Abstract
Protein kinase D (PKD) is a cytosolic protein, which upon binding to the trans-Golgi network (TGN) regulates the fission of transport carriers specifically destined to the cell surface. We have found that the first cysteine-rich domain (C1a), but not the second cysteine-rich domain (C1b), is sufficient for the binding of PKD to the TGN. Proline 155 in C1a is necessary for the recruitment of intact PKD to the TGN. Whereas C1a is sufficient to target a reporter protein to the TGN, mutation of serines 744/748 to alanines in the activation loop of intact PKD inhibits its localization to the TGN. Moreover, anti-phospho-PKD antibody, which recognizes only the activated form of PKD, recognizes the TGN-bound PKD. Thus, activation of intact PKD is important for binding to the TGN.
Keywords: C1 domain/Golgi/PH domain/PKD/vesicular transport
Introduction
Protein kinase D (PKD) is a cytosolic protein, which localizes to different cellular membranes depending on the cell type (Prestle et al., 1996; Matthews et al., 2000; Storz et al., 2000; Liljedahl et al., 2001). When bound to the trans-Golgi network (TGN), it regulates the fission of transport carriers that form from the TGN, which are ordinarily en route to the cell surface (Liljedahl et al., 2001). But how is PKD recruited specifically to the TGN? PKD has various domains that are reported to interact with lipids and proteins. These domains are composed of a hydrophobic region at the N-terminus, two cysteine-rich domains C1s (C1a and C1b), a pleckstrin homology (PH) domain and a C-terminal catalytic domain (CD). The C1 domains bind diacylglycerol (DAG), phorbol esters, lipid kinases, phosphatidylinositol 4-kinase (PI 4-K) and phosphatidylinositol-4-phosphate 5-kinase (PI-4P 5-K), 14-3-3 protein and Btk kinase (Valverde et al., 1994; Van Lint et al., 1995; Dieterich et al., 1996; Nishikawa et al., 1998; Hausser et al., 1999; Johannes et al., 1999). The PH domain binds protein kinase C (PKC) η and ε, and the trimeric G protein subunit Gβγ (Jamora et al., 1999; Waldron et al., 1999b). Deletion of the PH domain results in a marked increase in the basal activity of PKD, suggesting that the PH domain plays an inhibitory role in the regulation of its enzymatic activity (Iglesias and Rozengurt, 1998). The CD exhibits some similarity with members of the PKC family, but is more related to the kinase domain of the myosin light chain kinase of Dictyostelium and Ca2+/calmodulin-dependent kinase II (Waldron et al., 1999a). The replacement of lysine 618 with asparagine (K618N) in the ATP binding site of the CD renders the protein inactive as a kinase (Liljedahl et al., 2001). This kinase-dead form cannot bind and/or activate PI 4-K and PI-4P 5-K, but can still bind to the TGN (Nishikawa et al., 1998; Liljedahl et al., 2001).
When the kinase activity of PKD is compromised in cells, only transport from the TGN to the cell surface is affected (Liljedahl et al., 2001). The cargo in the TGN that is destined for the cell surface is packaged into transport carriers, but the carriers fail to undergo fission. They remain attached to the TGN and grow into long tubes. Other intracellular transport events are not affected under these conditions. Based on this information we have assigned PKD and its kinase activity a role in the fission of TGN-derived transport carriers. The obvious issues pertaining to PKD vis-à-vis membrane transport then are: (i) how is PKD recruited to the TGN; (ii) how does PKD regulate the fission of transport carriers? In this report we address the first of these two issues and reveal key determinants that are required for the recruitment of PKD to the TGN.
Results
Localization of wild-type PKD to the TGN
We have shown previously that a kinase-inactive form of PKD called PKD-K618N cannot bind ATP and localizes predominantly to the TGN. PKD-K618N overexpression causes tubule formation emanating from the perinuclear Golgi area. Both the TGN-specific protein TGN46 and vesicular stomatitis virus (VSV) G protein, a marker of the secretory pathway, are found in the PKD-K618N-containing tubes, suggesting that PKD-K618N localizes to the TGN (Liljedahl et al., 2001). But as TGN46 is a recycling molecule, one could argue that TGN46 trapped in the PKD-K618N-containing tubes is moving from the TGN to the cell surface and not from endosomes or from the cell surface to the TGN. Another issue is whether the localization of PKD-K618N to the TGN is aberrant and is due to its lack of kinase activity. In order to understand the mechanism by which PKD regulates the fission of TGN-derived transport carriers, we must first establish the mechanism of its localization. Thus, we have to address the following: (i) wild-type PKD also localizes to the TGN and (ii) wild-type PKD kinase activity is required exclusively for protein transport from the TGN to the cell surface. In other words, PKD on the TGN has to be in a functionally active form.
By immunofluorescence staining, wild-type PKD co-localizes with the TGN marker (Liljedahl et al., 2001). But as the expression of wild-type PKD does not block protein transport and there is no obvious tubulation of the TGN, determination of the precise location of wild-type PKD is technically more difficult than PKD-K618N.
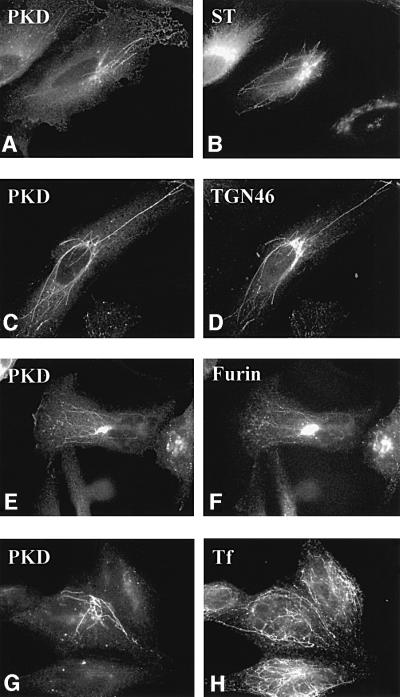
To help resolve these issues we have made use of the finding that short-term treatment with brefeldin A (BFA) causes different tubulation of the early Golgi cisternae, TGN and the endosomes (Lippincott-Schwartz et al., 1991; Wood et al., 1991). The tubulated Golgi cisternae, TGN and endosomes contain compartment-specific enzymes and, more interestingly, these tubulated membranes retain their compartmentation for a short duration. We have used this procedure to determine the localization of wild-type PKD within these membranes. HeLa cells were co-transfected with glutathione S-transferase (GST)-tagged PKD in combination with either enhanced green fluorescent protein (EGFP) tagged furin (a protein that cycles between TGN–cell surface–endosomes and the TGN) or GFP-tagged sialyltransferase (a resident enzyme of the TGN and trans-Golgi cisternae). A parallel experiment was carried out with HeLa cells expressing only GST-tagged PKD to determine its localization with the endogenous TGN46 and the internalized transferrin (a marker of the endosomal pathway). These cells were treated with BFA for 6–10 min, stained with the respective compartment-specific reagents and visualized by fluorescence microscopy. To localize wild-type PKD we used an anti-phospho-PKD-specific antibody. The results show that PKD-containing tubes caused by BFA treatment co-localize with TGN46 (Figure 1C and D) and furin (E and F). GST-tagged PKD containing tubes, however, do not co-localize with tubes containing GFP-tagged sialyltransferase (A and B) and transferrin (G and H). The following important conclusions can be derived from these results. (i) Wild-type PKD is recruited to the TGN but not to the earlier Golgi cisternae and the endosomes. (ii) The PKD-containing tubes exclude the Golgi-resident enzyme and only contain the cargo in transit to the cell surface. (iii) PKD does not dissociate from TGN upon short-term BFA treatment. This is an important finding as BFA inhibits the loading of GTP onto ADP ribosylation factor (ARF) and this is known to cause the dissociation of a number of peripheral Golgi-associated proteins such as COPI coats, spectrin, etc. (Robinson and Kreis, 1992; Beck et al., 1994). Thus, the mechanism by which PKD is attached to the TGN is different from that of other peripheral Golgi proteins involved in the formation of COPI vesicles.
Fig. 1. Wild-type PKD localizes to TGN. HeLa cells were transfected with GST–PKD (C, D, G and H) alone or co-transfected with GFP–sialyltransferase (A and B) or EGFP–furin (E and F). The cells were treated with BFA for 6 min (A and B) or 10 min (C–H), fixed and stained with anti-phospho-PKD (A, C, E and G), anti-TGN46 (D) and anti-transferrin (H) antibodies. For early endosome staining, as described in Materials and methods, the cells were incubated with pre-incubation medium for 1 h, followed by incubation with iron-saturated transferrin for 15 min prior to BFA treatment (G and H). BFA induced tubulation of the early Golgi cisternae, TGN and the endosomes. PKD co-localizes with TGN46 (C and D) and furin (E and F), but not sialyltransferase (A and B) and transferrin (G and H). Thus, PKD is contained in the portion of the TGN containing furin and TGN46. Interestingly, under these conditions, the peripheral Golgi proteins COPI coats are detached; PKD, however, remains attached to theTGN-derived tubes.
PKD localizes to specific domains within the TGN
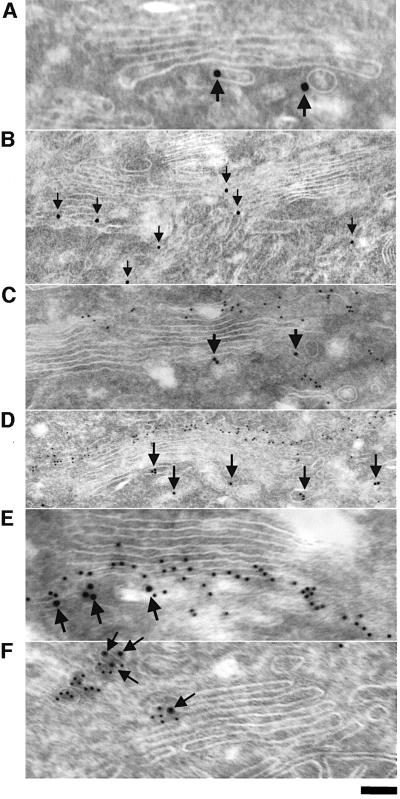
The fluorescence microscopy-based findings reveal that PKD wild type and PKD-K618N are bound to the TGN. PKD is present on specific domains of TGN and co-localizes with TGN46, but shows little overlap with the TGN and trans-Golgi-specific resident enzyme sialyltransferase. To examine the distribution of PKD in more detail we employed immunoelectron microscopy of a stable line of HeLa cells that express moderate but uniform levels of GST–FLAG-tagged PKD-K618N (Liljedahl et al., 2001). The cells were fixed and double labeled with antibodies against bona fide Golgi proteins and GST to visualize PKD-K618N. Our results show that PKD-K618N localizes to the tubules and large vesicles on the most distal (trans) side of the Golgi stacks (Figure 2A and B). GST–FLAG-tagged PKD-K618N does not co-localize with the cis-specific Golgi protein GM130 (Figure 2C and D), but shows partial co-localization with the trans and TGN enzyme galactosyltransferase (Figure 2E) and a very high level of co-localization with TGN46. These results fit well with data from fluorescence microscopy-based analysis and strengthen our conclusion that PKD-K618N localizes only to specific domains of TGN, which contain TGN46. It is quite possible that PKD-K618N (and PKD wild type) is recruited specifically to sites on TGN marked for the formation of transport carrier that are en route to the cell surface.
Fig. 2. PKD-K618N is localized to specific domains of the TGN. HeLa cells stably transfected with GST–FLAG-tagged PKD-K618N (GF17 cells) were fixed at steady state and prepared for immunogold labeling with anti-GST antibody to visualize PKD-K618N (10 nm particles, arrows), and the Golgi markers GM130 (for cis/medial compartment), galactosyl transferase (for trans-Golgi compartment and TGN) and TGN46 (for the TGN) (5 nm particles). (A and B) GST–FLAG-tagged PKD-K618N is located to the tubular-vesicular profiles attached to the trans side of the Golgi stack and rarely to its lateral side (B). (C and D) GST–FLAG-tagged PKD-K618N does not co-localize withGM-130. Gold labeling for GST–FLAG-tagged PKD-K618N (arrows) is located on the opposite side of the Golgi stack compared with GM130 (small 5 nm gold particles). (E) GST–FLAG-tagged PKD-K618N (arrows) is partially co-localized with the galactosyl transferase (small 5 nm gold particles). (F) GST–FLAG-tagged PKD-K618N (arrows) co-localizes with TGN46 (5 nm gold particles) on the trans side of the Golgi stack. Bar: (A, E, F), 70 nm; (B), 250 nm; (C), 150 nm; (D), 300 nm.
The first cysteine-rich domain (C1a) of PKD is essential and sufficient for recruitment to the TGN
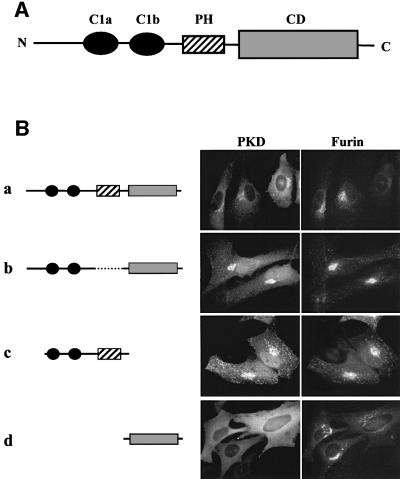
The known domains of PKD are shown schematically in Figure 3A. We made deletion mutants to determine which domain is important for the recruitment of PKD to the TGN. The constructs of PKD mutants shown in the left panels of Figure 3 were expressed with a GST tag at the N-terminus. Each construct was co-expressed with EGFP-tagged furin in HeLa cells, and 60 h post-infection the cells were stained with an anti-GST antibody and visualized by fluorescence microscopy. As shown in Figure 3B, wild-type PKD is localized to TGN and present as a diffusely dispersed protein in the cytoplasm. The Golgi-associated pool of PKD co-localizes with furin of the TGN. This morphological criterion was used to determine the requirements for PKD recruitment and effects on the organization of the TGN for the experiments described below.
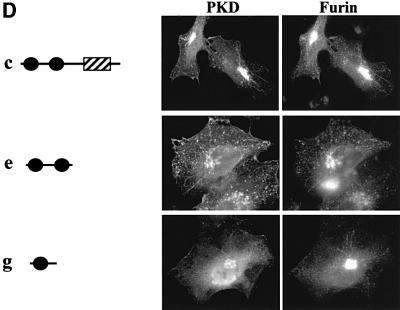
Fig. 3. The first cysteine-rich domain (C1a) is sufficient for TGN localization of a reporter molecule GST. (A) Schematic presentation of the domains of PKD. PKD is composed of two cysteine-rich domains (C1a and C1b), a PH domain and a CD at the C-terminus. (B) The indicated GST-tagged PKD domains and pEGFP-furin were transiently co-expressed in HeLa cells. Two days after transfection, the cells were stained with anti-GST antibody followed by Texas Red-conjugated secondary antibody to visualize GST–PKD domains. The results show that the PH domain and the CD of PKD are not required for TGN localization. (C) The indicated GST–PKD domains and pEGFP-furin were transiently co-expressed in HeLa cells. After 2 days, the cells were stained with anti-GST antibody followed by Texas Red-conjugated secondary antibody as shown in (B). The C1a domain of PKD is sufficient for TGN localization. (D) The indicated GST–PKD domains and pEGFP-furin were co-expressed transiently in HeLa cells. In these transient transfection experiments, the levels of the proteins being expressed vary. Whereas the co-localization of the GST–PKD and pEGFP-furin is seen in all cells expressing these two proteins, cells expressing higher levels of GST–PKD domains show tubulation and the tubules contain furin.
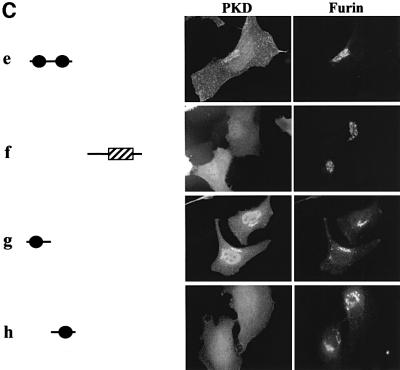
Of all the individual domains tested, GST-tagged PKDΔPH (b), GST-tagged C1a + C1b + PH (c), GST-tagged C1a + C1b (e) and GST-tagged C1a (g) were recruited to the TGN (Figure 3). At low levels of expression, these domains were found bound to TGN, but the TGN was not tubulated. The high levels of expression of these mutant proteins, which contain C1a domain, caused tubulation (Figure 3D). Thus, C1a is sufficient for recruitment to the TGN. Interestingly, the C1b domain by itself was not able to localize GST-tagged C1b to the TGN, and localized it to the plasma membrane more clearly than did other domains (h). This suggests that C1a and C1b have different functions for the localization of PKD (see Discussion).
A novel feature of PKD is the presence of a PH domain between the cysteine-rich domain and the CD. PH domains in general are recognized as important modules for protein–lipid and protein–protein interactions. The PH domain of the oxysterol binding protein (OSBP) is required for targeting to the Golgi membranes (Levine and Munro, 1998). The binding requires phosphatidylinositol-4,5-bisphosphate [PI-(4,5)P2] and an as yet to be identified component on the Golgi membranes. The GST-tagged PH domain of PKD, when expressed in HeLa cells, was not TGN associated (f). On the other hand, GST-tagged PKDΔPH, which lacks only the PH domain, was clearly TGN associated (b). Thus, in the case of PKD, the PH domain is not involved in targeting to the TGN.
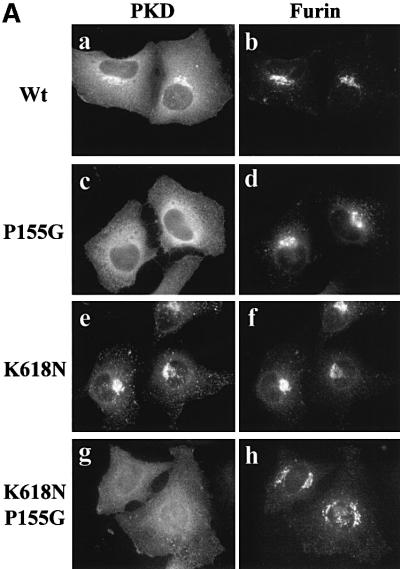
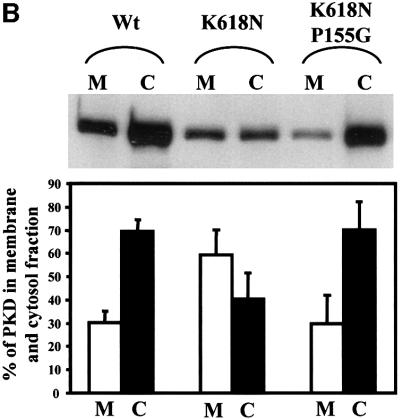
The C1a domain contains a proline at position 155. The conserved proline corresponding to this position is known to contribute to a high-affinity binding site for phorbol esters and DAG for many members of the PKC family of kinases (Zhang et al., 1995). To test whether proline 155 is required for the binding of C1a to the TGN, GST-tagged PKD in which proline 155 was replaced with glycine (P155G) was co-expressed with EGFP-tagged furin in HeLa cells. Staining with anti-GST antibody revealed that GST-tagged PKD-P155G was not associated with the TGN and was mostly cytosolic (Figure 4A, c). As shown previously, PKD-K618N exhibited more intense staining on the TGN than its wild-type counterpart (e). To test whether the P155G mutation also causes mislocalization of PKD-K618N, PKD-K618N/P155G was co-expressed with EGFP-tagged furin in HeLa cells. Staining with the anti-GST antibody showed that GST-tagged PKD-K618N/P155G was not TGN associated (g). The cells expressing GST-tagged PKD-K618N and GST-tagged PKD-K618N/P155G were homogenized and separated into a membrane and cytosol fraction by centrifugation. PKD was isolated from these fractions by adsorption with glutathione–beads, analyzed by SDS–PAGE followed by western blotting with anti-PKD antibody. The blots were quantitated (see Materials and methods) and both the western blot and the histogram of the quantitation are shown in Figure 4B. The results reveal that in cells expressing wild-type PKD, 70% of the total PKD was cytosolic and 30% membrane associated. In cells expressing GST-tagged PKD-K618N, the distribution was 40% cytosolic and 60% on membranes. In cells expressing the GST-tagged PKD-K618N/P155G, ∼70% of the total of this protein was cytosolic. Thus, a proline at position 155 in C1a is necessary for the TGN localization of PKD.
Fig. 4. Proline 155 in the first cysteine-rich domain (C1a) is necessary for TGN localization. (A) HeLa cells were co-transfected with pEGFP-furin and GST–wild-type PKD (a and b), GST–PKD-P155G (c and d), GST–PKD-K618N (e and f) or GST–PKD-K618N/P155G (g and h). The cells were stained with anti-GST antibody to localize GST–PKD and the results show that replacement of proline 155 in the first CRD in wild-type and the kinase-inactive PKD prevents the recruitment of the corresponding PKD to the TGN. (B) HeLa cells transfected with GST–wild-type PKD, GST–PKD-K618N or GST–K618N/P155G were separated into the membrane (M) and the cytosolic (C) fractions. GST-tagged PKD-derivatives were affinity purified from each fraction using glutathione–beads and western blotted with anti-GST antibodies. The corresponding bands were quantitated and the results show that replacing proline with glycine causes a quantitative loss in the recruitment of PKD to the membrane fraction. These data represent the mean ± SD of three independent experiments.
In summary, these results reveal that the C1a domain of PKD is essential and sufficient for recruitment to the TGN, and proline at position 155 in the C1a is important for this localization.
Activation of PKD through phosphorylation of serines 744/748 is required for efficient recruitment to the TGN
Whereas the C1a domain is sufficient to target GST to the TGN, do other regions of the PKD molecule play a role in the recruitment of intact PKD to the TGN? We have focused on the previous findings that serines 744/748 in the activation loop, which are highly conserved in many serine threonine kinases including the PKD, are required for activation of the corresponding kinase (Waldron et al., 1999a). Changing serines 744/748 to alanines causes a loss in the basal activity and phorbol ester-mediated activation of PKD. Replacing serines with glutamic acid results in the phorbol ester-independent activation of the kinase (Iglesias et al., 1998b). It therefore made sense to test whether activation of PKD is important for its subsequent translocation to the TGN.
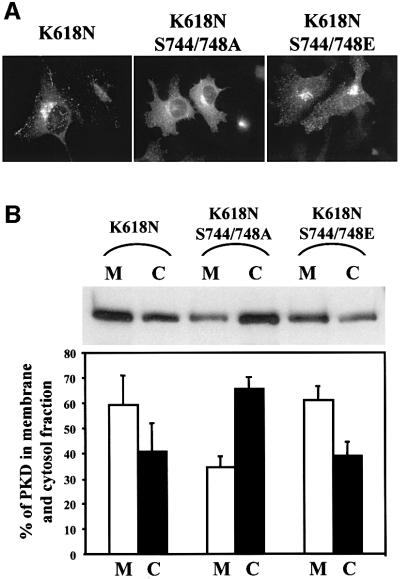
GST-tagged PKD-K618N in which serines 744/748 had been changed to alanines or glutamic acids were expressed in HeLa cells. The cells were visualized with anti-GST antibody to score the location of the expressed proteins. The results show that changing serine 744/748 to alanines causes a reduction in the amount of TGN-associated PKD-K618N and a clear increase in the amount of cytosolic PKD (Figure 5A). The amount of membrane-associated PKD-K618N in which the serines were replaced with alanine or glutamic acid was quantitated as described above. Of the total PKD-K618N, 60% was membrane associated. However, changing serines 744/748 to alanine shifted the equilibrium and 35% of the total kinase was found to be membrane associated (Figure 5B). Changing serines 744/748 to glutamic acids had no significant effect on the recruitment of PKD-K618N to the TGN. These results fit well with those obtained from the microscopy-based analysis shown in Figure 5A. In summary, activation of PKD through phosphorylation of serines 744/748 in PKD-K618N is important for the C1a domain-dependent recruitment to the TGN.
Fig. 5. Phosphorylation of serines 744/748 in the CD is required for the recruitment of intact PKD to the TGN. (A) HeLa cells were transfected with GST–PKD-K618N, GST–PKD-K618N/S744/748A or GST–PKD-K618N/S744/748E. The cells were stained with anti-GST antibody. Replacing serines 744/748 with alanines prevents the recruitment of PKD-K618N to the TGN. (B) HeLa cells transfected with the same expression vectors shown in (A) were fractionated into cytosolic (C) and membrane (M) fractions, and the quantity of PKD contained in these fractions was determined as described in the legend to Figure 4B. The results reveal that replacement of serines 744/748 with alanines quantitatively inhibits recruitment of PKD-K618N to the membrane fractions. These data represent the mean ± SD of three independent experiments.
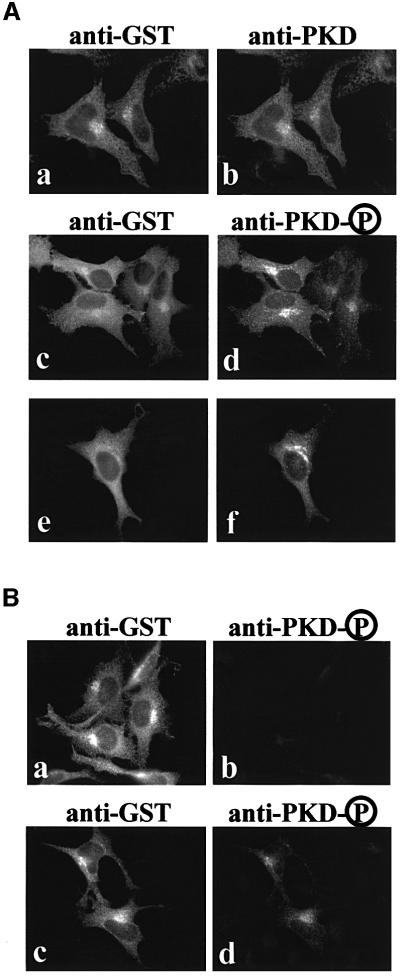
The activated form of PKD, phosphorylated at serine 744/748, undergoes autophosphorylation at serine 916. Phosphorylation of this residue is known to correlate well with the activation status of PKD (Matthews et al., 1999b). An antibody that recognizes the phosphorylated serine 916 has been generated and this antibody (anti-phospho-PKD) was used to address whether the TGN-attached PKD represents the activated form. HeLa cells were transfected with GST-tagged PKD-S744/748E (the constitutively activated form) or GST-tagged PKD-S744/748E/S916A (the form that is activated but cannot phosphorylate serine at position 916) to test the specificity of the anti-phospho-PKD antibody. The cells expressing these constructs were double stained with anti-GST and anti-phospho-PKD antibodies. As shown in Figure 6B, both versions of PKD bind to the TGN (a and c), but GST-tagged PKD-S744/748E/S916A was not recognized by the anti-phospho-PKD antibody (b and d). The anti-phospho-PKD antibody, therefore, recognizes specifically the form of PKD that contains phosphorylated serine 916. Moreover, autophosphorylation of serine 916 is not necessary for binding to the TGN (Figure 6B, a), which supports our finding that a kinase-inactive form of PKD (PKD-K618N) binds to the TGN. But is the TGN-associated pool of wild-type PKD in an activated form? GST-tagged PKD was expressed in HeLa cells and the cells were double stained with anti-GST and rabbit polyclonal anti-PKD antibody (Figure 6A, a and b) or double stained with anti-GST and rabbit polyclonal anti-phospho-PKD antibody (Figure 6A, c–f). The results show that TGN-associated PKD is recognized by the phospho-PKD antibody and is, therefore, present in an active form.
Fig. 6. The TGN-associated pool of PKD is in an active form. (A) HeLa cells were transfected with GST–wild-type PKD and double stained with anti-GST (a) and anti-PKD (b) or anti-GST (c and e) and anti-phospho-PKD (d and f) antibodies. Two different fields (c and d, e and f) are shown here for double staining with anti-GST and anti-phospho-PKD antibodies. The TGN-associated pool of PKD is highly reactive with the phospho-PKD antibody, which recognizes the activated form of PKD (d and f). (B) HeLa cells were transfected with GST–PKD-S744/748E/S916A (a and b) or GST–PKD-S744/748E (c and d) and double stained with anti-GST (a and c) and anti-phospho-PKD (b and d) antibodies. GST–PKD-S744/748E/S916A does not undergo autophosphorylation at serine 916 and is, therefore, not recognized by the phospho-PKD antibody (b), but GST antibody recognizes the TGN-attached pool of PKD (a), whereas expression of the constitutively activated form of PKD in which serines 744 and 748 are replaced with glutamic acid is recognized by both the GST and the phospho-PKD antibodies. These four images were taken under exactly the same conditions. The results confirm the specificity of the anti-phospho-PKD antibody.
Discussion
The mechanism by which kinases are activated and brought to the site of action involves a complex series of reactions. This ensures that the kinase is present in an active form, only when and where it is needed. We have focused on the process by which PKD is recruited to the TGN. Our results can be summarized as follows. Activation of PKD through the phosphorylation of serines 744/748 causes efficient recruitment to the TGN, which takes place via the first cysteine-rich domain (C1a). The TGN-associated pool of PKD is an activated form. PKD localizes to specific domains of the TGN and it remains a strong possibility that these domains are specialized regions of transport carrier formation from the TGN. The overall mechanism of attachment is independent of BFA-sensitive ARF recruitment, which is necessary for Golgi association of many peripherally associated proteins such as the COPI coats.
Our finding that proline 155 in C1a is required for the recruitment of PKD to the TGN might help reveal the connection between DAG, PKD and protein transport from the TGN to the cell surface. In proteins containing the C1 domain, this residue is known to be a high-affinity binding site for lipids, phorbol esters and DAG. Hurley and colleagues have shown that binding of DAG and phorbol esters ‘caps’ a hydrophilic site in the globular structure of the C1 domains in general (Zhang et al., 1995). This binding does not change the conformation of the domains to any great extent, but instead renders the domain into a contiguous hydrophobic region, which in turn promotes their insertion into the lipid bilayer. The involvement of proline 155 within the C1a domain in the recruitment of PKD to the TGN therefore fits well with the proposed mechanism by which C1 domains in general are translocated from the cytosol to the membranes upon receipt of an appropriate signal (Ron and Kazanietz, 1999). Interestingly, DAG is known to be required for the transport of proteins from the Golgi to the cell surface in yeast (Huijbregts et al., 2000). Thus, there is the potential that C1a-mediated interaction with DAG also plays a role in transport between the Golgi and the cell surface in animal cells. Whether DAG is a binding partner for PKD in the TGN membranes, or the binding of DAG promotes anchoring, via the C1 domains, through interactions with some other TGN-associated protein, and how DAG is produced in the TGN are the obvious new questions to be addressed.
The significance of the PH domain and the second cysteine-rich domain of PKD
When we realized that PKD contains a PH domain, our first thought was that this domain might play a role in the recruitment of PKD to the TGN. This is not an unreasonable supposition as proteins such as the OSBP are targeted to the Golgi membranes via the PH domain (Levine and Munro, 1998). However, in the case of PKD, its recruitment to the TGN does not require the PH domain. What then is the role of the PH domain of PKD? As mentioned previously, the binding of Gβγ to the PH domain of PKD activates this kinase (Jamora et al., 1999). Deletion of the PH domain also generates a constitutively activated form of the kinase (Iglesias and Rozengurt, 1998). The PH domain of PKD, therefore, is in a conformation or bound to other proteins that prevent its inadvertent activation. In other words, the PH domain may be an additional checkpoint in the overall activation of PKD.
Why is the C1b not involved in the recruitment of PKD to the TGN? Perhaps the two C1s in PKD have different roles or affinity for lipids regardless of the extensive similarity in overall sequence. This certainly appears to be the case with the C1 domains of PKCα, δ and PKD (Slater et al., 1996; Szallasi et al., 1996; Iglesias et al., 1998a; Matthews et al., 1999a; Medkova and Cho, 1999; Wang et al., 2001). In general, C1 domains contain ∼50 well-conserved amino acids. Position 22 from the N-terminus of these conserved amino acids is occupied by tryptophan. When changed to lysine or tyrosine, this highly conserved amino acid in PKCδ C1b has been reported to severely reduce the affinity to bind DAG without any obvious change in the affinity to bind phorbol dibutyrate (PDBu) (Wang et al., 2001). PKCα also has a tryptophan and tyrosine at the same position of the conserved tryptophan in the C1a and C1b domain, respectively, and it was reported that C1a and C1b have opposite affinities for DAG and phorbol esters (Slater et al., 1996). Mutation analysis showed that this residue in C1a, but not in C1b, is essential for the membrane penetration and activation of PKCα, although C1b has a higher affinity for phorbol esters (Medkova and Cho, 1999). C1b in PKD is responsible for the majority of PDBu binding, both in vitro and in vivo (Iglesias et al., 1998a; Matthews et al., 1999a). C1a of PKD contains the highly conserved tryptophan at position 22. However, in the C1b domain of PKD, this position is occupied by lysine. This could explain the differences in the affinities of C1a and C1b for binding DAG, and hence their affinities for the TGN and other cellular membranes. Another possibility that has to be examined carefully is whether this domain, along with the PH domain, is involved in recruiting other components that facilitate the activity of PKD in membrane fission. Cantley and colleagues (Nishikawa et al., 1998) have shown that two, wortmannin-insensitive PI kinases bind and/or are activated by PKD, and this reaction requires the cysteine-rich domain and kinase activity. One exciting possibility that we favor at present is that the serine phosphorylation in the activation loop of PKD triggers the recruitment via C1a to the TGN. Gβγ then binds to the PH domain, thus activating PKD to recruit other components such as the PI kinases. These reactions then lead, somehow, to the fission of TGN-derived transport carriers.
The new issues
Our findings tell us that the formation of transport carriers requires PKD, which in turn relies on the trimeric G protein subunit Gβγ for its activation. In addition, we propose that at least three additional components will be necessary for the completion of reaction involving Gβγ and PKD. These are: (i) DAG and the enzymes involved in the generation of DAG in the TGN; (ii) the wortmannin-insensitive PI kinases, which bind and/or are activated by the PKD; and (iii) a kinase that phosphorylates serines 744/748 in order to activate PKD for its subsequent recruitment to the TGN.
TGN is the main sorting station for proteins destined to other cellular sites. A tight regulation at this site is employed to generate carriers depending on the size, shape and the quantity of cargo to be transported. Little is known about this type of intracellular regulation and Gβγ-PKD-mediated interactions may just be the beginning of a complex ending.
Materials and methods
Cell culture and reagents
HeLa cells obtained from ATCC and a GF17 cell line expressing GST–FLAG-tagged PKD-K618N were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS) and antibiotics as described previously (Liljedahl et al., 2001). Drs Ian Trowbridge (Salk Institute) and Gary Thomas (Vollum Institute) kindly provided GFP-tagged sialyltransferase (pCMV3-ST-GFP) and furin (pEGFP-furin) expression plasmids, respectively. Rabbit polyclonal antibodies were obtained as follows: anti-GM130, a gift from Dr M.A.De Matteis (Mario Negri Sud Institute, Italy); anti-galactosyl-transferase, from Dr E.G.Berger (University of Zurich, Zurich, Switzerland); and a sheep polyclonal antibody against TGN46, from Dr V.Ponnambalam (Leeds University, Leeds, UK). Polyclonal antibody against GST was from Sigma for electron microscopy and from Pharmacia for all other experiments. Protein A conjugated with colloidal gold was from Dr J.Slot (Utrecht University, Utrecht, The Netherlands) and was used according to the manufacturer’s recommendations.
Construction of mutant plasmids
All mutants were constructed into pME-Py-GST vector, which has a GST tag at the N-terminus of fusion proteins, by PCR or site-directed mutagenesis. pME-Py-GST-PKD wild type and K618N (kinase-dead) were constructed by subcloning of a SalI–XbaI fragment from pGMEXT3-PKD wild type and K618N, respectively, into the same sites of pME-Py-GST vector (Liljedahl et al., 2001). For deletion mutants, the 537–951, 708–1154, 537–1154, 528–1866, 1107–1866 and 1866–2880 nucleotides of mouse PKD gene (DDBJ/EMBL/GenBank accession No. Z34524) were generated by PCR for C1a, C1b, C1a + C1b, C1a + C1b + PH, PH and CD, respectively, and subcloned into the EcoRI and XhoI restriction sites following the GST tag. pME-Py-GST-PKDΔPH was constructed by replacing the NotI–pflMI region of pME-Py-GST-PKD with the fragment excised from pcDNA3-PKDΔPH (Iglesias and Rozengurt, 1998). Serine 744, 748 and 916 and proline 155 point mutations were carried out following the protocol of the Quickchange Site-Directed Mutagenesis Kit (Stratagene). All mutants were verified by sequencing.
Transfections and antibodies
Transfections were carried out using the calcium phosphate method as described previously (Liljedahl et al., 2001) except for fractionation experiments (see below). For the production of polyclonal anti-PKD antibody, a synthetic peptide, EEREMKALSERVSIL, and for the production of polyclonal anti-phospho-PKD antibody, a phosphopeptide CERVS*IL, where S* is phosphorylated, corresponding to the C-terminal portion of PKD were coupled to keyhole limpet hemocyanin (KLH) for immunizing rabbits. Coupling was performed using the imject maleimide kit (Pierce). The antisera were partially purified by precipitation with 50% ammonium sulfate. The commercial rabbit polyclonal antibody against the C-terminus of PKD was also used for immunofluorescence staining (Santa Cruz Biotechnology). The polyclonal anti-phospho-PKD antibody was used at 1:2000 dilution. We could not see any difference between polyclonal antibodies against PKD made in our laboratory and the commercial preparation. Dr Vas Ponnambalam (University of Leeds) kindly provided the sheep polyclonal antibody against TGN46. The goat anti-human transferrin antibody was purchased from Caltag Laboratories. All secondary antibodies, Texas red-, fluorescein isothiocyanate- and Cy2-conjugated donkey anti-rabbit, -goat and -sheep IgG antibodies were purchased from Jackson ImmunoResearch.
Transferrin endocytosis assay
The cells were washed twice with phosphate-buffered saline (PBS) and incubated with pre-incubation medium [DMEM containing 0.5% fat-free bovine serum albumin (BSA)] for 1 h. The cells were incubated with DMEM containing 10% FCS and 40 µg/ml iron-saturated transferrin (Sigma) for 15 min, followed by another 10 min incubation after adding 2.5 µg/ml BFA. The cells were washed briefly three times with cold buffer made up of 0.5 M NaCl and 0.5% acetic acid, and with the pre-incubation buffer once, fixed and immunostained with goat anti-transferrin antibody.
Subcellular fractionation
A total of 1.5 × 107 cells in 400 µl of culture medium were electroporated with 30 µg each of the plasmids under 260 V and 960 µF using a Gene Pulser (Bio-Rad). Three days after electroporation, the cells were harvested, suspended in 1 ml of hypotonic solution made up of 20 mM Tris pH 7.4, 25 mM NaF, 5 mM Na4P2O7, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 µg/ml leupeptin, 0.2 µg/ml aprotinin and 0.2 µg/ml pepstatin for 10 min on ice, homogenized, added with 30 µl of 5 M NaCl and centrifuged at 10 000 g for 10 min. The supernatants were centrifuged at 100 000 g for 1 h to separate the cytosolic (supernatant) and membrane (pellet) fraction. The cytosolic fraction was incubated with EDTA and NP-40 at final concentrations of 1 mM and 1%, respectively. The pellets were resuspended in 1 ml of lysis buffer made up of 20 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA and 1% NP-40, and centrifuged at 100 000 g for 1 h. The cytosolic fractions and supernatants obtained from the pellet fractions were incubated with 30 µl of 50% glutathione–Sepharose suspension overnight. The glutathione–Sepharose was washed four times with 1 ml of lysis buffer and the precipitants were eluted with sample buffer, separated by SDS–PAGE electrophoresis and analyzed by western blotting using rabbit polyclonal antibody against PKD or goat polyclonal antibody against GST and horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) and Renaissance (NEN life Science Products, Inc.). For quantitation purposes, X-ray films were scanned by a scanner (Hewlett Packard) and analyzed using Kodak Digital Science 1D Image Analysis Software (Kodak).
Immunofluorescence and electron microscopy
For immunofluorescence microscopy, the cells were washed with PBS, fixed with 4% formaldehyde in PBS for 10 min at room temperature and incubated with blocking buffer (2.5% horse serum, 1% Triton X-100 in PBS). The cells were then incubated with the respective antibodies in blocking buffer for 40 min at room temperature followed by the appropriate secondary antibodies. For immunoelectron microscopy, a stable line of HeLa cells expressing GST–FLAG-tagged PKD-K618N was used to localize PKD-K618N (Liljedahl et al., 2001). The cells were processed for immunoelectron microscopy in ultrathin cryosections as described by Peters and Hunziker (2001). Sections were collected on butvar-coated nickel grids and incubated with polyclonal antibodies followed by protein A–gold. A rabbit anti-mouse immunoglobulin antibody was used as a bridging antibody when monoclonal antibodies or polyclonal antibodies from sheep were used. PKD was labeled with 10 nm gold particles, whereas Golgi markers were labeled with 5 nm particles.
Acknowledgments
Acknowledgements
Members of the Malhotra laboratory, D.J.Faulkner and Suzanne Pfeffer are thanked for useful discussions. We thank David Timberlake for help with quantitative analysis of the distribution of PKD-K618N. Y.M. is supported by a fellowship from the Uehara Memorial Foundation, Japan, J.V.L. is supported by the ‘Fonds voor wetenschappelijk Onderzoek-Vlaanderen’, the ‘Interuniversitiare Attractiepolen’ (P4/26) and by the Geconcerteerde Onderzoeksacties. G.V.B. and A.A.M. were supported by grants from the Italian Association for Cancer Research (AIRC, Milano, Italy) and Telethon Italy (grants n.E.0982, E.1105 and E.1249). The work in V.M.’s laboratory is supported by grants from the NIH.
References
- Beck K.A., Buchanan,J.A., Malhotra,V. and Nelson,W.J. (1994) Golgi spectrin: identification of an erythroid β-spectrin homolog associated with the Golgi complex. J. Cell Biol., 127, 707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich S., Herget,T., Link,G., Bottinger,H., Pfizenmaier,K. and Johannes,F.J. (1996) In vitro activation and substrates of recombinant, baculovirus expressed human protein kinase C µ. FEBS Lett., 381, 183–187. [DOI] [PubMed] [Google Scholar]
- Hausser A., Storz,P., Link,G., Stoll,H., Liu,Y.C., Altman,A., Pfizenmaier,K. and Johannes,F.J. (1999) Protein kinase C µ is negatively regulated by 14-3-3 signal transduction proteins. J. Biol. Chem., 274, 9258–9264. [DOI] [PubMed] [Google Scholar]
- Huijbregts R.P., Topalof,L. and Bankaitis,V.A. (2000) Lipid metabolism and regulation of membrane trafficking. Traffic, 1, 195–202. [DOI] [PubMed] [Google Scholar]
- Iglesias T. and Rozengurt,E. (1998) Protein kinase D activation by mutations within its pleckstrin homology domain. J. Biol. Chem., 273, 410–416. [DOI] [PubMed] [Google Scholar]
- Iglesias T., Matthews,S. and Rozengurt,E. (1998a) Dissimilar phorbol ester binding properties of the individual cysteine-rich motifs of protein kinase D. FEBS Lett., 437, 19–23. [DOI] [PubMed] [Google Scholar]
- Iglesias T., Waldron,R.T. and Rozengurt,E. (1998b) Identification of in vivo phosphorylation sites required for protein kinase D activation. J. Biol. Chem., 273, 27662–27667. [DOI] [PubMed] [Google Scholar]
- Jamora C., Yamanouye,N., Van Lint,J., Laudenslager,J., Vandenheede,J.R., Faulkner,D.J. and Malhotra,V. (1999) Gβγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell, 98, 59–68. [DOI] [PubMed] [Google Scholar]
- Johannes F.J., Hausser,A., Storz,P., Truckenmuller,L., Link,G., Kawakami,T. and Pfizenmaier,K. (1999) Bruton’s tyrosine kinase (Btk) associates with protein kinase C mu. FEBS Lett., 461, 68–72. [DOI] [PubMed] [Google Scholar]
- Levine T.P. and Munro,S. (1998) The pleckstrin homology domain of oxysterol-binding protein recognizes a determinant specific to Golgi membranes. Curr. Biol., 8, 729–739. [DOI] [PubMed] [Google Scholar]
- Liljedahl M., Maeda,Y., Colanzi,A., Ayala,I., Van Lint,J. and Malhotra,V. (2001) Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell, 104, 409–20. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan,L., Tipper,C., Amherdt,M., Orci,L. and Klausner,R.D. (1991) Brefeldin As effects on endosomes, lysosomes and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell, 67, 601–616. [DOI] [PubMed] [Google Scholar]
- Matthews S., Iglesias,T., Cantrell,D. and Rozengurt,E. (1999a) Dynamic re-distribution of protein kinase D (PKD) as revealed by a GFP–PKD fusion protein: dissociation from PKD activation. FEBS Lett., 457, 515–521. [DOI] [PubMed] [Google Scholar]
- Matthews S.A., Rozengurt,E. and Cantrell,D. (1999b) Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/protein kinase Cµ. J. Biol. Chem., 274, 26543–26549. [DOI] [PubMed] [Google Scholar]
- Matthews S.A., Iglesias,T., Rozengurt,E. and Cantrell,D. (2000) Spatial and temporal regulation of protein kinase D (PKD). EMBO J., 19, 2935–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medkova M. and Cho,W. (1999) Interplay of C1 and C2 domains of protein kinase C-α in its membrane binding and activation. J. Biol. Chem., 274, 19852–19861. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Toker,A., Wong,K., Marignani,P.A., Johannes,F.J. and Cantley,L.C. (1998) Association of protein kinase Cµ with type II phosphatidylinositol 4-kinase and type I phosphatidylinositol-4-phosphate 5-kinase. J. Biol. Chem., 273, 23126–23133. [DOI] [PubMed] [Google Scholar]
- Peters P.J. and Hunziker,W. (2001) Subcellular localization of Rab17 by cryo-immunogold electron microscopy in epithelial cells grown on polycarbonate filters. Methods Enzymol., 329, 210–225. [DOI] [PubMed] [Google Scholar]
- Prestle J., Pfizenmaier,K., Brenner,J. and Johannes,F.J. (1996) Protein kinase C µ is located at the Golgi compartment. J. Cell Biol., 134, 1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.S. and Kreis,T.E. (1992) Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell, 69, 129–138. [DOI] [PubMed] [Google Scholar]
- Ron D. and Kazanietz,M.G. (1999) New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J., 13, 1658–1676. [PubMed] [Google Scholar]
- Slater S.J., Ho,C., Kelly,M.B., Larkin,J.D., Taddeo,F.J., Yeager,M.D. and Stubbs,C.D. (1996) Protein kinase Cα contains two activator binding sites that bind phorbol esters and diacylglycerols with opposite affinities. J. Biol. Chem., 271, 4627–4631. [DOI] [PubMed] [Google Scholar]
- Storz P., Hausser,A., Link,G., Dedio,J., Ghebrehiwet,B., Pfizenmaier,K. and Johannes,F.J. (2000) Protein kinase Cµ is regulated by the multifunctional chaperon protein p32. J. Biol. Chem., 275, 24601–24607. [DOI] [PubMed] [Google Scholar]
- Szallasi Z., Bogi,K., Gohari,S., Biro,T., Acs,P. and Blumberg,P.M. (1996) Non-equivalent roles for the first and second zinc fingers of protein kinase C δ. Effect of their mutation on phorbol ester-induced translocation in NIH 3T3 cells. J. Biol. Chem., 271, 18299–18301. [DOI] [PubMed] [Google Scholar]
- Valverde A.M., Sinnett-Smith,J., Van Lint,J. and Rozengurt,E. (1994) Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc. Natl Acad. Sci. USA, 91, 8572–8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint J.V., Sinnett-Smith,J. and Rozengurt,E. (1995) Expression and characterization of PKD, a phorbol ester and diacylglycerol-stimulated serine protein kinase. J. Biol. Chem., 270, 1455–1461. [DOI] [PubMed] [Google Scholar]
- Waldron R.T., Iglesias,T. and Rozengurt,E. (1999a) Phosphorylation-dependent protein kinase D activation. Electrophoresis, 20, 382–290. [DOI] [PubMed] [Google Scholar]
- Waldron R.T., Iglesias,T. and Rozengurt,E. (1999b) The pleckstrin homology domain of protein kinase D interacts preferentially with the η isoform of protein kinase C. J. Biol. Chem., 274, 9224–9230. [DOI] [PubMed] [Google Scholar]
- Wang Q.J., Fang,T.W., Nacro,K., Marquez,V.E., Wang,S. and Blumberg,P.M. (2001) Role of hydrophobic residues in the C1b domain of protein kinase C δ on ligand and phospholipid interactions. J. Biol. Chem., 276, 19580–19587. [DOI] [PubMed] [Google Scholar]
- Wood S.A., Park,J.E. and Brown,W.J. (1991) Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell, 67, 591–600. [DOI] [PubMed] [Google Scholar]
- Zhang G., Kazanietz,M.G., Blumberg,P.M. and Hurley,J.H. (1995) Crystal structure of the cys2 activator-binding domain of protein kinase C δ in complex with phorbol ester. Cell, 81, 917–924. [DOI] [PubMed] [Google Scholar]