Abstract
Gene expression in skeletal muscles of adult vertebrates is altered profoundly by changing patterns of contractile work. Here we observed that the functional activity of MEF2 transcription factors is stimulated by sustained periods of endurance exercise or motor nerve pacing, as assessed by expression in trans genic mice of a MEF2-dependent reporter gene (desMEF2-lacZ). This response is accompanied by transformation of specialized myofiber subtypes, and is blocked either by cyclosporin A, a specific chemical inhibitor of calcineurin, or by forced expression of the endogenous calcineurin inhibitory protein, myocyte-enriched calcineurin interacting protein 1. Calcineurin removes phosphate groups from MEF2, and augments the potency of the transcriptional activation domain of MEF2 fused to a heterologous DNA binding domain. Across a broad range, the enzymatic activity of calcineurin correlates directly with expression of endogenous genes that are transcriptionally activated by muscle contractions. These results delineate a molecular pathway in which calcineurin and MEF2 participate in the adaptive mechanisms by which skeletal myofibers acquire specialized contractile and metabolic properties as a function of changing patterns of muscle contraction.
Keywords: calcineurin/exercise/MEF2/motor neuron/skeletal muscle
Introduction
Skeletal myofibers are large multinucleated cells that are directed to assume a variety of highly specialized phenotypes by signaling cues received during fetal development and by neural inputs received by adult muscles (Booth and Baldwin, 1996). Specialized myofiber phenotypes match biophysical and metabolic capabilities of different muscle groups to environmental demands, and are established by distinctive programs of gene expression that involve hundreds of individual genes. Specific genes that are stringently regulated in this manner encode isoforms of contractile proteins with different biophysical properties, enzymes of intermediary metabolism, cell surface receptors, intracellular signaling proteins, transcription factors and components of the cytoskeleton (Schiaffino and Reggiani, 1996; Williams and Neufer, 1996).
Recently, we proposed that calcineurin, a calcium/calmodulin-dependent protein phosphatase, serves an important signaling function linking different patterns of motor nerve activity to distinctive programs of gene expression that establish phenotypic diversity among skeletal myofibers (Chin et al., 1998). Specifically, we hypothesized that sustained increases in regulatory pools of intracellular calcium resulting from tonic patterns of motor neuron stimulation activate calcineurin, leading to increased transcription of genes expressed selectively in fatigue-resistant subtypes (Type I and IIa) of skeletal myofibers. Subsequent investigations provided evidence that calcineurin-dependent signals are transduced to relevant target genes in skeletal muscles by transcription factors of the NFAT and MEF2 gene families, and are amplified by concomitant activation of other calcium-regulated signaling cascades initiated by calmodulin-dependent protein kinases I and IV (reviewed in Olson and Williams, 2000). Support for this mechanistic hypothesis also has been presented from other laboratories (Bigard et al., 2000; Delling et al., 2000; Youn et al., 2000), but some investigators have questioned certain features of this proposed molecular mechanism (Calvo et al., 1999; Swoap et al., 2000).
Here we describe new and rigorous tests of this hypothesis, focusing specifically on the role of MEF2 transcription factors in transducing calcineurin-dependent signals arising as a consequence of muscle work to relevant target genes. The experiments used two different approaches to augment contractile activity in muscles of intact mice. Expression of a MEF2-dependent lacZ transgene (desMEF2-lacZ) is potently elevated by sustained periods of voluntary wheel running or low-frequency tonic pacing of the motor nerve, and is associated with changes in endogenous gene expression consistent with fiber type transformation induced by these stimuli. The effect of sustained periods of muscle contractions on enhancing the function of MEF2 proteins as transcriptional activators in these models is dependent on calcineurin. Induction of the desMEF2-lacZ transgene by running or nerve stimulation was blocked by concomitant administration of cyclosporin A, or in doubly transgenic mice produced by genetic crosses of desMEF2-lacZ mice with other transgenic lines engineered to express myocyte-enriched calcineurin interacting protein 1 (MCIP1), an endogenous calcineurin inhibitory protein. Other experiments performed in cultured cells or in vitro demonstrated that calcineurin and MEF2 form a physical complex, MEF2 becomes hypophosphorylated when calcineurin is active, and calcineurin augments the potency of the transcriptional activation domain (AD) of MEF2. Finally, direct measurements of calcineurin activity across a broad range revealed a dose–response relationship, with the induction of genes characteristic of fatigue-resistant myofiber subtypes (Type I and IIa).
Results
The transcriptional activation function of MEF2 is enhanced by spontaneous running in mice
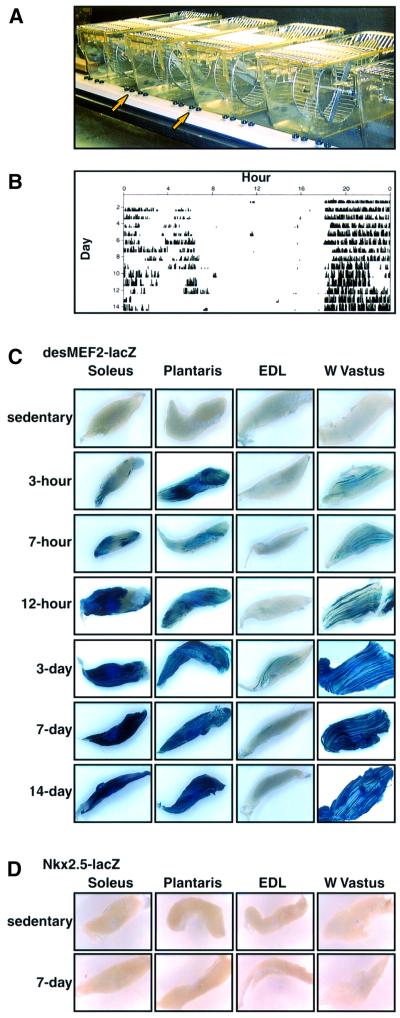
Transgenic mice carrying a MEF2-dependent lacZ indicator gene (desMEF2-lacZ) used for these experiments have been described previously (Naya et al., 1999). During embryonic and fetal life, the transgene is expressed in the developing heart, smooth muscle and skeletal muscles, consistent with the important role of MEF2 proteins in myogenic differentiation (Black and Olson, 1998). Surprisingly, expression of lacZ is not detectable in heart or most skeletal muscles of adult desMEF2-lacZ mice, despite clear evidence that MEF2 proteins are abundant and capable of binding DNA in these tissues (data not shown). We reported previously that among ∼100 animals, lacZ activity was detectable by whole-mount staining techniques only within soleus skeletal muscles of a subset (10–15%) of animals, and was uniformly undetectable in extensor digitorum longus (EDL), plantaris or white vastus (WV) muscles (Wu et al., 2000). This observation was consistent with the viewpoint that MEF2 is activated by muscle contractions, with a time–activity threshold that is reached in sedentary, cage-bound animals only in soleus, as this muscle is used to support the skeleton against gravity even in the absence of locomotor activity. However, the incomplete penetrance of the soleus-specific lacZ expression phenotype precluded a clear interpretation of these results.
Here we demonstrate that muscle contractions of a sustained and repeated nature augment the function of MEF2 as a transcriptional activator of target genes. When provided access to a running wheel (Figure 1A), mice voluntarily exercised almost continuously for up to 12 h during the nocturnal phase of their day–night cycle. Activity was monitored and quantified by counting wheel revolutions (Figure 1B). As little as 3 h of running was sufficient to generate levels of β-galactosidase activity detectable by whole-mount staining in hind limb muscles in 100% of desMEF2-lacZ animals (Figure 1C). The number of positively stained myofibers and the intensity of staining increased progressively with longer durations of running. Contraction-induced augmentation of the transcriptional activator function of MEF2 proteins, as assessed by this indicator transgene, was evident in soleus, plantaris and WV muscles, but not in EDL of running mice, indicating that the effect was produced only in those muscles recruited to power this particular form of locomotion. The EDL muscle is located in the anterior compartment of the distal hind limb and would be employed to ‘brake’ the body while descending an incline, but not for continuous assent associated with wheel running. As a negative control, a lacZ transgene controlled by the identical basal promoter (from hsp68), but linked to an enhancer element from the Nkx2.5 gene (Lien et al., 1999), showed no induction by wheel running in any muscle (Figure 1D).
Fig. 1. Voluntary wheel running stimulates the transcriptional activation function of MEF2. (A) Design of cages with running wheels. (B) Sample recording of 22 days of wheel-running activity, recorded as revolutions per minute and depicted by the height of the vertical bars. The recording is continuous and reads from left to right with each line representing a single day. All animals run throughout most of the nocturnal phase of each 12:12 h light:dark cycle (beginning daily at hour 18 here). (C) Whole-mount staining for β-galactosidase enzymatic activity in individual skeletal muscles [soleus, plantaris, EDL and white (W) vastus lateralis] from desMEF2-lacZ transgenic mice. Tissues were acquired from sedentary animals (0 h) and from mice undergoing voluntary wheel running for the indicated periods of time. Blue staining indicates augmented function of MEF2 as a transcriptional activator in muscles recruited for this locomotor activity (soleus, plantaris, W. vastus but not EDL). Similar results were observed in 3–6 animals assessed at each time point. (D) Absence of detectable β-galactosidase activity in muscles from sedentary or exercising mice carrying an Nkx2.5-lacZ transgene.
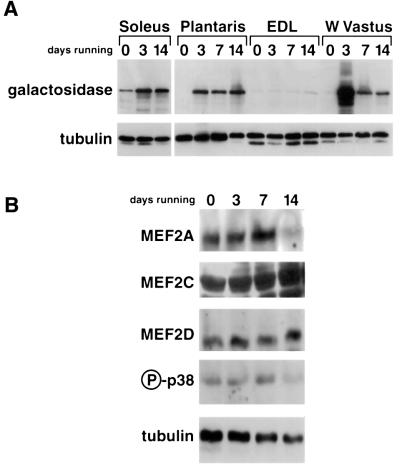
Similar results were evident when expression of the lacZ transgene was detected by immunoblot analysis using an antibody directed against β-galactosidase protein (Figure 2A). As shown in Figure 2B, increased transactivator function of MEF2 induced by running appears to be independent of changes in concentration of major MEF2 isoforms, or of the activation state of p38 MAP kinase, a signaling molecule known to augment MEF2 function (Zhao et al., 1999).
Fig. 2. Immunoblots of muscle extracts from sedentary and exercising desMEF2-lacZ mice. (A) Expression of the lacZ transgene was detected only in soleus muscles of sedentary animals (0 days), but was up-regulated by running (3–14 days) in muscles recruited for this locomotor activity (soleus, plantaris, W. vastus but not EDL). Each lane was loaded with muscle extracts prepared from a pool of five animals (10 muscles) and comparable recovery and transfer of each sample was assessed by anti-tubulin antibody. (B) Up-regulation of desMEF2-lacZ transgene expression in plantaris muscle by running (3–14 days) occurs without detectable changes in the abundance of major MEF2 isoforms or of the activated (phosphorylated) form of p38 MAPK.
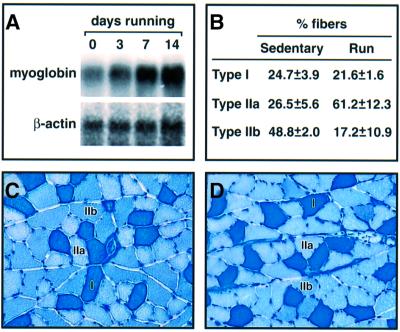
Enhanced transactivator function of MEF2 induced by spontaneous wheel running was associated with greater expression of endogenous genes known to be targets for MEF2 and known to be responsive to changes in contractile work. Myoglobin mRNA became progressively more abundant with an increased duration of nocturnal wheel running (Figure 3A). The proportion of myofibers expressing Type IIa myosin was increased in running animals, with a reciprocal decline in fibers expressing Type IIb myosin (Figure 3B–D). Thus, sustained periods of repeated muscle contractions associated with wheel running augment the transcriptional activator function of MEF2 proteins. This effect occurs independently of the pre-existing composition of the active muscle with respect to specialized myofiber subtypes, as soleus, plantaris and WV show similar responses. The induction of MEF2 function reflects localized neuromuscular activity rather than a systemic response to exercise, as it is limited to muscles recruited for this form of exercise.
Fig. 3. Voluntary wheel running alters expression of endogenous genes. (A) Expression of myoglobin mRNA assessed by northern blotting in plantaris muscles of sedentary (0 days) and exercising mice. β-actin mRNA provides a loading control. (B) Specialized myofiber subtypes assessed by myosin ATPase histochemistry reveal fiber type transformation (Type IIb to IIa) in plantaris muscles of running mice. Data represent the mean ± SD in five animals within each group. (C and D) Sample sections of metachromatic staining used for scoring the proportion of myofiber subtypes in sedentary (C) and running (D) mice.
MEF2 activation by muscle contractions is dependent upon calcineurin
Previous studies show that the transactivating function of MEF2 proteins can be regulated by several different signaling pathways. For example, p38 MAP kinase (MAPK) activates MEF2 by phosphorylating two threonine residues in the transcriptional AD of MEF2 (Zhao et al., 1999). However, we observed no up-regulation of the active phosphorylated form of p38 MAPK after wheel running (Figure 2B), suggesting that this pathway was not responsible for exercise-induced MEF2 activation. Calcineurin can activate MEF2 in skeletal muscle cells (Wu et al., 2000), and here we addressed the importance of this pathway for the induction of MEF2 function by muscle contractions.
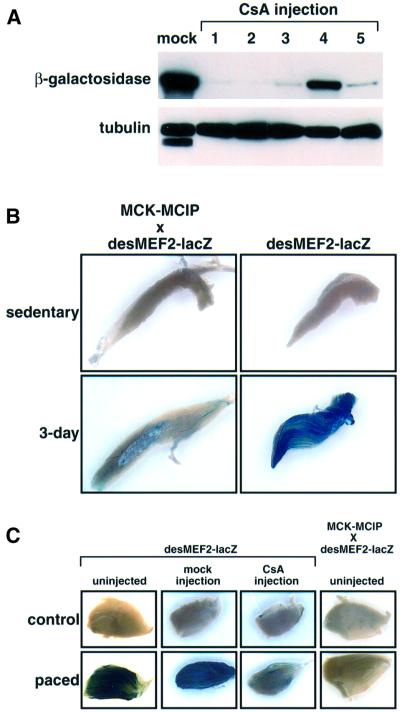
Expression of β-galactosidase is detectable by immunoblot analysis in the soleus muscles of sedentary desMEF2-lacZ mice, but not in EDL, plantaris or WV lateralis muscles (Figure 2A), a result we interpret to reflect a greater degree of tonic contractile activity of antigravity muscles. In five animals treated for 18 days by daily injection of cyclosporin A, a pharmacological inhibitor of calcineurin, expression of the lacZ transgene was uniformly reduced compared with levels found in a pool of soleus muscles from five animals treated with vehicle only (mock injection) (Figure 4A). This result indicates that the greater transactivating function of MEF2 in soleus muscles of sedentary animals is dependent on calcineurin activity.
Fig. 4. Enhanced transactivator function of MEF2 by muscle contractions is dependent on calcineurin activity. (A) Immunoblot of β-galactosidase expression in soleus muscles of sedentary desMEF2-lacZ mice injected either with vehicle (mock; pooled muscles from five animals) or with cyclosporin A (CsA; each lane represents an individual animal), a pharmacological inhibitor of calcineurin. Tubulin provides a loading control. (B and C) Histochemical detection of β-galactosidase expression in plantaris muscles of desMEF2-lacZ mice, and in animals overexpressing MCIP1, a protein inhibitor of calcineurin, in skeletal muscles. Similar results were observed in 3–6 animals within each group. (B) Up-regulation of MEF2 transactivator function by 3 days of running is prevented by expression of MCIP1. (C) Up-regulation of MEF2 transactivator function by 30 h of continuous motor nerve stimulation (10 Hz) is prevented by administration of CsA or by expression of MCIP1.
Because of systemic toxicity associated with the administration of cyclosporin A, which could impair running behavior, a different experimental strategy was required to assess the role of calcineurin in mediating contraction-dependent induction of MEF2 function in muscles of animals engaged in spontaneous wheel running. MCIP1 functions as an endogenous inhibitor of calcineurin (Rothermel et al., 2000, 2001; Yang et al., 2000). Transgenic mice were engineered to express an MCIP1 transgene under the control of the muscle creatine kinase (MCK) gene enhancer, thereby driving expression of MCIP1 selectively in skeletal muscles, in particular fast fibers in which the MCK gene is preferentially, but not exclusively, active. Doubly transgenic mice, which were generated by a genetic cross of desMEF2-lacZ animals to an MCK-MCIP1 transgenic line, failed to activate the MEF2-dependent indicator gene in the manner seen in the absence of MCIP1 overexpression (Figure 4B). The data indicate that calcineurin transduces signals arising as a consequence of muscle contraction to activate MEF2 and stimulate transcription of MEF2 target genes. The use of an MCIP1 transgene to inhibit calcineurin afforded more rigorous conclusions than those based on calcineurin antagonist drugs, as calcineurin is inhibited only within skeletal myofibers, without potentially confounding effects on motor neurons and without systemic toxicity.
As further proof that calcineurin is required for contraction-dependent activation of MEF2 in skeletal myofibers, desMEF2-lacZ and desMEF2-lacZxMCK- MCIP1 transgenic mice were subjected to chronic low-frequency electrical stimulation of the motor nerve. This stimulus is known to induce expression of genes characteristic of fatigue-resistant (Type I and IIa) myofibers in a manner similar to the effects of endurance exercise (Mayne et al., 1996; Romanul and Van der Meulen, 1966). Sustained periods (28–40 h) of continuous motor nerve stimulation at 10 Hz produced effects similar to wheel running on lacZ expression, and this response was abrogated either by administration of cyclosporin A (18 days before pacing) or by forced expression of MCIP1 (Figure 4C).
Molecular mechanisms of calcineurin-dependent activation of MEF2
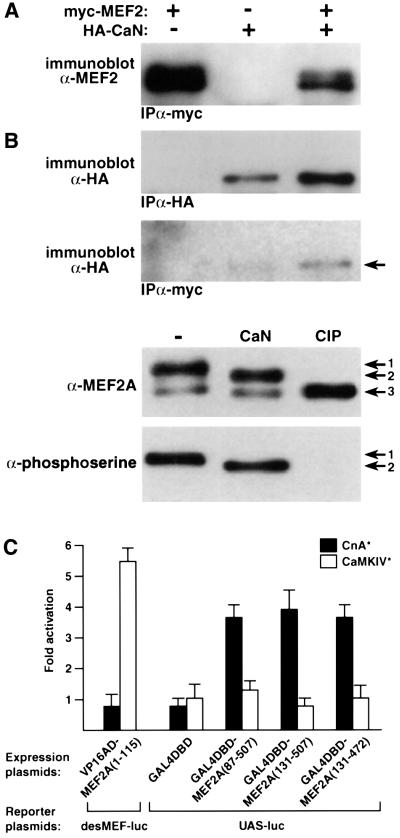
Hypophosphorylation of MEF2 accompanies its functional activation by calcineurin (Mao et al., 1999; Wu et al., 2000). Here we observed that calcineurin forms a physical complex with MEF2, and directly removes phosphate groups from serine residues within MEF2. Calcineurin is present in protein complexes precipitated from muscle cell extracts with antibodies directed against a c-myc epitope-tagged form of MEF2 (Figure 5A). Incubation of partially purified MEF2 with activated calcineurin in vitro converts the major band (Figure 5B, arrow 1) to a faster migrating form (Figure 5B, arrow 2), consistent with partial dephosphorylation, based on comparison with the effects of calf intestinal alkaline phosphatase (AP), which removes all phosphate groups from MEF2 (Figure 5B, arrow 3). The conclusion that calcineurin promotes partial dephosphorylation of MEF2, while certain phosphoserine residues are insensitive, is confirmed by probing the same blot with anti-phosphoserine antibody.
Fig. 5. Physical and functional interactions between MEF2 and calcineurin. (A) Calcineurin and MEF2 form a physical complex. Epitope-tagged forms of MEF2A (myc-MEF2) and calcineurin A (HA-CaN) were expressed in C2C12 myogenic cells, either alone or in combination, and complexes were precipitated with the indicated antibodies (α-HA or α-c-myc). Immunoprecipitated proteins were identified by immunoblots (α-HA or α-MEF2). (B) MEF2 is a substrate for calcineurin. Partially purified MEF2A was incubated in vitro with purified calcineurin (CaN) or calf intestinal phosphatase (CIP), and different phosphorylation states of MEF2 (arrows 1, 2 and 3) were identified by mobility during SDS–PAGE as detected by immunoblotting (α-MEF2A or α-phosphoserine antibodies). (C) Calcineurin-dependent augmentation of the transcriptional activation function of MEF2 is mediated through a C-terminal domain. The indicated segments of MEF2A protein (amino acids 1–507) were fused either to a heterologous transactivation domain (VP16 AD) or to a heterologous DNA binding domain (GAL4 DBD) and co-transfected into C2C12 myogenic cells with a luciferase reporter gene controlled by multimerized binding sites for either MEF2 (desMEF2) or GAL4 (UAS). The effects of constitutively active forms of calcineurin (CnA*) or calmodulin-dependent protein kinase IV (CaMKIV*) to alter basal levels of reporter gene expression were calculated as fold activation over basal levels (mean ± SD in 3–6 independent transfections). Data are normalized to β-galactosidase activity expressed from a CMV-lacZ plasmid co-transfected as a control for transfection efficiency.
The molecular mechanisms of calcineurin-dependent enhancement of the functional properties of MEF2 also were explored in co-transfection experiments using various regions of MEF2 fused either to the transcriptional AD of the herpes simplex viral protein VP16 or to the DNA binding domain (BD) of the yeast transcriptional regulatory protein GAL4. In cultured C2C12 myogenic cells, the ability of a VP16AD:MADS-MEF2 fusion protein (containing only the first 115 amino acids of MEF2 including the DNA BD) to activate expression of a luciferase reporter gene controlled by high-affinity MEF2 binding sites was enhanced by a constitutively active form of CaMKIV, as previously described (McKinsey et al., 2000), but not by constitutively active calcineurin (Figure 5C). This result indicates that calcineurin does not enhance either DNA binding by MEF2 or the recruitment of co-activator or co-repressor proteins to this region of MEF2. In contrast, expression of a luciferase reporter gene controlled by high-affinity GAL4 binding sites in the presence of three different GAL4BD:MEF2AD (C-terminal fragments) fusion proteins was stimulated by calcineurin, but not by CaMKIV (Figure 5C). This latter finding suggests that dephosphorylation of serine– threonine residues within the MEF2 AD by calcineurin is associated with enhanced transactivator function in a manner independent of effects on DNA binding.
Dose–response relationships between calcineurin activity and expression of endogenous MEF2 target genes
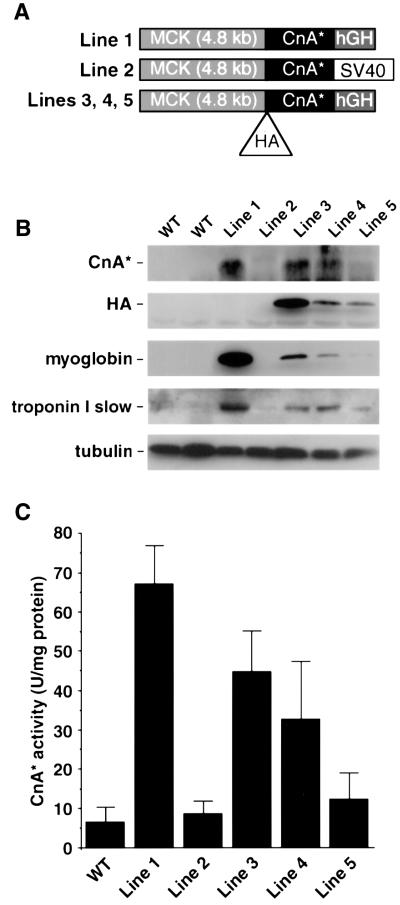
Relationships between the enzymatic activity of calcineurin in hindlimb muscles and expression of myoglobin and troponin I slow, endogenous genes known to be controlled by MEF2 activity (Grayson et al., 1998; Wu et al., 2000; Yan et al., 2001) and induced by exercise or motor nerve stimulation (Underwood and Williams, 1987) were assessed by the comparison of five independent lines of transgenic mice engineered to express a constitutively active form of calcineurin under the control of the MCK promoter. Three different transgene constructions were used, as shown in Figure 6A. Other phenotypes associated with two of these lines (lines 1 and 2 in Figure 6) have been reported elsewhere (Dunn et al., 2000; Naya et al., 2000), but the remaining three lines were generated de novo for this purpose, and the data presented here are entirely novel. Although all five transgenic lines contain an identical MCK promoter/enhancer fragment (Sternberg et al., 1988) and an identical protein coding segment that is translated to produce a truncated, constitutively active, 398 amino acid form of calcineurin A (CnA*), they differ markedly in expression of the CnA* transgene product (Figure 6B). Such highly divergent levels of transgene expression occur as a function of modulatory effects of flanking DNA at different chromosomal insertion sites, and possibly because of differences in 3′ UTR and polyadenylation sites. This broad range of CnA* concentrations afforded an opportunity to assess dose–response relationships between the enzymatic activity of calcineurin and expression of MEF2 target genes such as myoglobin and troponin I slow.
Fig. 6. Dose–response relationships between calcineurin activity and myoglobin gene expression in transgenic mice. (A) Diagram of transgene constructs used to generate five independent transgenic lines. (B) Representative immunoblot of WV lateralis muscle extracts from wild-type (WT) and transgenic MCK-CnA* lines using antibodies recognizing calcineurin, HA, myoglobin, troponin I slow and tubulin (loading control). (C) Calcineurin activity assay of dialyzed muscle extracts from plantaris muscles. One unit (U) is defined as the amount of enzyme that can release 1 pmol of phosphate from the substrate per minute. Each data point represents the mean value from at least three mice.
The enzymatic activity of the transgene-derived protein was assessed in the presence of 4 mM EGTA, a condition appropriate to measure the activity of the truncated form of calcineurin A, which has been engineered to function independently of calcium/calmodulin signaling. Conventional assays of calcineurin are based on the initial rate of dephosphorylation of a synthetic peptide substrate in the presence of saturating concentrations of calcium and calmodulin (Fruman et al., 1996). Under these latter conditions, the observed activity bears no predictable relationship to the activation state of endogenous calcineurin in vivo or to the level of CnA* protein that is expressed. The conventional assay measures the peak catalytic capacity of calcineurin recovered in the soluble cell fraction, but provides no information about the fraction of that pool that was active in the intact cell or tissue. In contrast, the new data provided here are directly relevant to the in vivo activity of the transgene product. As shown in Figure 6C, the line of MCK-CnA* transgenic mice reported by Naya et al. (2000) (line 1) expressed substantially greater CnA* activity than the mice described by Dunn et al. (2000) (line 2), whereas the newly generated lines 3–5 expressed intermediate levels. There was a direct correlation between immunoreactive CnA* protein and the catalytic activity of the enzyme (compare Figure 6B and C) across the five different MCK-CnA* transgenic lines.
Different levels of CnA* protein expression also correlated directly with up-regulation of troponin I slow and myoglobin gene expression (Figure 6B), which serve as molecular markers of the Type I and Type IIa (red, oxidative) myofiber subtypes and as MEF2 target genes. Increased expression of these marker genes as a dose-dependent function of transgene expression provides prima facie evidence for fiber type transformation driven by calcineurin.
Discussion
The principal finding of this paper is that the transcriptional activator function of MEF2 is up-regulated by a calcineurin-dependent pathway in response to contractile activity of skeletal muscles. This response requires sustained, repeated patterns of muscle contractions, but is independent of the antecedent specialization state (fiber type composition) of the muscle undergoing contractions. At a molecular level, new data show that calcineurin can dephosphorylate MEF2 directly and enhance the transactivator function of MEF2, in the absence of effects on DNA binding. New evidence from transgenic mice establishes a dose–response relationship between the enzymatic activity of calcineurin and expression of MEF2 target genes in skeletal muscles.
As MEF2 can act either as an activator or repressor of transcription under different circumstances (McKinsey et al., 2000), measurements of MEF2 mRNA and protein expression, or DNA binding activity, provide little information to evaluate the role of MEF2 in gene regulatory responses. The use of ‘MEF2 indicator mice’ (Naya et al., 1999), in which the expression of a lacZ transgene is dependent solely on MEF2 transactivator function, provides a readout of MEF2 activity that is suitable for physiological studies of intact animals. Here we observed that voluntary wheel running or motor nerve stimulation consistently activates MEF2 in skeletal muscles, with a time course consistent with a causal role in regulation of endogenous genes that respond to this physiological stimulus.
Kinetic features of contraction-dependent gene regulation in skeletal muscles, revealed by our observations, merit particular attention. As in classical stimulus– response paradigms, contractile activity of skeletal myofibers promotes transcriptional responses that can be grouped into temporal stages (e.g. immediate early, immediate late, delayed), each characterized by the activation–deactivation of specific sets of genes. A single acute bout of exercise or motor neuron pacing (minutes/hours) induces short-lived changes in expression of certain genes, but no stable alterations of muscle phenotype (Michel et al., 1994; Neufer et al., 1998; Pette and Vrbova, 1999). Remodeling of myofibers from one specialized phenotype to another is a process involving reprogramming of hundreds of genes, and occurs as a cumulative function of repeated, sustained periods of contractile work (days/weeks) (Booth and Baldwin, 1996; Mayne et al., 1996; Williams and Neufer, 1996). To review the kinetic features of desMEF2-lacZ expression, a few hours are sufficient to evoke a uniformly detectable response to muscle contractions, but maximal activation requires days to weeks. This time course parallels that of endogenous genes that establish specialized myofiber phenotypes (e.g. myoglobin, troponin I slow). It remains to be established that these responses of endogenous genes are driven directly by activated MEF2, but the present data support a causal role for MEF2 in this response.
Owing to the stable nature of β-galactosidase, it is difficult to examine the deactivation kinetics of MEF2 by monitoring transgene expression in desMEF2-lacZ animals. However, the inducing effects of 1 week of running are no longer detectable after 4 weeks of sedentary caging (data not shown), indicating that MEF2 is deactivated after running is terminated. This reversal of contraction-induced transgene activation upon a return to ambient activity resembles that of stable endogenous proteins of the sarcomere or mitochondria (Brown et al., 1989; Booth and Baldwin, 1996).
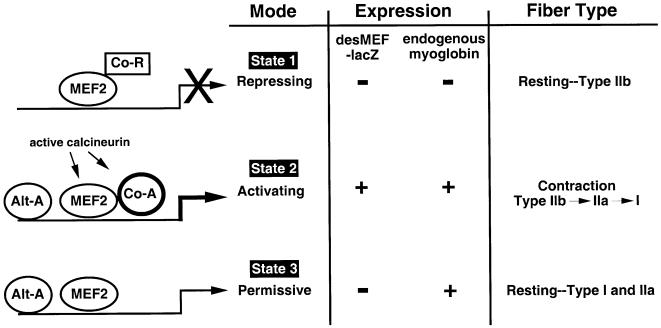
These new findings, in conjunction with other published work, are consistent with a three-state model for the role of MEF2 in controlling specialized programs of gene expression in skeletal muscles in response to different patterns of contractile work (Figure 7). During prolonged periods of muscle inactivity (state 1), calcineurin and CaMK are inactive, and MEF2 functions primarily as a transcriptional repressor in complex with HDAC proteins. The ability of MEF2 to function in this manner has been established in cultured cells (Miska et al., 1999; McKinsey et al., 2000). Thus, certain MEF2 target genes (e.g. myoglobin) are silenced in fast, glycolytic (Type IIb) fibers of sedentary animals. Other MEF2 target genes, however, could remain transcriptionally active, due to the influence of other transcriptional regulatory events that are sufficient to overcome direct repression by MEF2:HDAC complexes.
Fig. 7. Schematic model of the role of MEF2 in contraction-dependent gene regulation in skeletal muscles based on the functional state of MEF2 as a transcriptional regulator with respect to expression of desMEF2-lacZ and endogenous MEF2 target genes (e.g. myoglobin). State 1 depicts the condition found in resting Type IIb (fast, glycolytic) myofibers in which neither desMEF2-lacZ nor myoglobin is expressed. State 2 is present during or immediately following sustained periods of contractile activity, as evoked by voluntary wheel running or motor nerve stimulation in our experiments. Both the MEF2-dependent reporter gene and endogenous genes are induced. State 3 describes resting oxidative myofibers (Type I and IIa) that express endogenous myoglobin but do not show evidence for activated MEF2 function as assessed by the desMEF2-lacZ transgene. MEF2 serves repressive, activating or permissive roles in each state, respectively. The maintenance of state 3 requires periodic reinforcement by the mechanisms at work in state 2, or eventually the myofiber reverts to state 1. In addition to MEF2, symbols depict co-repressor (Co-R), co-activator (Co-A) and alternative activator (Alt-A) proteins. Co-A proteins are recruited to DNA by physical interactions with MEF2, while Alt-A proteins bind DNA at sites discrete from MEF2 binding elements. Specific features and implications of this model as discussed in the text.
State 2 in our model represents the condition found in actively contracting muscles, as demonstrated by activation of the desMEF2-lacZ transgene in our current experiments. Contractile work is associated with increases in intracellular calcium accessible to the relevant signaling molecules, resulting in activation of calcineurin and CaMK. Activated CaMKI or CaMKIV disrupts the MEF2:HDAC complex, thereby relieving repression, and activated calcineurin stimulates the transactivator function of MEF2. The net effect is up-regulation of previously repressed MEF2 target genes such as myoglobin and troponin I slow. Our present data show that calcineurin is necessary for this state transition, but the model predicts that CaMK, and probably other signaling inputs, are important as well. Individual MEF2 target genes are expected to have different thresholds for the intensity and duration of contractile activity required to drive their promoters from state 1 to state 2, based on differences in binding affinity for MEF2 of nucleotide motifs within promoter/enhancer regions (Wu et al., 2000), and on differences in signaling inputs transduced through other transcription factors. State 2 can be established in any myofiber, irrespective of the antecedent state of specialization (fiber type), although stimulus–response thresholds may differ among different fiber types.
State 3 represents the condition defined in our present experiments by Type I and IIa myofibers of sedentary animals that do not express the desMEF2-lacZ gene to measurable levels. MEF2 target genes such as myoglobin are expressed, even though the transactivator function of MEF2 cannot be detected by the indicator transgene. This apparent paradox can be explained in several ways. First, the condition we define as state 3 could occur because the half-life of mRNA and protein products of endogenous genes regulated by MEF2 (e.g. myoglobin) is long relative to the time period in which MEF2 is maintained in its transactivating mode by contractile activity. Thus, the effects of a period of contractile work on the expression of target genes could extend for hours or days beyond the time in which MEF2 is functioning as a transcriptional activator. A second explanation is that contractile activity promotes the elaboration and/or activation of other transcription factors (termed ‘alternative activators’ in Figure 7), the half-life of which extends beyond the period in which MEF2 is active. The effect is to prolong expression of endogenous MEF2 target genes (e.g. myoglobin) beyond the period in which MEF2 itself is active. It is important to note that the desMEF2-lacZ transgene is controlled solely by MEF2 and its associated co-activators and co-repressors, whereas endogenous genes are subject to control by other classes of transcription factors that employ different nucleotide sequence recognition motifs within promoter/enhancer regions of the relevant genes. Indeed, there is good evidence to support a role of other classes of transcription factors in the maintenance of specialized myofiber phenotypes (Spitz et al., 1997; O’Mahoney et al., 1998; Hughes et al., 1999; Murgia et al., 2000; Yan et al., 2001).
According to this conceptual model, state 2 requires the stimulus of contractile activity, the intensity and duration of which (and hence the resulting calcium signals) must be sufficient to convert MEF2 from repressor to transactivator mode. State 3 occurs when such periods of contractile activity are repeated at intervals longer than the half-life of transcriptional complexes formed in state 2, which may include alternative activators distinct from MEF2, or the products of relevant MEF2 target genes. In the absence of sufficiently sustained periods of contractile activity, myofibers revert to state 1. By introducing kinetic considerations, the model provides a plausible explanation for how calcineurin and MEF2 can play central regulatory roles in controlling the expression of genes that establish specialized myofiber subtype, even though MEF2 activation by contractions can be detected (transiently) in any myofiber subtype. The model also accounts for the fact that specialized myofiber subtypes that we propose to be dependent on a calcineurin/MEF2/NFAT signaling pathway are maintained at times when the transactivator function of MEF2 is undetectable.
With respect to the molecular mechanisms of MEF2 activation by calcineurin, either phosphorylation or dephosphorylation of MEF has been associated with enhancement of its transactivating function (Han et al., 1997; Mao et al., 1999; Yang et al., 1999; Zhao et al., 1999; Tamir and Bengal, 2000; Wu et al., 2000). The current studies of GAL4 and VP16 fusion proteins are consistent with an activation mechanism that involves dephosphorylation by calcineurin of inhibitory serine– threonine residues within the C-terminal transcriptional AD of MEF2. Calcineurin also may augment the function of MEF2 by mechanisms that are unrelated to direct dephosphorylation of MEF2. For example, direct binding of NFAT proteins to MEF2 may participate in calcineurin-dependent signaling via MEF2 binding sites (Blaeser et al., 2000).
In summary, MEF2 integrates multiple calcium- regulated signaling inputs important for gene regulation in skeletal muscles. Contractile activity enhances the function of MEF2 as a transcriptional activator by way of pathways that necessarily include the protein phosphatase calcineurin. New insights based on data presented here inspired a kinetic model concerning the role of a calcium/calcineurin/NFAT/MEF2 signaling module in the determination of specialized myofiber subtypes. The model serves to guide future work to elucidate the integration of multiple pathways and signaling molecules that cooperate in the control of specialized myofiber phenotypes.
Materials and methods
Plasmid constructs, tissue culture, cell transfection and reporter assay
A synthetic enhancer consisting of three copies of a high-affinity MEF2 binding site (Naya et al., 1999) was linked to an hsp68 minimal promoter in pGL3 (Promega) to generate the desMEF2-luciferase reporter. A c-myc-tagged MEF2A was subcloned from pCDNAI/amp-MEF2A (Black et al., 1995) into pCI-neo (Promega). Constitutively active forms of calcineurin and CaMKIV have been described elsewhere (O’Keefe et al., 1992; Cruzalegui and Means, 1993). PCR fragments representing different regions of MEF2A were inserted into pM and pVP16 vectors (Clontech) to generate GAL4 and VP16 fusion constructs. C2C12 myoblast cells were propagated, transfected and assayed for luciferase and β-galactosidase activities as described previously (Chin et al., 1998).
Immunoprecipitation and immunoblotting
C2C12 cells transfected with expression plasmids were lysed in modified RIPA buffer [50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM sodium vanadate, 1% NP-40, 0.5% sodium deoxycholate, 1 mM dithiothreitol (DTT) and proteinase inhibitor cocktail (Roche Molecular Biochemicals)]. c-myc- or HA-tagged proteins were immunoprecipitated with anti-c-myc or anti-HA antibody (Roche Molecular Biochemicals), followed by incubation with protein G linked to agarose beads (Roche Molecular Biochemicals). After washing the protein-complexed beads five times in RIPA buffer, the samples were boiled in SDS–PAGE loading buffer, loaded onto an SDS–PAGE gel and transferred to nitrocellulose paper following standard immunoblotting procedures. For phosphatase treatment, c-myc–MEF2A was immunoprecipitated from transfected cell lysates using c-myc antibody coupled to protein G–agarose beads. The immunoprecipitant was incubated with recombinant human calcineurin (BIOMOL), 0.6 µM calmodulin, 1 mM calcium for 1 h at 30°C or with calf intestine AP (Roche Molecular Biochemicals) for 1 h at 37°C with continuous shaking. The samples were processed for immunoblotting as described above. Antibodies against calcineurin (Transduction Laboratories), β-galactosidase (Promega), MEF2A (Santa Cruz Biotechnology), MEF2C (gift from John Schwarz, University of Texas Medical School at Houston, Houston, TX), MEF2D (Transduction Laboratories), Phospho-p38 MAP kinase (Cell Signaling Technology), phosphoSerine (Zymed Laboratories Inc.), α-tubulin (Sigma), myoglobin (Dako) and troponin I slow (Santa Cruz) were used for immunoblot analysis.
RNA isolation and analysis
Total RNA was prepared from mouse skeletal muscles using RNA STAT-60 (Tel-Test Inc.) following the manufacturer’s instructions. Northern blots were performed with 20 µg of total RNA in each lane and probed in Ultrahyb (Ambion) with labeled myoglobin or β-actin cDNA.
β-galactosidase staining and metachromatic fiber typing of skeletal myofibers
Dissected muscles were fixed with 2% paraformaldehyde/0.1% glutaraldehyde in phosphate-buffered saline (PBS) on ice for 30–45 min, followed by washing and X-gal staining (5 mM ferrocyanide, 5 mM ferricyanide, 2 mM MgCl2, 1 mg/ml X-gal, 0.01% sodium deoxycholate, 0.02% NP-40) for 1–12 h. Fiber typing was performed with a metachromatic dye–ATPase method (Ogilvie et al., 1990).
Transgenic mice
Mice carrying the desMEF2-lacZ transgene have been described previously (Naya et al., 1999). New transgenic lines (F2 hybrid: C57Bl/6 × SJL) were generated using the MCK gene enhancer/promoter (Sternberg et al., 1988) linked to a cDNA encoding a HA-tagged form of human MCIP1 protein (Rothermel et al., 2000). In addition, five independent lines of transgenic mice were engineered to express a constitutively active form of calcineurin (CnA*) under the control of the MCK enhancer/promoter. Two of these lines have been described elsewhere (Dunn et al., 2000; Naya et al., 2000). In three new lines, the calcineurin transgene product included a HA epitope tag for convenient assessment of transgene expression. All experiments involving animals were reviewed and approved by the Institutional Animal Care and Research Advisory Committee.
Voluntary wheel running and electrical pacing of the mouse hindlimb muscles
For running experiments, mice (F7 hybrid: C57Bl/6 × C3H/He) were individually housed in cages (15 × 32 cm) equipped with running wheels (11 cm in diameter). Animals were maintained on a 12:12 h cycle of light and dark, and wheel-running activity was monitored continuously with a Dataquest Acquisition and Analysis System (Data Sciences International). For nerve pacing studies, miniature neuromuscular stimulators were purchased from Dr Jonathan Jarvis at the University of Liverpool, UK (jcj@liverpool.ac.uk). The stimulators were implanted under the dorsal skin of anesthetized mice and leads were tunneled subcutaneously to the sciatic nerve. After the animals recovered from anesthesia, the circuit was activated by flashes of light transmitted through the skin to deliver supramaximal pulses of 0.2 ms duration at a frequency of 10 Hz. Adult mice were injected subcutaneously with cyclosporin A (25 mg/kg) or vehicle daily for 18 days.
Enzymatic assay for constitutively active calcineurin
The assay was performed in a buffer containing 50 mM Tris–HCl pH 7.5 with 100 mM NaCl, 0.5 mM DTT, 100 µg/ml bovine serum albumin, 500 nM okadaic acid, 0.1 mM RII phosphopeptide (DLDVPIPGRFDRRVpSVAAE; BIOMOL) and 3 mM EGTA. Dialyzed muscle protein extract was added and incubated for 20 min at 30°C, then 500 µl of Biomol Green solution (BIOMOL) were added and further incubated at room temperature for 30 min before OD620 measurement. Standard curves were constructed with free phosphate. Recombinant human calcineurin (BIOMOL) provided a positive control. One unit is defined as the enzyme activity that can release 1 pmol of phosphate from the substrate per minute.
Acknowledgments
Acknowledgements
We thank Dr Joseph Garcia for advising us on the voluntary wheel running system and April Hawkins for assistance with transgenic mouse. This work was supported by grants from the National Institutes of Health, the Donald W.Reynolds Cardiovascular Clinical Research Center, the Robert A.Welch Foundation and the Texas Higher Education Coordinating Board.
References
- Bigard X., Sanchez,H., Zoll,J., Mateo,P., Rousseau,V., Veksler,V. and Ventura-Clapier,R. (2000) Calcineurin co-regulates contractile and metabolic components of slow muscle phenotype. J. Biol. Chem., 275, 19653–19660. [DOI] [PubMed] [Google Scholar]
- Black B.L. and Olson,E.N. (1998) Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol., 14, 167–196. [DOI] [PubMed] [Google Scholar]
- Black B.L., Martin,J.F. and Olson,E.N. (1995) The mouse MRF4 promoter is trans-activated directly and indirectly by muscle-specific transcription factors. J. Biol. Chem., 270, 2889–2892. [DOI] [PubMed] [Google Scholar]
- Blaeser F., Ho,N., Prywes,R. and Chatila,T.A. (2000) Ca(2+)-dependent gene expression mediated by MEF2 transcription factors. J. Biol. Chem., 275, 197–209. [DOI] [PubMed] [Google Scholar]
- Booth F.W. and Baldwin,K.M. (1996) Muscle plasticity: energy demand and supply processes. In Bowell,L.B. and Shepard,J.T. (eds), The Handbook of Physiology: Integration of Motor, Circulatory, Respiratory and Metabolic Control During Exercise. American Physiology Society, Bethesda, MD, pp. 1075–1123.
- Brown J.M., Henriksson,J. and Salmons,S. (1989) Restoration of fast muscle characteristics following cessation of chronic stimulation: physiological, histochemical and metabolic changes during slow- to-fast transformation. Proc. R. Soc. Lond. B Biol. Sci., 235, 321–346. [DOI] [PubMed] [Google Scholar]
- Calvo S., Venepally,P., Cheng,J. and Buonanno,A. (1999) Fiber-type-specific transcription of the troponin I slow gene is regulated by multiple elements. Mol. Cell. Biol., 19, 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin E.R. et al. (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev., 12, 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzalegui F.H. and Means,A.R. (1993) Biochemical characterization of the multifunctional Ca2+/calmodulin-dependent protein kinase type IV expressed in insect cells. J. Biol. Chem., 268, 26171–26178. [PubMed] [Google Scholar]
- Delling U., Tureckova,J., Lim,H.W., De Windt,L.J., Rotwein,P. and Molkentin,J.D. (2000) A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol. Cell. Biol., 20, 6600–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S.E., Chin,E.R. and Michel,R.N. (2000) Matching of calcineurin activity to upstream effectors is critical for skeletal muscle fiber growth. J. Cell Biol., 151, 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman D.A., Pai,S.Y., Klee,C.B., Burakoff,S.J. and Bierer,B.E. (1996) Measurement of calcineurin phosphatase activity in cell extracts. Methods, 9, 146–154. [DOI] [PubMed] [Google Scholar]
- Grayson J., Bassel-Duby,R. and Williams,R.S. (1998) Collaborative interactions between MEF-2 and Sp1 in muscle-specific gene regulation. J. Cell. Biochem., 70, 366–375. [PubMed] [Google Scholar]
- Han J., Jiang,Y., Li,Z., Kravchenko,V.V. and Ulevitch,R.J. (1997) Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature, 386, 296–299. [DOI] [PubMed] [Google Scholar]
- Hughes S.M., Chi,M.M., Lowry,O.H. and Gundersen,K. (1999) Myogenin induces a shift of enzyme activity from glycolytic to oxidative metabolism in muscles of transgenic mice. J. Cell Biol., 145, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien C.L., Wu,C., Mercer,B., Webb,R., Richardson,J.A. and Olson,E.N. (1999) Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development, 126, 75–84. [DOI] [PubMed] [Google Scholar]
- Mao Z., Bonni,A., Xia,F., Nadal-Vicens,M. and Greenberg,M.E. (1999) Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science, 286, 785–790. [DOI] [PubMed] [Google Scholar]
- Mayne C.N., Sutherland,H., Jarvis,J.C., Gilroy,S.J., Craven,A.J. and Salmons,S. (1996) Induction of a fast-oxidative phenotype by chronic muscle stimulation: histochemical and metabolic studies. Am. J. Physiol., 270, C313–C320. [DOI] [PubMed] [Google Scholar]
- McKinsey T.A., Zhang,C.L., Lu,J. and Olson,E.N. (2000) Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature, 408, 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J.B., Ordway,G.A., Richardson,J.A. and Williams,R.S. (1994) Biphasic induction of immediate early gene expression accompanies activity-dependent angiogenesis and myofiber remodeling of rabbit skeletal muscle. J. Clin. Invest., 94, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska E.A., Karlsson,C., Langley,E., Nielsen,S.J., Pines,J. and Kouzarides,T. (1999) HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J., 18, 5099–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia M., Serrano,A.L., Calabria,E., Pallafacchina,G., Lomo,T. and Schiaffino,S. (2000) Ras is involved in nerve-activity-dependent regulation of muscle genes. Nature Cell Biol., 2, 142–147. [DOI] [PubMed] [Google Scholar]
- Naya F.J., Wu,C., Richardson,J.A., Overbeek,P. and Olson,E.N. (1999) Transcriptional activity of MEF2 during mouse embryogenesis monitored with a MEF2-dependent transgene. Development, 126, 2045–2052. [DOI] [PubMed] [Google Scholar]
- Naya F.J., Mercer,B., Shelton,J., Richardson,J.A., Williams,R.S. and Olson,E.N. (2000) Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem., 275, 4545–4548. [DOI] [PubMed] [Google Scholar]
- Neufer P.D., Ordway,G.A. and Williams,R.S. (1998) Transient regulation of c-fos, α B-crystallin and hsp70 in muscle during recovery from contractile activity. Am. J. Physiol., 274, C341–C346. [DOI] [PubMed] [Google Scholar]
- Ogilvie R.W. and Feedback,D.L. (1990) A metachromatic dye–ATPase method for the simultaneous identification of skeletal muscle fiber types I, IIA, IIB and IIC. Stain Technol., 65, 231–241. [DOI] [PubMed] [Google Scholar]
- O’Keefe S.J., Tamura,J., Kincaid,R.L., Tocci,M.J. and O’Neill,E.A. (1992) FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature, 357, 692–694. [DOI] [PubMed] [Google Scholar]
- O’Mahoney J.V., Guven,K.L., Lin,J., Joya,J.E., Robinson,C.S., Wade,R.P. and Hardeman,E.C. (1998) Identification of a novel slow-muscle-fiber enhancer binding protein, MusTRD1. Mol. Cell. Biol., 18, 6641–6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E.N. and Williams,R.S. (2000) Calcineurin signaling and muscle remodeling. Cell, 101, 689–692. [DOI] [PubMed] [Google Scholar]
- Pette D. and Vrbova,G. (1999) What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve, 22, 666–677. [DOI] [PubMed] [Google Scholar]
- Romanul F.C. and Van der Meulen,J.P. (1966) Reversal of the enzyme profiles of muscle fibres in fast and slow muscles by cross-innervation. Nature, 212, 1369–1370. [DOI] [PubMed] [Google Scholar]
- Rothermel B., Vega,R.B., Yang,J., Wu,H., Bassel-Duby,R. and Williams,R.S. (2000) A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J. Biol. Chem., 275, 8719–6725. [DOI] [PubMed] [Google Scholar]
- Rothermel B.A. et al. (2001) Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc. Natl Acad. Sci. USA, 98, 3328–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S. and Reggiani,C. (1996) Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol. Rev., 76, 371–423. [DOI] [PubMed] [Google Scholar]
- Spitz F., Salminen,M., Demignon,J., Kahn,A., Daegelen,D. and Maire,P. (1997) A combination of MEF3 and NFI proteins activates transcription in a subset of fast-twitch muscles. Mol. Cell. Biol., 17, 656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E.A., Spizz,G., Perry,W.M., Vizard,D., Weil,T. and Olson, E.N. (1988) Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine kinase gene. Mol. Cell. Biol., 8, 2896–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap S.J., Hunter,R.B., Stevenson,E.J., Felton,H.M., Kansagra,N.V., Lang,J.M., Esser,K.A. and Kandarian,S.C. (2000) The calcineurin-NFAT pathway and muscle fiber-type gene expression. Am. J. Physiol. Cell Physiol., 279, C915–C924. [DOI] [PubMed] [Google Scholar]
- Tamir Y. and Bengal,E. (2000) Phosphoinositide 3-kinase induces the transcriptional activity of MEF2 proteins during muscle differentiation. J. Biol. Chem., 275, 34424–34432. [DOI] [PubMed] [Google Scholar]
- Underwood L.E. and Williams,R.S. (1987) Pretranslational regulation of myoglobin gene expression. Am. J. Physiol., 252, C450–C453. [DOI] [PubMed] [Google Scholar]
- Williams R.S. and Neufer,P.D. (1996) Regulation of gene expression in skeletal muscle by contractile activity. In Rowell L.B. and Shepard,J.T. (eds), The Handbook of Physiology: Integration of Motor, Circulatory, Respiratory and Metabolic Control During Exercise. American Physiology Society, Bethesda, MD, pp. 1124–1150.
- Wu H. et al. (2000) MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J., 19, 1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Serrano,A.L., Schiaffino,S., Bassel-Duby,R. and Williams,R.S. (2001) Regulatory elements governing transcription in specialized myofiber subtypes. J. Biol. Chem., 276, 17361–17366. [DOI] [PubMed] [Google Scholar]
- Yang J., Rothermel,B., Vega,R.B., Frey,N., McKinsey,T.A., Olson,E.N., Bassel-Duby,R. and Williams,R.S. (2000) Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ. Res., 87, E61–E68. [DOI] [PubMed] [Google Scholar]
- Yang S.H., Galanis,A. and Sharrocks,A.D. (1999) Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol. Cell. Biol., 19, 4028–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn H.D., Chatila,T.A. and Liu,J.O. (2000) Integration of calcineurin and MEF2 signals by the coactivator p300 during T-cell apoptosis. EMBO J., 19, 4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., New,L., Kravchenko,V.V., Kato,Y., Gram,H., di Padova,F., Olsen,E.N., Ulevitch,R.J. and Han,J. (1999) Regulation of the MEF2 family of transcription factors by p38. Mol. Cell. Biol., 19, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]