Abstract
This investigation was designed to determine the change in cell numbers in the trigeminal ganglia following unilateral section of one of its peripheral branches. In 9 young adult cats, under general anesthesia, the inferior alveolar nerve was transected. In 3 of the animals the cut ends were reapposed and in the other 6 regeneration was blocked. After 15 weeks the trigeminal ganglia were removed, and the number of neurons present estimated by counting nucleoli in every third section. The counts were compared with those obtained from 3 unoperated control animals. The mean number of cells in the ganglia of control animals was 14,324 with no statistically significant difference between sides. There was no significant difference between counts from opposite sides of cats whose nerves were allowed to regenerate. In the animals in which regeneration was prevented the mean count on the operated side was 13,874 and on the unoperated 18,374. These differences were statistically significant and appeared to result from an increase in cell counts on the unoperated side rather than a reduction in the counts on the operated side. This may be explained by the presence of neurons with inconspicuous nucleoli in normal ganglia, which are stimulated to enlarge and become more prominent following peripheral nerve injury. This change could occur on both sides and would mask cell loss on the operated side and produce an apparent increase in the count on the unoperated side.
Full text
PDF

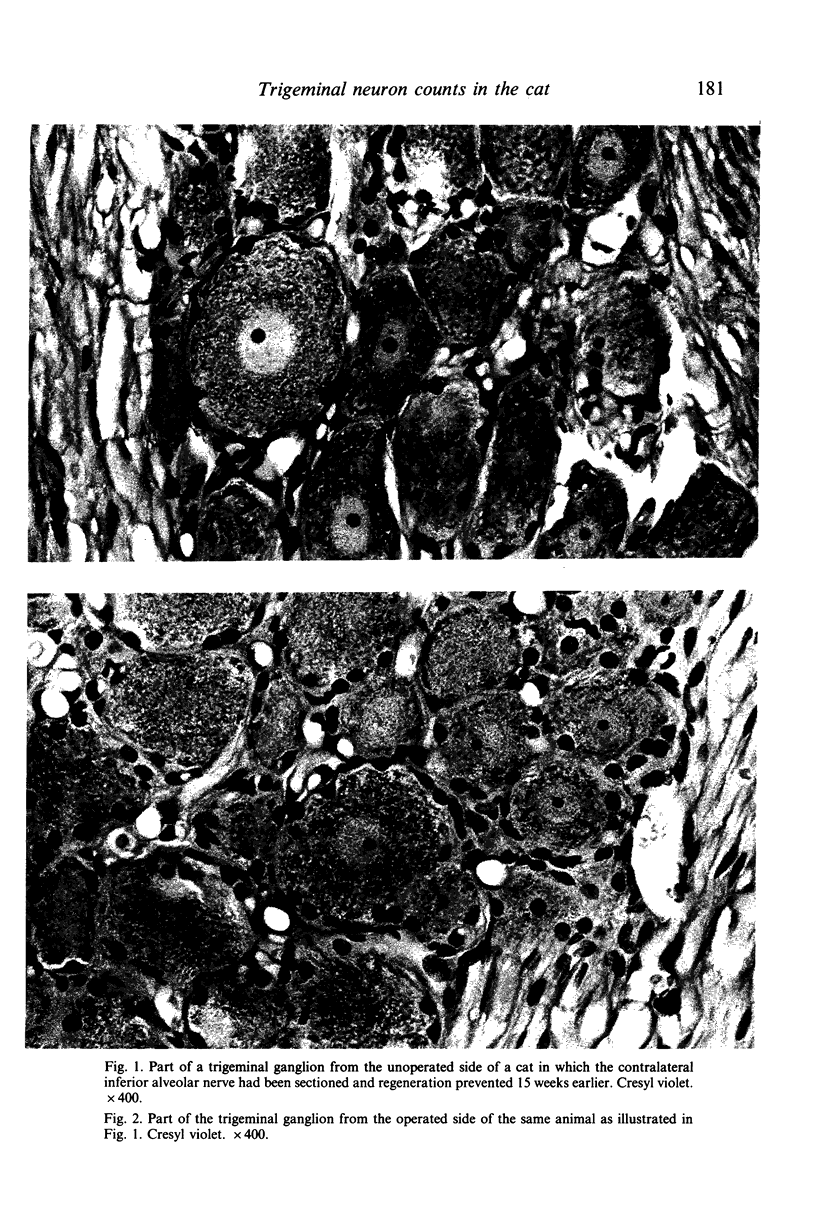
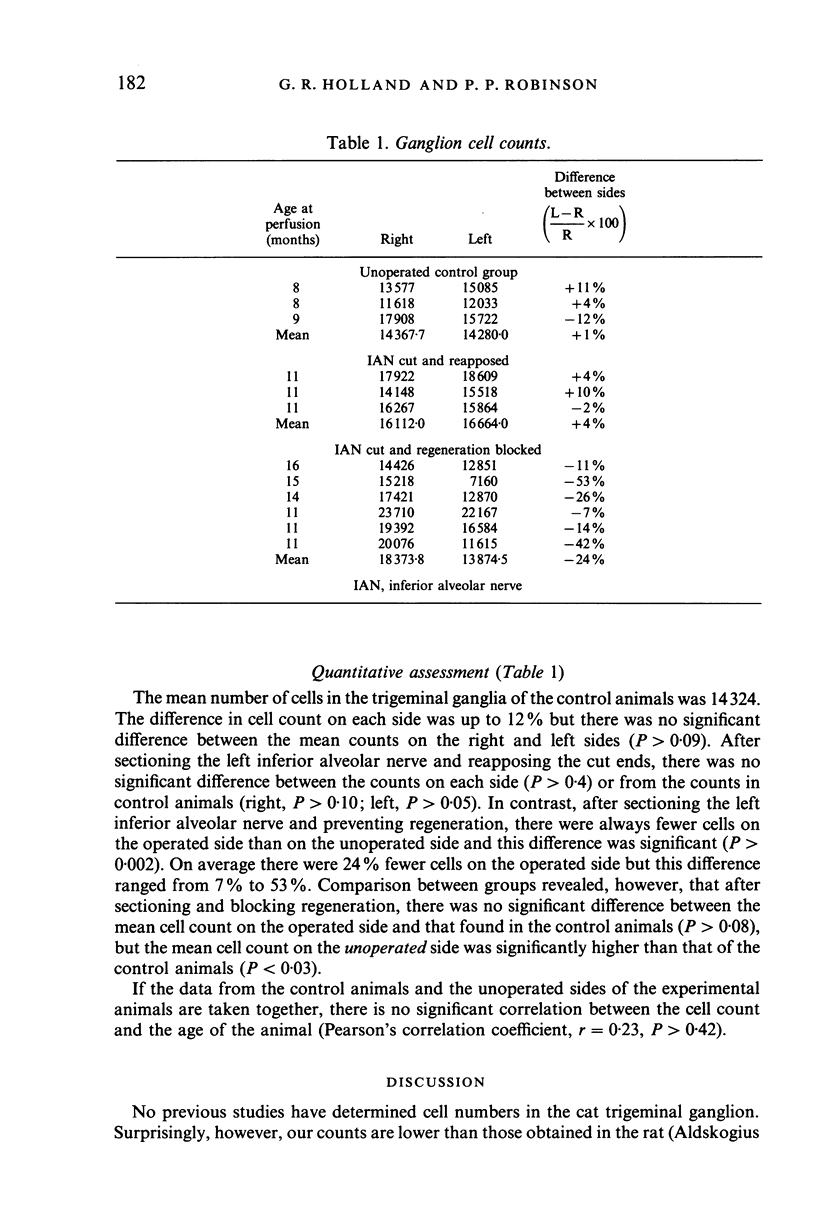




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldskogius H., Arvidsson J., Grant G. The reaction of primary sensory neurons to peripheral nerve injury with particular emphasis on transganglionic changes. Brain Res. 1985 Sep;357(1):27–46. doi: 10.1016/0165-0173(85)90006-2. [DOI] [PubMed] [Google Scholar]
- Aldskogius H., Arvidsson J. Nerve cell degeneration and death in the trigeminal ganglion of the adult rat following peripheral nerve transection. J Neurocytol. 1978 Apr;7(2):229–250. doi: 10.1007/BF01217921. [DOI] [PubMed] [Google Scholar]
- Aldskogius H., Risling M. Effect of sciatic neurectomy on neuronal number and size distribution in the L7 ganglion of kittens. Exp Neurol. 1981 Nov;74(2):597–604. doi: 10.1016/0014-4886(81)90194-1. [DOI] [PubMed] [Google Scholar]
- Arvidsson J., Ygge J., Grant G. Cell loss in lumbar dorsal root ganglia and transganglionic degeneration after sciatic nerve resection in the rat. Brain Res. 1986 May 14;373(1-2):15–21. doi: 10.1016/0006-8993(86)90310-0. [DOI] [PubMed] [Google Scholar]
- Berger R. L., Byers M. R. Dental nerve regeneration in rats. II. Autoradiographic studies of nerve regeneration to molar pulp and dentin. Pain. 1983 Apr;15(4):359–375. doi: 10.1016/0304-3959(83)90072-6. [DOI] [PubMed] [Google Scholar]
- CAMMERMEYER J. DIFFERENTIAL RESPONSE OF TWO NEURON TYPES TO FACIAL NERVE TRANSECTION IN YOUNG AND OLD RABBITS. J Neuropathol Exp Neurol. 1963 Oct;22:594–616. doi: 10.1097/00005072-196310000-00003. [DOI] [PubMed] [Google Scholar]
- CAVANAUGH M. W. Quantitative effects of the peripheral innervation area on nerves and spinal ganglion cells. J Comp Neurol. 1951 Apr;94(2):181–219. doi: 10.1002/cne.900940203. [DOI] [PubMed] [Google Scholar]
- Chiaia N. L., Hess P. R., Rhoades R. W. Preventing regeneration of infraorbital axons does not alter the ganglionic or transganglionic consequences of neonatal transection of this trigeminal branch. Brain Res. 1987 Nov;433(1):75–88. doi: 10.1016/0165-3806(87)90066-6. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E., Chung K., Greenwood D., Hulsebosch C. E. An empirical method for converting nucleolar counts to neuronal numbers. J Neurosci Methods. 1984 Dec;12(2):125–132. doi: 10.1016/0165-0270(84)90011-6. [DOI] [PubMed] [Google Scholar]
- Devor M., Govrin-Lippmann R., Frank I., Raber P. Proliferation of primary sensory neurons in adult rat dorsal root ganglion and the kinetics of retrograde cell loss after sciatic nerve section. Somatosens Res. 1985;3(2):139–167. doi: 10.3109/07367228509144581. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Wall P. D., Goedert M., Emson P. C. Nerve growth factor counteracts the neurophysiological and neurochemical effects of chronic sciatic nerve section. Brain Res. 1985 Apr 15;332(1):131–141. doi: 10.1016/0006-8993(85)90396-8. [DOI] [PubMed] [Google Scholar]
- Fried K., Erdélyi G. Inferior alveolar nerve regeneration and incisor pulpal reinnervation following intramandibular neurotomy in the cat. Brain Res. 1982 Jul 29;244(2):259–268. doi: 10.1016/0006-8993(82)90084-1. [DOI] [PubMed] [Google Scholar]
- Gobel S., Binck J. M. Degenerative changes in primary trigeminal axons and in neurons in nucleus caudalis following tooth pulp extirpations in the cat. Brain Res. 1977 Aug 26;132(2):347–354. doi: 10.1016/0006-8993(77)90427-9. [DOI] [PubMed] [Google Scholar]
- Grant G., Arvidsson J. Transganglionic degeneration in trigeminal primary sensory neurons. Brain Res. 1975 Sep 23;95(2-3):265–279. doi: 10.1016/0006-8993(75)90106-7. [DOI] [PubMed] [Google Scholar]
- Hofer M. M., Barde Y. A. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988 Jan 21;331(6153):261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- Holland G. R., Robinson P. P. A morphological study of the collateral reinnervation of the cat's canine tooth. Exp Neurol. 1987 Dec;98(3):489–498. doi: 10.1016/0014-4886(87)90258-5. [DOI] [PubMed] [Google Scholar]
- Horch K. W., Lisney S. J. On the number and nature of regenerating myelinated axons after lesions of cutaneous nerves in the cat. J Physiol. 1981;313:275–286. doi: 10.1113/jphysiol.1981.sp013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Smith M. E. Effect of nerve section on beta-endorphin and alpha-melanotropin immunoreactivity in motor nerves of normal and dystrophic mice. Neurosci Lett. 1988 Sep 23;92(1):1–7. doi: 10.1016/0304-3940(88)90732-x. [DOI] [PubMed] [Google Scholar]
- Jänig W., McLachlan E. On the fate of sympathetic and sensory neurons projecting into a neuroma of the superficial peroneal nerve in the cat. J Comp Neurol. 1984 May 10;225(2):302–311. doi: 10.1002/cne.902250213. [DOI] [PubMed] [Google Scholar]
- Otto D., Unsicker K., Grothe C. Pharmacological effects of nerve growth factor and fibroblast growth factor applied to the transectioned sciatic nerve on neuron death in adult rat dorsal root ganglia. Neurosci Lett. 1987 Dec 16;83(1-2):156–160. doi: 10.1016/0304-3940(87)90233-3. [DOI] [PubMed] [Google Scholar]
- Owen D. J., Logan A., Robinson P. P. A role for nerve growth factor in collateral reinnervation from sensory nerves in the guinea pig. Brain Res. 1989 Jan 9;476(2):248–255. doi: 10.1016/0006-8993(89)91245-6. [DOI] [PubMed] [Google Scholar]
- Rhoades R. W., Fiore J. M., Math M. F., Jacquin M. F. Reorganization of trigeminal primary afferents following neonatal infraorbital nerve section in hamster. Brain Res. 1983 Apr;283(2-3):337–342. doi: 10.1016/0165-3806(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Robinson P. P. Regenerating nerve fibres do not displace the collateral reinnervation of cat teeth. Brain Res. 1984 Sep 24;310(2):303–310. doi: 10.1016/0006-8993(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Robinson P. P. Reinnervation of teeth, mucous membrane and skin following section of the inferior alveolar nerve in the cat. Brain Res. 1981 Sep 14;220(2):241–253. doi: 10.1016/0006-8993(81)91215-4. [DOI] [PubMed] [Google Scholar]
- Rotshenker S., Tal M. The transneuronal induction of sprouting and synapse formation in intact mouse muscles. J Physiol. 1985 Mar;360:387–396. doi: 10.1113/jphysiol.1985.sp015623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassburg M. Morphologic reaction of the trigeminal ganglion after experimental surgery on the maxillodental region. J Oral Surg. 1967 Mar;25(2):107–114. [PubMed] [Google Scholar]
- Sugimoto T., Gobel S. Primary neurons maintain their central axonal arbors in the spinal dorsal horn following peripheral nerve injury: an anatomical analysis using transganglionic transport of horseradish peroxidase. Brain Res. 1982 Sep 30;248(2):377–381. doi: 10.1016/0006-8993(82)90598-4. [DOI] [PubMed] [Google Scholar]
- Waite P. M. Rearrangement of neuronal responses in the trigeminal system of the rat following peripheral nerve section. J Physiol. 1984 Jul;352:425–445. doi: 10.1113/jphysiol.1984.sp015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall P. D., Gutnick M. Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp Neurol. 1974 Jun;43(3):580–593. doi: 10.1016/0014-4886(74)90197-6. [DOI] [PubMed] [Google Scholar]
- Ygge J., Aldskogius H., Grant G. Asymmetries and symmetries in the number of thoracic dorsal root ganglion cells. J Comp Neurol. 1981 Nov 1;202(3):365–372. doi: 10.1002/cne.902020306. [DOI] [PubMed] [Google Scholar]
- Ygge J., Aldskogius H. Intercostal nerve transection and its effect on the dorsal root ganglion. A quantitative study on thoracic ganglion cell numbers and sizes in the rat. Exp Brain Res. 1984;55(3):402–408. doi: 10.1007/BF00235270. [DOI] [PubMed] [Google Scholar]




