinski, C. A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol.1 (4), 337–341 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Oduselu, G. O. et al. Homology modelling and molecular Docking studies of selected substituted benzo [d] imidazol-1-yl) methyl) benzimidamide scaffolds on Plasmodium falciparum adenylosuccinate lyase receptor. Bioinform Biol. Insights. 13, 1177932219865533. 10.1177/1177932219865533 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luo, B., Yan, D., Yan, H. & Yuan, J. Cytochrome P450: implications for human breast cancer. Oncol. Lett.22 (1), 548. 10.3892/ol.2021.12809 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mokhosoev, I. M., Astakhov, D. V., Terentiev, A. A. & Moldogazieva, N. T. Human cytochrome p450 cancer-related metabolic activities and gene polymorphisms: a review. Cells. 13(23), ; (1958). 10.3390/cells13231958 (2024). [DOI] [PMC free article] [PubMed]
- 75.Hajji, M. et al. GC/MS and LC/MS analysis, and antioxidant and antimicrobial activities of various solvent extracts from Mirabilis Jalapa tubers. Process. Biochem.45 (9), 1486–1493 (2010). [Google Scholar]
- 76.Ainsworth, E. A. & Gillespie, K. M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc.2 (4), 875–877 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Arvouet-Grand, A., Vennat, B., Pourrat, A. & Legret, P. Standardisation d’un extrait de propolis et identification des principauxconstituants [Standardization of propolis extract and identification of principal constituents]. J. De Pharma De Belgique. 49 (6), 462–468 (1994). [PubMed] [Google Scholar]
- 78.Price, M. L. & Butler, L. G. Rapid visual Estimation and spectrophotometric determination of tannin content of sorghum grain. J. Agri Food Chem.25 (6), 1268–1273 (1977). [Google Scholar]
- 79.Prieto, P., Pineda, M. & Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem.269 (2), 337–341 (1999). [DOI] [PubMed] [Google Scholar]
- 80.Brand-Williams, W., Cuvelier, M. E. & Berset, C. L. W. T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol.28 (1), 25–30 (1995). [Google Scholar]
- 81.Smirnoff, N. & Cumbes, Q. J. Hydroxyl radical scavenging activity of compatible solutes. Phytochem28 (4), 1057–1060 (1989). [Google Scholar]
- 82.Damien Dorman, H. J., Deans, S. G., Noble, R. C. & Surai, P. Evaluation in vitro of plant essential oils as natural antioxidants. J. Essent. Oil Res.7 (6), 645–651 (1995). [Google Scholar]
- 83.Oyaizu, M. Studies on products of Browning reaction antioxidative activities of products of Browning reaction prepared from glucosamine. Japanese J. Nutri Dietetics. 44 (6), 307–315 (1986). [Google Scholar]
- 84.Benzie, I. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem.239 (1), 70–76 (1996). [DOI] [PubMed] [Google Scholar]
- 85.Freshney, R. I. Culture of animal cells in A Manual of Basic Technique and Specialized Applications. 832 (Wiley-Blackwell, (2015).
- 86.Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65 (1–2), 55–63 (1983). [DOI] [PubMed] [Google Scholar]
- 87.Kim, M. J. et al. Citrus reticulata Blanco induces apoptosis in human gastric cancer cells SNU-668. Nutr. Cancer. 51 (1), 78–82 (2005). [DOI] [PubMed] [Google Scholar]
- 88.Wlodkowic, D., Skommer, J. & Darzynkiewicz, Z. Flow cytometry-based apoptosis detection. Methods Mol. Biol.559, 19–32 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The nucleotide sequence of fungal ITSs zone has been deposited to the NCBI GenBank Nucleotide database (Baltimore, USA) and published with the Accession number PP024880.1.










