Abstract
To obtain a better understanding of how hippocampal neurons selectively target proteins to axons, we assessed whether any of the large cytoplasmic regions of neuronal sodium channel Nav1.2 contain sufficient information for axonal compartmentalization. We show that addition of the cytoplasmic C-terminal region of Nav1.2 restricted the distribution of a dendritic–axonal reporter protein to axons. The analysis of mutants revealed that a critical segment of nine amino acids encompassing a di-leucine-based motif mediates axonal compartmentalization of chimera. In addition, the Nav1.2 C-terminus is recognized by the clathrin endocytic pathway both in non-neuronal cells and the somatodendritic domain of hippocampal neurons. The mutation of the di-leucine motif located within the nine amino acid sequence to alanines resulted in the loss of chimera compartmentalization in axons and of internalization. These data suggest that selective elimination by endocytosis in dendrites may account for the compartmentalized distribution of some proteins in axons.
Keywords: axonal sorting/endocytosis/hippocampal neurons/sodium channel
Introduction
Neurons possess the remarkable ability to selectively target proteins to distinct axonal or somatodendritic domains. This leads to the asymmetric organization of the neuronal plasma membrane which is essential for vectorial communication. The mechanisms by which hippocampal neurons target proteins to dendrites are starting to be unraveled. Carrier vesicles containing dendritic proteins are sorted at the level of the trans-Golgi network (for review see Winckler and Mellman, 1999) and are excluded from axons (Burack et al., 2000). At present, three major groups of sorting signals that account for the vectorial trafficking of dendritic proteins have been identified. The first group includes tyrosine-based signals (Jareb and Banker, 1998), whereas the second group comprises di-leucine-based motifs (Poyatos et al., 2000). It is noteworthy that both of these sorting signals are also involved in protein targeting to the basolateral membrane of epithelial cells, such as the MDCK line (for review see Mellman, 1996). Thus, neurons can recognize the same motifs for somatodendritic targeting that epithelial cells use for basolateral targeting. In addition, novel somatodendritic sorting sequences falling outside these two defined groups have recently been characterized (West et al., 1997a; Stowell and Craig, 1999; Lim et al., 2000; Ruberti and Dotti, 2000).
Whereas information on dendritic targeting signals is emerging, much less is known about the mechanisms that lead to the polarized distribution of axonal proteins in hippocampal neurons (Winckler and Mellman, 1999; Burack et al., 2000). Most of the proteins that are directed to apical membranes in epithelial cells are distributed both on dendrites and axons when expressed in hippocampal neurons, indicating that apical sorting signals are not recognized as axonal determinants (Jareb and Banker, 1998). It is thought that the sorting of proteins to axons is under the control of specific sequence motifs distinct from those ensuring dendritic targeting, but little information is available about their structure (Winckler and Mellman, 1999). In addition, the possibility remains open that mechanisms downstream of carrier vesicle transport operate to target proteins to axons. A recent study (Burack et al., 2000) involving the visualization of carrier vesicles containing green fluorescent protein-tagged NgCAM (neuron–glial cell adhesion molecules) has shown that this axonal membrane protein follows a non-vectorial trafficking pathway and is transported in vesicles within both axons and dendrites. This suggests that vesicles containing axonal proteins are not able to fuse with the dendritic membrane and are only inserted in axonal membranes. Alternatively, it has been proposed (Stowell and Craig, 1999; Winckler and Mellman, 1999; Burack et al., 2000) that insertion of axonal proteins into the somatodendritic domain occurs, but is followed by selective elimination; however, this has never been demonstrated.
One of the major physiological roles of neuronal voltage-gated sodium channels is to generate action potentials at the axon hillock/initial segment, and to ensure propagation along myelinated or unmyelinated fibers to nerve terminals (Stuart et al., 1997; Catterall, 2000). Localization studies have shown that a member of the voltage-gated sodium channel family, Nav1.2, is preferentially distributed on fibers in adult rat brain (Westenbroek et al., 1989; Gong et al., 1999). Biochemical, developmental and genetic studies suggest that the cytoskeletal linker protein ankyrin G could play an important role in sodium channel clustering at the initial segment (for review see Bennett and Lambert, 1999). However, the upstream mechanisms that initially direct sodium channels to the axonal membrane remain poorly understood.
During the early stages of the establishment of neuronal polarity, Nav1.2 was found to be preferentially distributed on axons of developing hippocampal neurons, and to display a high density at the initial axonal segment (Dargent et al., 1998). Molecular characterization in developing hippocampal neurons indicated that Nav1.2, the pore-forming α subunit, is associated with the auxiliary β2 subunit, but not the β1 subunit, in good agreement with previous studies (Scheinman et al., 1989; Gong et al., 1999). The observation that a myc-tagged β2 subunit expressed by transfection in hippocampal neurons was uniformly distributed on dendrites and axons (B.Sampo, J.A.Boudier, E.Carlier, F.Jullien, J.L.Boudier, A.Le Bivic, R.A.Maue and B.Dargent, submitted) eliminated the possibility that the sodium channel β2 subunit carries a dominant axonal sorting signal and suggested that Nav1.2 contains intrinsic information for axonal compartmentalization. Therefore, the goal of the present study was to examine this hypothesis. A strategy based on the expression of chimeras in hippocampal neurons demonstrated that the cytoplasmic C-terminal domain of Nav1.2 restricted the distribution of a dendritic–axonal reporter protein to axons. The analysis of mutants revealed a region of nine amino acids encompassing a di-leucine-based motif within the proximal region of the Nav1.2 C-terminus that determines axonal compartmentalization.
Results
The C-terminal cytoplasmic domain of Nav1.2 contains an axonal determinant
In preliminary experiments, we expressed Nav1.2 tagged with a myc epitope in COS-7 cells. After cell permeabilization, significant immunostaining was observed intracellularly, but no labeling was revealed on non-permeabilized cells using an antibody directed against an extracellular epitope (not shown), indicating that the level of surface expression was too low for detection by immunofluorescence. Similar results were obtained when Nav1.2 was transfected in hippocampal neurons. To circumvent this problem, and taking into account the fact that targeting/clustering motifs have been identified within cytoplasmic regions of potassium channels (Zito et al., 1997; Lim et al., 2000) and of the AMPA receptor (Ruberti and Dotti, 2000), we addressed the question as to whether any of the large intracellular regions of Nav1.2 contain axonal sorting and/or clustering signals. We therefore constructed chimeric proteins (Figure 1B) in which the cytoplasmic tail of the human CD4 receptor, a type I membrane protein, was replaced by loop I–II, loop II–III or the C-terminal cytoplasmic tail of Nav1.2. In parallel, we replaced the cytoplasmic N-terminus of the human transferrin receptor (hTfR), a type II membrane protein, with that of Nav1.2. The CD4–Nav1.2 loop III–IV chimera was not generated. This region is involved in fast channel inactivation, is relatively highly conserved (Catterall, 2000) and is thus unlikely to contain subtype-specific sorting signals.
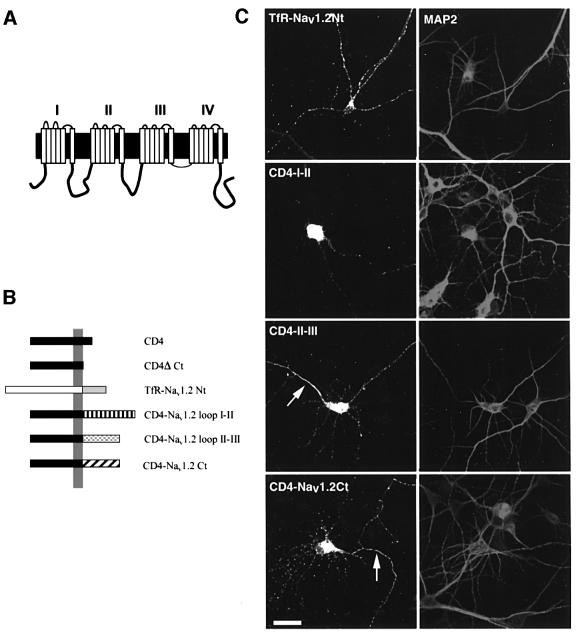
Fig. 1. Expression of chimeric proteins containing the cytoplasmic regions of Nav1.2 in hippocampal neurons. (A) Schematic representation of the neuronal sodium channel Nav1.2. The N- and C-termini are located intracellularly. Four homologous domains (I–IV), each containing six transmembrane segments, are connected by large cytoplasmic loops (I–II, II–III and III–IV). (B) Schematic representation of the chimeric proteins. Because of the topology of the intracellular regions of Nav1.2, two types of chimera were generated. The N-terminus of the human transferrin receptor, a type II membrane protein, was replaced by the N-terminus of Nav1.2 (hTfR–Nav1.2Nt). The other chimeras were composed of the extracellular and transmembrane regions of human CD4 receptor and loop I–II (CD4–I–II), loop II–III (CD4–II–III) and the C-terminal region of Nav1.2 (CD4–Nav1.2Ct). (C) Expression of the chimeras in hippocampal neurons. The constructs indicated were transfected into seven DIV hippocampal neurons. Two days later, neurons were fixed, permeabilized, and double stained for MAP2 and either hTfR or CD4. The N-terminal chimera is localized only in the somatodendritic domain, while the loop I–II chimera is mostly concentrated in soma, and to a lower extent throughout the dendrites. Note that the chimera containing loop II–III was concentrated within the axon initial segment (arrow), whereas only the CD4–Nav1.2Ct chimera was immunodetected all along the axon (arrow). Bar, 50 µm.
We first verified using either an anti-hTfR antibody or an anti-CD4 antibody that the chimeric proteins were efficiently transported and inserted into the plasma membrane of non-permeabilized COS-7 cells (not shown, but see Figure 4). Chimeras were next transiently expressed in hippocampal neurons and their expression was analyzed by immunostaining either before or after cell permeabilization to detect surface and total distribution. Discrimination between axonal and somatodendritic domains was based on staining for MAP2, a somatodendritic marker, and on morphological criteria. Axons were identified as MAP2-negative processes. Figure 1C shows chimera distribution in permeabilized hippocampal neurons. The TfR–Nav1.2Nt chimera was visualized only in the somatodendritic domain. CD4–I–II was mainly localized within the soma and some labeling was detected in dendrites but not in axons. CD4–II–III was also distributed in the soma and within the axonal initial segment. A lower staining was visualized in dendrites and in the distal region of axons. CD4–Nav1.2Ct was detected within the soma and all along axons. Vesicular staining in dendrites was also observed.
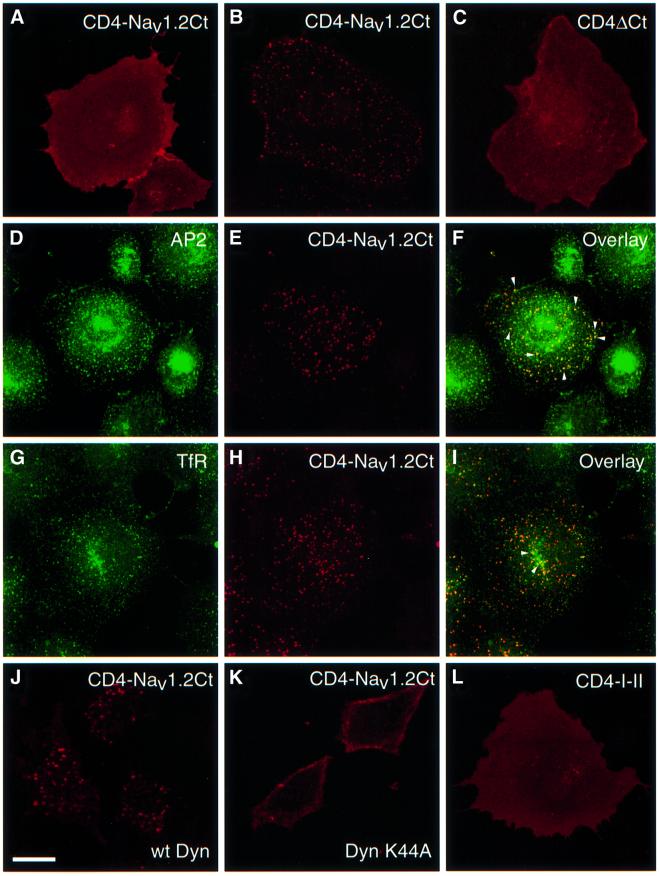
Fig. 4. Internalization of CD4–Nav1.2Ct through the clathrin-dependent pathway in non-neuronal cell lines. COS-7 cells were transfected with CD4–Nav1.2Ct (A and B), CD4ΔCt (C) and CD4–I–II (L) constructs. Living cells were subjected to an immunoendocytosis assay for 20 min. CD4–Nav1.2Ct showed a diffuse membrane staining after the pre-labeling step with an anti-CD4 antibody at 4°C (A). Following 20 min of incubation at 37°C, antibody-prelabeled CD4–Nav1.2Ct (B) displayed a punctate intracellular distribution, whereas CD4ΔCt (C) and CD4–I–II (L) were confined to the cell surface. (D–I) Following an immunoendocytosis assay, COS-7 cells expressing CD4–Nav1.2Ct were exposed to either anti-AP2 (E) or anti-transferrin receptor (H) monoclonal antibodies. Vesicles containing CD4–Nav1.2Ct co-localized with AP2 (arrowheads, F) and with transferrin receptor (arrowheads, I). (J and K) HeLa cells expressing wild-type dynamin (J) or mutant K44A dynamin (K) were transfected with CD4–Nav1.2Ct and an immunoendocytosis assay similar to that described above. A representative confocal section of CD4–Nav1.2Ct staining for each condition is shown. In HeLa cells expressing wild-type dynamin, CD4–Nav1.2Ct is efficiently endocytosed (J, confocal section), whereas in cells expressing mutant dynamin the chimeric protein remains in the plasma membrane (K, confocal section). Bar, 25 µm.
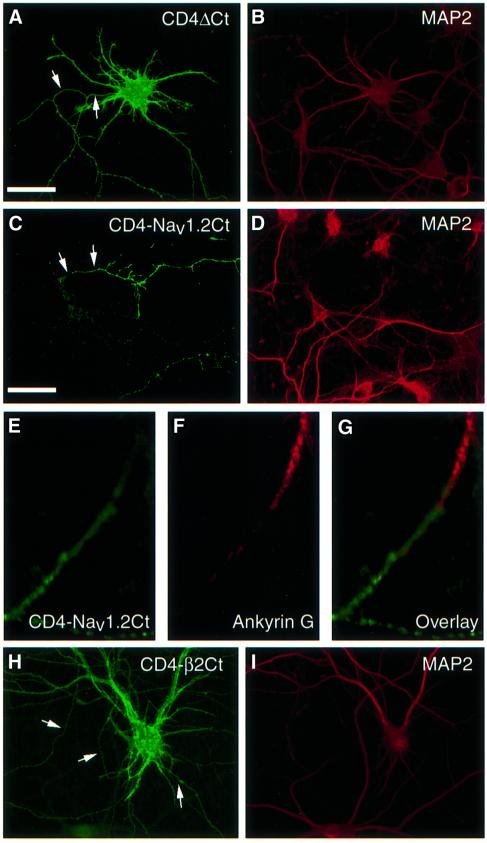
We next examined the surface expression of the chimeras at the steady state on non-permeabilized hippocampal neurons. hTfRΔNt shows a non-polarized distribution when expressed in hippocampal neurons (West et al., 1997a). TfR–Nav1.2Nt was visualized on the somatodendritic domain, but not in axons (100% of hTfR-positive cells, n = 30, three independent experiments; not shown), indicating that this chimera is not able to enter axons. CD4ΔCt was detected both in the somatodendritic and axonal membranes (Figure 2A), whereas CD4–I–II and CD4–II–III chimeras were not immunodetected at the plasma membrane (not shown), in contrast to what was observed in COS-7 cells. As shown in Figure 2C, the addition of the C-terminal region of Nav1.2 to CD4ΔCt restricted the surface distribution of the chimeric protein to axons. CD4-labeled axons were thinner and much longer than dendrites, and extended far beyond the cell body of transfected cells, forming numerous branched processes. The polarized surface expression of CD4–Nav1.2Ct was very reproducible (Table I; Figure 3B), and its distribution was more pronounced distally than proximally (Figure 2E). Endogenous Nav1.2 subunits are preferentially distributed on axons of developing hippocampal neurons, and in particular at the initial segment where they co-localize with ankyrin G (Bennett and Lambert, 1999). A direct comparison of the immunostaining of endogenous Nav1.2 with that of CD4–Nav1.2Ct could not be carried out because the only anti-Nav1.2 antibody that provides satisfactory staining in our hands is directed against the C-terminus (Gong et al., 1999). Therefore, we used an antibody against ankyrin G, a marker of the initial axonal segment. Double immunostaining showed that CD4–Nav1.2Ct was not concentrated at the initial segment, but was generally located in more distal regions of the axon than ankyrin G (Figure 2E–G). Finally, when the cytoplasmic tail of CD4 was replaced by the cytoplasmic C-terminus of the auxiliary β2 subunit, the corresponding chimera CD4–β2Ct was uniformly distributed on dendrites and axons (Figure 2H).
Fig. 2. Addition of the C-terminus of Nav1.2 restricts the surface distribution of CD4 to axons. Seven DIV hippocampal neurons were transfected with CD4ΔCt (A), CD4–Nav1.2Ct (C and E) and CD4–β2Ct (H) constructs. After 48 h, neurons were fixed and incubated with an anti-CD4 antibody prior to permeabilization, then exposed either to an antibody against MAP2, a somatodendritic marker (B, D and I) or to an anti-ankyrin G antibody (F). CD4ΔCt (A) was distributed both in dendrites and axons (arrows). In contrast, addition of the Nav1.2 C-terminus (C) restricts chimera expression to axons (arrows), whereas the addition of β2 C-terminus did not (H). The expression of CD4–Nav1.2Ct (green; E) was detected in axonal domains distal to ankyrin G (red; G), which is localized in the initial segment. Bar, 50 µm.
Table I. Differential surface distribution of CD4 chimeras containing the C-terminal region of Nav1.1, Nav1.2 and Nav1.6.
| Axonal | Somatodendritic | Non-polarized | |
|---|---|---|---|
| CD4–Nav1.1 | 31 ± 8 | 69 ± 8 | |
| CD4–Nav1.2 | 89 ± 0.5 | 11 ± 0.5 | |
| CD4–Nav1.6 | 82 ± 18 | 27 ± 12 |
Data (mean ± SD) are expressed as percentages, taking as 100% the total population of transfected neurons, i.e. CD4-positive neurons from three (CD4–Nav1.1, n = 50; CD4–Nav1.6, n = 81) and six (CD4–Nav1.2, n = 50) different experiments.
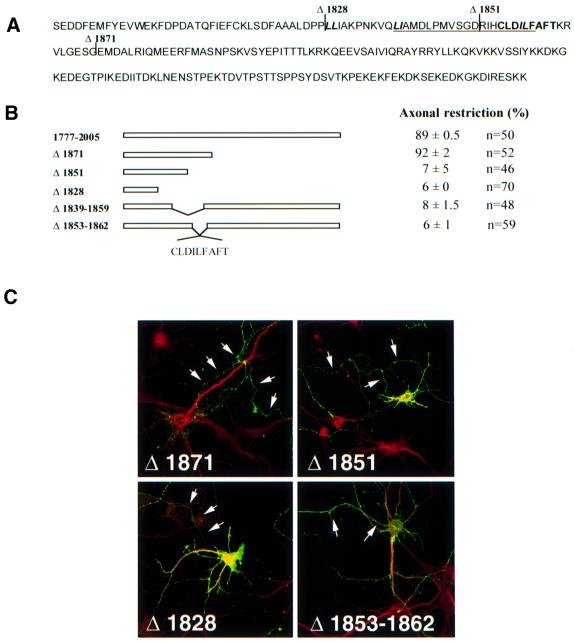
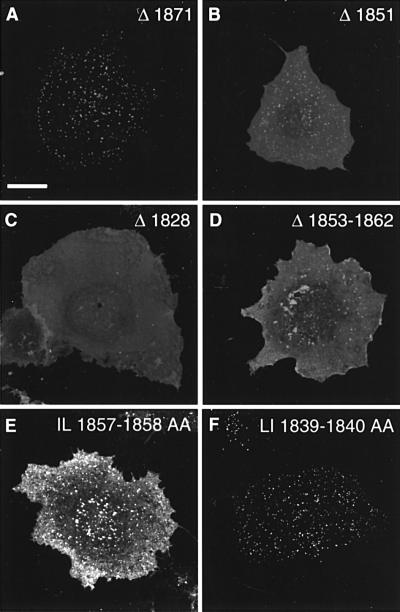
Fig. 3. The proximal region of the Nav1.2 C-terminus governs the axonal compartmentalization of the CD4 chimera. (A) Amino acid sequence of the Nav1.2 C-terminus (1777–2005) showing the major deletions (Δ1828, Δ1851 and Δ1871). Internal deletion Δ1839–1859 is underlined and Δ1853–1862 is in bold. Di-leucine motifs are represented in italics. (B) Schematic representation of mutations generated in the C-terminus of Nav1.2. The percentage of transfected CD4-positive hippocampal neurons with axonal polarity is indicated, taking as 100% the total population of transfected neurons, i.e. CD4-positive neurons. Hippocampal neurons were considered as showing restricted axonal expression when a cell process displayed immunoreactivity to anti-CD4 antibodies, but staining was absent at the surface of dendrites and soma. Data are represented as the mean ± SD from six different experiments and n represents the total number of neurons analyzed for each chimera. (C) Surface expression of CD4–Nav1.2Ct mutants. Seven DIV hippocampal neurons were transfected with the indicated constructs. Two days later, they were double stained for CD4 (green) and MAP2 (red). The surface distribution of the truncated mutant Δ1871 was restricted to axons (arrows), indicating that the C-terminal extremity of Nav1.2 is not required for the polarized expression of the chimera. In contrast, the Δ1851 and Δ1828 mutants were distributed on soma and dendrites as well as in axons. The internal deletion of nine amino acids between 1853 and 1862 resulted in the loss of CD4–Nav1.2Ct restriction in axons.
To ascertain further that the C-terminus of Nav1.2 contains specific information for axonal compartmentalization, we generated chimeras in which the C-terminus of CD4 was replaced by that of two other neuronal sodium channel types: Nav1.1 or Nav1.6 (Goldin, 1999). Table I shows that these chimeras displayed distinct steady-state distributions when expressed in hippocampal neurons. CD4–Nav1.1Ct was distributed on the somatodendritic and axonal membranes (69% of cells), whereas CD4–Nav1.6Ct was found to be located in the somatodendritic domain (82% of cells). Altogether, these results indicate that the C-terminus of Nav1.2 carries specific information that restricts the expression of an axonal–dendritic reporter protein to axons.
Identification of the axonal determinant located within the C-terminal region of Nav1.2
The next step was to identify the molecular determinant(s) of the C-terminal of Nav1.2 implicated in the polarized expression of CD4–Nav1.2Ct. With this aim, we generated truncated mutants by PCR (Δ1974, Δ1915, Δ1892, Δ1871, Δ1851 and Δ1828; Figure 3B). The surface distribution of the mutant proteins was analyzed by immunostaining in transfected hippocampal neurons. Deletion of the C–terminal domain distal to residues 1974, 1915, 1892 or 1871 did not alter the compartmentalized expression of chimeric proteins. The results obtained with the Δ1871 chimera are illustrated (Figure 3); they demonstrate an absence of staining in dendrites, whereas an axonal localization was still observed. This indicates that the distal C-terminal region (residues 1871–2005) of the Nav1.2 subunit does not contain axonal determinants. In contrast, mutants Δ1851 and Δ1828 displayed a non-polarized distribution, as judged by their surface expression detected on both dendrites and axons (Figure 3B and C). Thus, a critical determinant located between amino acids 1851 and 1871 governs the polarized expression pattern of CD4–Nav1.2Ct. To define this determinant more precisely, we constructed two internal deletions (Δ1839–1859 and Δ1853–1862; Figure 3). Both mutants showed a non-compartmentalized expression in transfected seven DIV hippocampal neurons (Figure 3B and C). Thus, a motif of nine amino acids governs the axonal compartmentalization of CD4–Nav1.2Ct.
CD4–Nav1.2Ct chimera undergoes internalization through the clathrin-dependent pathway
At least two distinct pathways may underlie the polarized expression of CD4–Nav1.2Ct in hippocampal neurons. The protein could be delivered directly to axons. However, in permeabilized cells, dendrites were positive for CD4 staining (Figure 1C). Therefore, it is possible that CD4–Nav1.2Ct is initially transported and inserted into both domains, but is retrieved from the dendritic plasma membrane while it is retained in the axonal membrane. To examine whether the C-terminal tail of Nav1.2 is recognized by components of the endocytotic pathway, we first applied an immuno-endocytosis assay to COS-7 cells expressing the constructs. When the surface population of CD4–Nav1.2Ct was pre-labeled with an anti-CD4 antibody at 4°C, followed by a 20 min incubation at 37°C, it was found to be located exclusively in intracellular vesicles visualized by confocal microscopy (Figure 4A and B). The disappearance of diffuse membrane staining indicated that antibody-labeled CD4–Nav1.2Ct was efficiently retrieved from the plasma membrane. The typical endocytotic pattern was already detected after 10 min at 37°C and was resistant to an acid wash (not shown). Other chimeric proteins such as CD4ΔCt, CD4–I–II (Figure 4C and L) and CD4–β2Ct (not shown) were not internalized. We then examined whether the internalization of CD4–Nav1.2Ct was mediated by the clathrin-dependent pathway. Figure 4D–F shows that vesicles containing CD4–Nav1.2Ct co-localized with adaptor protein 2 (AP2), which is involved in clathrin-coated pit assembly (Marsh and McMahon, 1999). Co-staining with the transferrin receptor, a marker of recycling endosomes (Figure 4G–I), and with the early endosome antigen 1 (EEA1; not shown) was also observed. To confirm these findings, experiments were carried out in HeLa cell lines expressing either wild-type dynamin II or a defective dynamin mutant (K44A; Schmid, 1997). After 10 min of incubation at 37°C, CD4–Nav1.2Ct was efficiently internalized in cells expressing wild-type dynamin (Figure 4J). In contrast, the localization of the chimeric protein was restricted to the plasma membrane in the mutant cell line, due to the inability of dynamin K44A to retrieve clathrin-coated vesicles (Figure 4K). These data strongly indicate that at least one internalization motif located in the C-terminal region of Nav1.2 is efficiently recognized by the clathrin-dependent endocytotic machinery in non-neuronal cells.
We next evaluated the possibility that CD4–Nav1.2Ct was also internalized when expressed in hippocampal neurons. A similar endocytosis assay was applied to transfected neurons, with the exception that the anti-CD4 antibody was added directly to the culture medium at 37°C. When visualized by confocal microscopy, antibody-labeled CD4–Nav1.2Ct was detected in vesicles distributed throughout dendrites and the cell body (Figure 5A). Some vesicles were also visualized in the proximal regions of axons, but not in more distal segments. In contrast, CD4ΔCt (Figure 5B) was not internalized, its localization being confined to the neuronal plasma membrane, like CD4–β2Ct (not shown). The presence of internalized CD4–Nav1.2Ct in endosomes throughout the somatodendritic domain was further confirmed by co-localization either with EEAI, a marker of somatodendritic early endosomes (Wilson et al., 2000) (Figure 5C–E), or with transferrin receptor and AP2 (not shown). Thus, these observations indicate that CD4–Nav1.2Ct undergoes internalization in the somatodendritic domain of hippocampal neurons.
Fig. 5. Internalization of CD4–Nav1.2Ct in the somatodendritic domain of hippocampal neurons. Seven DIV hippocampal neurons were transfected with CD4–Nav1.2Ct (A) or CD4ΔCt constructs (B). Following an immunoendocytosis assay, cells were fixed, permeabilized and incubated with anti-MAP2 antibody (red). CD4–Nav1.2Ct-containing vesicles (green) are distributed throughout dendrites (arrows) and the soma (box). Some punctate staining also appeared to be concentrated in the proximal portion of the axon (asterisk). In contrast, internalization of CD4ΔCt was not detected. Inserts represent 0.5 µm confocal sections of neuronal cell bodies showing the presence (A) or absence (B) of endocytotic vesicles. The axons of transfected cells are indicated by arrowheads. Hippocampal neurons expressing CD4–Nav1.2Ct (D) were subjected to an immunoendocytosis assay as described above. Following fixation and permeabilization, cells were exposed to an anti-EEA1 antibody (C). The overlay (E) shows that vesicles containing CD4–Nav1.2Ct (red) are also positive for EEA1 (green; arrows). Bar, 25 µm.
A di-leucine motif acts as an axonal determinant and an internalization signal
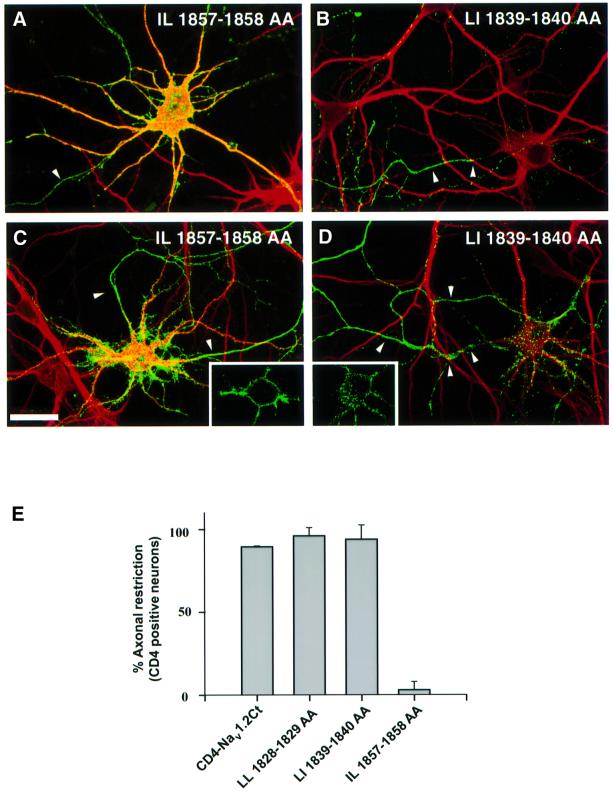
The nine amino acid sequence that is critical for CD4–Nav1.2Ct restriction to axons encompasses a potential di-leucine internalization motif (IL 1857–1858; Figure 3). Therefore, we next addressed a possible relationship between the internalization of CD4– Nav1.2Ct and its restriction to axonal membranes at steady state. We first analyzed the ability of truncated and internal mutants to undergo internalization in COS-7 cells (Figure 6). Confocal microscopy analysis showed that the Δ1871 mutant was still efficiently internalized in COS-7 cells (Figure 6A), and no significant cell surface signal was detectable 20 min after pre-labeling, as previously observed with wild-type CD4–Nav1.2Ct (Figure 4B). In contrast, expression of the mutant Δ1828 was confined to the cell surface (Figure 6C). In the case of the Δ1851 mutant, both surface and internalized populations were identified (Figure 6B), reflecting slower internalization kinetics compared with Δ1871 or the wild-type CD4–Nav1.2Ct sequence. The internal deletion of nine amino acids (Δ1853–1862) greatly reduced chimera internalization (Figure 6D). A chimera containing an internal deletion between amino acids 1839 and 1859 was also endocytosed less efficiently in COS-7 cells (not shown). To confirm further the involvement of IL 1857–1858 in CD4–Nav1.2Ct internalization, this motif was replaced by alanines. The internalization of the corresponding mutant (IL 1857–1858 AA) was strongly affected (Figure 6E), as compared with CD4–Nav1.2Ct (Figure 4B). Confocal analysis indicated that most of the punctate staining observed for the mutant IL 1857–1858 AA was located at the plasma membrane. We next examined whether this substitution influences the polarized expression of CD4–Nav1.2Ct in hippocampal neurons. As shown in Figure 7A and E, the IL 1857–1858 AA mutant displayed a non-polarized steady-state distribution, as visualized by the presence of somatodendritic and axonal surface staining. Importantly, mutation of LI 1839–1840 to alanines did not influence CD4–Nav1.2Ct internalization in COS-7 cells (Figure 6F) and its axonal restriction in hippocampal neurons (Figure 7B and E). Similar results were obtained when the LL 1829–1830 motif was replaced by alanines (Figure 7E). The results obtained with site-directed mutants, which are in good agreement with the properties of the truncated mutant (Figure 3), indicated that the di-leucine motif IL 1857–1858 governs chimera compartmentalization in axons. The ability of mutant IL 1857–1858 AA to be internalized in somatodendritic domains was impaired, as visualized by confocal microscopy (Figure 7C), whereas vesicles containing the LI 1839–1840 AA mutant were distributed in soma and dendrites (Figure 7D). Altogether, our data indicate that the di-leucine motif IL 1857–1858 mediates CD4–Nav1.2Ct compartmentalization in axons and its elimination in soma and dendrites by endocytosis.
Fig. 6. Involvement of a di-leucine motif in internalization of CD4– Nav1.2Ct in COS-7 cells. COS-7 cells expressing the CD4–Nav1.2Ct mutants indicated were submitted to an immunoendocytosis assay. The truncated Δ1871 (A) mutant showed a punctate and predominantly intracellular distribution, similar to that observed with wild-type CD4–Nav1.2Ct (Figure 4B). The Δ1851 mutant (B) was localized predominantly at the plasma membrane and displayed some punctate staining. In contrast, the Δ1828 mutation (C) impaired internalization; note the diffuse membrane staining. Mutant Δ1853–1862 (D) was mainly confined to the plasma membrane as with Δ1851. The substitution of the di-leucine motif at position 1857–1858 with alanines (E) strongly affected internalization, whereas replacement of LI 1839–1849 with alanines was ineffective (F).
Fig. 7. Involvement of a di-leucine motif in the axonal compartmentalization of CD4–Nav1.2Ct in hippocampal neurons. (A and B) Surface expression of CD4–Nav1.2Ct mutants in which the di-leucine motifs located in the proximal region have been replaced by alanines. Seven DIV hippocampal neurons were transfected with the constructs indicated. Two days later, they were double stained for CD4 (green) and MAP2 (red). The mutation of the IL 1857–1858 motif resulted in the loss of CD4–Nav1.2Ct compartmentalization in axons (A), whereas mutation of LI 1839–1840 was ineffective (B). Bar, 50 µm. (C and D) Hippocampal neurons expressing site-directed CD4–Nav1.2Ct mutants were submitted to an immunoendocytosis assay. Confocal microscopy analysis shows that IL 1857–1858 AA (C) mutant was confined to the cell surface, whereas vesicles containing LI 1839–1840 AA (D) are distributed throughout soma and dendrites. Inserts represent 0.5 µm confocal sections of neuronal cell bodies showing the absence (C) or presence (D) of endocytotic vesicles. The axons of transfected cells are indicated by arrowheads. (E) Involvement of IL 1857–1858 in the axonal compartmentalization of CD4–Nav1.2Ct. For each of the site-directed mutants indicated, the percentage of transfected CD4-positive hippocampal neurons with axonal polarity is indicated, taking as 100% the total population of transfected neurons, i.e. CD4-positive neurons. Hippocampal neurons were considered as showing restricted axonal expression when a cell process displayed immunoreactivity to anti-CD4 antibodies, but staining was absent at the surface of dendrites and soma. Data are represented as the mean ± SD from three different experiments, where n represents the total number of neurons analyzed for each mutant (CD4–Nav1.2Ct, n = 50; LL 1828–1829 AA, n = 60; LI 1839–1840 AA, n = 76; IL 1857–1858 AA, n = 159).
Discussion
Information relevant to ion channel trafficking has recently been obtained from expression studies in heterologous systems (Zerangue et al., 1999; Bichet et al., 2000). However, in neurons, the molecular determinants implicated in ion channel compartmentalization into subdomains of the plasma membrane are less well documented. This is in part due to the complex oligomeric or heteromeric composition of ion channels, which hampers dissection of targeting and/or clustering signals. For example, the AMPA receptor, an exogeneous GluR1 subunit lacking a somatodendritic targeting signal, was still appropriately sorted to dendrites because of its association with endogenous subunits (Ruberti and Dotti, 2000). To circumvent these difficulties, a strategy based on chimera construction has recently been developed (Zito et al., 1997; Lim et al., 2000; Ruberti and Dotti, 2000). In the present study, an analysis of the distribution of chimeric proteins expressed in hippocampal neurons revealed specific features of the intracellular regions of Nav1.2, and provides new insights into the mechanisms that underlie the compartmentalization of axonal proteins in neurons.
A motif in the cytoplasmic C-terminus of Nav1.2 acts as an axonal determinant and as an internalization signal
We demonstrate here that the addition of the Nav1.2 C-terminus to the extracellular and transmembrane segment of a reporter protein was able to restrict the surface distribution of the chimeric protein to axons, unlike the other intracellular regions tested (the Nav1.2 N-terminus, loops I–II and II–III). The fact that CD4 chimeras composed of the C-terminus of Nav1.1, Nav1.6 or β2 exhibited either a non-polarized or a somatodendritic distribution further supports the idea that the C-terminus of Nav1.2 carries information for axonal compartmentalization.
An important aspect of our work is the demonstration that a critical di-leucine motif within the proximal region of the Nav1.2 C-terminus (IL 1857–1858) acts as an axonal determinant and as an internalization signal. Interestingly, this sequence is distinct from those used by mGluR7 (Stowell and Craig, 1999) and by VAMP2 (West et al., 1997b). This type of motif also mediates basolateral sorting of membrane proteins in epithelial cells (Winckler and Mellman, 1999), whereas in hippocampal neurons it has been shown that a di-leucine motif in the glycine transporter is used as a somatodendritic targeting signal (Poyatos et al., 2000). The maintenance of the glycine transporter in dendrites may be mediated through PDZ protein interactions, impairing internalization and subsequent redistribution in axons. In the case of CD4–Nav1.2Ct, it is conceivable that the chimera is inserted into dendrites, but as it lacks a retention signal, it would be rapidly retrieved by endocytosis and subsequently transported to axons.
From our study, it can also be concluded that the C-terminal regions of neuronal sodium channels such as Nav1.1, Nav1.2 and Nav1.6 carry distinct information recognized by cultured hippocampal neurons. The di-leucine motif (IL 1857–1858 of Nav1.2Ct) is present in the other neuronal sodium channels, but its substitution by alanines did not modify the steady-state distribution of CD4–Nav1.6Ct (71% of CD4-positive cells exhibiting a somatodendritic surface staining; n = 71) or CD4–Nav1.1Ct (100% of CD4-positive cells exhibiting a non-polarized surface staining; n = 29). How can this differential distribution be explained? A comparison of the C-termini of Nav1.1, Nav1.2 and Nav1.6 shows that the distal regions are less homologous than the proximal ones. One possibility is that because of differences in the distal regions, the C-termini of Nav1 subunits assume distinct conformations, modulating the accessibility of motifs. Alternatively, the C-termini may interact differentially with endogenous partners expressed in hippocampal neurons (Gee et al., 1998). Such interactions may be required for dendritic/axonal localization, as recently observed with type 5 metabotropic glutamate receptor (Ango et al., 2000).
Elimination in dendrites and retention in axons can account for the compartmentalized distribution
Several studies have highlighted the fact that hippocampal neurons possess distinct somatodendritic and axonal endosomal subcompartments (Parton et al., 1992; Hemar et al., 1997; West et al., 1997b; Prekeris et al., 1999; Wilson et al., 2000), but their respective roles in sorting are still poorly understood. From our data, it is conceivable that, at steady state, the absence of immunodetectable surface CD4–Nav1.2Ct in dendrites could reflect an efficient elimination process. In other words, the equilibrium between surface and internalized protein populations would be displaced in favor of the internalized population in the somatodendritic domain, whereas the opposite situation occurs in the axonal domain. Consistent with this hypothesis are the extensive somatodendritic endosomal network (Parton et al., 1992; Prekeris et al., 1999; Wilson et al., 2000) and our data demonstrating that the C-terminus of the Nav1.2 subunit is efficiently recognized by a somatodendritic endocytic pathway. Furthermore, analysis of CD4–Nav1.2Ct mutants revealed that a di-leucine motif (IL 1857–1858) is critical for internalization, and its abrogation resulted in an axonal and somatodendritic distribution. It is also important to mention that efficient retrieval of certain proteins in dendrites may involve unidentified factors that potentiate internalization, as recently reported from studies on the AMPA receptor (Man et al., 2000).
How can the steady-state distribution of CD4–Nav1.2Ct in axons be explained? One possibility is that internalization could be prevented by anchoring at the plasma membrane via direct or indirect interactions of the Nav1.2 C-terminal domain with an endogenous axonal partner. For instance, abrogation of a somatodendritic/clustering determinant perturbs the dendritic localization of the Kv2.1 potassium channel in hippocampal neurons (Lim et al., 2000), whereas PSD-95 suppresses internalization of Kv1.4 by clustering the channel at the cell surface (Jugloff et al., 2000). A potential candidate for CD4–Nav1.2Ct retention is ankyrin B (Scotland et al., 1998), but this kind of interaction remains to be demonstrated. Alternatively, we cannot exclude the possibility that axonal and somatodendritic endosomal pathways are differentially regulated (Prekeris et al., 1999). Whatever the mechanisms that prevent CD4–Nav1.2Ct removal in axons, our data demonstrate that an elimination mechanism operating at the level of the plasma membrane can be implicated in the sorting of some axonal proteins, as proposed by Burack et al. (2000).
Implications for sodium channel organization and trafficking
Our strategy based on chimera expression in hippocampal neurons does not allow us to exclude the possibility that a transmembrane or extracellular region of the Nav1.2 subunit plays a role in sodium channel compartmentalization and organization in discrete domains of the neuronal plasma membrane. However, the following information, relevant to the trafficking of neuronal sodium channels, can be drawn from our study. The identification of an internalization motif located in the C-terminus of Nav1.2 is consistent with previous studies carried out in our laboratory showing that endogenous sodium channels undergo acitivity-regulated endocytosis in fetal rat brain neurons (Dargent et al., 1994). It is tempting to speculate that neuronal sodium channels can be recognized by AP2 and AP3, as observed for VAMP2, a synaptic vesicle protein (Shi et al., 1998). Our study also indicates that the C-terminus of Nav1.2 does not contain sufficient information for sodium channel localization at the axonal initial segment, because the steady-state distribution of CD4–Nav1.2Ct was distinct from that of the cytoskeletal linker ankyrin G. This observation may be explained by an efficient internalization at the initial segment where there is a high expression of amphiphysin II and dynamin I (Marsh and McMahon, 1999), and by the inability of the Nav1.2 C-terminus to interact with ankyrin G. An additional possibility is that components involved in protein trafficking to axonal initial segments differ from those involved in protein sorting to more distal regions of axons. Nav1.2 accumulation at axonal initial segments may be governed by specific interactions between other intracellular regions of the channel and specific components of this subdomain of the axon. The cytoplasmic loops linking domains I–II and II–III could contain motifs of this kind. We have as yet been unable to address this issue because CD4–I–II and CD4–II–III chimeras were not detected at the plasma membrane in non-permeabilized hippocampal neurons, but we are currently undertaking a complementary strategy to obtain this kind of information. Finally, the inability of the CD4–I–II chimera to reach the cell surface in hippocampal neurons may be due to the presence of a potential retention motif (RKR) within Nav1.2 loop I–II, as recently identified in the α (Kir 6.1/2) and β (SUR1) subunits of KATP potassium channels (Zerangue et al., 1999). It is also possible that the transport of CD4–I–II depends on interactions with synaptotagmin (Sampo et al., 2000) and on its phosphorylation state (Li et al., 1992).
In conclusion, our study supports the recently proposed models in which the neuronal plasma membrane plays an important role in protein sorting through selective elimination (Kelly, 1999; Burack et al., 2000). In this scheme of events, the endocytotic machinery and proteins involved in downstream transport would contribute to the determination of the final destination of proteins. Electrophysiological and localization studies have demonstrated that sodium channel subtypes are differentially distributed in adult rat brain (Goldin, 1999; Catterall, 2000). As sodium channels can be internalized in non-polarized fetal neurons (Dargent et al., 1994), it is possible to hypothesize that dynamic and complex processes, involving different endocytotic pathways and distinct retention mechanisms, specific to either dendrites or axons, control the density of sodium channels in discrete domains and subdomains of the plasma membrane, and thus regulate neuronal excitability.
Materials and methods
Plasmid construction
The cDNAs encoding Nav1.1 and Nav1.6 were gifts from A.L.Goldin (University of California, Irvine, CA), Nav1.2 was from H.Lester (California Institute of Technology, Pasadena, CA) and rat brain sodium channel β2 subunit was from Robert A.Maue (Darmouth Medical School, Hannover, NH). Human CD4 cDNA was donated by J.Mérot (Centre d’Etude Atomique, Saclay, France) and the human transferrin receptor was provided by L.Kühn (Lausanne, Switzerland). CD4ΔCt (amino acids 1–396) was generated by PCR and cloned into PCB6 with EcoRI cloning sites. CD4 chimeras containing intracellular domains of Nav1.2 (I–II, amino acids 428–753; II–III, amino acids 984–1203; Ct, amino acids 1777–2005) or the C-terminal region of β2 (amino acids 181–215), Nav1.1 (amino acids 1780–2009) and Nav1.6 (amino acids 1763–1976) were constructed by sequential PCR amplifications using Expand High Fidelity Taq DNA polymerase (Roche Molecular Biochemicals). PCR products were cloned into PCB6 using KpnI–XbaI or EcoRI cloning sites. Chimeric DNA encoding the N-terminus of Nav1.2 (amino acids 1–124) and the human transferrin receptor (amino acids 62–760) was generated in the same way and inserted as an XbaI–XbaI fragment into PCB6 vector. Chimeric CD4–Nav1.2Ct proteins with deletions were generated by PCR by adding a stop codon in the antisense primer. CD4–Nav1.2Ct internal deletions (Δ1853–1862 and Δ1839–1859) were also generated by sequential PCR amplification. Site-directed mutants were constructed by sequential PCR amplification using oligonucleotides containing the desired mutation. All constructions were verified by DNA sequencing.
Cell culture and transfection
Primary hippocampal neurons were prepared from embryonic day 18 rats according to Goslin and Banker (1989), with slight modifications as previously described (Berton et al., 2000). Two methods were employed for the transfection of hippocampal neurons, which gave identical results. A calcium phosphate protocol was applied (Xia et al., 1996), with minor modifications as previously described (Berton et al., 2000). In addition we used Effectene according to the manufacturer’s instructions (Qiagen).
HeLa cells stably expressing wild-type dynamin II or dynamin K44A were a gift of S.Schmid (The Scripps Research Institute, La Jolla, CA). HeLa cells and COS-7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)–Glutamax I (Gibco-BRL) containing 10% fetal bovine serum. Tetracycline (1 µg/ml) was added to the culture medium of HeLa cells to prevent dynamin expression, but was omitted before transfection. Cells were transfected at a confluence of 40% with Fugene-6 (Roche Molecular Biochemicals), according to the manufacturer’s instructions. Transfected cells were processed for immunofluorescence 2 days after transfection.
Immunofluorescence
Surface expression of chimeras in hippocampal neurons was assayed as follows. Cultured neurons were fixed in 4% paraformaldehyde for 20 min. Non-specific binding was blocked with 0.22% gelatin in 0.1 M phosphate buffer (PB). Then, cells were incubated for 1 h with either an anti-CD4 antibody (polyclonal or monoclonal) or with a monoclonal anti-human transferrin receptor (clone B3/25; Roche). Following a permeabilization step (0.066% saponin, 0.22% gelatin in PB), hippocampal neurons were incubated either with a polyclonal anti-MAP2 antibody (Diez-Guerra and Avila, 1995), a monoclonal anti-MAP2 antibody (1:400; Sigma) or a monoclonal anti-ankyrin G antibody (7.5 µg/ml; Zymed Laboratories). For detection of surface and intracellular chimeric proteins, cells were fixed and permeabilized before addition of primary antibodies. The secondary antibodies were TRITC-conjugated donkey anti-rabbit or anti-mouse (1:200; Jackson ImmunoResearch Laboratories) or anti-mouse and anti-rabbit antibodies conjugated to Alexa 488 (1:400; Molecular Probes). Coverslips were mounted in Mowiol.
Endocytosis assay
COS-7 and HeLa cells were exposed at 4°C to the primary antibody diluted in DMEM–HEPES medium (Gibco-BRL) supplemented with 0.1% bovine serum albumin (BSA), washed extensively, and then transferred to 37°C pre-heated DMEM medium and returned to the air/CO2 incubator. Following the incubation time indicated, cells were fixed, permeabilized and subjected to secondary antibody binding. For double immunostaining, a monoclonal anti-AP2 antibody (1:200; Affinity Bio Reagents), a monoclonal anti-EEA1 antibody (1:400; Transduction Laboratories) or a monoclonal anti-transferrin receptor antibody (1:400; Zymed Laboratories) was added to permeabilized cells followed by secondary antibodies. Hippocampal neurons were incubated with either a polyclonal or monoclonal anti-CD4 antibody in glial conditioned medium supplemented with 0.1% BSA for 30 min at 37°C, washed and processed as described above.
Confocal microscopy
Labeling was viewed with a confocal laser scanning microscope (Leica TCS) equipped with an argon–krypton laser (488, 568 and 647 nm excitation lines). For double staining, light emitted from the two fluorophores was detected sequentially. Band-pass filters were chosen to select each emission. Images were reconstructed from a series of optical sections taken in the x–y plane from consecutive z positions, 0.45–0.5 µm apart, using the standard microscope software (Leica Scanware). Original fields were made up of 512 × 512 pixels. Images were processed for presentation with Adobe Photoshop.
Acknowledgments
Acknowledgements
We are grateful to colleagues for the generous gifts of cDNA, HeLa cells and antibodies. We thank Virginie Fico and Véronique Cornet for technical assistance, Michael Seagar, André Le Bivic, Jean Mérot, Agnés Hémar and Christian Vannier for discussion and critical reading of the manuscript. J.J.G. was supported initially by an INSERM postdoctoral fellowship and then by a grant from the Association Francaise contre les Myopathies.
References
- Ango F., Pin,J.P., Tu,J.C., Xiao,B., Worley,P.F., Bockaert,J. and Fagni,L. (2000) Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J. Neurosci., 20, 8710–8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. and Lambert,S. (1999) Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J. Neurocytol., 28, 303–318. [DOI] [PubMed] [Google Scholar]
- Berton F., Cornet,V., Iborra,C., Garrido,J., Dargent,B., Fukuda,M., Seagar,M. and Marqueze,B. (2000) Synaptotagmin I and IV define distinct populations of neuronal transport vesicles. Eur. J. Neurosci., 12, 1294–1302. [DOI] [PubMed] [Google Scholar]
- Bichet D., Cornet,V., Geib,S., Carlier,E., Volsen,S., Hoshi,T., Mori,Y. and De Waard,M. (2000) The I–II loop of the Ca2+ channel α1 subunit contains an endoplasmic reticulum retention signal antagonized by the β subunit. Neuron, 25, 177–190. [DOI] [PubMed] [Google Scholar]
- Burack M.A., Silverman,M.A. and Banker,G. (2000) The role of selective transport in neuronal protein sorting. Neuron, 26, 465–472. [DOI] [PubMed] [Google Scholar]
- Catterall W.A. (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron, 26, 13–25. [DOI] [PubMed] [Google Scholar]
- Dargent B., Paillart,C., Carlier,E., Alcaraz,G., Martin-Eauclaire,M.F. and Couraud,F. (1994) Sodium channel internalization in developing neurons. Neuron, 13, 683–690. [DOI] [PubMed] [Google Scholar]
- Dargent B, Mouret,I., Shah,R.M., Spooner,E.T., Boudier,J.A., Le Bivic,A., Maue,R. and Sampo,B. (1998) Targeting of the voltage-dependent sodium channel to the axon of cultured hippocampal neurons. Soc. Neurosci. Abstr., 24, 1078. [Google Scholar]
- Diez-Guerra F.J. and Avila,J. (1995) An increase in phosphorylation of microtubule-associated protein 2 accompanies dendrite extension during the differentiation of cultured hippocampal neurones. Eur. J. Biochem., 227, 68–77. [DOI] [PubMed] [Google Scholar]
- Gee S.H., Madhavan,R., Levinson,S.R., Caldwell,J.H., Sealock,R. and Froehner,S.C. (1998) Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J. Neurosci., 18, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A.L. (1999) Diversity of mammalian voltage-gated sodium channels. Ann. NY Acad. Sci., 868, 38–50. [DOI] [PubMed] [Google Scholar]
- Gong B., Rhodes,K.J., Bekele-Arcuri,Z. and Trimmer,J.S. (1999) Type I and type II Na+ channel α-subunit polypeptides exhibit distinct spatial and temporal patterning and association with auxiliary subunits in rat brain. J. Comp. Neurol., 412, 342–352. [PubMed] [Google Scholar]
- Goslin K. and Banker,G. (1989) Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell Biol., 108, 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemar A., Olivo,J.C., Williamson,E., Saffrich,R. and Dotti,C.G. (1997) Dendroaxonal transcytosis of transferrin in cultured hippocampal and sympathetic neurons. J. Neurosci., 17, 9026–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jareb M. and Banker,G. (1998) The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron, 20, 855–867. [DOI] [PubMed] [Google Scholar]
- Jugloff D.G., Khanna,R., Schlichter,L.C. and Jones,O.T. (2000) Internalization of the Kv1.4 potassium channel is suppressed by clustering interactions with PSD-95. J. Biol. Chem., 275, 1357–1364. [DOI] [PubMed] [Google Scholar]
- Kelly R.B. (1999) Deconstructing membrane traffic. Trends Cell Biol., 9, M29–M33. [PubMed] [Google Scholar]
- Li M., West,J.W., Lai,Y., Scheuer,T. and Catterall,W.A. (1992) Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron, 8, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Lim S.T., Antonucci,D.E., Scannevin,R.H. and Trimmer,J.S. (2000) A novel targeting signal for proximal clustering of the Kv2.1 K+ channel in hippocampal neurons. Neuron, 25, 385–397. [DOI] [PubMed] [Google Scholar]
- Man Y.H., Lin,J.W., Ju,W.H., Ahmadian,G., Liu,L., Becker,L.E., Sheng,M. and Wang,Y.T. (2000) Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron, 25, 649–662. [DOI] [PubMed] [Google Scholar]
- Marsh M. and McMahon,H.T. (1999) The structural era of endocytosis. Science, 285, 215–220. [DOI] [PubMed] [Google Scholar]
- Mellman I. (1996) Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol., 12, 575–625. [DOI] [PubMed] [Google Scholar]
- Parton R.G., Simons,K. and Dotti,C.G. (1992) Axonal and dendritic endocytic pathways in cultured neurons. J. Cell Biol., 119, 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyatos I., Ruberti,F., Martinez-Maza,R., Gimenez,C., Dotti,C.G. and Zafra,F. (2000) Polarized distribution of glycine transporter isoforms in epithelial and neuronal cells. Mol. Cell. Neurosci., 15, 99–111. [DOI] [PubMed] [Google Scholar]
- Prekeris R., Foletti,D.L. and Scheller,R.H. (1999) Dynamics of tubulovesicular recycling endosomes in hippocampal neurons. J. Neurosci., 19, 10324–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberti F. and Dotti,C.G. (2000) Involvement of the proximal C-terminus of the AMPA receptor subunit GluR1 in dendritic sorting. J. Neurosci., 20, RC78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampo B., Tricaud,N., Leveque,C., Seagar,M., Couraud,F. and Dargent,B. (2000) Direct interaction between synaptotagmin and the intracellular loop I–II of neuronal voltage-sensitive sodium channels. Proc. Natl Acad. Sci. USA., 97, 3666–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinman R.I., Auld,V.J., Goldin,A.L., Davidson,N., Dunn,R.J. and Catterall,W.A. (1989) Developmental regulation of sodium channel expression in the rat forebrain. J. Biol. Chem., 264, 10660–10666. [PubMed] [Google Scholar]
- Schmid S.L. (1997) Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem., 66, 511–548. [DOI] [PubMed] [Google Scholar]
- Scotland P., Zhou,D., Benveniste,H. and Bennett,V. (1998) Nervous system defects of AnkyrinB (–/–) mice suggest functional overlap between the cell adhesion molecule L1 and 440 kD AnkyrinB in premyelinated axons. J. Cell Biol., 143, 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G., Faundez,V., Roos,J., Dell’Angelica,E.C. and Kelly,R.B. (1998) Neuroendocrine synaptic vesicles are formed in vitro by both clathrin-dependent and clathrin-independent pathways. J. Cell Biol., 143, 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell J.N. and Craig,A.M. (1999) Axon/dendrite targeting of metabotropic glutamate receptors by their cytoplasmic C-terminal domains. Neuron, 22, 525–536. [DOI] [PubMed] [Google Scholar]
- Stuart G., Spruston,N., Sakmann,B. and Hausser,M. (1997) Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci., 20, 125–131. [DOI] [PubMed] [Google Scholar]
- West A.E., Neve,R.L. and Buckley,K.M. (1997a) Identification of a somatodendritic targeting signal in the cytoplasmic domain of the transferrin receptor. J. Neurosci., 17, 6038–6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A.E., Neve,R.L. and Buckley,K.M. (1997b) Targeting of the synaptic vesicle protein synaptobrevin in the axon of cultured hippocampal neurons: evidence for two distinct sorting steps. J. Cell Biol., 139, 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek R.E., Merrick,D.K. and Catterall,W.A. (1989) Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron, 3, 695–704. [DOI] [PubMed] [Google Scholar]
- Wilson J.M., de Hoop,M., Zorzi,N., Toh,B.H., Dotti,C.G. and Parton,R.G. (2000) EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells and fibroblasts. Mol. Biol. Cell, 11, 2657–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B. and Mellman,I. (1999) Neuronal polarity: controlling the sorting and diffusion of membrane components. Neuron, 23, 637–640. [DOI] [PubMed] [Google Scholar]
- Xia Z., Dudek,H., Miranti,C.K. and Greenberg,M.E. (1996) Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci., 16, 5425–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N., Schwappach,B., Jan,Y.N. and Jan,L.Y. (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron, 22, 537–548. [DOI] [PubMed] [Google Scholar]
- Zito K., Fetter,R.D., Goodman,C.S. and Isacoff,E.Y. (1997) Synaptic clustering of Fascilin II and Shaker: essential targeting sequences and role of Dlg. Neuron, 19, 1007–1016. [DOI] [PubMed] [Google Scholar]