Abstract
Specimens of skull and palate were taken from 7 beagle dogs after perfusion and the periosteum examined by light and electron microscopy. Three zones were evident in the periosteum, differing in terms of the proportion of cells, fibres and matrix. Zone I consisted of osteoblasts adjacent to the bone surface and a supraosteoblast layer of smaller, compact cells, Zone II was a relatively translucent zone with numerous capillaries and Zone III consisted of cells intermingled with collagen fibrils. Quantification of the relative volumes of tissue components using stereology indicated significant differences between the three zones in each bony site, but not between the two sites. Measurement of numerical density, surface density, profile cross-sectional area, cell volume and cell surface/volume ratio of fibroblast-like cells revealed marked differences between these cells in Zone I and the other two zones; it is possible that the fibroblast-like cells seen in Zone I represent osteoprogenitor cells. Zone II represents the classical cambial layer and contains the majority of the vascular elements present in the periosteum. Zone III contains large volumes of fibroblasts and collagen fibrils and corresponds to the classical fibrous layer. The similarity of this zonal organisation in different regions suggests that periosteum has a consistent structure in membrane bone.
Full text
PDF

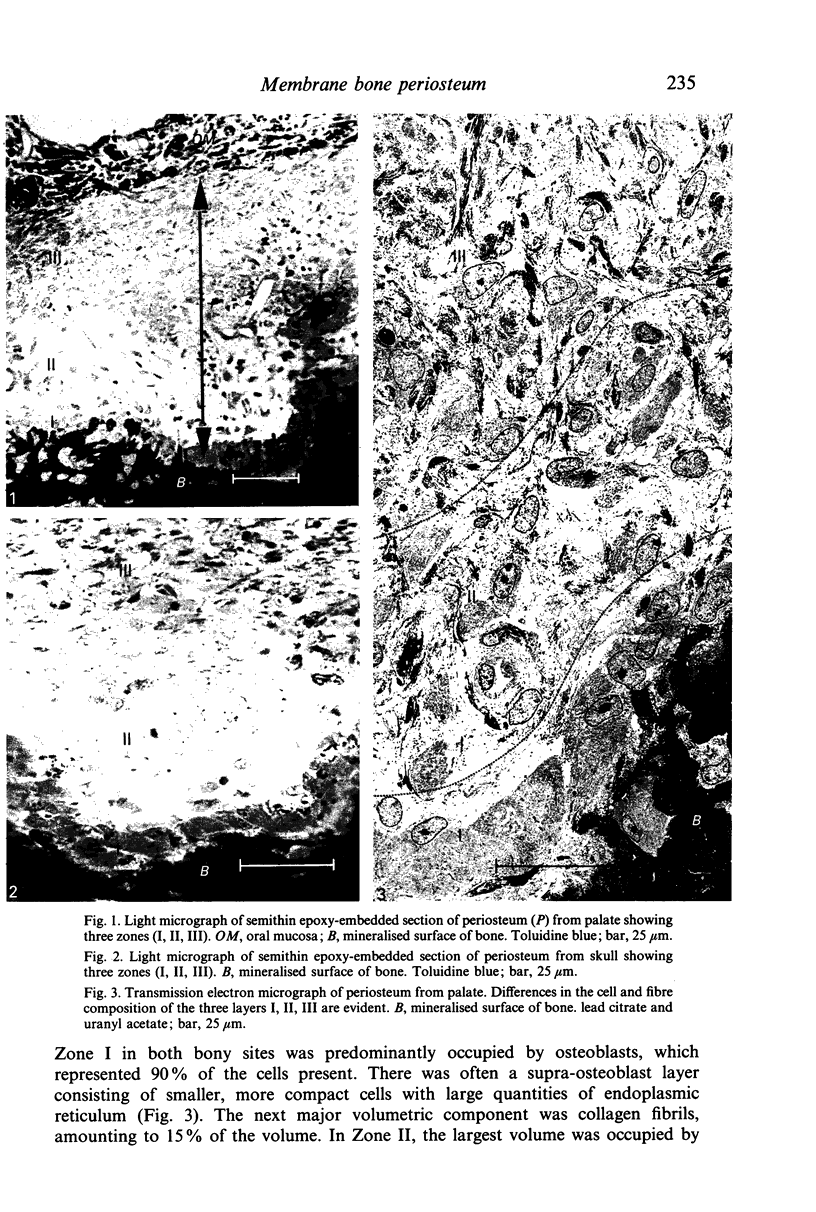
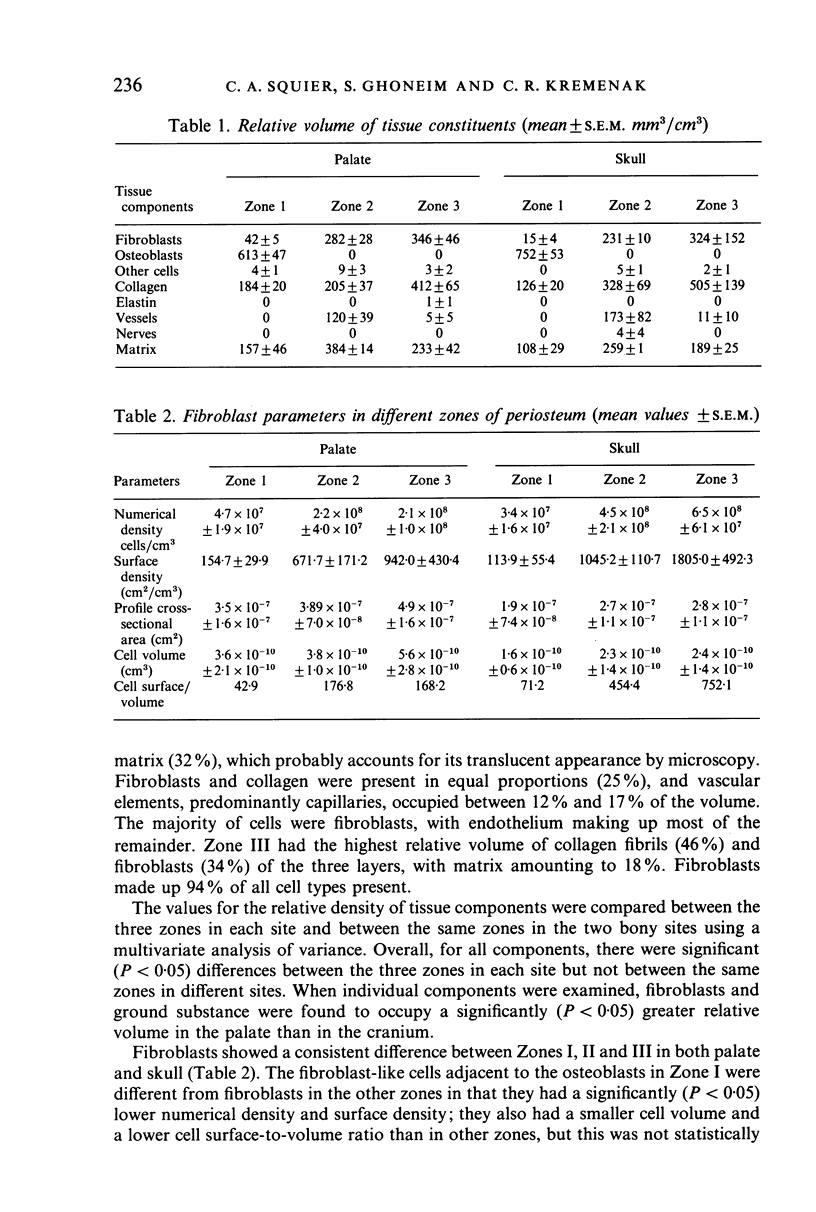



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitasalo K., Lehtinen R. The influence of a free periosteal transplant on bone healing in the irradiated tibia. A radiological histological and biochemical study on rabbits. Scand J Plast Reconstr Surg. 1985;19(3):237–244. doi: 10.3109/02844318509074509. [DOI] [PubMed] [Google Scholar]
- Barro W. B., Latham R. A. Palatal periosteal response to surgical trauma. Plast Reconstr Surg. 1981 Jan;67(1):6–16. doi: 10.1097/00006534-198101000-00002. [DOI] [PubMed] [Google Scholar]
- Canalis R. F., Burstein F. D. Osteogenesis in vascularized periosteum. Interactions with underlying bone. Arch Otolaryngol. 1985 Aug;111(8):511–516. doi: 10.1001/archotol.1985.00800100059007. [DOI] [PubMed] [Google Scholar]
- Finley J. M., Acland R. D., Wood M. B. Revascularized periosteal grafts--a new method to produce functional new bone without bone grafting. Plast Reconstr Surg. 1978 Jan;61(1):1–6. doi: 10.1097/00006534-197801000-00001. [DOI] [PubMed] [Google Scholar]
- Habal M. B., Maniscalco J. E. Observations on the ultrastructure of the pericranium. Ann Plast Surg. 1981 Feb;6(2):103–111. [PubMed] [Google Scholar]
- Holtrop M. E. The ultrastructure of bone. Ann Clin Lab Sci. 1975 Jul-Aug;5(4):264–271. [PubMed] [Google Scholar]
- Müller W. Stereology of inflammatory connective tissue infiltrates in oral mucosa. Pathol Res Pract. 1980;166(2-3):271–289. doi: 10.1016/S0344-0338(80)80135-X. [DOI] [PubMed] [Google Scholar]
- Perko M. A. Two-stage closure of cleft palate (progress report). J Maxillofac Surg. 1979 Feb;7(1):46–80. [PubMed] [Google Scholar]
- Ritsilä V., Alhopuro S., Gylling U., Rintala A. The use of free periosteum for bone formation in congenital clefts of the maxilla. A preliminary report. Scand J Plast Reconstr Surg. 1972;6(1):57–60. doi: 10.3109/02844317209103460. [DOI] [PubMed] [Google Scholar]
- Satoh T., Tsuchiya M., Harii K. A vascularised iliac musculo-periosteal free flap transfer: a case report. Br J Plast Surg. 1983 Jan;36(1):109–112. doi: 10.1016/0007-1226(83)90025-5. [DOI] [PubMed] [Google Scholar]
- Schroeder H. E., Münzel-Pedrazzoli S. Correlated morphometric and biochemical analysis of gingival tissue. Morphometric model, tissue sampling and test of stereologic procedures. J Microsc. 1973 Dec;99(3):301–329. doi: 10.1111/j.1365-2818.1973.tb04629.x. [DOI] [PubMed] [Google Scholar]
- Simpson A. H. The blood supply of the periosteum. J Anat. 1985 Jun;140(Pt 4):697–704. [PMC free article] [PubMed] [Google Scholar]
- Skoog T. The use of periosteal flaps in the repair of clefts of the primary palate. Cleft Palate J. 1965 Oct;2:332–339. [PubMed] [Google Scholar]
- Squier C. A., Kremenak C. R. Quantitation of the healing palatal mucoperiosteal wound in the beagle dog. Br J Exp Pathol. 1982 Oct;63(5):573–584. [PMC free article] [PubMed] [Google Scholar]
- TONNA E. A., CRONKITE E. P. Autoradiographic studies of cell proliferation in the periosteum of intact and fractured femora of mice utilizing DNA labeling with H3-thymidine. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:719–721. doi: 10.3181/00379727-107-26733. [DOI] [PubMed] [Google Scholar]
- Tang X. M., Chai B. F. Ultrastructural investigation of osteogenic cells. Chin Med J (Engl) 1986 Dec;99(12):950–956. [PubMed] [Google Scholar]
- Tenenbaum H. C., Palangio K. G., Holmyard D. P., Pritzker K. P. An ultrastructural study of osteogenesis in chick periosteum in vitro. Bone. 1986;7(4):295–302. doi: 10.1016/8756-3282(86)90211-5. [DOI] [PubMed] [Google Scholar]
- Tonna E. A. Response of the cellular phase of the skeleton to trauma. Periodontics. 1966 May-Jun;4(3):105–114. [PubMed] [Google Scholar]
- WEIBEL E. R., GOMEZ D. M. A principle for counting tissue structures on random sections. J Appl Physiol. 1962 Mar;17:343–348. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]





