Abstract
Stabilization of hypoxia inducible factor 1α (HIF-1α), a biomarker of hypoxia, in hypoxic tumors mediates a variety of downstream genes promoting tumor angiogenesis and cancer cell survival as well as invasion, and compromising therapeutic outcome. In this study, dynamic contrast enhanced (DCE)-MRI with a biodegradable macromolecular MRI contrast agent was used to non-invasively assess the antiangiogenic effect of a RGD-targeted multifunctional lipid ECO/siHIF-1α nanoparticles in a mouse HT29 colon cancer model. The RGD-targeted ECO/siHIF-1α nanoparticles resulted in over 50% reduction in tumor size after intravenous injection at a dose of 2.0 mg-siRNA/kg every three days for three weeks as compared to a saline control. DCE-MRI revealed significant decline in vascularity and over a 70% reduction in the tumor blood flow, permeability-surface area product, and plasma volume fraction vascular parameters in the tumor treated with the targeted ECO/siHIF-1α nanoparticles. The treatment with targeted ECO/siRNA nanoparticles resulted significant silence of HIF-1α expression at the protein level, which also significantly suppressed the expression of VEGF, Glut-1, HKII, PDK-1, LDHA, and CAIX, which are all important players in the tumor angiogenesis, glycolytic metabolism, and pH-regulation. By possessing the ability to illicit a multi-faceted effect on tumor biology, silencing HIF-1α with RGD-targeted ECO/siHIF-1α nanoparticles has great promise as a single therapy or in combination with traditional chemotherapy or radiation strategies to improve cancer treatment.
Keywords: multifunctional lipid, DCE-MRI, siRNA, HIF-1, angiogenesis
Graphical Abstract

INTRODUCTION
Tumor hypoxia plays a central role in angiogenesis, proliferation, and resistance to cancer therapies. This state of oxygen deprivation often arises when aggressive tumors outgrow their vascular supply, and tend to develop an abnormal vascular network comprised largely of disorganized, heterogeneous, and structurally immature blood vessels that lack the proper functionality to maintain constant perfusion and oxygen tension throughout the entire lesion 1–6. Although the inefficient supply of oxygen would suggest an eventual decline in tumor proliferation, numerous studies have demonstrated that intratumoral hypoxia in various types of cancer is actually associated with a highly proliferative and aggressive phenotype that is mediated by the transcription factor hypoxia inducible factor-1α (HIF-1α). The up-regulation of HIF-1α expression plays an essential role in tumor progression by inducing the transcription of various downstream genes that guide tumors to adapt and survive under hypoxic conditions.
One mechanism by which HIF-1α contributes to cancer progression is by initiating the production and release of pro-angiogenic factors to expand the vascular network and meet the demand for nutrients and oxygen by the growing tumor. It is also responsible for switching cellular metabolism from an oxidative process to one that is highly glycolytic, as wells as imparting protective measures against glycolytic acid buildup, both of which provide selective growth advantages for tumor cells in the stromal tissue. In addition, hypoxic tumors that express high levels of HIF-1α are typically characterized by their increased risk of metastatic invasion and apoptotic resistance. Consequently, tumor hypoxia, under the guidance of HIF-1α, is associated with a that is also largely resistant to various radiation and chemotherapy treatments 7. Therefore, silencing HIF-1α constitutes a promising strategy for future cancer therapies. One way to accomplish this is by developing a novel gene delivery platform that takes advantage of RNA interference (RNAi).
RNAi is an inherent post-transcriptional gene regulatory process in cells, comprised of a complex set of enzymatic machinery that is able to induce sequence-specific degradation of target mRNA transcripts through the use of small interfering RNA (siRNA). The power of RNAi is now being harnessed for the development of new anti-cancer therapies that regulate the expression of cancer-related genes. Due to the ease of siRNA synthesis, RNAi can be utilized to regulate the expression of potentially any protein in the cell, regardless of where it is located. Such versatility is particularly attractive for cancer therapy because it allows for robust silencing of molecular targets that may not be compatible with conventional treatment modalities, including small-molecular inhibitors and monoclonal antibodies 8, 9.
However, the therapeutic potential of RNAi is challenged by a number of barriers that impede the delivery of therapeutic siRNA first into the tumor site, and then into individual cancer cells 10. In order to maximize performance, siRNA needs to be formulated in a system that can protect it from serum nucleases in circulation, promote intracellular escape from the endocytic pathway, and then facilitate cytosolic delivery 11, 12. We recently introduced a new multifunctional pH-sensitive lipid carrier ECO that is able to electrostatically complex with siRNA and form stable nanoparticles with the aid of hydrophobic interactions and disulfide crosslinking. Upon cellular uptake in vitro, ECO/siRNA nanoparticles have demonstrated the ability to enhance endosomal escape, cytosolic siRNA release, and robust gene silencing events 13–16. ECO also allows for facile modification of siRNA nanoparticles with tumor-specific targeting agents for enhanced delivery of therapeutic siRNA following systemic administration. Intravenous injections of RGD-targeted ECO/siβ3 nanoparticles have recently been shown to effectively silence the expression of β3-integrin expression in tumors, alleviating primary tumor burden, and significantly inhibiting metastasis of triple negative breast cancer in mouse tumor models17.
In this study, we investigated the effectiveness of targeted ECO nanoparticles of an anti-HIF-1α siRNA (siHIF-1α) in antiangiogenic cancer therapy in a HT29 mouse colon cancer model. Dynamic contrast enhanced MRI (DCE-MRI) using the biodegradable macromolecular contrast agent GODP was employed to noninvasively and quantitatively assess the efficacy of the RGD-ECO/determine the anatomical and physiological changes of the tumor vasculature in response to the RNAi treatment 18. Parametric values related to blood flow, vascular permeability, and plasma volume were calculated by applying the adiabiatic approximation to the tissue homogeneity (AATH) model. The overall changes observed in the tumor vasculature with DCE-MRI were verified by analyzing the concurrent effects on CD31 and VEGF protein expression throughout the tumor tissue, the latter of which is known to be a downstream target of the HIF-1α transcription factor. The effect of HIF-1α silencing on the expression of multiple downstream targets involved in tumor metabolism and pH regulation was also investigated in this study 19.
MATERIALS AND METHODS
Materials
Anti-HIF-1α siRNA (siHIF-1α) (sense sequence: 5′-UCACCAAAGUUGAAUCAGAdTdT-3′, anti-sense sequence 5′-UCUGAUUCAACUUUGGUGAdTdT-3′) and negative control siRNA (siCon) (sense sequence: 5′-UUAGCGUAGAUGUAAUGUGdTdT-3′, antisense sequence: 5′-CACAUUACAUCUACGCUAA-3′) were purchased from Dharmacon (Lafayette, CO). AlexaFluor-647 labeled siHIF-1α siRNA was purchased from Qiagen (Valencia, CA). Cyclic RGD (Arg-Gly-Asp-D-Phe-Lys) and cyclic RAD (Arg-Gly-Asp-D-Phe-Lys) peptides were purchased from Peptides International Inc (Louisville, KY). The hetereobifunctional polyethylene glycol linker MAL-PEG-SCM (MW=5 kDa) was supplied by Creative PEGWorks (Winston Salem, NC). Primary antibodies for GLUT-1, CAIX, and HIF-1α were purchase from Novus Biologicals (Littleton, CO). The primary antibodies for VEGF and CD31 were purchased from Abcam (Cambridge, MA), while those for LDHA, PDK1, HKII, and β-actin were purchased from Cell Signaling (Danvers, MA). The secondary antibodies Dk-anti-Rb-HRP and Dk-anti-Rb-Alexa647 were purchased from Jackson ImmunoResearch (West Grove, PA). Pimonidazole hypoxia stain was supplied by Hypoxyprobe Inc (Burlington MA). The HT29 human colon adenocarcinoma cell line was purchased from ATCC (Manassas, VA), and athymic nude mice were purchased from Charles River Laboratories.
Targeted ECO/siRNA Nanoparticle formulation
The ECO cationic lipid carrier was synthesized using a solid phase reaction scheme that has been previously reported 13. The targeting peptides cyclic RGD (c-RGDfK) and RAD (c-RADfK) were conjugated to the hetereobifunctional polyethylene glycol (PEG) linker MAL-PEG-SCM (MW=5 kDa) to yield Mal-PEG(5000)-RGD/RAD (RGD/RAD-PEG) conjugates. Stock solutions of the RGD/RAD-PEG conjugates and siRNA were prepared in nuclease-free water, while the ECO lipid was dissolved in ethanol. To formulate the particles, the maleimide groups on the peptide-PEG conjugates were first reacted with the free thiols on ECO at a 2.5:100 molar ratio (PEG:ECO) for 30 min. siRNA was then added to the mixture and stirred for another 30 min to allow for nanoparticle formation. Each formulation was prepared at an N/P ratio of 10, whereby the final volume for injection was 200 μL and the ratio of ethanol:water was 1:20. Chemical structures of ECO, RGD, Mal-PEG(5000)-NHS, and the RGD-PEG conjugate, as well as a schematic of the final nanoparticle are displayed in Figure 1.
Figure 1.

Structures of the multifunctional amino lipid ECO, cyclic RGD peptide, Mal-PEG(5000)-NHS, and Mal-PEG(5000)-RGD (RGD-PEG) (A) used for the preparation of GRD targeted ECO/siRNA nanoparticles (B).
Mouse model and siRNA delivery with ECO/siRNA nanoparticles
A mouse model bearing subcutaneous HT29 colon adenocarcinoma flank xenografts was developed in accordance a protocol approved by the Institutional Animal Care and Use Committee for Case Western Reserve University. A total of 5×105 cells were inoculated into athymic nude mice in a 250 μL volume of Matrigel (BD Bioscienes). Tumors were allowed to grow to approximately 0.5 – 1.0 cm in diameter for two weeks before the treatment experiments. A total of 2 groups consisting of 3 mice each with HT29 xenografts with a size of approximately 1 cm in diameter were used for assess tumor siRNA delivery efficiency of the RGD- and RAD-targeted ECO-siRNA nanoparticles. The nanoparticles were intravenously injected with a siRNA dose of 2 mg/kg. The siRNA was labeled with AlexaFluor-647 and tumor siRNA accumulation was assessed using a Maestro fluorescence imaging system (PerkinElmer) after the mice were sacrificed at 24 hours post-injection.
Tumor treatment with RGD-ECO/siHIF-1α delivery system
The anti-tumor efficacy of the RGD targeted ECO/siHIF-1α nanoparticles was investigated in a mouse model bearing subcutaneous HT29 colon adenocarcinoma flank xenografts with a size of approximately 0.5 cm in diameter. The mice (4 mice each group) were either treated with RGD-targeted ECO/siHIF-1α, RAD-targeted ECO/siHIF-1α, or RGD-targeted ECO/siCon nanoparticles. Another group received a phosphate-buffered saline (PBS) control therapy. Treatments were carried out by tail-vein injections of the therapeutic regimen on days 0, 3, 6, 9, 12, 15, and 18. All mice were sacrificed on day 21 after the start of treatment after DCE-MRI. Nanoparticles were administered at a dose of 2 mg/kg of siRNA. Tumor growth was measured at several time points during the therapy with a caliper, and volumes were calculated using the formula V=(1/6)πD12D22, where D1 and D2 were two diameters measured along perpendicular axes of the tumor lesion.
Dynamic contrast enhanced magnetic resonance imaging
On day 21, DCE-MRI was performed on each mouse from both the saline control and RGD-targeted ECO/siHIF-1α treatment groups. Each mouse was catheterized and injected with a bolus of GODP, a newly designed biodegradable macromolecular gadolinium-based contrast agent, at the start of the imaging sequence. A T1-weighted 3D-FLASH gradient echo (GE) sequence was used for the DCE-MRI data acquisition on a 7T pre-clinical scanner (Bruker). Sequential MR images of the tumor were acquired before, during, and after the injection of the contrast agent using a imaging protocol described in a previous publication 18.
The signal intensity of the MR images of the regions of interest was determined and converted into concentration of the contrast agent by using the variable flip angle technique 20. Concentration-time curves were formulated for both the tumor tissue and a major artery near the lesion, which served as the arterial input function for pharmacokinetic modeling. These concentration-time curves were parametrically curve-fitted to the AATH model to calculate blood flow (Fp), permeability-surface area product (PS), and plasma volume fraction (Vp) in the tumor. The concentration-time curves from the tumor were also integrated in order to determine the extent of contrast agent accumulation within the lesion. This calculation was performed over the entire duration of the scan, and also over only the first 90 seconds, in order to obtain total area-under-the-curve (AUC) and initial AUC (iAUC) measurements, respectively.
Immunofluorescence and western blotting
After completing DCE-MRI acquisition, each mouse from the control and RGD-targeted ECO/HIF-1α groups was intravenously injected with 60 mg/kg of pimonidazole, an established marker for the detection of tissue hypoxia. The tumors from these mice were resected one hour after pimonidazole administration in order to determine changes in HIF-1α, Glut-1, HKII, PDK-1, LDHA, CAIX, VEGF, CD31, and hypoxia using western blotting and immunofluorescence analysis. The western blots from all 4 mice in each group are presented throughout this paper. Image J software was utilized to quantify relative protein expression from the blots. H&E staining was also performed to determine the extent of necrosis in each treatment group.
Statistical Analysis
Statistical analyses were performed using unpaired, two-tailed Student’s t-tests with a 95% confidence interval, assuming equal variances. Probability values of p<0.05 were considered to be significant.
RESULTS
Tumor targeting and therapeutic efficacy of RGD targeted ECO/siHIF-α nanoparticles
RGD-targeted ECO/siRNA nanoparticles were designed to target αvβ3 integrins highly expressed on HT-29 cancer cells for efficient delivery of anti-HIF-1α siRNA. Figure 2A shows fluorescence images of the accumulation of the fluorescently labeled siRNA in HT-29 tumors 24 hours after intravenous injection of the RGD- and RAD-targeted ECO/siRNA nanoparticles. RGD-targeted ECO/siRNA nanoparticles were able to deliver siRNA more efficiently into tumors than the non-specific RAD-targeted counterparts via systemic administration. Consequently, multiple intravenous injections of the RGD-targeted ECO/siHIF-1α nanoparticles resulted in more effective tumor inhibition than the RAD-targeted ECO/siHIF-1α nanoparticles, saline, and RGD-targeted nanoparticles bearing a non-specific control siRNA (siCon), Figure 2B. The RGD targeted ECO/siHIF-1α nanoparticles were able to significantly reduce the size of the primary lesion by 54.9% in comparison to the saline control tumors by the end of the treatment period (p = 0.001). The RAD -targeted ECO/siHIF-1α nanoparticles also resulted in a 32.5% reduction in size as compared to the saline control (p = 0.005). The difference in the tumor growth rates between the RGD- and RAD-targeted ECO/siHIF-1α nanoparticles was also significant (p = 0.009). The RGD-targeted ECO/siCon nanoparticles did not show any significant changes in the tumor growth rate as compared to the PBS control.
Figure 2.
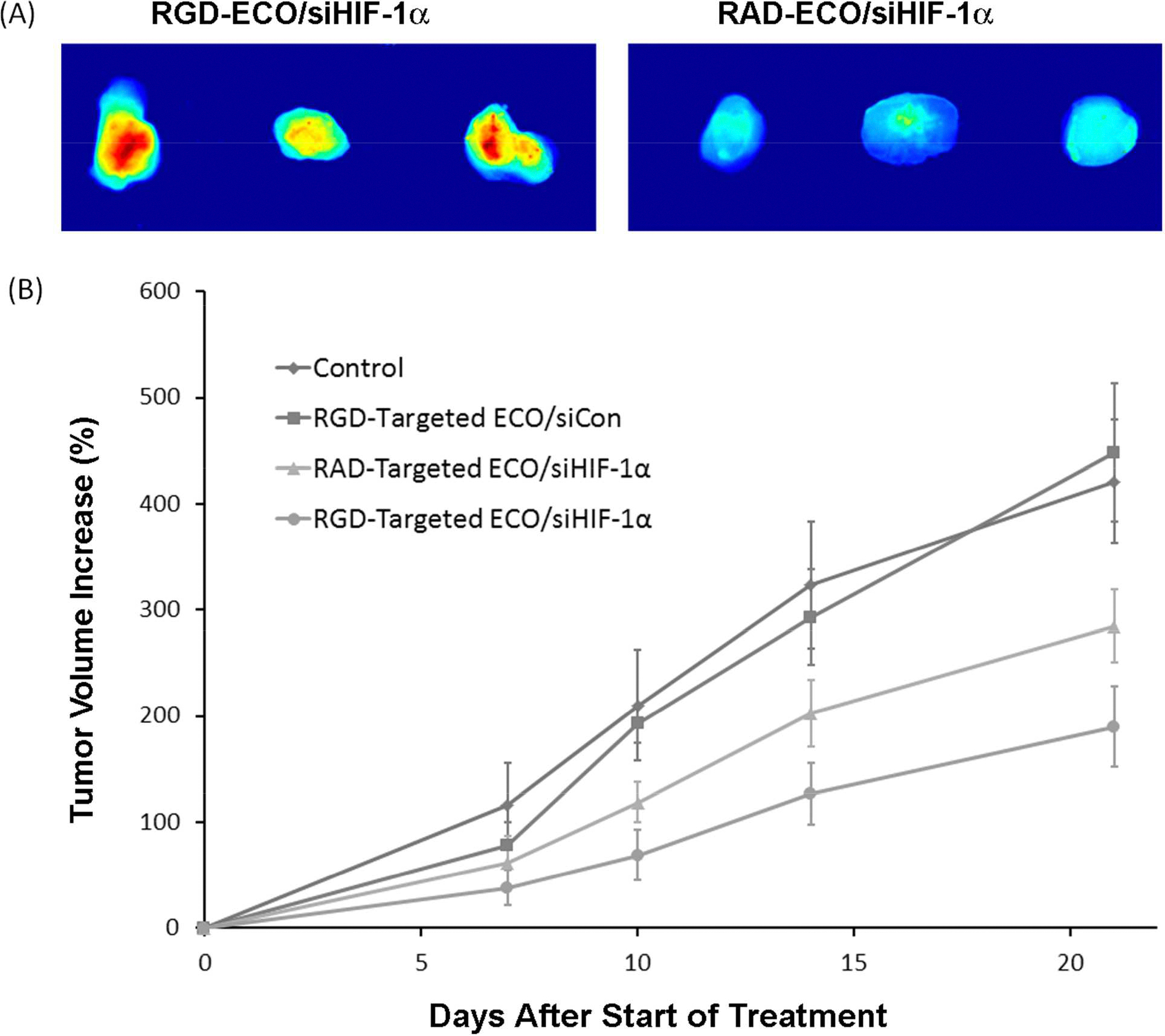
Fluorescence images showing the tumor accumulation of RGD- and RAD-targeted ECO/siRNA nanoparticles of siRNA labeled with Alexa Fluor-647 = 24 hours after intravenous injection (A). Tumor growth of the mice bearing HT-29 colon cancer xenografts after intravenous injection of RGD targeted ECO/siHIF-1α, RAD-targeted ECO/siHIF-1α, and RGD-targeted ECO/siCon nanoparticles at a dose of 2.0 mg-siRNA/kg and PBS (control) every three days.
DCE-MRI reveals anti-angiogenic effects of RGD targeted ECO/siHIF-1α nanoparticles
DCE-MRI was performed to assess tumor vascularity after the treatment with RGD targeted ECO/siHIF-1α nanoparticles in comparison with the PBS control at the end of the treatment. Tumor blood flow (Fp), permeability-surface area product (PS), and plasma volume fraction (Vp) were calculated from the DCE-MRI data using the AATH kinetic model. The treatment with the RGD targeted ECO/siHIF-1α nanoparticles resulted in significant reduction in the average Fp, PS, and Vp values as compared to those treated with saline (Figure 3A). Respectively, the average Fp, PS, and Vp values were 71.2%, 75.3%, and 73.2% lower in the siHIF-1α treated group (p = 0.002, p = 0.003, p = 0.03). In concert with these changes, average total area-under-the-curve (AUC) and initial area-under-the-curve (iAUC) measurements were also significantly decreased by 70.1% (p = 0.003) and 66.9% (p = 0.001) in the treatment group, respectively (Figure 3B,C). The results implicate that tumor vascularity and angiogenesis were significantly suppressed by the RNAi therapy
Figure 3.

Average tumor blood flow (Fp), permeability-surface area product (PS), and plasma volume fraction (Vp) in response to the treatment with RGD-targeted ECO/siHIF-1α nanoparticles and PBS determined by DCE-MRI using the AATH tracer kinetic model (A). The apparent regression in tumor vasculature was corroborated by significant decreases in both the average AUC (B) and iAUC (C) of tumor contrast uptake kinetics.
Pixel-by-pixel data analysis further revealed the changes of tumor vascular parameters throughout the tumor tissues after the treatment. Figure 4 shows the parametric maps that tumors treated with PBS possessed more extensive vascular networks than those treated with targeted ECO/siHIF-1α nanoparticles, as evidenced by the greater Vp values throughout the tumor tissue. The Vp maps suggested that the tumor vasculature in the siHIF-1α treated group was predominately confined to the periphery, and largely absent within the core tissue. Similar spatial distributions were observed in the Fp, PS, and AUC maps, suggesting a high degree of vascular collapse in the tumor core following the treatment with RGD targeted ECO/siHIF-1α nanoparticles. The result was supported by the histological analysis of the tumor tissues in Figure 5, which shows substantially more necrosis in the core of the siHIF-1α treated tumors than in those of the control group. More importantly, the necrotic tissue appears to coincide with areas of low vascularity in the DCE-MRI parametric maps, implying that the anti-angiogenic behavior of the RGD targeted ECO/siHIF-1α nanoparticles promoted severe vascular regression, and that the chronic lack of blood vessels potentially induced prolific cell death and necrosis throughout the tumor. Overall, the RGD targeted ECO/siHIF-1α nanoparticles demonstrated significant anti-angiogenic properties that may have contributed to the anti-tumor activity.
Figure 4.

Color coded tumor parametric maps constructed from pixel-by-pixel analyses of DCE-MRI data of the tumors treated with RGD-targeted ECO/siHIF-1α nanoparticles and PBS.
Figure 5.

H&E staining reveals that the tumors treated with RGD-targeted ECO/siHIF-1α nanoparticles contain much larger areas of necrotic tissue than the control tumors (A) and magnified images show the structural differences in the H&E stains between the viable and necrotic tissue (B).
RGD targeted ECO/siHIF-1α nanoparticles silences the expression of HIF-1α and other downstream genes
After completion of DCE-MRI, the tumors from the groups treated with saline and RGD-targeted ECO/siHIF-1α nanoparticles were removed for analyzing the expression of HIF-1α and other downstream gene targets. Figure 6 shows HIF-1α expression in the tumor tissues as determined by western blotting and immunohistochemistry. Pixel analysis of western blots revealed that the RNAi therapy was able to significantly reduce HIF-1α expression by 52.7% as compared to the control (P < 0.05). These results were corroborated by a decrease in IHC staining for this protein.
Figure 6.
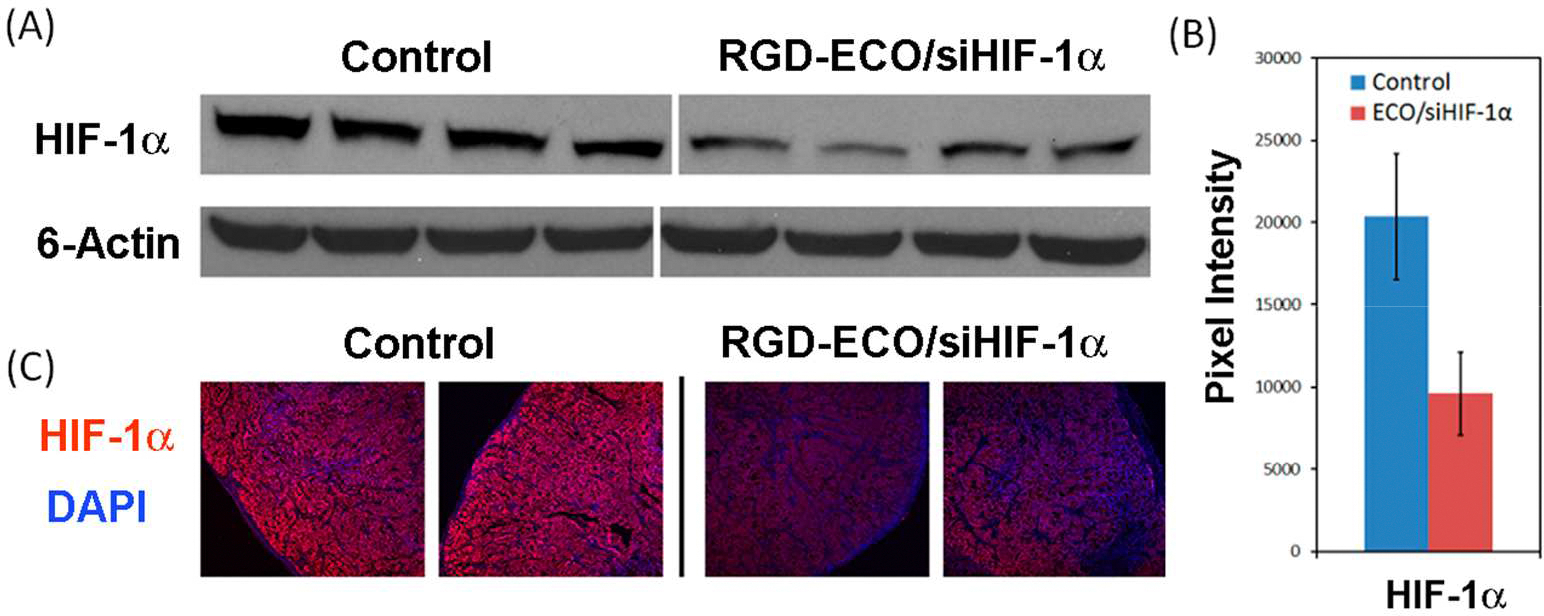
Silence of HIF-1α protein expression in the tumors treated with RGD-targeted ECO/siHIF-1α nanoparticles and PBS determined by Western blotting analysis (A and B) and immunohistochemistry staining (C).
The treatment with RGD-targeted ECO/siHIF-1α nanoparticles also resulted in significant down-regulation of vascular endothelial growth factor (VEGF), one of the most prominent pro-angiogenic factors secreted by tumor cells, Figure 7A–C. A 49.8% reduction of VEGF was observed in the tumors treated with siHIF-1α as compared to the control (p = 0.01), validating the anti-angiogenic activity of RGD-targeted ECO/siHIF-1α nanoparticles. This observation was supported by the reduction of CD31 expression, a biomarker of tumor vasculature. CD31 protein expression was reduced by 67.1% (p < 0.001) in response to the siHIF-1α treatment as compared to the control. The decline in CD31 was verified by immunohistochemistry, which showed that the blood vessel density was less in the siHIF-1α treated tumors than the control tumors, demonstrating that the RNAi therapy effectively triggered vascular regression (Figure 7D). The confocal images of CD31 expression in Figure 7D reveals that RGD-targeted ECO/siHIF-1α nanoparticles greatly inhibited tumor vascularity in both the peripheral and interior regions of the tumors, and compromised the tumor vasculature to the point where it was largely absent throughout the tumor core. Since a decrease in blood vessel density usually impairs tissue oxygenation, it was not surprising to see from the pimonidazole staining in Figure 7E that the lower levels of CD31 expression in the siHIF-1α treated tumors corresponded to greater levels of tumor hypoxia. The results demonstrated that systemic delivery of RGD-targeted ECO/siHIF-1α nanoparticles was effective to down-regulate the expression of VEGF associated with tumor angiogenesis and resulted in significant inhibition of tumor angiogenesis and proliferation.
Figure 7.

Down-regulation of VEGF and CD31 in the tumors treated with RGD-targeted ECO/siHIF-1α nanoparticles and PBS determined by Western blotting (A, B) and VEGF (C) and CD31 (D) by immunohistochemistry. Tumor hypoxia exhibited by the pimonidazole staining (E). All IHC images presented here are at 10× magnification.
Silencing of HIF-1α in the tumor resulted in significant down-regulation of several other down stream genes, including glucose transporter-1 (Glut-1), hexokinase II (HKII), pyruvate dehydrogenase kinase-1 (PDK-1), and lactate dehydrogenase-A (LDHA) associated with tumor glycolysis, Figure 8. Glycolysis is a prominent energy producing process in a wide variety of tumors by which cells oxidize glucose to rapidly generate ATP for the demanding metabolic needs of actively growing tumors. It serves as the primary source of energy to provide a selective growth advantage over normal cells that experience hypoxic changes in the tumor microenvironment. Overexpression of Glut-1 by tumor cells enhances the rate of glucose uptake, which is subsequently metabolized by HKII mediated phosphorylation to entrap it inside the cells. In response to HIF-1α silencing, the levels Glut-1, HKII, PDK-1, and LDHA were reduced by 28.6% (p = 0.004), 36.4% (p = 0.003), 59.3% (p = 0.003), and 41.5% (p = 0.005), respectively, as compared to the control. Down-regulation of these proteins was further validated by immunohistochemistry. Silencing these mediators of glycolysis may have facilitated a decline in the metabolic rate of the HT29 tumor cells, and thus contributed to the inhibition of tumor growth.
Figure 8.

Silence of Glut-1, HKII, PDK1, and LDHA in the tumors treated with RGD-targeted ECO/siHIF-1α nanoparticles and PBS determined by Western blotting (A) and immunohistochemistry (B).
HIF-1α is also known to promote the transcription and expression of several pH regulators in order to counteract the intracellular buildup of acid created by glycolytic metabolism. Carbonic anhydrase IX (CAIX) is up-regulated by HIF-1α to play a significant role in pH regulation. The protein analysis in Figure 9 revealed that silencing of HIF-1α with RGD-targeted ECO/siHIF-1α nanoparticles was able to down-regulate CAIX expression by 53.9% (p = 0.001). Such a change also contributed to tumor growth inhibition by affecting pH homeostasis in HT29 cancer cells.
Figure 9.
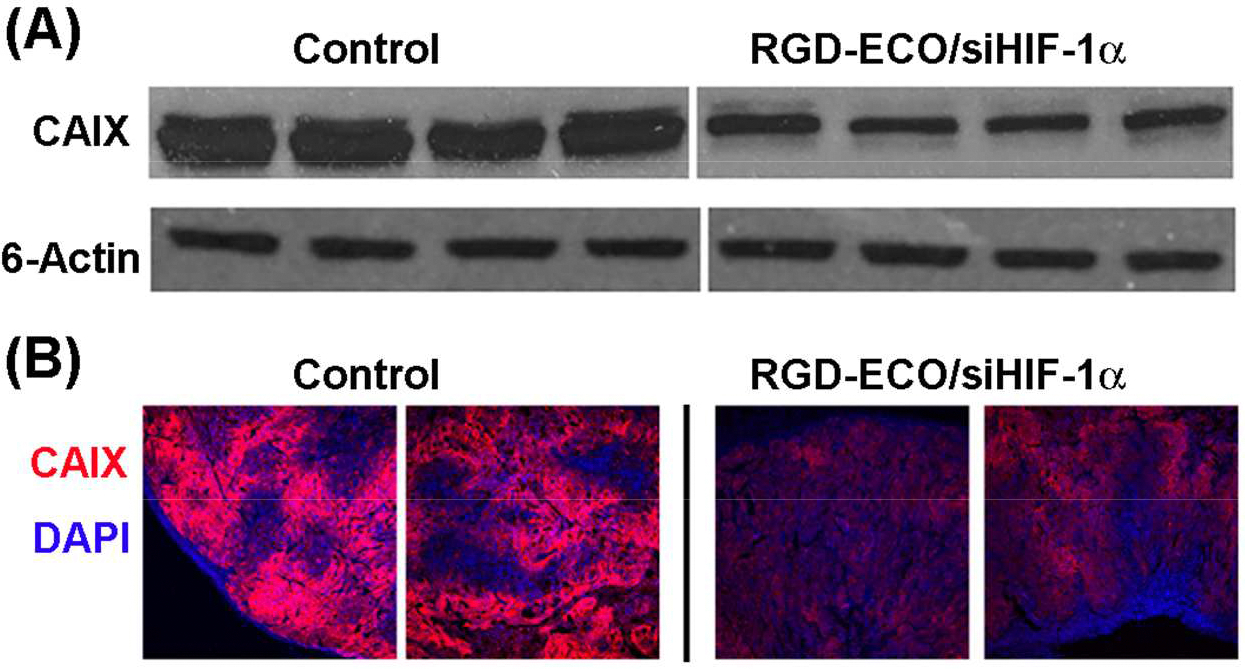
Silence of the expression of CAIX in the tumors treated with RGD-targeted ECO/siHIF-1α nanoparticles and PBS determined by Western blotting (A) and immunohistochemistry (B).
DISCUSSION
The expression of HIF-1α in hypoxic tumors has specifically led to the transcriptional expression of genes involved in angiogenesis, glycolytic metabolism, pH regulation, anti-apoptotic survival, and metastasis, all of which contribute to an increase in tumor grade, chemotherapy and radiation resistance, and poor clinical outcomes 21. The interplay of HIF-1α and tumor hypoxia throughout cancer progression establishes this transcription factor as a formidable target for RNAi-based cancer therapeutics, especially since the hypoxia-inducible response pathway is a common feature amongst a wide variety of tumors. We and others previously showed that down-regulation of HIF-1α with RNAi was effective to suppress tumor proliferation in various tumor models 22–24. In this study, DCE-MRI was employed for the first time to analyze the effect of silencing HIF-1α on tumor angiogenesis. The RGD-ECO/siHIF-1α nanoparticle therapy presented here demonstrates the ability to silence the expression of intratumoral HIF-1α expression, and as a result, significantly enhance anti-tumor activity by inhibiting tumor angiogenesis and the expression of numerous downstream oncogenes.
DCE-MRI is a non-invasive diagnosing imaging tool that is able to characterize angiogenesis by enabling the quantitative assessment of anatomical and physiological changes in tumor vascularity. This rapid imaging technique profiles the intravenous passage of a contrast agent through a region of interest, generating concentration-time curves that are parametrically fitted to pharmacokinetic models that describe the flux of contrast agent through the interstitial and vascular spaces of a tumor. Here, we utilized the AATH model able to calculate changes in the blood flow, permeability, and volume fraction of the vasculature in the tumor tissue, all of which can serve as important biomarkers for monitoring the therapeutic response following RGD-ECO/siHIF-1α therapy 18, 25. Parametric curve-fitting was performed on a macroscopic level by using average MR signal intensities from the entire tumor, and also on a pixel-by-pixel basis to assess changes in spatial heterogeneity of the vasculature over time. Previous, we reported that DCE-MRI with the biodegradable macromolecular MRI contrast agent GODP is able to accurately monitor and quantify tumor angiogenesis18. GODP is a new polydisulfide-based MRI contrast agent possessing stable macrocyclic Gd(III) chelates. It behaves as a macromolecular agent during DCE-MRI acquisition, and then rapidly degrades so that it can be excreted and cleared from the body to minimize potential toxic side effects.
DCE-MRI with GODP non-invasively revealed that the RGD-ECO/siHIF-1α nanoparticle therapy significantly impaired tumor angiogenesis, as evidenced by the reduction in average Fp, PS, and Vp, values derived from the AATH model, relative to those obtained with the saline control treatment 25, 26. Such changes in tumor vascularity were supported by significant decreases in the semi-quantitative AUC and iAUC area-under-the-curve calculations. This was expected since iAUC is known to parallel the combined effects of Fp and PS, while the AUC measurement can additionally reflect changes in tumor Vp. Nevertheless, the Fp, PS, Vp, and AUC parametric maps also demonstrated the apparent anti-angiogenic effects of the RNAi therapy by showing that much of the tumor tissue was largely devoid of any functional vasculature compared to the control. It also appears that the tumor vasculature in the RGD-ECO/siHIF-1α group was predominately confined to the periphery, suggesting a high degree of vascular collapse in the tumor core following the treatment. The reduction in these tumor vascular parameters was validated by reduced CD31 staining and concurrent increases in tumor hypoxia and necrosis throughout the core tissue.
The anti-angiogenic effects of the RGD-ECO/siHIF-1α nanoparticle therapy correlated with significantly lower intratumoral levels of VEGF, which is one of the most prominent pro-angiogenic growth factors in the tumor microenvironment 27–30. Although VEGF has been identified as a therapeutic target for cancer treatment, monoclonal antibodies designed to bind and inhibit this growth factor have not been able to achieve consistent, long-term curative anti-tumor effects. Previous reports have documented that an initial period of tumor regression from such anti-angiogenic therapies are often compromised by the onset of elevated tumor hypoxia and subsequent activation of the HIF-1α response pathway18, 31. This relapse is often associated with highly invasive cancer phenotypes that are typically refractory to future drug treatments 21, 32, 33.
Similarly, in this study, pimonidazole staining revealed that the decrease in tumor vasculature resulted in significantly increased levels of tumor hypoxia following treatment with RGD-ECO/siHIF-1α. However, despite the observed elevation increase in tumor hypoxia, the RNAi therapy was still able to maintain HIF-1α suppression and inhibit tumor progression. This is in stark contrast to a prior DCE-MRI study where we showed that although an anti-angiogenic therapy was able to reduce tumor vascularity, it did not necessarily reduce the tumor growth rate, primarily due to a concurrent increase in HIF-1α protein expression. We believe targeting HIF-1α directly may constitute a far superior option to treating cancer than traditional anti-angiogenic cancer therapies that solely inhibit growth factors like VEGF. By silencing HIF-1α through the use of RNAi therapy, it is possible to not only deplete VEGF expression and suppress tumor vascularity, but also prevent the subsequent induction of HIF-1α that may result from impaired tissue oxygenation. Avoiding potential increases in HIF-1α expression will likewise eliminate the variety of adaptations that help cancer cells survive tumor hypoxia and aid their transformation into very aggressive phenotypes.
In addition to its effects on tumor angiogenesis, the RGD-ECO/siHIF-1α therapy also had a significant effect on several other downstream targets associated with tumor metabolism (Glut-1, HKII, PDK-1, and LDHA) and pH regulation (CAIX). Inhibiting the production of these proteins may have contributed to the significant anti-tumor activity observed here by preventing the rapid production of ATP required to support the energetic needs of the tumor cells under hypoxic conditions, which relies predominantly on glycolytic metabolism as opposed to oxidative phosphorylation. In addition, dampening the glycolytic flux is known to reduce the biosynthesis of macromolecular building blocks and NADPH ROS scavengers from the pentose phosphate pathway 34–37. The latter is particularly important, especially since previous studies have demonstrated that the modulation of PDK-1 and LDHA activity in tumors is known to promote cell death through the induction of redox stress. PDK-1 inhibition can promote the generation of ROS in low oxygenated environments by enabling pyruvate to enter the TCA cycle, while reduced LDHA activity may also lead to greater ROS production following the elevation of NADH/NAD+ ratios within the cell 36. Nevertheless, inhibiting glycolytic metabolism can prevent tumor progression by slowing the production of lactate, which plays a major role in tumor growth and metastasis by enhancing the immune escape, angiogenic expansion, and antioxidant capacity of the tumor lesion 38, 39. Meanwhile, other reports have suggested that HKII knockdown can also play a major role in regulating cancer survival by sensitizing tumors to caspase-mediated cell death 40, 41. Modulating CAIX expression has the ability to control tumor proliferation by disrupting the normal pH regulatory mechanisms that counteract and extrude the accumulation of the potentially toxic acid load that arise from glycolysis within cancer cells. Such acidity in the tumor microenvironment has also proven critical for enzymatic degradation and remodeling of the extracellular matrix to facilitate tumor progression 42. Taken together, suppression of all these metabolic and pH regulators, in addition to the observed anti-angiogenic effects, through HIF-1α inhibition may have synergistically contributed to the significant anti-tumor activity observed in this study.
Due to its variety of roles in tumor progression, HIF-1α is a potentially powerful therapeutic target that has the ability to be extremely advantageous in the development of future anti-cancer therapies. This study showed that RNAi is a highly efficient gene regulatory mechanism that can effectively down-regulate HIF-1α expression in hypoxic tumors with the proper siRNA delivery system. The lipid carrier ECO possesses the requisite multifunctionality to overcome the barriers of efficient cytosolic delivery of therapeutic siRNA and facilitate RNAi-mediated suppression. This agent also exhibits a good safety profile for prolonged and repeated systemic administrations when complexed with siRNA, and can be easily modified to incorporate tumor-specific targeting moieties. Although the targeted RGD-ECO/siHIF-1α nanoparticle therapy has shown substantial anti-tumor activity on its own, future efforts should also be devoted to exploring its use as part of a combination regimen. Combinatorial approaches to fighting cancer are currently gaining momentum in cancer therapy because aggressive tumors possess a multitude of inherent and adaptive mechanisms that compromise and resist a wide range of therapeutic strategies. Not surprisingly, dozens of clinical trials have demonstrated that antineoplastic chemotherapy and radiotherapy failure are related to the survival and multi-drug resistance mechanisms empowered by HIF-1α 43–46. Due to the efficacy of RGD-targeted ECO/siHIF-1α nanoparticles at silencing HIF-1α and its downstream transcriptional targets, combination with other therapies has the potential to overcome some of the major biological obstacles facing current standard-of-care options.
CONCLUSION
Systemic administration of RGD-targeted multifunctional ECO/siHIF-1α nanoparticles significantly inhibited tumor proliferation in mice bearing HT-29 colon cancer xenografts via RNAi-mediated suppression of the HIF-1α transcription factor. DCE-MRI with the biodegradable macromolecular contrast agent GODP revealed that silencing HIF-1α expression severely inhibited angiogenesis and induced a substantial decline in vascular permeability, flow rate, and plasma volume fraction throughout the tumor lesion. The RGD-ECO/siHIF-1α therapy regulated intratumoral levels of VEGF and reduced the protein expression of several important downstream genes associated with glycolytic metabolism and pH regulation. The RGD-ECO/siHIF-1α nanoparticle therapy is a promising candidate for the efficacious treatment of a variety of human cancers either alone or in combination with traditional chemotherapy and radiotherapy.
Acknowledgement
Research support was provided, in part, by grants from the National Institutes of Health to Z-R.L. (EB00489 and CA194518), and by a National Science Foundation Graduate Research Fellowship to M.D.G. (DGE-0951783). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.”
REFERENCES
- 1.Majmundar AJ; Wong WJ; Simon MC Hypoxia-inducible factors and the response to hypoxic stress. Molecular cell 2010, 40, (2), 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL HIF-1: using two hands to flip the angiogenic switch. Cancer metastasis reviews 2000, 19, (1–2), 59–65. [DOI] [PubMed] [Google Scholar]
- 3.Vaupel P The role of hypoxia-induced factors in tumor progression. The oncologist 2004, 9 Suppl 5, 10–7. [DOI] [PubMed] [Google Scholar]
- 4.Liao D; Johnson RS Hypoxia: a key regulator of angiogenesis in cancer. Cancer metastasis reviews 2007, 26, (2), 281–90. [DOI] [PubMed] [Google Scholar]
- 5.Gillies RJ; Gatenby RA Hypoxia and adaptive landscapes in the evolution of carcinogenesis. Cancer metastasis reviews 2007, 26, (2), 311–7. [DOI] [PubMed] [Google Scholar]
- 6.Baluk P; Hashizume H; McDonald DM Cellular abnormalities of blood vessels as targets in cancer. Current opinion in genetics & development 2005, 15, (1), 102–11. [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003, 3, (10), 721–32. [DOI] [PubMed] [Google Scholar]
- 8.Pecot CV; Calin GA; Coleman RL; Lopez-Berestein G; Sood AK RNA interference in the clinic: challenges and future directions. Nature reviews. Cancer 2011, 11, (1), 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miele E; Spinelli GP; Miele E; Di Fabrizio E; Ferretti E; Tomao S; Gulino A Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. International journal of nanomedicine 2012, 7, 3637–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuhorn IS; Engberts JB; Hoekstra D Gene delivery by cationic lipid vectors: overcoming cellular barriers. European biophysics journal : EBJ 2007, 36, (4–5), 349–62. [DOI] [PubMed] [Google Scholar]
- 11.Xu W; Pan R; Zhao D; Chu D; Wu Y; Wang R; Chen B; Ding Y; Sadatmousavi P; Yuan Y; Chen P Design and evaluation of endosomolytic biocompatible peptides as carriers for siRNA delivery. Mol Pharm 2015, 12, (1), 56–65. [DOI] [PubMed] [Google Scholar]
- 12.Ma D; Tian S; Baryza J; Luft JC; DeSimone JM Reductively Responsive Hydrogel Nanoparticles with Uniform Size, Shape, and Tunable Composition for Systemic siRNA Delivery in Vivo. Mol Pharm 2015, 12, (10), 3518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malamas AS; Gujrati M; Kummitha CM; Xu R; Lu ZR Design and evaluation of new pH-sensitive amphiphilic cationic lipids for siRNA delivery. J Control Release 2013, 171, (3), 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gujrati M; Vaidya A; Lu ZR Multifunctional pH-Sensitive Amino Lipids for siRNA Delivery. Bioconjug Chem 2016, 27, (1), 19–35. [DOI] [PubMed] [Google Scholar]
- 15.Wang XL; Ramusovic S; Nguyen T; Lu ZR Novel polymerizable surfactants with pH-sensitive amphiphilicity and cell membrane disruption for efficient siRNA delivery. Bioconjug Chem 2007, 18, (6), 2169–77. [DOI] [PubMed] [Google Scholar]
- 16.Gujrati M; Malamas A; Shin T; Jin E; Sun Y; Lu ZR Multifunctional cationic lipid-based nanoparticles facilitate endosomal escape and reduction-triggered cytosolic siRNA release. Mol Pharm 2014, 11, (8), 2734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parvani JG; Gujrati MD; Mack MA; Schiemann WP; Lu ZR Silencing beta3 Integrin by Targeted ECO/siRNA Nanoparticles Inhibits EMT and Metastasis of Triple-Negative Breast Cancer. Cancer Res 2015, 75, (11), 2316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malamas AS; Jin E; Zhang Q; Haaga J; Lu ZR Anti-angiogenic Effects of Bumetanide Revealed by DCE-MRI with a Biodegradable Macromolecular Contrast Agent in a Colon Cancer Model. Pharm Res 2015, 32, (9), 3029–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J; Schmid T; Schnitzer S; Brune B Tumor hypoxia and cancer progression. Cancer letters 2006, 237, (1), 10–21. [DOI] [PubMed] [Google Scholar]
- 20.Yankeelov TE; Luci JJ; Lepage M; Li R; Debusk L; Lin PC; Price RR; Gore JC Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: a reference region model. Magnetic resonance imaging 2005, 23, (4), 519–29. [DOI] [PubMed] [Google Scholar]
- 21.Poon E; Harris AL; Ashcroft M Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev Mol Med 2009, 11, e26. [DOI] [PubMed] [Google Scholar]
- 22.Wang XL; Xu R; Wu X; Gillespie D; Jensen R; Lu ZR Targeted systemic delivery of a therapeutic siRNA with a multifunctional carrier controls tumor proliferation in mice. Mol Pharm 2009, 6, (3), 738–46. [DOI] [PubMed] [Google Scholar]
- 23.Gillespie DL; Aguirre MT; Ravichandran S; Leishman LL; Berrondo C; Gamboa JT; Wang L; King R; Wang X; Tan M; Malamas A; Lu ZR; Jensen RL RNA interference targeting hypoxia-inducible factor 1alpha via a novel multifunctional surfactant attenuates glioma growth in an intracranial mouse model. J Neurosurg 2015, 122, (2), 331–41. [DOI] [PubMed] [Google Scholar]
- 24.Liu XQ; Xiong MH; Shu XT; Tang RZ; Wang J Therapeutic delivery of siRNA silencing HIF-1 alpha with micellar nanoparticles inhibits hypoxic tumor growth. Mol Pharm 2012, 9, (10), 2863–74. [DOI] [PubMed] [Google Scholar]
- 25.St Lawrence KS; Lee TY An adiabatic approximation to the tissue homogeneity model for water exchange in the brain: II. Experimental validation. J Cereb Blood Flow Metab 1998, 18, (12), 1378–85. [DOI] [PubMed] [Google Scholar]
- 26.Sourbron SP; Buckley DL Tracer kinetic modelling in MRI: estimating perfusion and capillary permeability. Physics in medicine and biology 2012, 57, (2), R1–33. [DOI] [PubMed] [Google Scholar]
- 27.Azam F; Mehta S; Harris AL Mechanisms of resistance to antiangiogenesis therapy. European journal of cancer 2010, 46, (8), 1323–32. [DOI] [PubMed] [Google Scholar]
- 28.Paez-Ribes M; Allen E; Hudock J; Takeda T; Okuyama H; Vinals F; Inoue M; Bergers G; Hanahan D; Casanovas O Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell 2009, 15, (3), 220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapisarda A; Melillo G Role of the hypoxic tumor microenvironment in the resistance to anti-angiogenic therapies. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy 2009, 12, (3), 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keunen O; Johansson M; Oudin A; Sanzey M; Rahim SA; Fack F; Thorsen F; Taxt T; Bartos M; Jirik R; Miletic H; Wang J; Stieber D; Stuhr L; Moen I; Rygh CB; Bjerkvig R; Niclou SP Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proceedings of the National Academy of Sciences of the United States of America 2011, 108, (9), 3749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X; Jeong EK; Emerson L; Hoffman J; Parker DL; Lu ZR Noninvasive evaluation of antiangiogenic effect in a mouse tumor model by DCE-MRI with Gd-DTPA cystamine copolymers. Mol Pharm 2010, 7, (1), 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ria R; Catacchio I; Berardi S; De Luisi A; Caivano A; Piccoli C; Ruggieri V; Frassanito MA; Ribatti D; Nico B; Annese T; Ruggieri S; Guarini A; Minoia C; Ditonno P; Angelucci E; Derudas D; Moschetta M; Dammacco F; Vacca A HIF-1alpha of bone marrow endothelial cells implies relapse and drug resistance in patients with multiple myeloma and may act as a therapeutic target. Clin Cancer Res 2014, 20, (4), 847–58. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W; Shi X; Peng Y; Wu M; Zhang P; Xie R; Wu Y; Yan Q; Liu S; Wang J HIF-1alpha Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer. PLoS One 2015, 10, (6), e0129603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenza GL Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol 2009, 19, (1), 12–6. [DOI] [PubMed] [Google Scholar]
- 35.Hamanaka RB; Chandel NS Targeting glucose metabolism for cancer therapy. The Journal of experimental medicine 2012, 209, (2), 211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dang CV; Hamaker M; Sun P; Le A; Gao P Therapeutic targeting of cancer cell metabolism. Journal of molecular medicine 2011, 89, (3), 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swietach P; Vaughan-Jones RD; Harris AL Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer metastasis reviews 2007, 26, (2), 299–310. [DOI] [PubMed] [Google Scholar]
- 38.Hirschhaeuser F; Sattler UG; Mueller-Klieser W Lactate: a metabolic key player in cancer. Cancer research 2011, 71, (22), 6921–5. [DOI] [PubMed] [Google Scholar]
- 39.De Saedeleer CJ; Copetti T; Porporato PE; Verrax J; Feron O; Sonveaux P Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PloS one 2012, 7, (10), e46571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathupala SP; Ko YH; Pedersen PL Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 2006, 25, (34), 4777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf A; Agnihotri S; Micallef J; Mukherjee J; Sabha N; Cairns R; Hawkins C; Guha A Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. The Journal of experimental medicine 2011, 208, (2), 313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb BA; Chimenti M; Jacobson MP; Barber DL Dysregulated pH: a perfect storm for cancer progression. Nature reviews. Cancer 2011, 11, (9), 671–7. [DOI] [PubMed] [Google Scholar]
- 43.Broxterman HJ; Gotink KJ; Verheul HM Understanding the causes of multidrug resistance in cancer: a comparison of doxorubicin and sunitinib. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy 2009, 12, (4–5), 114–26. [DOI] [PubMed] [Google Scholar]
- 44.Fulda S; Debatin KM HIF-1-regulated glucose metabolism: a key to apoptosis resistance? Cell cycle 2007, 6, (7), 790–2. [DOI] [PubMed] [Google Scholar]
- 45.Dewhirst MW; Cao Y; Moeller B Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nature reviews. Cancer 2008, 8, (6), 425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semenza GL Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer cell 2004, 5, (5), 405–6. [DOI] [PubMed] [Google Scholar]


