Abstract
The p85-associated phosphatidylinositol (PI) 3-kinase/Akt pathway mediates the oestradiol-induced S-phase entry and cyclin D1 promoter activity in MCF-7 cells. Experiments with Src, p85α and Akt dominant-negative forms indicate that in oestradiol-treated cells these signalling effectors target the cyclin D1 promoter. Oestradiol acutely increases PI3-kinase and Akt activities in MCF-7 cells. In NIH 3T3 cells expressing ERα, a dominant-negative p85 suppresses hormone stimulation of Akt. The Src inhibitor, PP1, prevents hormone stimulation of Akt and PI3-kinase activities in MCF-7 cells. In turn, stimulation of Src activity is abolished in ERα-expressing NIH 3T3 fibroblasts by co-transfection of the dominant-negative p85α and in MCF-7 cells by the PI3-kinase inhibitor, LY294002. These findings indicate a novel reciprocal cross-talk between PI3-kinase and Src. Hormone stimulation of MCF-7 cells rapidly triggers association of ERα with Src and p85. In vitro these proteins are assembled in a ternary complex with a stronger association than that of the binary complexes composed by the same partners. The ternary complex probably favours hormone activation of Src- and PI3-kinase-dependent pathways, which converge on cell cycle progression.
Keywords: cell cycle/cross-talk/oestradiol receptor/PI3-kinase/Src
Introduction
Phosphatidylinositol (PI) 3-kinases mediate the action of numerous growth factors as indicated by the levels of phospholipid products of PI3-kinases that are increased by growth factors or oncogenic transformation (Tocker and Cantley, 1997). Class Ia PI3-kinase consists of a 110 kDa catalytic subunit (α, β or δ) and a p85 regulatory/adapter subunit that plays roles in protein–protein interactions through a series of molecular domains (Stein and Waterfield, 2000). Class Ia PI3-kinase can be activated by tyrosine kinase-coupled receptors through the binding of p85 and by GTP-bound Ras, which interacts with the catalytic subunit (for a review see Vanhaesebroeck et al., 2001). Activation of PI3-kinase by Src-family kinases through binding of the p85 subunit to the Src-SH3 domain has also been reported (Pleiman et al., 1994). The biological effects of PI3-kinase activation are due to the PI3-kinase downstream targets (Stein and Waterfield, 2000). Protein kinase B (PKB), also called Akt, is a well known target of PI3-kinase and regulates multiple biological functions, including gene expression, cell cycle, survival, insulin-induced metabolic signals, endocytosis and vesicular trafficking, cell transformation and oncogenesis (for a review see Coffer et al., 1998).
In the present paper we report that in MCF-7 cells oestradiol rapidly stimulates a p85-regulated PI3-kinase and Akt, increases cyclin D1 expression and its promoter activity as well as the S-phase entry of cells. The relevance of PI3-kinase in cell cycle progression and the ability of activated PI3-kinase to promote entry into the S phase led us to examine the role of the PI3-kinase signalling in oestradiol-induced DNA synthesis. By combined use of molecules selectively interfering with the PI3-kinase pathway we demonstrate such a role.
Although several studies have suggested that cyclin D1 is involved in the steroid-dependent growth of both normal and malignant mammary epithelial cells, the molecular mechanism by which oestrogens control cyclin D1 is at present poorly defined (Planas-Silva et al., 1999). It has been reported that PI3-kinase promotes the S-phase entry by increasing cyclin D1 transcription and translation, preventing its degradation and reducing p27kip1 levels (for a review see Vanhaesebroeck et al., 2001). In this report we have addressed the role of several extra-nuclear effectors on oestradiol-induced cyclin D1 promoter activity. Our findings show that the PI3-kinase pathway controls the oestradiol-stimulated cyclin D1 transcription and the progression through the G1–S phase of the cell cycle.
The dominant-negative Src also interferes with the hormonal stimulation of cyclin D1 transcription, suggesting that Src might play a role in the PI3-kinase pathway activation by oestradiol. Such a role was investigated. Hormone stimulation of PI3-kinase and PI3-kinase-dependent Akt was prevented by a Src inhibitor. In turn, PI3-kinase inhibition abolishes the oestradiol-induced Src activation. These findings reveal the hormone regulation of a novel reciprocal cross-talk between Src and PI3-kinase. Oestradiol treatment of MCF-7 cells rapidly induces association of oestradiol receptor (ER) α, with Src and p85. It is likely that hormonal induction of this association facilitates the oestradiol activation of the two signalling members and their reciprocal communication. As a corollary of the present report, experiments with NIH 3T3 cells transfected with hERα cDNA further support the view that classic steroid receptors, universally known as transcriptional activators, once occupied by agonists, also activate extra-nuclear signalling pathways.
Results
The PI3-kinase/Akt pathway triggers oestradiol-dependent S-phase entry of MCF-7 cells
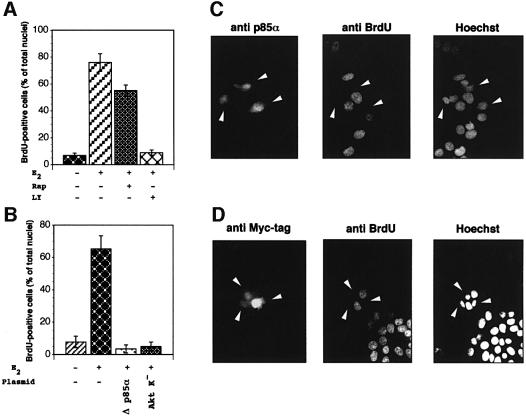
To investigate the role of PI3-kinase in the oestradiol-induced DNA synthesis of target cells, we first used the chemical inhibitor, LY294002. Quiescent MCF-7 cells were pre-incubated in the absence or presence of the inhibitor at a concentration (20 µM) that completely inhibits the activity of p110α (Vlahos et al., 1994). Figure 1A shows that LY294002 abolished the hormonal stimulation of bromodeoxyuridine (BrdU) incorporation. Since LY294002 also inhibits other enzymes such as target of rapamycin kinase (TOR) and DNA-dependent protein kinase (Hartley et al., 1995; Brunn et al., 1996), the effect of the specific TOR inhibitor, rapamycin, on the oestradiol-induced BrdU incorporation of MCF-7 cells was evaluated. Many cells treated with rapamycin were still able to enter the S phase when stimulated by oestradiol (Figure 1A). Rapamycin was a much weaker inhibitor of the S-phase entry than LY294002; therefore, it is likely that the two inhibitors have different targets. It is noteworthy that treatment of MCF-7 cells with either LY294002 or rapamycin did not affect BrdU incorporation in the absence of hormone (not shown).
Fig. 1. Inhibition of the PI3-kinase/Akt pathway prevents the oestradiol-dependent S-phase entry of MCF-7 cells. (A) Quiescent MCF-7 cells on coverslips were left unstimulated or stimulated with 10 nM oestradiol in the absence or presence of either 20 µM LY294002 or 50 nM rapamycin. One hundred µM BrdU was added and 24 h later cells were fixed and stained. Several coverslips were analysed. DNA synthesis was calculated by the formula: percentage of BrdU-positive cells = (number of BrdU-positive cells/number of total cells) × 100. Results of more than three independent experiments have been averaged; mean and SEM are shown. (B) Quiescent MCF-7 cells on coverslips were untransfected or transfected with either Δp85α or Myc-tagged Akt K– expressing plasmids. Cells were left unstimulated or stimulated with 10 nM oestradiol. BrdU was added and after 24 h, coverslips were analysed for DNA synthesis in transfected cells. This was calculated by the following formula: percentage of BrdU-positive cells = (number of transfected BrdU-positive cells/number of transfected cells) × 100. For each plasmid, data are derived from at least 400 scored cells. Results of more than two independent experiments have been averaged; mean and SEM are shown. (C and D) Representative fields from experiments in (A) and (B) are presented. Fluorescence in the left panels is from reactivity with either the anti-p85 antibody (left panel in C) or the anti-Myc tag antibody (left panel in D). White arrowheads mark the cells transfected with either Δp85α (left panel in C) or Akt K– (left panel in D) expressing plasmids. The central panels show staining with anti-BrdU antibody. Hoechst 33258 nuclear staining is presented in the right panels. The construct expressing the pUSEAmp empty plasmid was also transfected into quiescent MCF-7 cells together with the green fluorescent protein (GFP)-expressing plasmid (from Clontech, CA), as transfection marker. Cells were left unstimulated or stimulated with oestradiol. BrdU was added and after 24 h coverslips were analysed for DNA synthesis. In the presence of oestradiol, 52.3% of the GFP-expressing MCF-7 cells entered the S phase, whereas the steroid-stimulated BrdU incorporation of non-transfected cells was 59%. BrdU incorporation of unstimulated cells was, in each case, <10%.
The effect of a dominant-negative regulatory subunit of PI3-kinase (Δp85α; Dhand et al., 1994) on the oestradiol-stimulated S-phase entry of MCF-7 cells was then analysed. Quiescent MCF-7 cells were transfected with a construct expressing Δp85α and 10 nM oestradiol was added to the medium. Cells were labelled with BrdU, fixed and stained. Different experiments were performed and coverslips were analysed. The number of BrdU-positive cells expressing Δp85α was compared with the number of BrdU-positive non-transfected cells from the same coverslips. Data were pooled and statistically analysed. The results in Figure 1B show that <10% of non-transfected cells incorporated BrdU in the absence of oestradiol. As expected, addition of the hormone to the medium strongly increased this number. Interestingly, over-expression of Δp85α completely prevented oestradiol-stimulated BrdU incorporation (Figure 1B). Overexpression of the pSG5 empty plasmid did not affect BrdU incorporation of MCF-7 cells in the absence or presence of oestradiol (not shown).
The serine/threonine kinase Akt/PKB is a major target of PI3-kinase and its activation through phosphorylation of Thr308/Ser473 mediates many of the downstream cellular effects of PI3-kinase (Franke et al., 1997). To investigate the role of Akt in the oestradiol-induced MCF-7 cell cycle progression we used a plasmid expressing the Myc-tagged kinase-dead Akt with Lys179 changed to methionine (Akt K–; Kohn et al., 1996) in transient transfection experiments. After transfection, cells were stimulated with 10 nM oestradiol, then pulsed with BrdU. Cells were finally fixed, stained and multiple coverslips were analysed. Overexpression of Akt K– like that of Δp85α suppressed the oestradiol-induced S-phase entry. Transfection with the empty plasmid (pUSEamp) did not interfere with BrdU incorporation of cells (see legend to Figure 1).
Images from one experiment with Δp85α (Figure 1C) or Akt K– (Figure 1D) were captured and shown. Left panels show cells expressing either Δp85α (in C) or the Myc-tagged Akt K– (in D). These cells are marked with arrows. The same cells did not incorporate BrdU when visualized with anti-BrdU antibody (middle panels in C and D). Staining of cell nuclei with Hoechst is shown in the right panels.
In conclusion, the PI3-kinase/Akt pathway mediates the effect of oestradiol in driving the G1–S progression of target cells.
Regulation of cyclin D1 expression in oestradiol stimulated MCF-7 cells by the PI3-kinase/Akt pathway
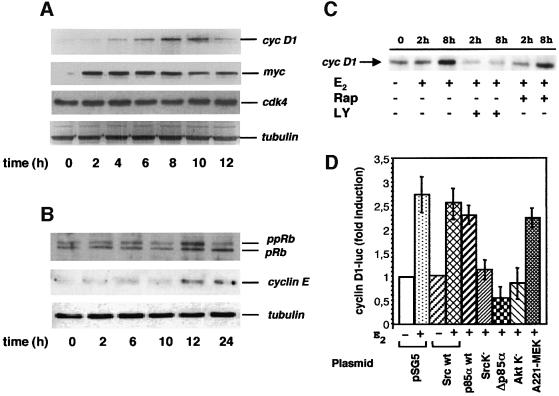
Oestradiol induces proliferation of target tissues by stimulating progression through the G1-phase of the cell cycle (Sutherland et al., 1983). To investigate this we analysed the oestradiol-induced expression of immediate genes in quiescent MCF-7 cells. Most of the cells were quiescent and such a condition was monitored by BrdU incorporation into newly synthesized DNA (see legend to Figure 2). Oestradiol was added to the medium and lysates were analysed by immunoblot with appropriate antibodies. The treatment of quiescent cells with oestradiol induced cyclin D1 expression with a peak detected after 8 h of hormonal stimulation (Figure 2A). When the same filter was re-probed with anti-Myc antibody a more rapid oestradiol-dependent induction of Myc expression was detected (Figure 2A). This is in agreement with previous reports (Dubik et al., 1987; van der Burg et al., 1989). Cdk4 expression was unaffected by oestradiol, indicating that hormonal stimulation did not indiscriminately affect the expression of genes involved in the cell cycle control (Figure 2A). A western blot with anti-tubulin antibody was developed as a protein loading control.
Fig. 2. Effect of multiple extra-nuclear effectors on the oestradiol-induced cyclin D1 expression. (A and B) Quiescent MCF-7 cells were left untreated or treated with 10 nM oestradiol for the indicated times. Proteins from cell lysates were resolved on SDS–PAGE, then transferred to nitrocellulose filters. Endogenous cyclin D1, Myc and Cdk4 expression (A) hyper-phosphorylated pRb and cyclin E (B) were revealed using the appropriate antibodies. Filters in (A and B) were re-probed with anti-α tubulin mouse monoclonal antibody (clone DM1A from Sigma) as a protein loading control. MCF-7 cells utilized for the experiments presented in (A and B) were also analysed for BrdU incorporation. In unstimulated cells, incorporation was observed in <15% of total cells, whereas in the presence of hormone it was detected in 82% of total cells. (C) Quiescent MCF-7 cells were left untreated or treated with 10 nM oestradiol for the indicated times in the absence or presence of either 20 µM LY294002 or 50 nM rapamycin. Endogenous cyclin D1 expression was analysed by immunoblot of cell lysates. (D) Quiescent MCF-7 cells were co-transfected with a cyclin D1 reporter construct (pGL2-D1-luc) along with the indicated construct. After 24 h, cells were left untreated or treated with 10 nM oestradiol. After 7 h of hormone stimulation, the reporter activity was analysed. The luciferase activity was normalized using β-gal as an internal control. Data are representative of at least two independent experiments. Mean and SEM are shown.
In Figure 2B the effect of oestradiol on quiescent MCF-7 cells is correlated with western blot analysis of retinoblastoma (Rb) and cyclin E. The upper section in panel B shows that lysates from untreated MCF-7 cells exhibited low levels of both, hypo- and hyper-phosphorylated forms of Rb. After 12 h of oestradiol treatment, when the first cells entered S phase (not shown), both, hypo- and hyper-phosphorylated forms of Rb increased. After 24 h, when a large number of the oestradiol-stimulated cells (82%; see legend to Figure 2) had progressed into S phase, the hyper-phosphorylated form of Rb decreased. When the same filter was re-probed with anti-cyclin E antibody, oestradiol-dependent induction of cyclin E expression was detected after 12 and 24h of hormonal stimulation (middle section in Figure 2B). The same filter was incubated with anti-tubulin antibody as a control (lower section in Figure 2B).
The effect of two signalling inhibitors on the oestradiol-induced cyclin D1 expression was then analysed. Figure 2C shows that the PI3-kinase inhibitor LY294002 completely inhibited cyclin D1 expression, whereas the effect of the TOR inhibitor rapamycin was weak. The effects of the two inhibitors mirror those of the same inhibitors on the hormone-induced S-phase entry of MCF-7 cells (Figure 1A).
Regulation of the oestradiol-induced cyclin D1 transcription in response to the expression of various extra-nuclear effectors was then studied. In transient transfection experiments a reporter construct encompassing 1.8 kb proximal to the transcriptional start site of the human cyclin D1 was employed. Such a construct has been previously utilized to verify the requirement of the PI3-kinase/Akt pathway for the serum-induced cyclin D1 expression as well as the S-phase entry of NIH 3T3 cells (Gille and Downward, 1999). In a preliminary experiment the oestradiol effect on the reporter gene transcription was followed in a time course experiment in MCF-7 cells (not shown). Luciferase activity was stimulated by oestradiol treatment of cells, with a 3-fold higher peak than the basal value after 7 h of treatment. The effect of wild type (wt) as well as dominant-negative forms of signalling pathway effectors on this peak was analysed. Upon oestradiol stimulation of either Src wt- or p85 wt-expressing cells a 2.53 and 2.47-fold increase of luciferase activity over the basal level, respectively, was observed (Figure 2D). These values correlate well with the 2.7-fold increase of Luc-reporter cyclin D1 promoter observed in MCF-7 cells transfected with the empty pSG5 plasmid (Figure 2D). A strong decrease of luciferase activity was observed when MCF-7 cells were transfected with the same amount of plasmids expressing the dominant-negative form of either Src (Src K–), or p85α (Δp85α) or Akt (Akt K–). Only a negligible inhibitory effect on the oestradiol-induced cyclin D1 transcription was observed after transfection of the kinase-inactive MAP/erk kinase (MEK) (A221 MEK-1). It has also been verified using immunoblot analysis that similar amounts of the various wild-type and dominant-negative effectors were expressed (not shown).
All together these results demonstrate that Src, PI3-kinase and Akt, in contrast with MEK-1, are particularly important in the activation of cyclin D1 transcription by oestradiol.
Oestradiol activates Akt through p85α-regulated PI3-kinase
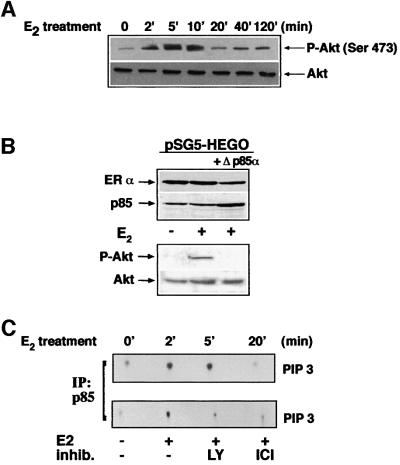
Since our data on the oestradiol-induced S-phase entry and cyclin D1 promoter stimulation indicate a key role for Akt/PKB, we analysed the hormone action on Akt activity (Figure 3). Figure 3A shows that oestradiol stimulates this activity. Stimulation was initially observed after 2 min of hormonal treatment. It reached a peak after 5 min and decreased towards the basal levels after 20 min. When the same filter was re-probed with anti-Akt antibody, similar amounts of the protein were detected (lower section of panel A).
Fig. 3. Oestradiol activation of Akt is mediated by p85α-regulated PI3-kinase. (A) Quiescent MCF-7 cells were left untreated or treated with 10 nM oestradiol for the indicated times. Proteins from cell lysates were resolved on SDS–PAGE, then transferred to nitrocellulose filters. In the upper section immunoblot with anti-P-Akt (Ser473) is shown. The same filter was re-probed with anti-Akt antibody and shown in the lower section. (B) NIH 3T3 fibroblasts were transfected with hERα alone (1st and 2nd lanes) or together with Δp85α (3rd lane) expressing plasmids. Cells were made quiescent, then left unstimulated or stimulated for 5 min with 10 nM oestradiol. Lysates were analysed for expression of either ERα or p85 (upper section) and for P-Akt (lower section). The same filter was re-probed with anti-Akt antibody. (C) Upper section: quiescent MCF-7 cells were left untreated or treated with oestradiol for the indicated times. Lower section: cells were left untreated or treated for 5 min with oestradiol in the absence or presence of the indicated compounds. LY294002 (20 µM) was added 10 min before hormonal stimulation and ICI 182 780 (10 µM) together with oestradiol. PI3-kinase activity of p85 immunoprecipitates was assayed and radiolabelled phospholipids were visualized by autoradiography.
The role of ERα and p85α-associated PI3-kinase in the oestradiol-induced activation of Akt was analysed using NIH 3T3 fibroblasts. These cells were made oestradiol-responsive by transient transfection with hERα cDNA and activation of Akt by oestradiol was followed in cells not expressing or expressing Δp85α. ERα expression confers hormonal responsiveness in terms of Akt activation and this activation was abolished by co-expression of the dominant-negative form of p85α (Figure 3B, lower panel). When the same filter was re-probed with anti-Akt antibody, similar amounts of Akt were detected in the loaded samples. The upper panels in B show that similar amounts of ERα were expressed in co-transfected cell extracts; over-expression of the Δp85α was shown by immunoblot analysis (compare the third lane with the other two lanes).
Consistent with the view that Akt is a target of PI3-kinase, Figure 3C shows that oestradiol treatment of quiescent MCF-7 cells increased the PI3-kinase activity. This activity was assayed by measuring the production of PtdIns-3P by the p85 immunoprecipitates. An increase was observed after 2 min of hormonal treatment, reaching a peak after 5 min and decreased to the basal level after 20 min (upper section). In addition, the PI3-K inhibitor, LY294002, as well as the ER antagonist, ICI 182 780, inhibited this increase (lower section).
In conclusion, oestradiol stimulates the p85α-regulated PI3-kinase/Akt pathway.
Cross-talk between PI3-kinase and Src
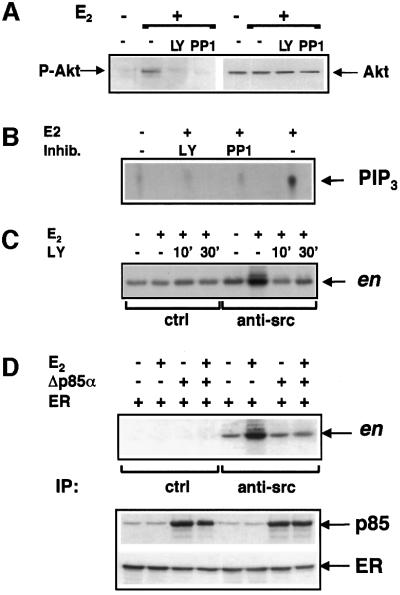
In addition to LY294002, the Src-inhibitor PP1 (Hanke et al., 1996) abolished the 5 min oestradiol-induced Akt activation in MCF-7 cells (left section of Figure 4A). No change of the protein level was detected by re-probing the same filter with anti-Akt antibody (right section of Figure 4A). Thus, not only PI3-kinase, but also Src regulates the activity of Akt. Like Akt activation, oestradiol-induced activation of PI3-kinase in p85 immunoprecipitates was inhibited not only by LY294002, but also by PP1 (Figure 4B).
Fig. 4. Reciprocal regulation of PI3-kinase and Src activities stimulated by oestradiol. (A and B) Quiescent MCF-7 were untreated or treated with 10 nM oestradiol for 5 min in the absence or presence of the indicated inhibitors. LY294002 (20 µM) and PP1 (10 µM) were added to the cell medium 10 min before hormone stimulation. In panel A lysates were analysed for P-Akt (left section). The same filter was re-probed with anti-Akt antibody (right section). In panel B PI3-kinase activity of p85 immunocomplexes was assayed and radiolabelled phospholipids revealed by autoradiography. (C) Quiescent MCF-7 were untreated or treated with 10 nM oestradiol for 3 min in the absence or presence of 20 µM LY294002. Two different times of pre-incubation with the inhibitor (10 and 30 min) were used. Src activity of Src immunocomplexes (right half four lanes) was assayed using acidified enolase (en) as a substrate. Left half four lanes show Src activity of proteins immunoprecipitated by control antibodies. (D) NIH 3T3 fibroblasts were transfected with ERα expressing plasmid alone or together with the Δp85α construct. Cells were made quiescent, then left untreated or treated for 3 min with 10 nM oestradiol. In the upper section: lysates were immunoprecipitated with anti-Src antibody and Src activity was assayed. In the lower section: lysates were analysed for either p85 or ERα expression by immunoblot.
In turn, the effect of PI3-kinase-inhibition on the oestradiol-induced Src activation was analysed. Pre-treatment of MCF-7 cells with LY294002 for two different times (10 and 30 min) prevented Src activation induced by oestradiol (Figure 4C).
The role of p85α in the oestradiol-stimulated Src activation was finally verified in NIH 3T3 fibroblasts expressing hERα. Src activation was observed after 3 min of hormonal treatment. Interestingly, co-expression of Δp85α completely inhibits this stimulation. Src activation was undetected in proteins immunoprecipitated by control antibodies. Expression of ERα and Δp85α in fibroblasts was verified by immunoblots of cell lysates (Figure 4D, lower section).
Taken together our findings show that oestradiol-stimulated PI3-kinase and Src regulate each other.
Oestradiol induces interaction of ERα with Src and PI3-kinase in MCF-7 cells
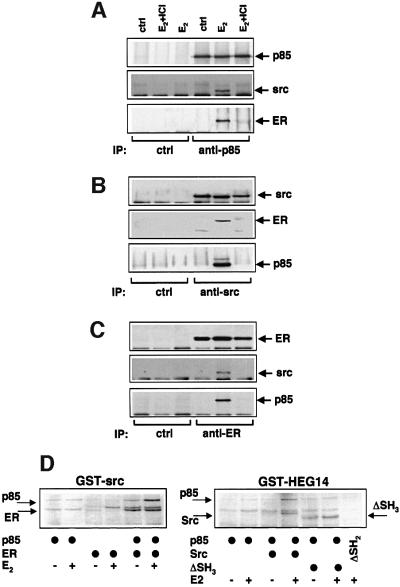
Quiescent MCF-7 cells were left unstimulated or stimulated with 10 nM oestradiol for 3 min. Cell lysates were immunoprecipitated with either anti-p85α (Figure 5A) or anti-Src (Figure 5B) or anti-ERα (Figure 5C) antibodies. Each immunoprecipitate was analysed by immunoblot with either anti-p85α, or anti-ERα or anti-Src antibodies. Results in Figure 5 show that whatever the antibody used, oestradiol treatment triggered co-immunoprecipitation of Src, PI3-kinase and ERα. The ER antagonist, ICI 182 780, disrupted such an interaction. No association was detected in control immunoprecipitates.
Fig. 5. Oestradiol-induced assembly of Src–PI3-kinase–ERα complex. Quiescent MCF-7 cells were left unstimulated or stimulated for 5 min with 10 nM oestradiol. Lysates were immunoprecipitated with either anti-p85 (A), or anti-Src (B) or anti-ER (C) antibodies. Immunocomplexes were analysed by immunoblot with antibodies against the indicated proteins. (D) Either GST–Src (left section) or GST–HEG14 (right section) was incubated in the absence or presence of 10 nM oestradiol with the indicated 35S-labelled proteins. In vitro interaction with GST fusion proteins was performed as described in Materials and methods. Proteins were eluted by Laemmli sample buffer, resolved on SDS–PAGE, then finally revealed by autoradiography.
Association between ERα, Src and PI3-kinase was further investigated in vitro by pull-down experiments with glutathione S-transferase (GST)-fusion protein constructs. Either bovine wild-type [35S] p85α, or human wild-type [35S] ERα were incubated with GST–Src (Figure 5D, left section). As expected from our previous report (Migliaccio et al., 2000), ERα interacted with GST–Src in the presence of hormone (lanes 3 and 4); a weak, hormone-independent interaction was observed between p85α and GST–Src (lanes 1 and 2). Since assembly of this binary complex was not observed in MCF-7 cells, it might be that p85 and Src association is detectable only at the high Src concentrations used in vitro. Interestingly, when p85α was incubated together with the ERα, an oestradiol-dependent increase of its association with GST–Src was observed (lanes 5 and 6). Pull-down experiments using GST–HEG14 fusion protein (HEG14 is the C-terminal half of the HEGO including the hormone binding domain of ERα) were carried out (Figure 5D, right section). The weak interaction between p85α and GST–HEG14 detected in the presence of hormone (lanes 1 and 2) was reinforced by simultaneous association of Src (lanes 3 and 4). Use of SH3 lacking-Src (ΔSH3) reduced to the basal level the interaction between p85α and GST–HEG14, although ΔSH3-Src was still able to associate with the fusion protein in a hormone-sensitive way (lanes 4 and 5). ERα interacts with the SH2 domain of Src (Migliaccio et al., 2000); therefore, we used Src deleted of the SH2 domain (ΔSH2) as a control for the in vitro experiments. The absence of interaction between ΔSH2 and GST–HEG14 in the presence of hormone (lane 6) confirmed the specificity of the observed associations.
In conclusion, the in vitro data reinforce the view that oestradiol triggers the assembly of a stable ERα/p85α/Src complex in MCF-7 cells.
Discussion
When oestradiol enters the cells, it binds nuclear receptors, which dimerize and interact with DNA sequences to regulate gene transcription (Beato et al., 1995; Mangelsdorf et al., 1995; Parker and White, 1996). Alternatively, rather than interacting directly with DNA, ERS bind DNA-associated transcription factors stimulating or repressing transcription (McKenna et al., 1999). The transcriptional activity of the ERS can be modulated in the absence of ligand through receptor phosphorylation by kinases regulated by peptide hormones, such as EGF (Power et al., 1991; Pietras et al., 1995). Alternate oestrogen actions are very rapid and non-genomic (Wehling, 1997; Revelli et al., 1998; McEwen and Alves, 1999).
We have recently identified a novel non-genomic action of oestradiol depending on the ability of the ERα or β to interact with Src and activate the Src/Shc/Ras/Erk pathway (Migliaccio et al., 1996, 2000). In addition to oestradiol, also progestins (Migliaccio et al., 1998) and androgens (Migliaccio et al., 2000) activate the same pathway although with some remarkable differences in the initial steps leading to the hormonal Src activation. Interference with the pathway activation abolishes the hormone-dependent growth (Castoria et al., 1999). Oestradiol activation of the same pathway protects osteoblasts from apoptotic stimuli (Kousteni et al., 2001), thus indicating that the Src/Ras/Erks stimulation can exert different effects, depending on the environmental conditions.
It has recently been observed that oestradiol activates the PI3-kinase/AKT pathway in endothelial cells, whose stimulation leads to increase of nitric oxide synthesis (Simoncini et al., 2000). Activation of the same pathway by oestradiol has also been implicated in neuroprotection (Honda et al., 2000).
The importance of PI3-kinase as a controller of cell growth is recognized (Carpenter and Cantley, 1996). Nevertheless, little is known about how PI3-kinases affect growth (Poser et al., 2000). In the present report inhibition of oestradiol-stimulated DNA synthesis of MCF-7 cells by a PI3-kinase inhibitor, LY294002, or transfected Δp85α is detected. The latter finding points to the involvement of class Ia PI3-kinase.
Much evidence demonstrates a critical role for PI3-kinase in cyclin D1 regulation (for a review see Vanhaesebroeck et al., 2001). We now observe that oestradiol increases the cyclin D1 expression of MCF-7 cells. Such an increase starts after the early oestradiol-induced Myc expression and precedes pRb phosphorylation. It is noteworthy that similar findings have been previously reported in MCF-7 cells whose quiescence was obtained by either treatment with anti-oestrogens (Musgrove et al., 1994) or serum starvation (Foster and Wimalasema, 1996). In our experiments cells were made quiescent removing steroids from serum by charcoal treatment. The finding that Δp85α and Akt K– expression in MCF-7 cells suppresses oestradiol activation of the cyclin D1 promoter proves that, at least in part, oestradiol increases cyclin D1 expression stimulating cyclin D1 transcription via PI3-kinase/Akt pathway. In this regard it should be noted that for the first time it has been observed that oestradiol stimulates cyclin D1 transcription through signalling effectors.
PI3-kinase has different targets (Rameh and Cantley, 1999). Among them, Akt received the most attention because of its role in a number of key biological functions, such as regulation of cell survival or death (Marte and Downward, 1997) and cell cycle progression (Coffer et al., 1998). We now observe that acute oestradiol treatment of MCF-7 cells or NIH 3T3 cells transfected with hERα cDNA activates Akt. In addition, oestradiol treatment of MCF-7 cells stimulates the p85-regulated PI3-kinase activity assayed by lipid production. In agreement with the causal association of the activation of the two enzymes, the PI3-kinase inhibitor, LY294002, abolishes the hormonal stimulation of Akt in MCF-7 cells and Δp85α expression suppresses the oestradiol Akt stimulation in NIH 3T3 cells transiently transfected with hERα cDNA.
The mechanism behind Akt activation in response to PI3-kinase stimulation has been at least in part elucidated. The lipids produced by PI3-kinase at the membrane result in recruitment of Akt via its pleckstrin homology domain. Akt is then fully activated at the membrane through phosphorylation (Marte and Downward, 1997). It is now observed that PP1, which inhibits the hormone-stimulated Src activity (Migliaccio et al., 1998), also prevents the PI3-kinase and Akt activation in oestradiol-treated MCF-7 cells. A Src/PI3-kinase/Akt signalling pathway has been proposed in osteoblasts and dendritic cells (Wong et al., 1999). Our findings indicate that a similar pathway is activated by oestradiol in MCF-7 cells. Activation of this pathway targets cyclin D1 transcription. The possibility that Src regulates the transcriptional level of cyclin D1 via MAP-kinases is unlikely, since the negative mutant of MEK-1 has a negligible effect in our reporter transcription assay. It has been reported that MEK activation triggers the promoter as well as the expression of cyclin D1 (Lavoie et al., 1996). This effect has been attributed to the sequential activation of the Raf/Mek/Erk pathway (Lavoie et al., 1996). However, it is likely that in oestradiol-stimulated MCF-7 cells the Mek/Erk pathway may be involved in the control of other steps acting on cyclin D1–cdk-4 activation. The role of MEK/Erk pathway in post-translational regulation of cyclin D1–cdk-4 complex assembly and activation has also been described (Cheng et al., 1998).
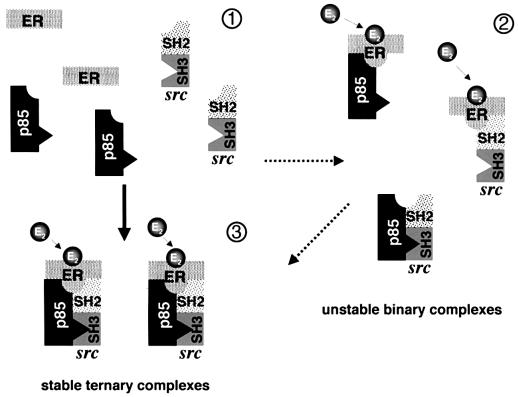
Surprisingly, hormonal Src activation is suppressed in NIH 3T3 cells by expression of Δp85α and in MCF-7 cells by LY294002. In turn, PP1 prevents lipid synthesis by oestradiol-activated PI3-kinase as well as Akt activation. These findings indicate the existence of a novel reciprocal cross-talk between Src and PI3-kinase regulated by oestradiol. ERα directly interacts with the Src-SH2 domain (Migliaccio et al., 2000) or p85 (Simoncini et al., 2000). We now observe that the two signalling members in MCF-7 cells simultaneously interact with ERα upon hormone stimulation. It is noteworthy that association between Src kinase family members and different PI3-kinases has previously been reported (Gutkind et al., 1990; Corey et al., 1993; Liu et al., 1993; Pleiman et al., 1994; Lopez-Ilasaca et al., 1997). Pull-down experiments show that oestradiol stimulates the assembly of the ERα–Src–p85 ternary complex. This complex shows a stronger association than that of each of the binary complexes made by the same molecules. The Src-SH3 domain is required for a stable assembly of the in vitro hormone-induced ternary complex. Interaction between the SH3 domain of the two Src-family members, Lyn and Fyn (Pleiman et al., 1994) or v-Src (Liu et al., 1993) with the p85 subunit of PI3-kinase has previously been observed. Taken together, our data suggest that in addition to direct interaction of ERα-HBD (where HBD is hormone binding domain) with Src and p85, association between Src and p85 mediated by the Src-SH3 domain stabilizes the ternary complex (this hypothesis is presented in Figure 6). This complex is thought to facilitate the simultaneous activation of Src and PI3-kinase and might be responsible for the novel Src–PI3-kinase reversible cross-talk.
Fig. 6. Hypothetical model of the association of ERα with Src and p85 triggered by oestradiol in MCF-7 cells. In the absence of hormone ERα does not interact with p85 or Src (1). Oestradiol triggers immediate association of the three proteins (3). Pull-down experiments support the assembly of the ternary complex. They also show that unstable binary complexes (2) are formed. They might represent a transition towards the more stable ternary complex.
Materials and methods
Constructs
cDNA coding the wild type hERα (HEG0) was cloned into pSG5 (Tora et al., 1989). cDNA coding the kinase-inactive form of Src (Lys259 changed to methionine) was also cloned into pSG5 (Barone and Courtneidge, 1996). cDNAs coding the kinase-dead MEK-1 (Ser221 changed to alanine; A221-MEK1) in pEXV3, and the wt p85a as well as its dominant-negative form (Δp85α) in pSG5 were used. Myc-His tagged dominant-negative Akt (K179M) in pUSEAmp as well as the empty pUSEAmp plasmid were from UBI (Lake Placid, NY). The luciferase reporter pGL2-luc D1 contained 1.8 kb pairs of the human cyclin D1 promoter. Chicken full-length Src as well as Src-ΔSH3 were cloned into pSGT. Src cDNA was subcloned into the plasmid vector pGEX-2TK (Pharmacia) and the resulting pGEX-Src construct was used to generate a GST–Src fusion protein (Migliaccio et al., 2000). Src-ΔSH2 in pRSP was subcloned into pSG5 mammalian expression vector (Migliaccio et al., 2000).
Cell culture and transfection techniques
Human mammary cancer-derived MCF-7 cells were routinely grown as reported (Castoria et al., 1999). For the S-phase entry analysis, they were seeded onto gelatine-precoated coverslips at 40% of confluence, then made quiescent using charcoal-treated serum and media lacking phenol-red (Castoria et al., 1999) for 3 days. Cells were then either left unstimulated or stimulated for 24 h with 10 nM oestradiol. The effect of inhibitors on steroid-stimulated S-phase entry was analysed using either LY294002 (20 µM) or rapamycin (50 nM). Both inhibitors were from Calbiochem (CA). When indicated, quiescent cells on coverslips were transfected using Superfect (Qiagen, GmbH). Three micrograms of the construct were used and 18 h after transfection the cells were left unstimulated or stimulated for 24 h with 10 nM oestradiol. For cyclin D1 promoter analysis, MCF-7 cells plated at 30% of confluence were made quiescent, then co-transfected by Superfect with 3 µg of luciferase reporter pGL2-lucD1 construct in combination with 0.5 µg of the indicated plasmids. In each case the empty pSG5 (2 µg) was added as a carrier. Twenty-four hours later, transfected cells were unstimulated or stimulated with 10 nM oestradiol for the indicated times. Lysates were prepared and the luciferase activity measured using a luciferase assay system (Promega). The results were corrected using CH110-expressed β-galactosidase activity (Amersham Pharmacia Biotech). Mouse fibroblasts NIH 3T3 were cultured as reported (Castoria et al., 1999). They were co-transfected by Superfect with 4 µg of pSG5-hERα. All other constructs were used at 1 µg. Two micrograms of the empty pSG5 were in each case co-transfected as a carrier. After 12 h, transfected cells were made quiescent as described (Castoria et al., 1999) and maintained under this condition for an additional 24 h. Cells were then either left unstimulated or stimulated with the oestradiol (10 nM) for the indicated times.
Immunofluorescence and DNA synthesis analysis
DNA synthesis was assayed by 6 h-pulse with 100 µM BrdU (Boehringer). After in vivo labelling, cells on coverslips were fixed, permeabilized and stained. Δp85α and Myc-His tagged Akt K– were visualized using diluted [1:100 in phosphate-buffered saline (PBS)] either anti-p85α or anti-Myc tag (both from UBI) rabbit polyclonal antibodies. Diluted (1:200 in PBS containing 0.2% bovine serum albumin) anti-rabbit Texas Red-conjugated antibodies (Jackson Laboratories) were added. After extensive washings in PBS, BrdU incorporation into newly synthesized DNA was assessed (Castoria et al., 1999). Nuclei were stained with Hoechst 33258 (Sigma) and coverslips inverted and finally mounted in Moviol (Calbiochem). Slides were analysed using an Axiophot fluorescent microscope (Zeiss). Significant fields were captured and processed using a KS300 software (Zeiss).
Production of recombinant proteins and protein–protein interaction assay
The GST–HEG14 and GST–Src fusion proteins were produced as reported (Abbondanza et al., 1998; Migliaccio et al., 2000). They were extracted and purified on glutathione–Agarose beads (Fluka) according to the same reports. The matrix with the adsorbed fusion proteins was utilized for protein–protein interaction assay. Coupled in vitro transcription–translation reactions were used to produce 35S-labelled human ERα, bovine p85α wild-type as well as chicken Src and Src-derivatives (either ΔSH2 or ΔSH3) in rabbit reticulocyte lysate (Promega). For protein–protein interaction assay, the matrix with the adsorbed fusion proteins was incubated with the radiolabelled proteins. Incubation was performed for 1 h at room temperature by gentle shaking in PBS containing 0.2% Triton X-100, 10 µg/ml leupeptin, 10 µg/ml antipain, 10 µg/ml pepstatin and 1mM phenylmethyl sulfonylfluoride (PMSF), in the absence or presence of hormone. Beads were washed 3× in the same buffer and proteins eluted by Laemmli sample buffer. They were finally resolved on SDS–PAGE and protein bands revealed by fluorography.
Immunoprecipitation and kinase assays
Unless otherwise stated, cell lysates were prepared as described (Migliaccio et al., 1996) and protein concentrations measured using a Bio-Rad protein assay kit (Bio-Rad, CA). ERα was immunoprecipitated from cell lysates (1 mg/ml of protein) using the rat monoclonal H222 antibody as previously described (Migliaccio et al., 1998). Lysates (1 mg/ml of protein) were used for Src immunoprecipitation, which was performed using the mouse monoclonal anti-Src antibody (clone 327 from Calbiochem), as reported (Migliaccio et al., 1998). Src activity was assayed using enolase as a substrate (Migliaccio et al., 1998). For PI3-kinase immunoprecipitation, lysates (1 mg/ml of protein) were incubated at 4°C for 2 h with 1 µg of anti-p85 rabbit polyclonal antibodies (from UBI). Immunocomplexes were finally precipitated by 30 µl of Protein A–Sepharose CL4B (Pharmacia Biotech) pre-equilibrated with lysis buffer. Protein A–Sepharose beads were washed 3× with 1 ml of lysis buffer and immunocomplexes reduced in Laemmli sample buffer. For the PI3-kinase assay, lysates (2 mg/ml of protein) were incubated at 4°C for 2 h using 2 µg of anti-p85 rabbit polyclonal antibodies (from UBI). Immunocomplexes were precipitated by 30 µl of Protein A–Sepharose CL4B (Pharmacia Biotech) pre-equilibrated with lysis buffer. Protein A–Sepharose beads were washed 4× with 1 ml of lysis buffer and PI3-kinase activity assayed as described (Whitman et al., 1985). PI (4,5) P2 (from Lipid Products, Guildford, Surrey) was used as a substrate. Phospholipids were resolved by thin layer chromatography in CHCl3/MEOH/H2O/NH4OH (60:47:11.3:2) buffer and radiolabelled lipids were finally visualized by autoradiography. For P-Akt (Ser473) and Akt detection, cell lysates were prepared in 20 mM Tris (pH 7.5) containing 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 mM PMSF, 1 µg/ml leupeptin and 10 µg/ml aprotinin.
Electrophoresis and immunoblotting
SDS–PAGE (12% gels) and electrotransfer to nitrocellulose filters were made as described (Migliaccio et al., 1998). ERα was detected using the rat monoclonal H222 antibody (Di Domenico et al., 1996). Human cyclin D1 was revealed using anti-cyclin D1 mouse monoclonal antibody (clone AM29 from Zymed Laboratories Inc., CA). c-Myc was detected using anti-c-Myc mouse monoclonal antibody (clone 9E10 from Zymed Laboratories). For Cdk4 detection, the rabbit polyclonal antibody (c-22 from Santa Cruz, CA) was used. Cyclin E was revealed using rabbit polyclonal antibody (M 20 from Santa Cruz). For pRb detection, proteins were separated by SDS–PAGE in a 10% gel. Immunoblot was performed using the anti-pRb mouse monoclonal antibody (G3-245 from Pharmingen, CA). p85 was immunoblotted using rabbit polyclonal antibody (from UBI). Anti-MEK-1 rabbit polyclonal antibody (C-18 from Santa Cruz) was employed to detect MEK-1. To assay Akt phosphorylation, proteins from cell lysates were reduced and resolved (40 µg/lane) on SDS–PAGE (12% gels). They were transferred to nitrocellulose filters using 25 mM Tris base, 0.2 M glycine, 20% methanol buffer (pH 8.5). Filters were probed using either anti P-Akt (Ser473) or anti-Akt rabbit polyclonal antibodies (both from New England BioLabs, MA). Immunoreactive proteins were revealed by the enhanced chemiluminescence detection system (Amersham).
Acknowledgments
Acknowledgements
We thank P.Chambon for the HEG0 expressing plasmid, J.Downward for the A221-MEK-1, Δp85α expressing plasmids and NIH 3T3 fibroblasts, B.Vanhaesebroeck for the wild-type p85α expressing plasmid, R.Assoian for the luciferase reporter pGL2-luc D1 construct, S.Gonfloni for pRSP-ΔSH2, S.A.Courtneidge for pSGT-Src and pSGT-ΔSH3, C.Abbondanza for GST-HEG14. The H222 anti-ERα monoclonal antibody was a gift from G.Greene. Zeneca (Italy) provided the antioestrogen ICI 182 780. The technical assistance of Flavia Vitale and Monica Doria as well as the editorial assistance of Gian Michele La Placa are also acknowledged. We also thank the ‘Centro di Microscopia ed Analisi di immagini’ dell’Istituto Pascale–Napoli. This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.), Ministero dell’Università e della Ricerca Scientifica (Cofinanziamento MURST 1999 e 2000-Fondi 40 e 60%) and Consiglio Nazionale delle Ricerche (C.N.R.- Agenzia 2000). M.V.B. is recipient of a Fondazione Italiana per la Ricerca sul Cancro scholarship.
References
- Abbondanza C. et al. (1998) Interaction of vault particles with estrogen receptor in the MCF-7 breast cancer cell. J. Cell Biol., 141, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone M.V. and Courtneidge,S.A. (1996) Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature, 378, 509–512. [DOI] [PubMed] [Google Scholar]
- Beato M., Herrlich,P. and Schutz,G. (1995) Steroid-hormone receptors: many actors in search of a plot. Cell, 83, 851–857. [DOI] [PubMed] [Google Scholar]
- Brunn G.J., Williams,J., Sabers,C., Wiederrecht,G., Lawrence,J.C.,Jr and Abraham,R.T. (1996) Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J., 15, 5256–5267. [PMC free article] [PubMed] [Google Scholar]
- Carpenter C.L. and Cantley,L.C. (1996) Phosphoinositide kinases. Curr. Opin. Cell Biol., 8, 153–158. [DOI] [PubMed] [Google Scholar]
- Castoria G., Barone,M.V., Di Domenico,M., Bilancio,A., Ametrano,D., Migliaccio,A. and Auricchio,F. (1999) Non-transcriptional action of estrogen and progestin triggers DNA synthesis. EMBO J., 18, 2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M., Sexl,V., Sherr,C.J. and Roussel,M.F. (1998) Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1). Proc. Natl Acad. Sci. USA, 95, 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffer P.J., Jin,J. and Woodgett,J.R. (1998) Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J., 335, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey S., Eguinoa,A., Puyana-Theall,K., Bolen,J.B., Cantley,L., Mollinedo,F., Jackson,T.R., Hawkins,P.T. and Stephens,L.R. (1993) Granulocyte macrophage-colony stimulating factor stimulates both association and activation of phosphoinositide 3OH-kinase and src-related tyrosine kinase(s) in human myeloid derived cells. EMBO J., 12, 2681–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand R. et al. (1994) PI 3-kinase: structural and functional analysis of intersubunit interactions. EMBO J., 13, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico M., Castoria,G., Bilancio,A., Migliaccio,A. and Auricchio,F. (1996) Estradiol activation of human colon carcinoma-derived Caco-2 cell growth. Cancer Res., 56, 4516–4521. [PubMed] [Google Scholar]
- Dubik D., Dembinski,T.C. and Shiu,R.P. (1987) Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res., 47, 6517–6521. [PubMed] [Google Scholar]
- Foster J.S. and Wimalasema,J. (1996) Estrogen regulates activity of cyclin-dependent kinases and retinoblastoma protein phosphorylation in breast cancer cells. Mol. Endocrinol., 10, 488–498. [DOI] [PubMed] [Google Scholar]
- Franke T.F., Kaplan,D.R. and Cantley,L.C. (1997) PI3K: downstream AKTion blocks apoptosis. Cell, 88, 435–437. [DOI] [PubMed] [Google Scholar]
- Gille H. and Downward,J. (1999) Multiple Ras effector pathways contribute to G1 cell cycle progression. J. Biol. Chem., 274, 22033–22040. [DOI] [PubMed] [Google Scholar]
- Gutkind J.S., Lacal,P.M. and Robbins,K.C. (1990) Thrombin-dependent association of phosphatidylinositol-3 kinase with p60c-src and p59fyn in human platelets. Mol. Cell. Biol., 10, 3806–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke J.H., Gardner,J.P., Dow,R.L., Changeliau,P.S., Brisselte,W.H., Weringer,E.J., Pollok,B.A. and Connelly,P.A. (1996) Discovery of a novel, potent and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem., 271, 695–701. [DOI] [PubMed] [Google Scholar]
- Hartley K.O. et al. (1995) DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell, 82, 849–856. [DOI] [PubMed] [Google Scholar]
- Honda K., Sawada,H., Kihara,T., Urushitani,M., Nakamizo,T., Akaike,A. and Shimohama,S. (2000) Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J. Neurosci. Res., 60, 321–327. [DOI] [PubMed] [Google Scholar]
- Kohn A.D., Summers,S.A., Birnbaum,M.J. and Roth,R.A. (1996) Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem., 27, 31372–31378. [DOI] [PubMed] [Google Scholar]
- Kousteni S. et al. (2001) Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell, 104, 719–730. [PubMed] [Google Scholar]
- Lavoie J.N., L’Allemain,G., Brunet,A., Muller,R. and Pouyssegur,J. (1996) Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J. Biol. Chem., 27, 20608–20616. [DOI] [PubMed] [Google Scholar]
- Liu X., Marengere,L.E., Koch,C.A. and Pawson,T. (1993) The v-Src SH3 domain binds phosphatidylinositol 3-kinase. Mol. Cell. Biol., 13, 5225–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ilasaca M., Crespo,P., Pellici,P.G., Gutkind,J.S. and Wetzker,R. (1997) Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science, 275, 394–397. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J. et al. (1995) The nuclear receptor superfamily: the second decade. Cell, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marte B.M. and Downward,J. (1997) PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem. Sci., 22, 355–358. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. and Alves,S.E. (1999) Estrogen actions in the central nervous system. Endocr. Rev., 20, 279–307. [DOI] [PubMed] [Google Scholar]
- McKenna N.J., Lanz,R.B. and O’Malley,B.W. (1999) Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev., 20, 321–344. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Di Domenico,M., Castoria,G., de Falco,A., Bontempo,P., Nola,E. and Auricchio,F. (1996) Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J., 15, 1292–1300. [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A., Piccolo,D., Castoria,G., Di Domenico,M., Bilancio,A., Lombardi,M., Gong,W., Beato,M. and Auricchio,F. (1998) Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen. EMBO J., 17, 2008–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A. et al. (2000) Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J., 19, 5406–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove E.A., Lee,C.S., Buckley,M.F. and Sutherland,R.L. (1994) Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc. Natl Acad. Sci. USA, 91, 8022–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M.G. and White,R. (1996) Nuclear receptors spring into action. Nature Struct. Biol., 3, 113–115. [DOI] [PubMed] [Google Scholar]
- Pietras R.J. et al. (1995) HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene, 10, 2435–2446. [PubMed] [Google Scholar]
- Planas-Silva M.D., Donaher,J.L. and Weinberg,R.A. (1999) Functional activity of ectopically expressed estrogen receptor is not sufficient for estrogen-mediated cyclin D1 expression. Cancer Res., 59, 4788–4792. [PubMed] [Google Scholar]
- Pleiman C.M., Hertz,W. and Cambier,J.C. (1994) Activation of PI3-kinase by Src-family kinase binding to the p85 subunit. Science, 263, 1609–1612. [DOI] [PubMed] [Google Scholar]
- Poser S., Impey,S., Trinh,K., Xia,Z. and Storm,D.R. (2000) SRF-dependent gene expression is required for PI3-kinase-regulated cell proliferation. EMBO J., 19, 4955–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R.F., Mani,S.K., Codina,J., Conneely,O.M. and O’Malley,B.W. (1991) Dopaminergic and ligand-independent activation of steroid hormone receptor. Science, 254, 1636–1639. [DOI] [PubMed] [Google Scholar]
- Rameh L.E. and Cantley,L.C. (1999) The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem., 274, 8347–8350. [DOI] [PubMed] [Google Scholar]
- Revelli A., Massobrio,M. and Tesarik,J. (1998) Nongenomic actions of steroid hormones in reproductive tissues. Endocr. Rev., 19, 3–17. [DOI] [PubMed] [Google Scholar]
- Simoncini T., Hafezi-Moghadam,A., Brazil,D.P., Ley,K., Chin,W.W. and Liao,J.K. (2000) Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH-kinase. Nature, 407, 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R.C. and Waterfield,M.D. (2000) PI3-kinase inhibition: a target for drug development? Mol. Med. Today, 6, 347–357. [DOI] [PubMed] [Google Scholar]
- Sutherland R.L., Hall,R.E. and Taylor,I.W. (1983) Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells. Cancer Res., 43, 3998–4006. [PubMed] [Google Scholar]
- Tocker A. and Cantley,L.C. (1997) Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature, 387, 673–676. [DOI] [PubMed] [Google Scholar]
- Tora L., Mullick,A., Metzger,D., Ponglikitmongkol,M., Park,I. and Chambon,P. (1989) The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J., 8, 1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burg B., van Selm-Miltenburg,A.J., de Laat,S.W. and van Zoelen,E.J. (1989) Direct effects of estrogen on c-fos and c-myc protooncogene expression and cellular proliferation in human breast cancer cells. Mol. Cell. Endocrinol., 64, 223–228. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Leevers,S.J., Ahmadi,K., Timms,J., Katso,R., Driscoll,P.C., Woscholski,R., Parker,P.,J. and Waterfield,M.D. (2001) Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem., 70, 535–602. [DOI] [PubMed] [Google Scholar]
- Vlahos C.J., Matter,W.F., Hui,K.Y. and Brown,R.F. (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem., 269, 5241–5248. [PubMed] [Google Scholar]
- Wehling M. (1997) Specific, nongenomic actions of steroid hormones. Annu. Rev. Physiol., 59, 365–393. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan,D.R., Schaffhausen,B., Cantley,L. and Roberts, T.M. (1985) Association of phosphatidylinositol kinase activity with polyoma middle T component for transformation. Nature, 315, 239–242. [DOI] [PubMed] [Google Scholar]
- Wong B.R., Besser,D., Kim,N., Arron,J.R., Vologodskaia,M., Hanafusa,H. and Choi,Y. (1999) TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell, 4, 1041–1049. [DOI] [PubMed] [Google Scholar]