Abstract
P2X receptors are ATP-gated ion channels in the plasma membrane, but activation of the P2X7 receptor also leads to rapid cytoskeletal re-arrangements such as membrane blebbing. We identified 11 proteins in human embryonic kidney cells that interact with the rat P2X7 receptor, by affinity purification followed by mass spectroscopy and immunoblotting [laminin α3, integrin β2, β-actin, α-actinin, supervillin, MAGuK, three heat shock proteins, phosphatidylinositol 4-kinase and receptor protein tyrosine phosphatase-β (RPTPβ)]. Activation of the P2X7 receptor resulted in its dephosphorylation. Whole-cell recordings from cells expressing P2X7 receptors showed that this markedly reduced subsequent ionic currents and it also slowed membrane bleb formation. By mutagenesis, we identified Tyr343 in the putative second transmembrane domain as the site of phosphorylation. Thus, we have identified a P2X7 receptor signalling complex, some members of which may initiate cytoskeletal rearrangements following receptor activation. Others, such as RPTPβ, might exert feedback control of the channel itself through its dephosphorylation.
Keywords: ion channel/P2X receptors/receptor protein tyrosine phosphatase-β/signalling complex/tyrosine phosphorylation
Introduction
P2X receptors form a family of ATP-gated ion channels extensively distributed throughout the cells of vertebrates. They are ligand-gated ion channels, each with distinct pharmacological and/or physiological properties. They form as homomers and/or heteromers, and current biochemical evidence suggests that the channel has three or perhaps six subunits (Nicke et al., 1998; North and Surprenant, 2000). The P2X7 receptor subunit has several features that set it apart from other members of the family (Surprenant et al., 1996). Co-immunoprecipitation experiments indicate that it is the only subunit that does not heteropolymerize with other P2X subunits (Torres et al., 1999). It is primarily localized to epithelia and immune cells, particularly antigen-presenting cells (Collo et al., 1997; Mutini et al., 1999). Receptor activation requires concentrations of ATP that are 10–100 times higher than those required to activate other P2X receptors, but the agonist affinity and maximum response can also be modulated 5- to 100-fold by alterations in external monovalent and divalent cations (Surprenant et al., 1996; Rassendren et al., 1997; Michel et al., 1999; Gudipaty et al., 2001). The ionic currents through P2X7 receptors show considerable plasticity, in the sense that repeated applications of the agonist result in prominent changes in the amplitude and time course of the current elicited by subsequent applications (Surprenant et al., 1996; Rassendren et al., 1997; Hibell et al., 2000).
Activation of native ATP receptors in macrophages and macrophage-like cell lines, in which P2X7 subunits are predominately expressed, is fundamentally different from that observed for other ion channels because it initiates several cellular consequences further downstream. These include alterations in the cell morphology (Cohn and Parks, 1967), interleukin-1β release (Ferrari et al., 1997a; Solle et al., 2001), NFκB activation (Ferrari et al., 1997b), phospholipase D activation (Humphreys and Dubyak, 1996), cytolysis and/or apoptosis (Di Virgilio, 2000). The morphological changes are readily seen with heterologous expression of P2X7 receptors in HEK293 cells, where large membrane blebs appear within 30–60 s of activating the receptor (Virginio et al., 1999). The changes in cell morphology are not seen with other P2X receptors, suggesting that they may result from direct coupling to cytoskeletal proteins rather than from the flow of ionic current. In support of this notion is our recent observation that rat P2X7 receptors heterologously expressed in HEK293 cells as well as native P2X7 receptors in rat peritoneal macrophage appeared as a strongly bound multimeric complex, which seemed unlikely to be comprised solely of P2X7 receptor subunits (Kim et al., 2001).
Here we have identified 11 proteins that interact with the rat P2X7 receptor. These are the extracellular matrix protein laminin α3, the membrane-spanning proteins integrin β2 and receptor protein tyrosine phosphatase β (RPTPβ) and eight intracellular proteins [α-actinin 4, β-actin, supervillin, three heat shock proteins (Hsp90, Hsc71 and Hsp70), phosphatidylinositol 4-kinase 230 (PI4K) and membrane-associated guanylate kinase P55 (MAGuK)]. We further show that the P2X7 receptor becomes dephosphorylated at Tyr343 upon activation, and that this strongly inhibits the membrane current and retards membrane blebbing. These results suggest that RPTPβ and other associated proteins are key molecular elements in downstream signalling from P2X7 receptors to the cytoskeleton, and that one aspect of that signalling is feedback control of the channel function.
Results
Identification of interacting proteins
The receptor complex was isolated by immunoprecipitation with ecto-P2X7 antibody (Ab) using purified membrane fractions of HEK293 cells stably expressing the rat P2X7 receptor (Kim et al., 2001). When the receptor complex was separated by SDS–PAGE and stained with Coomassie Blue, numerous bands ranging in size from <25 to 250 kDa were apparent (Figure 1A, lane 4). These were not present in membrane extracts bound to the γ-bind G-Sepharose used in the immunoprecipitations nor from those immunoprecipitated with anti-P2X2 Ab (Figure 1A, lanes 2 and 3). Bands indicated by arrowheads in Figure 1A were excised, subjected to peptic digest with trypsin, endoprotease Arg-C or Asp-N, and identified by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectroscopy (MS). One example of results obtained for the protein identified as heat shock protein Hsp70 is shown in Figure 1A. Eleven proteins were unambiguously identified (Table I). These comprised of the extracellular matrix protein laminin α3, three cytoskeletal proteins (α-actinin 4, β-actin, supervillin) and an integrin subunit (β2), one scaffolding protein (MAGuK), three chaperones (Hsp90, Hsp70, Hsc71) and two signalling proteins (RPTPβ and PI4K). N-terminal amino acid sequencing was also used to identify the IgG bands indicated in Figure 1A.
Fig. 1. Proteomic characterization of P2X7 receptor protein complex. (A) Protein staining of SDS–PAGE membrane extracts from HEK cells stably expressing P2X7 receptors. Lane 1 is total membrane extract; lanes 2 and 3 are negative controls showing membrane extracts bound to γ-bind G-Sepharose and after immunoprecipitation with an anti-P2X2 Ab, respectively. Lane 4 shows protein staining after immunoprecipiteads show position of bands excised for mass spectroscopy. IgG bands were identified by N-terminal amino acid sequencing. Right-hand panel shows MALDI-TOF spectrum of the 70 kDa protein band after digestion with trypsin; ‘T’ labels peptide from trypsin autolysis. The remaining peaks match peptides from Hsp70. (B) Blotting with antibodies to β-actin, α-actinin, integrin β2, P2X7 receptor, Hsp70, Hsc71, Hsp90, supervillin, laminin α3 and MAGuK-p55 (as indicated) from membrane fractions (a) from HEK293 cells stably expressing P2X7 receptors or (b) from HEK293 cells transiently expressing P2X7-EE receptor. In each case, lane 1 is solubilized membrane fraction, lane 2 is negative control with mouse IgG, and lane 3 is from IP with ecto-P2X7 or EE Ab. (C) Blots with anti-RPTPβ Ab. Lane 1 is membrane fraction, lane 2 is IP with ecto-P2X7 Ab from P2X7-expressing cells (stable transfection), lane 3 is IP with anti-EE Ab from P2X7-EE-expressing cells (transient transfection), and lane 4 is negative control with mouse IgG. (D) Co-expression of P2X7-EE and PI4K-HA cDNA (lanes 3 and 4). Lane 1, P2X7-EE cDNA only; lane 2, PI4K-HA cDNA only. Lanes 1 and 3, IP with anti-EE Ab; lanes 2 and 4, IP with anti-HA Ab; membranes blotted with anti-P2X7 or anti-HA as indicated.
Table I. Proteins identified in the P2X7 receptor complex by MALDI-TOF MS and confirmed by western blotting.
| Protein name | Abbreviation | Molecular weight (kDa) | SwissProt/Trembl | Peptidesa |
|---|---|---|---|---|
| Extracellular | ||||
| laminin α3 chain | 187 | Q16787 | 7 (2, 3)b | |
| Transmembrane | ||||
| integrin β2 | 85 | P05107 | 9 | |
| protein tyrosine phosphatase β | RPTPβ | 222 | P23467 | 8 (2, 2)b |
| Intracellular | ||||
| β-actin | 42 | P02570 | 9 | |
| α-actinin 4 | 105 | O43707 | 6 | |
| heat shock cognate 71 kDa protein | Hsc71 | 71 | P11142 | 15 |
| heat shock 70 kDa protein 1 | Hsp70 | 70 | P08107 | 16 |
| heat shock protein HSP90-β | Hsp90 | 83 | P08238 | 9 |
| phosphatidylinositol 4-kinase 230 | PI4K | 231 | Q9UPG2 | 8 (3, 4)b |
| MAGUK P55 subfamily member 3 | MAGuK | 66 | Q13368 | 6 |
| supervillin | 201 | O60611 | 9 (3, 1)b |
aThe number of peptides matched following trypsin digestion.
bNumbers in parentheses indicate endo-Arg-C and endo-Asp-N digestions.
We next used immunoblotting to provide direct biochemical evidence for physical association of these proteins within the P2X7 receptor complex. We pulled down the receptor complex from HEK293 cells stably expressing the P2X7 receptor using ecto-P2X7 Ab or from HEK293 cells transiently transfected with a C-terminal epitope-tagged P2X7 receptor cDNA (P2X7-EE) using anti-EE Ab. We then blotted antibodies to the 11 identified proteins (Figure 1B–D). All eleven proteins indi vidually co-immunoprecipitated with the P2X7 receptor (Figure 1B–D; Table I).
Tyrosine phosphorylation of the P2X7 receptor
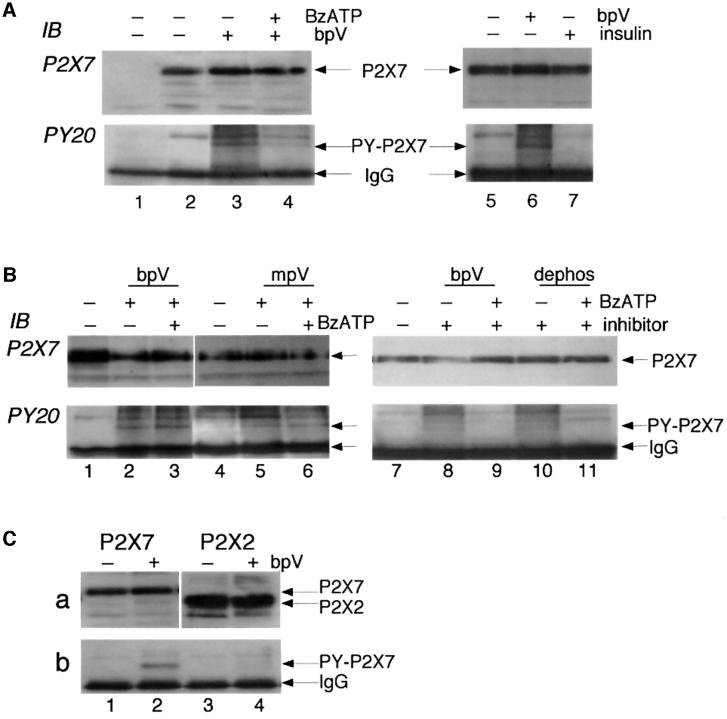
The identification of RPTPβ as an associated protein in the P2X7 receptor complex allowed the possibility that the receptor itself could be phosphorylated on tyrosine(s). Therefore, we incubated P2X7-expressing HEK cells for 5–30 min in the presence or absence of the pervanadate analogue bisperoxo-(1,10-phenanthrolone)-oxovanadate (bpV), a cell-permeable protein tyrosine phosphatase inhibitor (Posner et al., 1994). We immunoprecipitated the receptor complex using the ecto-P2X7 Ab for stable cell lines or the anti-EE Ab for transiently transfected cells (mouse IgG was the negative control). The immunoprecipitated proteins were separated by SDS– PAGE, transferred to PVDF membranes and detected using the anti-phosphotyrosine antibody PY20. A phosphotyrosine protein running at the expected size of the P2X7 receptor subunit was detected; this was directly confirmed as the P2X7 receptor by stripping the membrane and re-probing with a C-terminal anti-P2X7 receptor Ab (Figure 2A). Although equal amounts of P2X7 receptor subunit protein were immunoprecipitated from untreated and bpV-treated cells, only the P2X7 receptor from bpV-treated cells showed detectable levels of tyrosine phosphorylation (Figure 2A, lanes 3 and 6). We found that 5 min incubation with bpV (100 µM) was sufficient to induce tyrosine phosphorylation of the receptor (data not shown; n = 3). We also examined the concentration dependence of this tyrosine phosphatase inhibitor. Incubation of cells for 10 min with 10, 100 or 500 µM bpV (but not 1 or 3 µM) resulted in detectable tyrosine phosphorylation of the P2X7 receptor (n = 3). When P2X7 receptors were activated by exposing cells to the ATP analogue 2′,3′-O-(benzoyl-4-benzoyl)ATP (BzATP, 100 µM) for 10 min prior to and during the 10 min incubation with bpV, significantly lower levels of tyrosine phosphorylation of the receptor were detected (Figure 2A, lane 4). However, no decrease in levels of tyrosine phosphorylation of the P2X7 receptor occurred if cells were first exposed to bpV for 10 min after which BzATP (in the continued presence of bpV) was added for 10 min (Figure 2B, lanes 2 and 3). ATP (1 mM) produced similar results to those obtained with BzATP (n = 3). These results suggest a tonic activity of a tyrosine phosphatase at the P2X7 receptor, which is significantly increased upon activation of the receptor by agonists.
Fig. 2. Tyrosine phosphorylation of P2X7 receptor. (A) Membrane extracts from untransfected (lane 1, negative control) or P2X7 receptor-expressing HEK293 cells were immunoprecipitated with ecto-P2X7 Ab, and phosphotyrosine incorporation into the P2X7 protein was detected by probing with anti-phosphotyrosine Ab PY20. The blot was then stripped and re-probed with C-terminal anti-P2X7 Ab to confirm that the phosphotyrosine bands (lanes 3, 4 and 6) were the P2X7 receptor. No tyrosine phosphorylation was detected in control P2X7-expressing cells (lanes 2 and 5) but after 10 min incubation with the tyrosine phosphatase inhibitor bpV (100 µM) phosphotyrosine was clearly detected (lanes 3 and 6). The level was much reduced if the P2X7 receptor was activated for 10 min with BzATP (100 µM) prior to incubation with bpV (lane 4). (B) Similar experiments using phosphatase inhibitors mpV (100 µM, lanes 5 and 6) or 3,4-dephostatin (100 µM, lanes 10 and 11) also show tyrosine phosphorylation of P2X7 subunit in the presence of the phosphatase inhibitors (lanes 5 and 10), which is reduced when BzATP is added prior to application of phosphatase inhibitor (lane 11), but not when BzATP is added after the phosphatase inhibitor (lanes 3 and 6). (C) Tyrosine phosphorylation occurs on P2X7 receptor but not P2X2 receptor. Similar experiment to others performed on HEK cells transiently transfected with P2X7-EE (lanes 1 and 2) or P2X2-EE (lanes 3 and 4) receptors. Immunoprecipitation was with anti-EE Ab in the absence or presence of bpV as indicated. Anti-PY20 blotting detected phosphotyrosine-P2X7 after bpV treatment (b, lane 2) but no tyrosine phosphorylation of P2X2 receptor (b, lanes 3 and 4). Stripping and re-probing with C-terminal anti-P2X7 (a, lanes 1 and 2) or anti-P2X2 (a, lanes 3 and 4) confirms tyrosine phosphorylation of P2X7 but not P2X2 receptor even though there is greater expression of P2X2 protein.
We obtained similar results with other protein tyrosine phosphatase inhibitors; these were the pervanadate analogue monoperoxo-(picolinato)-oxovanadate (mpV), and the structurally unrelated inhibitor 3,4-dephostatin (Figure 2B). With these phosphatase inhibitors we observed that activation of the P2X7 receptor prior to but not during or after phosphatase inhibition also resulted in decreased levels of tyrosine phosphorylation of the receptor (Figure 2B, lanes 5 and 6 for BzATP simultaneous with mpV, lanes 10 and 11 for BzATP prior to 3,4-dephostatin). Because bpV can also activate insulin receptor kinase (Posner et al., 1994) and because some HEK293 cells have been reported to express insulin receptors (Kellerer et al., 1997), we asked whether exposure to insulin (100 nM for 30 min) might lead to P2X7 receptor tyrosine phosphorylation. It did not (Figure 2A, lane 7).
Tyrosine phosphorylation was not generalized to other members of the P2X receptor family. We transfected maximal concentrations of cDNA encoding either P2X7-EE or P2X2-EE receptors into HEK293 cells, immmunoprecipitated with anti-EE Ab, and separated the proteins on SDS–PAGE. The amount of P2X2 receptor protein was always 3- to 5-fold greater than that of P2X7 receptor protein (Figure 2C; n = 4). However, no tyrosine phosphorylation of the P2X2 receptor could be detected in the control or bpV-treated cells (Figure 2C).
Functional modulation of P2X7 receptor by tyrosine phosphorylation
To test for a possible functional consequence of P2X7 receptor phoshorylation, we recorded whole-cell currents from HEK293 cells expressing P2X7 receptors during repeated applications of BzATP or ATP. Brief (4 s duration, 2 min intervals) applications of agonist at high concentrations (100–300 µM BzATP; 1–3 mM ATP) evoked inward currents that showed a marked run-down in amplitude; during the run-down the current became distinctly biphasic, consisting of an initial transient and subsequent smaller sustained current (Figure 3A). The run-down depended on the total duration of agonist application and not on duration of whole-cell recording (i.e. dialysis) or intracellular pipette solution. That is, the peak current declined approximately exponentially (time constant 4.6 ± 0.2 min, n = 9), and at steady-state it had run-down to 21 ± 2% of initial peak current (Figure 3A and C). When BzATP was initially applied for 30 s, the peak amplitude of the current evoked by the test (4 s duration) application 2 min later was 18 ± 4% (n = 4). Moreover, no obvious differences in the run-down were apparent when the patch pipette contained NaCl, KCl, Na-aspartate, K-aspartate, CsCl, or when intracellular calcium was strongly or weakly buffered using 4 mM BAPTA/10 mM EGTA or 0.1 mM EGTA/0.5 mM CaCl2, respectively. There were no significant differences in the time course of development or steady-state level of run-down from cells, in which the agonist was first applied 0.5 or 12 min after establishing whole-cell conditions (n = 4), nor were there significant differences if an ATP-regenerating cocktail (Rosenmund and Westbrook, 1993) was included in the patch pipette (n = 8). Finally, the run-down of the currents did not depend on the direction of current flow; the kinetics of the responses and the time-course of the run-down were the same at –60 mV (inward current) and 30 mV (outward current, n = 4). When using sub-maximal concentrations (i.e. 30 µM BzATP or 500 µM ATP), inward currents increased in amplitude and deactivated more slowly with repeated applications (‘run-up’ data not shown). This current run-up has been described in detail in previous studies (Surprenant et al., 1996; Hibell et al., 2000) and has not been further examined in the present study.
Fig. 3. Functional evidence for use-dependent tyrosine dephosphorylation of P2X7 receptor. (A) Currents in response to successive applications of 100 µM BzATP (4 s duration, indicated by bars) at 2 min intervals; note the marked change in kinetics of the current. (B) Currents recorded from another cell in response to successive applications of 100 µM BzATP in the presence of the tyrosine phosphatase inhibitor bpV (100 µM). (C) Summary of results from all experiments as illustrated in (A) and (B) (n = 43 for each point in control and 7–13 for each point in any of the phosphatase inhibitors). (D) Similar experiments performed on P2X2-expressing HEK cells. Minimal run-down occurs with successive stimuli and is unaltered by bpV (100 µM; n = 6–12 for each point). All results were obtained from HEK cells stably expressing P2X7 (A–C) or P2X2 (D) receptors.
The run-down was not observed when any one of the three phosphatase inhibitors was present in the superfusion solution (Figure 3B and C). Repeated applications of agonist elicited currents with similar kinetics, and their peak amplitude showed a small (5–20%) increase with successive applications (Figure 3B and C). Additionally, the current elicited by the first application of BzATP (100 µM) was up to 3-fold greater in cells treated with phosphatase inhibitors than in control cells (peak amplitudes were: control 1720 ± 145 pA, bpV-treated 3000 ± 239 pA, n = 47 for each; Figure 3B). The agonist half-maximal concentrations (EC50 values) were not significantly different (n = 4) in the presence of tyrosine phosphatase inhibitors. The effect of the phosphatase inhibitors was fully reversible; within 6–20 min of washout the current evoked by BzATP (100 µM) had run-down by approximately the same amount (32 ± 12% of initial current amplitude, n = 9). The EC50 for bpV was 5 ± 0.4 µM with a Hill slope of 3.3 ± 0.7 (n = 6; see Materials and methods).
When P2X2 receptors are expressed in HEK293 cells, repeated applications of high agonist concentrations also produce a run-down of the evoked current, although this is of much lower magnitude than at the P2X7 receptor and occurs without any change in the kinetics of the response. Application of bpV (100 µM; up to 16 min) did not alter the amplitude or kinetics of run-down of the P2X2 currents (Figure 3D; n = 6). The lack of effect of phosphatase inhibitors on ATP-evoked currents at P2X2 receptors is in keeping with the lack of tyrosine phosphorylation of this protein observed with immunoblots and suggests that this modulation is likely to be P2X7 receptor specific.
Our experiments indicate that the resting P2X7 receptor is phosphorylated on tyrosine but we did not identify any associated tyrosine kinase by affinity purification. Several other channels can be tyrosine phosphorylated by Src family kinases [e.g. Kv1.3 potassium channels (Holmes et al., 1996), maxi-K channels (Ling et al., 2000), γ-aminobutyric acid receptors (Moss et al., 1995), NMDA receptors (Yu et al., 1997; Zheng et al., 1998; Vissel et al., 2001; Yang and Leonard, 2001)]. However, inclusion of c-Src in the patch pipette (n = 7) or co-expression of v-Src and P2X7 receptor cDNAs (n = 7) did not significantly alter the time course, kinetics or extent of current run-down produced by 100 µM BzATP. The peak amplitude of the initial responses was also not significantly different from control responses. Moreover, c-Src did not co-immunoprecipitate with P2X7 receptors in immunoblot experiments (data not shown).
Identification of Tyr343 as a substrate for tyrosine phosphorylation
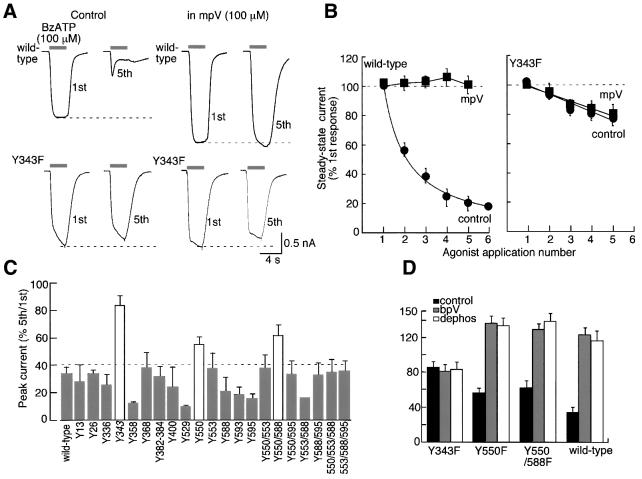
There are two tyrosines in the short intracellular N-terminus of the rat P2X7 receptors and 12 in the longer intracellular C-termini of the protein; only one of these (Tyr13) is conserved among all P2X receptors. We first mutated each of these 14 tyrosines to phenylalanine and compared the amplitudes, kinetics and run-down of the currents, as well as the effects of bpV (100 µM), using our standard protocol (4 s duration of 100 µM BzATP at 2 min intervals). All mutants were approximately equally expressed in the plasma membrane, as assessed by immunohistochemistry using ecto-P2X7 Ab and confocal microscopy (Kim et al., 2001). Only Y550F differed significantly from the wild type in showing minimal change in current kinetics with repeated stimuli and less run-down (Figure 4C). However, the actions of bpV and 3,4-dephostatin were the same as for the wild-type receptor (Figure 4D), and the P2X7[Y550F] receptor protein was equally tyrosine phosphorylated in the presence of bpV (Figure 5B). Several of these tyrosines were also mutated in pairs and triplets but no significant differences from the wild type were observed of those that expressed well (Figure 4C).
Fig. 4. Mutation of Tyr343 prevents run-down of current and effect of phosphatase inhibitors. (A) Currents recorded from wild type (upper traces) and Y343F (lower traces) in response to BzATP (100 µM). Left traces are from cells in normal solution and right traces are cells in mpV (100 µM). (B) Current amplitude at end of 4 s duration application of BzATP as a function of agonist application number for all experiments with HEK cells transiently transfected with wild type (left; n = 12–24) and Y343F (right; n = 6 each) P2X7 receptor. (C) Summary of tyrosine mutagenesis of P2X7 receptor. Histogram shows BzATP-evoked current as a percentage of amplitude of 5th/1st application; only Y343F and Y550F or Y550/588F (white bars) show significantly less run-down than wild type. (D) Phosphatase inhibitors (bpV and dephostatin) have no effect on Y343F but affect Y550F and Y550/588F to the same extent as at the wild-type receptor; data are plotted as in (C); n = 3 for each mutant, 13 for wild type.
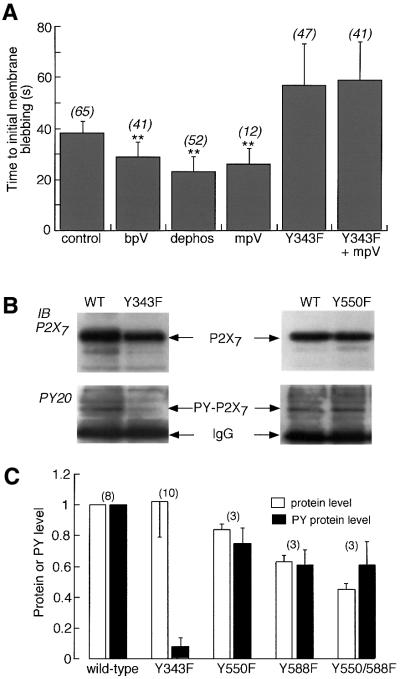
Fig. 5. P2X7 receptor signalling to the cytoskeleton is modulated by phosphorylation of Tyr343. (A) Time to initial membrane blebbing in response to application of 100 µM BzATP. Phosphatase inhibitors (100 µM) significantly decreased time to initial blebbing (p < 0.0001) in wild type but not in Y343F mutant. Numbers in parentheses are number of cells. (B) Tyrosine phosphorylation of P2X7 receptor is markedly reduced in Y343F mutant. Immunoblots for wild type and Y550F or wild type and Y343F are shown. In each case anti-EE Ab was used for immunoprecipitation from cells transiently transfected with P2X7-EE receptor. Tyrosine phosphorylation was then detected with anti-PY-20 Ab, after which the membrane was stripped and re-probed with anti-P2X7 Ab. In all cases bpV (100 µM) was present for 10 min prior to membrane extraction. Tyrosine phosphorylation of wild type and Y550F was observed, but not Y343F. (C) Densitometric analysis of wild-type and mutant P2X7 receptors. Each protein band (P2X7, open bars; tyrosine phosphorylated P2X7, closed bars) was scanned using GeneGenius and raw data were normalized to that of wild type.
There are also two tyrosines in the second hydrophobic domain, which is presumed to be membrane spanning (Figure 6). The Y336F (and Y336C) mutants were similar in their properties to wild-type receptors. However, P2X7 receptors with point mutations Y343F or Y343C showed no significant run-down of the current with repeated agonist applications, showed no change in the kinetics of the current and showed no effect of bpV, mpV or 3,4-dephostatin (Figure 4). In these mutated receptors, the initial response to BzATP was approximately the same as the wild-type receptor. Immunoblotting detected little or no tyrosine phosphorylation of P2X7[Y343F] receptor (Figure 5B and C).
Fig. 6. Schematic summary of P2X7 receptor signalling complex. (A) Depiction of P2X7 receptor and interacting proteins identified. Laminin is a heterotrimeric extracellular matrix molecule; the α3 subunit is found in laminin-5, laminin-6 and laminin-7 (Colognato and Yurchenko, 2000). RPTPβ indicates the 230 kDa form of RPTPβ; the extracellular part of the molecule has an N-terminal carbonic anhydrase domain and 16 fibronectin type 3 domains, and the intracellular part has one or two tyrosine phosphatase catalytic domains (Fischer et al., 1991). Integrin β2 is a subunit of hetero dimeric integrin receptors Mac1 (cd11b, αMβ2) and LFA (cd11a, αLβ2); its cytoplasmic C-terminus can bind to α-actinin (Sampath et al., 1998). α-actinin indicates α-actinin 4, an actin-bundling protein. This forms a reciprocal homodimer, with the two N-terminal actin binding domains at either end. It also has two calponin homology domains, four spectrin repeats and two EF hands. Supervillin is also an F-actin bundling protein that associates with the plasma membrane; it has multiple actin-binding domains and a villin/gelsolin homology domain (Wulfkuhle et al., 1999). PI4K is phosphatidylinositol 4-kinase 230; its domains include an N-terminus SH3 domain, two proline-rich regions, two leucine-zippers, a pleckstrin homology domain and the C-terminal catalytic domain (Gehrmann and Heilmeyer, 1998). MAGuK is membrane-associated guanylate kinase P55 subfamily 3; it contains PDZ, SH3 and GUK domains (Dimitratos et al., 1999). Hsp90 is heat shock protein 90-β. Hsp70 is heat shock protein 70 kDa 1. Hsc71 is heat shock cognate 71 kDa protein. See Table I and text for further information. (B) Depiction of the second hydrophobic domain of the P2X7 receptor (Thr325–Asn356) as a membrane-spanning α-helix. Residues are coloured as follows: blue, hydrophobicity index >2.5 (probably lipid facing); red, polar or charged (protein- or pore-facing); black, non-polar. Some residues are partially hidden; the complete sequence is TGGKFDIIQLVVYIGSTLSYFGLATVCIDLIIN. The first two-thirds of the helix might span the membrane and expose Tyr343 (arrow) at the inner juxtamembrane region. Residues conserved among all P2X subunits are shown in bold (Gly328, G329, Lys330, Phe331, Gly345 and Asp353).
We asked whether phosphorylation at Tyr343 might also be involved in the other signals from the P2X7 receptor, independent of the flow of ionic current. We measured the time to appearance of the first membrane bleb in response to sustained application of 100 µM BzATP (Virginio et al., 1999) in the wild type and in all the tyrosine mutated receptors; only Y550F and Y343F showed altered kinetics of initial blebbing (Figure 5A). For the wild-type receptor, tyrosine phosphatase inhibitors all reduced the time to the appearance of membrane blebbing. In the Y343F receptor, this time was increased and it was not affected by mpV (Figure 5A).
Discussion
P2X7 receptor signalling complex
Activation of the P2X7 receptor not only opens a cation-permeable ion channel, it also results in the activation of several disparate downstream signalling pathways and re-arrangement of the cytoskeleton (see Introduction). We have now identified 11 proteins that form part of a P2X7 receptor signalling complex when it is expressed in HEK293 cells. In broad terms these comprise structural proteins (extracellular, laminin α3; transmembrane, integrin β2 subunit; cytoskeletal, β-actin, α-actinin 4, supervillin, Hsp90, Hsp70, Hsc71, MAGuK) and signalling proteins (PI4K and RPTPβ). These are listed in Table I and summarized schematically in Figure 6A. Whereas some of these proteins have also been found in other ion channel complexes (e.g. the NMDA receptor complex; Husi et al., 2000; Sheng and Pak, 2000), several have not previously been described to associate with membrane ion channels (e.g. PI4K, supervillin, integrin β2).
Laminin α3 may associate directly with the ectodomain of the P2X7 receptor but it is also possible that it is found in the complex because of its binding to integrin β2. Laminins containing the α3 subunit are known to bind to integrins α3β1, α6β4 and α6β1 (Colognato and Yurchenco, 2000), although there is only limited evidence for the involvement of β2 integrins (Bohnsack et al., 1990). Integrin β2 is predominantly expressed by leukocytes, and an 11-amino acid sequence in the C-terminus interacts directly with α-actinin (Sampath et al., 1998). α-actinin and supervillin both interact directly with β-actin. α-actinin head-to-tail dimers cross-link the fibres of β-actin into a regular array and are also involved in anchoring membrane proteins to the cytoskeleton (see below). Supervillin is also an F-actin binding protein in the villin/gelsolin family (Pestonjamasp et al., 1997).
MAGuK is encoded by the gene discs large homolog 3 (dlg3); it is a scaffolding protein containing several binding domains (PDZ, SH3, GK; Dimitratos et al., 1999). Some MAGuK family members have been implicated in the anchoring of other ion channels to the cytoskeleton. Thus, the C-terminal sequences of several potassium channels and ligand-gated channels bind directly to PDZ and SH3 domains of MAGuK (Sheng and Pak, 2000). The P2X7 receptor does not have such a C-terminal sequence but it does have proline-rich regions that might engage the SH3 domain of MAGuK; one of these (Pro450-Pro-Ile-Pro) forms a canonical SH3-binding sequence. A further possibility is that α-actinin 4 directly anchors the P2X7 receptor to β-actin. The glutamate receptor NR1 subunit has been shown to bind to α-actinin 2 by a region 860–866 (KNLQDRK) in its C-terminal domain (Krupp et al., 1999); a homologous sequence appears in the P2X7 receptor at residues 419–425 (KSLQDVK). The heat shock proteins Hsp90 and Hsp70 are well known to associate with each other, although this requires further proteins including Hop (Caplan, 1999). They could be involved in the P2X7 receptor complex through their association with actin (Koyasu et al., 1986).
PI4K catalyses the addition of phosphate to the 4-position of phosphatidylinositol (PI), which is the first committed step in the formation of further bis-, tris- and tetra-phosphorylated forms. Clearly, the formation of PI 4,5-bisphosphate (PIP2) could provide the P2X7 receptor with the ability to signal downstream through inositol trisphosphate and diacylglycerol. PIP2 generation would also provide a mechanism for the activation of phospholipase D resulting from P2X7 receptor activation (Humphreys and Dubyak, 1996). PI4Ks have a well conserved domain structure (Gehrmann and Heilmeyer, 1998) and this suggests at least two ways in which PI4K could associate directly with the P2X7 receptor. The proline-rich region of the P2X7 receptor could bind to the N-terminal SH3 domain of PI4K, or it could interact through leucine-zippers. PI4K has two leucine-zipper domains, with five and four leucines. We note that the C-terminus of the P2X7 receptor has a region that is predicted to be α-helical (beginning at Leu512) and that might provide one partner to a leucine-zipper interaction.
The last of the 11 proteins identified in the present work was RPTPβ. This prompted us to examine the possibility that the P2X7 receptor itself might be a substrate for tyrosine phosphorylation and dephosphorylation and that this would be reflected in altered channel function.
Receptor dephosphorylation and regulation of channel function
Our biochemical studies show that tyrosine phosphorylation of the P2X7 receptor protein itself can be observed in cells exposed to tyrosine phosphatase inhibitors for as little as 5–10 min, but that activation of P2X7 receptors prior to addition of phosphatase inhibitors yielded a much reduced level of tyrosine phosphorylation of the receptor. This implies that the liganded receptors cannot be readily phosphorylated. Extracellular ATP has previously been shown to dephosphorylate proteins on tyrosine in the mouse leukaemia cell line L1210, and vanadate prevented downstream signalling to apoptosis (Bronte et al., 1996). One of the proteins dephosphorylated in these experiments was a 76 kDa protein perhaps corresponding to a P2X7 receptor subunit.
Our functional studies indicate that under the present experimental conditions a phosphatase, perhaps RPTPβ, is tonically active at low levels to dephosphorylate the unliganded P2X7 receptor and that this activity is much increased upon receptor activation. This dephosphorylation results in the strong run-down of the current and a reduced ability to couple to cytoskeletal changes (e.g. membrane blebbing). Essentially similar, if less marked, effects on membrane currents of tyrosine phosphatase inhibitors have recently been reported for the NMDA receptor heterologously expressed in HEK cells; run-down of currents at high glutamate concentrations was also found to be due to a use-dependent tyrosine dephosphorylation of the NMDA receptor, which was independent of ion flux (Vissel et al., 2001). The voltage-gated sodium channel has been shown recently to associate with RPTPβ; inhibition of the enzyme causes a 6 mV hyperpolarizing shift in the voltage dependence of inactivation and thus inhibits the peak current (Ratcliffe et al., 2000). In that study, RPTPβ was found to interact with both extracellular and intracellular regions of the sodium channel but its target tyrosine was not identified.
We made amino acid substitutions of phenylalanine for tyrosine at each of sixteen positions (Figure 4). In only two cases did we observe an alteration in the phenotype based on agonist-evoked currents and time to membrane blebbing; these were Y550F and Y343F. Tyr550 probably does not play a significant role in tyrosine phosphorylation of the receptor because this protein was still tyrosine phosphorylated on immunoblots, and agonist-evoked current run-down, which could be prevented by tyrosine phosphatase inhibitors, was still observed. In contrast, little or no tyrosine phosphorylation of P2X7[Y343F] protein was detected; in this mutant, currents showed only P2X2 receptor-like run-down and this was not affected by tyrosine phosphatase inhibitors. The phosphatase inhibitors also did not alter the time to initial membrane blebbing in the P2X7[Y343F] receptor. Such results provide fairly conclusive evidence that Tyr343 is the major residue that is dephosphorylated by receptor activation.
The involvement of Tyr343 in the action of the phosphatase inhibitors was at first surprising because it had previously been thought to be situated well within the second transmembrane domain of the receptor. Tyrosine at this position is found only in the P2X7 receptor; the other subunits have isoleucine, leucine, serine or tryptophan. One must consider the possibility that Tyr343 is not directly phosphorylated and that the mutation Y343F (and Y343C) results in a conformational change of the protein such that the run-down of the current is prevented. This seems very unlikely because mutations of no other tyrosine prevented the action of the phosphatase inhibitors. Therefore, Tyr343 should itself be accessible to kinase and phosphatase. Hydrophobicity plots indicate that a segment of ∼26 amino acids (Phe328–Ile355) is likely to contribute to the second transmembrane of the P2X7 receptor. Secondary structure prediction algorithms indicate that this is likely to be helical and that the first two-thirds of it would be strongly amphipathic. One face would be exposed to lipid and one side, containing Tyr343, exposed to protein or water. Figure 6B depicts schematically how this might result in accessibility of Tyr343 to phosphorylation.
In the case of the NMDA receptor subunits NR1 and NR2, tyrosine residues were identified as playing a role in run-down of the membrane current with repeated applications (Vissel et al., 2001). In that case also, the run-down was not observed with NMDA subunits in which critical tyrosine residues had been substituted by phenylalanine residues. The tyrosines were also located at the interface between the final transmembrane domain and the beginning of the C-terminal tail, and the run-down of the current resulted from association with an adaptor protein (Vissel et al., 2001). It seems possible that a similar interaction between this part of the P2X7 receptor and an associated protein could underlie the similar functional picture. Phosphorylation at Tyr343 might be necessary for the interaction of another protein with the P2X7 receptor because Y343F mutation also showed an impaired ability to couple to cytoskeletal elements and bring about blebbing formation.
Significance of P2X7 receptor signalling complex
There are now several cases known of interactions between membrane ion channels and specific cytoskeletal elements (Sheng and Pak, 2000). In those cases, the emphasis has been largely on ‘inside-out’ signalling. That is to say, the results have been interpreted in terms of the associated proteins and the cytoskeleton altering the distribution and function of the channels. The P2X7 receptor is unique because it has been well characterized as initiating ‘outside-in’ signalling (see Introduction). Activation of the P2X7 receptor itself rapidly engages the cytoskeleton and brings about changes in cell shape and form. The present experiments have identified some of the initial molecular steps in this process and they have also shown how it might be controlled through feedback alterations in receptor phosphorylation of an identified tyrosine located at the inner membrane interface. They provide a framework for the further analysis of the increasing distinction between the flow of ionic current and direct protein–protein interactions in the cell physiology of P2X7 receptors.
Materials and methods
Purification of P2X7 receptor complex by immunoprecipitation
The receptor complex was isolated from HEK293 cells stably expressing rat P2X7 receptor using immunoprecipitation with ecto-P2X7 Ab (Kim et al., 2001). In brief, the receptor complex was incubated with solubilization buffer [20 mM dodecylmaltoside (DM) in phosphate-buffered saline (PBS) containing protease inhibitors] from purified membrane fractions, followed by pre-absorption of background proteins with γ-bind G-Sepharose, incubation with ecto-P2X7 Ab and subsequent γ-bind G-Sepharose precipitation. After overnight incubation at 4°C, the resins were washed with 10 vols of solubilization buffer and proteins were separated from the resin by boiling for 5 min in SDS sample buffer.
In-gel digestion and MALDI-TOF MS
The in-gel digestion of the proteins was performed with some modifications according to Shevchenko et al. (1996). Receptor complex samples were separated by SDS–PAGE. The Coomassie-stained gel pieces were decoloured by repeated cycles of dehydration with 50% acetonitrile (ACN) in 50 mM NH4HCO3 pH 8.4 and hydration with 50 mM NH4HCO3. The destained protein bands were shrunk by dehydration in ACN and dried in a vacuum centrifuge. The protein bands were reduced with 10 mM dithiothreitol (DTT) in 100 mM NH4HCO3 for 1 h at 56°C. For alkylation of proteins, the DTT solution was replaced by 55 mM iodoacetic acid in 100 mM NH4HCO3 and incubated for 1 h in the dark. The reduced, alkylated gel pieces were washed with 100 mM NH4HCO3, dehydrated with ACN, swollen in 100 mM NH4HCO3, and shrunk again by ACN. The liquid phase was removed, and the gel pieces were completely dried in a vacuum centrifuge. The gel pieces were incubated overnight at 37°C with endoprotease Asp-N (50 mM sodium phosphate buffer pH 8.0), endoprotease Arg-C (100 mM Tris–HCl pH 7.6, 10 mM CaCl2, 5 mM DTT, 0.5 mM EDTA) or trypsin (50 mM Tris–HCl pH 7.6, 1 mM CaCl2). Protease-digested supernatants were adjusted to 0.1% trifluoroacetic acid (TFA) by addition of 10% TFA. The peptide fragments were desalted, concentrated with ZipTipC18 and finally eluted with matrix solution (α-cyano-cinnamic acid in 50% ACN/0.1% TFA). Samples were prepared using dried-drop method, by dropping sample-matrix solution directly onto target plate. Mass spectra were obtained using a TofSpecE MALDI-TOF mass spectrometer (Micromass, Manchester, UK) equipped with a reflectron mode for increased mass accuracy in positive ion. Mass spectra were either externally calibrated or mass converted using internal calibrants with MassLynx program. Proteins were identified based on matching the MS data with mass values of calculated peptide fragments using ProFound (prowl.rockefeller.edu), PeptIdent (www.expasy.ch) and Peptide Mass (www.expasy.ch).
Immunoblot analysis
Immunoprecipitated P2X7 complex proteins were separated by SDS– PAGE and western blots were performed as described previously (Kim et al., 2001). For detection of tyrosine-phosphorylated proteins, the following modifications were used. Cells were lysed immediately, solubilized with solubilization buffer containing 20 mM DM, 500 µM bpV and protease inhibitors (Complete™ EDTA-free) in PBS for 1 h at 4°C, followed by immunoprecipitation with either ecto-P2X7 Ab, anti-EE Ab or mouse IgG as control. Membranes were immunoblotted with either anti-phosphotyrosine Ab PY20 or polyclonal anti-P2X7 receptor Ab. For western blotting of anti-phosphotyrosine Ab, blocking buffer [1% bovine serum albumin (BSA), 10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20] was used. To examine protein–protein interaction between PI4K230 (HA-tagged) and P2X7 receptor, co-transfection of these cDNAs was performed, after which immunoprecipitation with anti-EE or anti-HA Ab and blot with anti-HA Ab or C-terminal polyclonal P2X7 Ab was carried out. When appropriate, the membranes were stripped with Restore™ western blot stripping buffer and reprobed with a different antibody. Protein bands on films were semi-quantitatively analysed by densitometric scanning via GeneSnap and GeneTools software (Syngene, Cambridge, UK). All experiments were repeated 3–10 times with similar results obtained throughout.
Mutagenesis and electrophysiology
Mutations were introduced into the rat P2X7 cDNA with a Glu-Glu (GLNEYMPME) tag at its C-terminus (P2X7-EE) in pcDNA3.1+, by the ‘quick change mutagenesis’ method using Pfu Turbo® polymerase (Stratagene). Sequences were verified for the entire open reading frame, by ABI Big Dye® sequencing (PE Biosystems). Standard whole-cell recordings and measurements of time to membrane blebbing were performed as described previously (Virginio et al., 1999). BzATP (100 µM) was typically applied for 4 s, at 2 min intervals. Inhibition by bpV was quantitated by measuring the amplitude of the current evoked by the sixth application of BzATP as a fraction of the amplitude of the current evoked by the first application (x); this was done in the presence of bpV (x ≈ 1) and after washout of bpV (x′ < 1). The fraction x′/x was normalized to the change in current observed with a maximal concentration of bpV (100 µM) and the results fit with a logistic function. All data are shown as mean ± SEM and tests for significance were by Mann–Whitney non-parametric analysis.
Chemicals and antibodies
DM, bpV, mpV and 3,4-dephostatin were purchased from Calbiochem (San Diego, CA), Complete™, endoprotease Asp-N, endoprotease Arg-C and trypsin (sequencing grade) were from Roche (Indianapolis, IN), ZipTipC18 from Millipore (Bedford, MA), γ-bind G-Sepharose from Amersham Pharmacia (Uppsala, Sweden), Restore™ western blot stripping buffer from Pierce (Rockford, IL). All other chemicals were from Sigma. Anti-RPTPβ, anti-Hsp90 and anti-phosphotyrosine (PY20) antibodies were from Transduction Laboratories (San Diego, CA), anti-β-actin and anti-α-actinin from Sigma, anti-Hsp70 from Calbiochem, anti-Hsc70 from Alexis Biochemicals (San Diego, CA), anti-integrin β2 from Oncogene Research Products (Cambridge, MA), anti-EE and anti-HA from BabCo (Berkeley, CA), C-terminal anti-P2X7 and C-terminal anti-P2X2 (polyclonals) from Alomone (Jerusalem, Israel), and ecto-P2X7 (monoclonal) as described previously (Kim et al., 2001). Anti-p55 MAGuK Ab (Ruff et al., 1991) was supplied by Dr A.Chishti (Tufts University), anti-laminin α3 (Hirosaki et al., 2000) by Dr K.Miyazaki (Yokohama City University), anti-supervillin (Pestonjamasp et al., 1997) by Dr E.Luna (University of Massachusetts, Worcester) and HA-tagged PI4K230 cDNA (Gehrmann et al., 1999) by Professor L.Heilmeyer (Ruhr-Universitat Bochum).
Acknowledgments
Acknowledgements
We thank M.Dickman and D.Hornby for training and help with MALDI-TOF MS. We thank D.Estoppey, G.Evans and J.Bailey for transfections, cell culture and molecular biology assistance. We are especially grateful to E.Luna, A.Chishti, K.Miyazaki and L.Heilmeyer for providing us with antibodies and cDNAs. This work was supported by grants from the Wellcome Trust.
References
- Bohnsack J.F., Akiyama,S.K., Damsky,C.H., Knape,W.A. and Zimmerman,G.A. (1990) Human neutrophil adherence to laminin in vitro. Evidence for a distinct neutrophil integrin receptor for laminin. J. Exp. Med., 171, 1221–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V., Macino,B., Zambon,A., Rosato,A., Mandruzzato,S., Zanovello,P. and Collavo,D. (1996) Protein tyrosine kinase inhibitors and phosphatases control apoptosis induced by extracellular adenosine 5′-triphosphate. Biochem. Biophys. Res. Commun., 218, 344–351. [DOI] [PubMed] [Google Scholar]
- Caplan A.J. (1999) Hsp90’s secrets unfold: new insights from structural and functional studies. Trends Cell Biol., 9, 262–268. [DOI] [PubMed] [Google Scholar]
- Cohn Z.A. and Parks,E. (1967) The regulation of pinocytosis in mouse macrophages. III. The induction of vesicle formation by nucleosides and nucleotides. J. Exp. Med., 125, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G., Neidhart,S., Kawashima,E., Kosco-Vilbois,M., North,R.A. and Buell,G. (1997) Tissue distribution of the P2X7 receptor. Neuropharmacology, 36, 1277–1283. [DOI] [PubMed] [Google Scholar]
- Colognato H. and Yurchenco,P.D. (2000) Form and function: the laminin family of heterotrimers. Dev. Dyn., 218, 213–234. [DOI] [PubMed] [Google Scholar]
- Dimitratos S.D., Woods,D.F., Stathakis,D.G. and Bryant,P.J. (1999) Signaling pathways are focused at specialized regions of the plasma membrane by scaffolding proteins of the MAGuK family. BioEssays, 21, 912–921. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. (2000) Dr Jekyll/Mr Hyde: the dual role of extracellular ATP. J. Auton. Nerv. Syst., 81, 59–63. [DOI] [PubMed] [Google Scholar]
- Ferrari D., Chiozzi,P., Falzoni,S., Dal Susino,M., Melchiorri,L., Baricordi,O.R. and Di Virgilio,F. (1997a) Extracellular ATP triggers IL-1β release by activating the purinergic P2Z receptor of human macrophages. J. Immunol., 159, 1451–1458. [PubMed] [Google Scholar]
- Ferrari D., Wesselborg,S., Bauer,M.K. and Schulze-Osthoff,K. (1997b) Extracellular ATP activates transcription factor NF-κB through the P2Z purinoreceptor by selectively targeting NF-κB p65. J. Cell Biol., 139, 1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E.H., Charbonneau,H. and Tonks,N. (1991) Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane proteins. Science, 253, 401–406. [DOI] [PubMed] [Google Scholar]
- Gehrmann T. and Heilmeyer,L.M.,Jr (1998) Phosphatidylinositol 4-kinases. Eur. J. Biochem., 253, 357–370. [DOI] [PubMed] [Google Scholar]
- Gehrmann T., Gulkan,H., Suer,S., Herberg,F.W., Balla,A., Vereb,G., Mayr,G.W. and Heilmeyer,L.M.,Jr (1999) Functional expression and characterization of a new human phosphatidylinositol 4-kinase PI4K230. Biochim. Biophys. Acta, 1437, 341–356. [DOI] [PubMed] [Google Scholar]
- Gudipaty L., Humphreys,B.D., Buell,G. and Dubyak,G.R. (2001) Regulation of P2X7 nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am. J. Physiol., 280, C943–C953. [DOI] [PubMed] [Google Scholar]
- Hibell A.D., Kidd,E.J., Chessell,I.P., Humphrey,P.P. and Michel,A.D. (2000) Apparent species differences in the kinetic properties of P2X7 receptors. Br. J. Pharmacol., 130, 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosaki T., Mizushima,H., Tsubota,Y., Moriyama,K. and Miyazaki,K. (2000) Structural requirement of carboxyl-terminal globular domains of laminin α3 chain for promotion of rapid cell adhesion and migration of laminin 5. J. Biol. Chem., 275, 22495–22502. [DOI] [PubMed] [Google Scholar]
- Holmes T.C., Fadool,D.A. and Levitan,I.B. (1996) Tyrosine phosphoryl ation of the Kv1.3 potassium channel. J. Neurosci., 16, 1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys B.D. and Dubyak,G.R. (1996) Induction of the P2z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-γ in the human THP-1 monocytic cell line. J. Immunol., 157, 5627–5637. [PubMed] [Google Scholar]
- Husi H., Ward,M.A., Choudhary,J.S., Blackstock,W.P. and Grant,S.G. (2000) Proteomic analysis of NMDA receptor–adhesion protein signaling complexes. Nature Neurosci., 3, 661–669. [DOI] [PubMed] [Google Scholar]
- Kellerer M., Mushack,J., Mischak,H. and Haring,H.U. (1997) Protein kinase C (PKC) ε enhances the inhibitory effect of TNFα on insulin signaling in HEK293 cells. FEBS Lett., 418, 119–122. [DOI] [PubMed] [Google Scholar]
- Kim M., Spelta,V., Sim,J., North,R.A. and Surprenant,A. (2001) Differential assembly of rat purinergic P2X7 receptor in immune cells of the brain and periphery. J. Biol. Chem., 276, 23262–23267. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Nishida,E., Kadowaki,T., Matsuzaki,F., Iida,K., Kasuga,M., Sakai,H. and Yahara,I. (1986) Two mammalian heat shock proteins, Hsp90 and Hsc100, are actin binding proteins. Proc. Natl Acad. Sci. USA, 83, 8054–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp J.J., Vissel,B., Thomas,C.G., Heinemann,S.F. and Westbrook,G.L. (1999) Interactions of calmodulin and α-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J. Neurosci., 19, 1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S., Woronuk,G., Lev,S. and Braun,A.P. (2000) Enhanced activity of a large conductance calcium-sensitive K channels in the presence of Src tyrosine kinase. J. Biol. Chem., 275, 30683–30689. [DOI] [PubMed] [Google Scholar]
- Michel A.D., Chessell,I.P. and Humphrey,P.P. (1999) Ionic effects on human recombinant P2X7 receptor function. Naunyn Schmiedebergs Arch. Pharmakol., 359, 102–109. [DOI] [PubMed] [Google Scholar]
- Moss S.J., Gorrie,G.H., Amato,A. and Smart,T.G. (1995) Modulation of GABA-A receptors by tyrosine phosphorylation. Nature, 377, 344–348. [DOI] [PubMed] [Google Scholar]
- Mutini C., Falzoni,S., Ferrari,D., Chiozzi,P., Morelli,A., Baricordi,O.R., Collo,G., Ricciardi-Castagnoli,P. and Di Virgilio,F. (1999) Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J. Immunol., 163, 1958–1965. [PubMed] [Google Scholar]
- Nicke A., Baumert,H.G., Rettinger,J., Eichele,A., Lambrecht,G., Mutschler,E. and Schmalzing,G. (1998) P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J., 17, 3016–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R.A. and Surprenant,A. (2000) Pharmacology of cloned P2X receptors. Annu. Rev. Pharmacol. Toxicol., 40, 563–580. [DOI] [PubMed] [Google Scholar]
- Pestonjamasp K.N., Pope,R.K., Wulfkuhle,J.D. and Luna,E.J. (1997) Supervillin (p205): a novel membrane-associated, F-actin-binding protein in the villin/gelsolin superfamily. J. Cell Biol., 139, 1255–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner B.I. et al. (1994) Peroxovanadium compounds. A new class of potent phosphotyrosine inhibitors which are insulin mimetics. J. Biol. Chem., 269, 4596–4604. [PubMed] [Google Scholar]
- Rassendren F., Buell,G.N., Virginio,C., Collo,G., North,R.A. and Surprenant,A. (1997) The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem., 272, 5482–5486. [DOI] [PubMed] [Google Scholar]
- Ratcliffe C.E., Qu,Y., McCormick,K.A., Tibbs,V.C., Dixon,J.E., Scheuer,T. and Catterall,W.A. (2000) A sodium channels signalling complex: modulation by associated receptor tyrosine phosphatase β. Nature Neurosci., 3, 437–444. [DOI] [PubMed] [Google Scholar]
- Rosenmund C. and Westbrook,G. (1993) Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron, 10, 805–814. [DOI] [PubMed] [Google Scholar]
- Ruff P., Speicher,D.W. and Chishti,A. (1991) Molecular identification of a major palmitoylated erythrocyte membrane protein containing the src homology 3 motif. Proc. Natl Acad. Sci. USA, 88, 6595–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath R., Gallagher,P.J. and Pavalko,F.M. (1998) Cytoskeletal interactions with the leukocyte integrin β2 cytoplasmic tail. Activation-dependent regulation of associations with talin and α-actinin. J. Biol. Chem., 273, 33588–33594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M. and Pak,D.T. (2000) Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu. Rev. Physiol., 62, 755–778. [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Wilm,M., Vorm,O. and Mann,M. (1996) Mass spectrometric sequencing of proteins from silver-stained poly-acrylamide gels. Anal. Chem., 66, 850–858. [DOI] [PubMed] [Google Scholar]
- Solle M., Labasi,J., Perregaux,D.G., Stam,E., Petrushova,N., Koller,B.H., Griffiths,R.J. and Gabel,C.A. (2001) Altered cytokine production in mice lacking P2X(7) receptors. J. Biol. Chem., 276, 125–132. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Rassendren,F., Kawashima,E., North,R.A. and Buell,G. (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science, 272, 735–738. [DOI] [PubMed] [Google Scholar]
- Torres G.E., Egan,T.M. and Voigt,M.M. (1999) Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J. Biol. Chem., 274, 6653–6659. [DOI] [PubMed] [Google Scholar]
- Virginio C., MacKenzie,A., North,R.A. and Surprenant,A. (1999) Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J. Physiol., 519, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissel B., Krupp,J.J., Heinemann,S.F. and Westbrook,G.L. (2001) A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nature Neurosci., 4, 587–596. [DOI] [PubMed] [Google Scholar]
- Wulfkuhle J.D., Donina,I.E., Stark,N.H., Pope,R.K., Pestonjamasp,K.N., Niswonger,M.L. and Luna,E.J. (1999) Domain analysis of supervillin, an F-actin bundling plasma membrane protein with functional nuclear localization signals. J. Cell Sci., 112, 2125–2136. [DOI] [PubMed] [Google Scholar]
- Yang M. and Leonard,J.P. (2001) Identification of mouse NMDA receptor subunit NR2A C-terminal tyrosine sites phosphorylated by co-expression with v-Src. J. Neurochem., 77, 580–588. [DOI] [PubMed] [Google Scholar]
- Yu X.-M., Askalan,R., Keil,G.J. and Salter,M.W. (1997) NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science, 275, 674–678. [DOI] [PubMed] [Google Scholar]
- Zheng F., Gingrich,M.B., Traynelis,S.F. and Conn,P.J. (1998) Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nature Neurosci., 1, 185–191. [DOI] [PubMed] [Google Scholar]