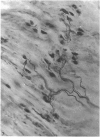
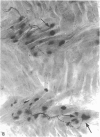
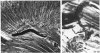
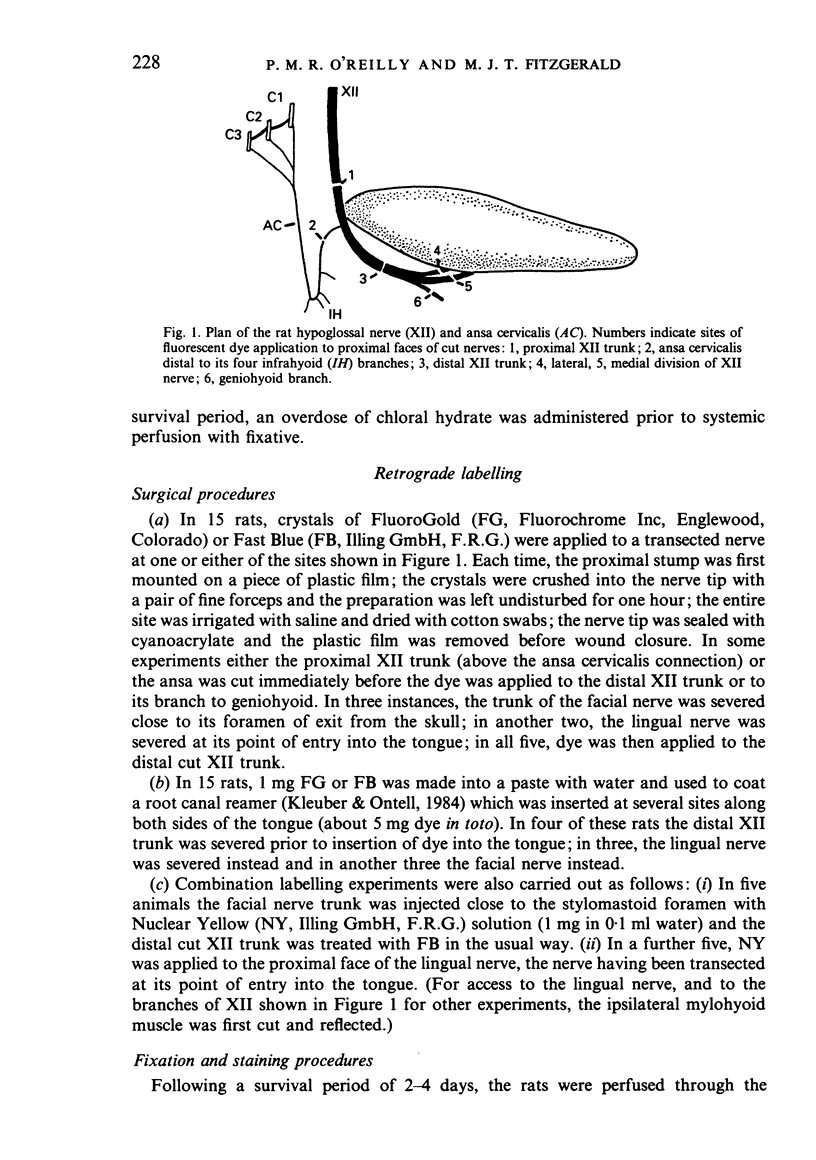
Abstract
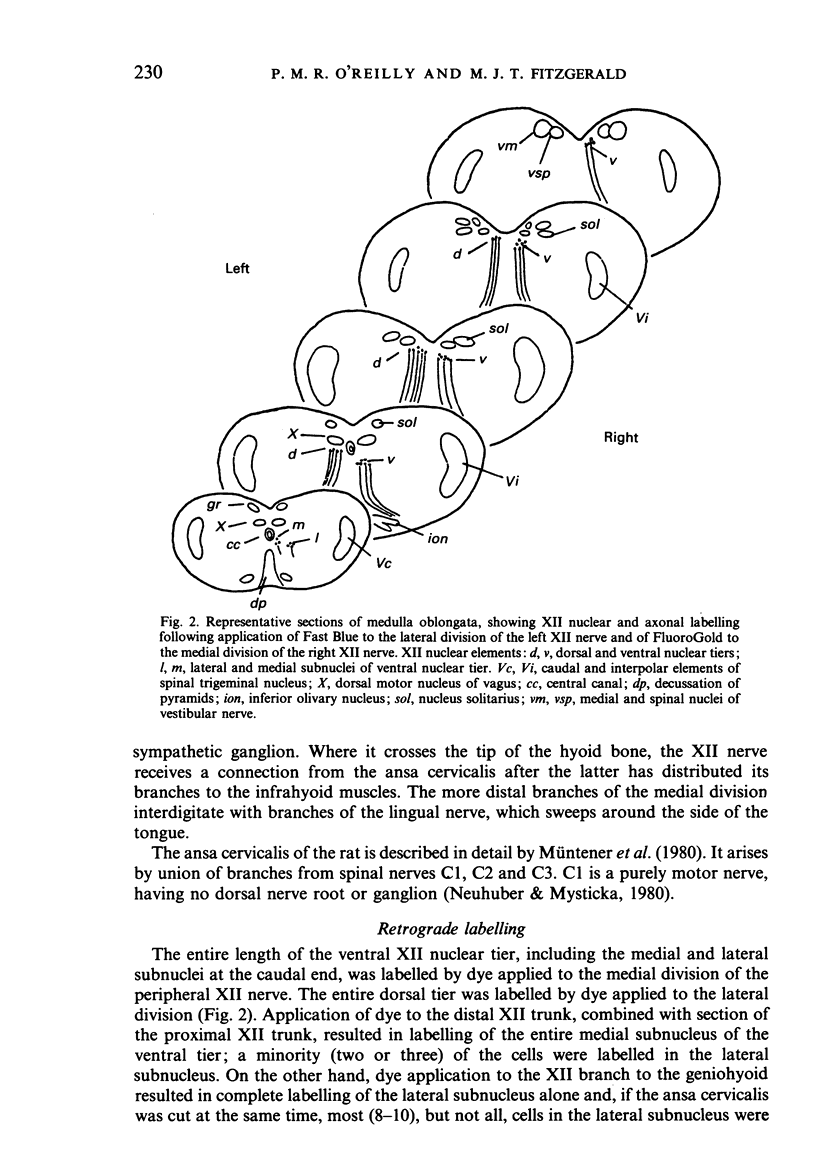
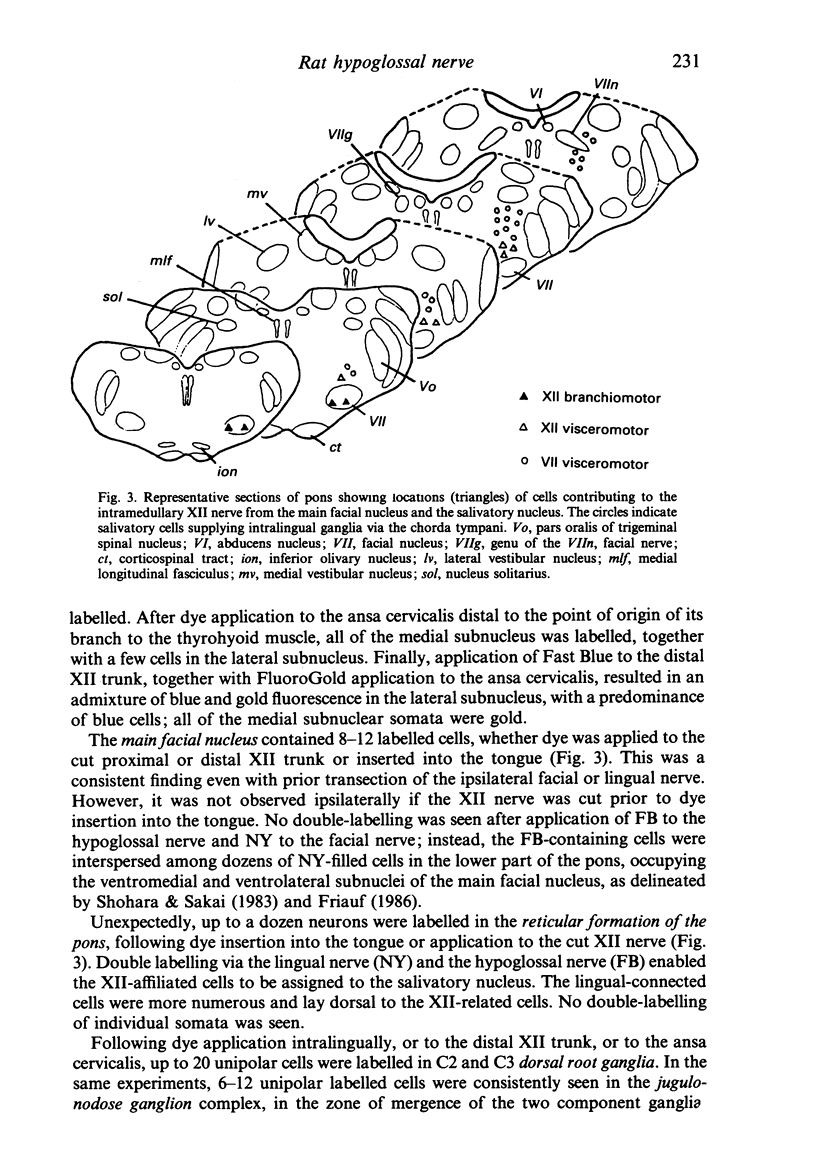
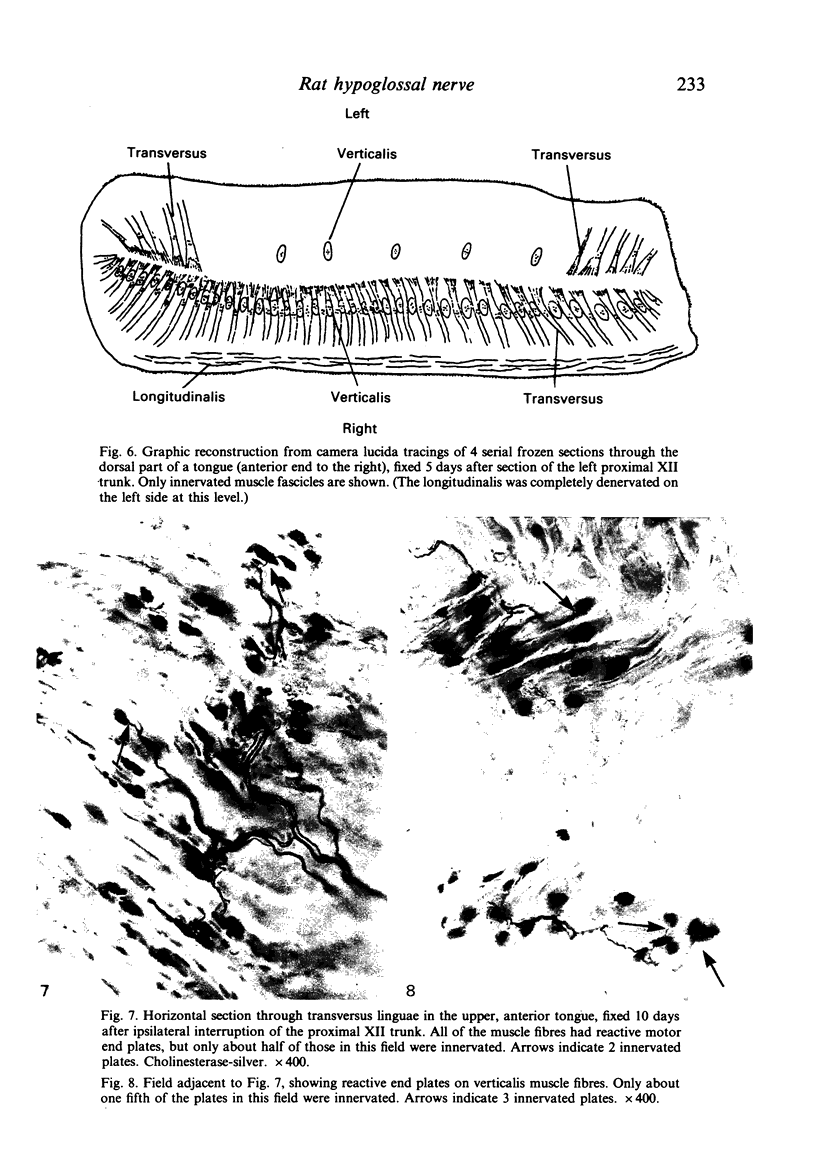
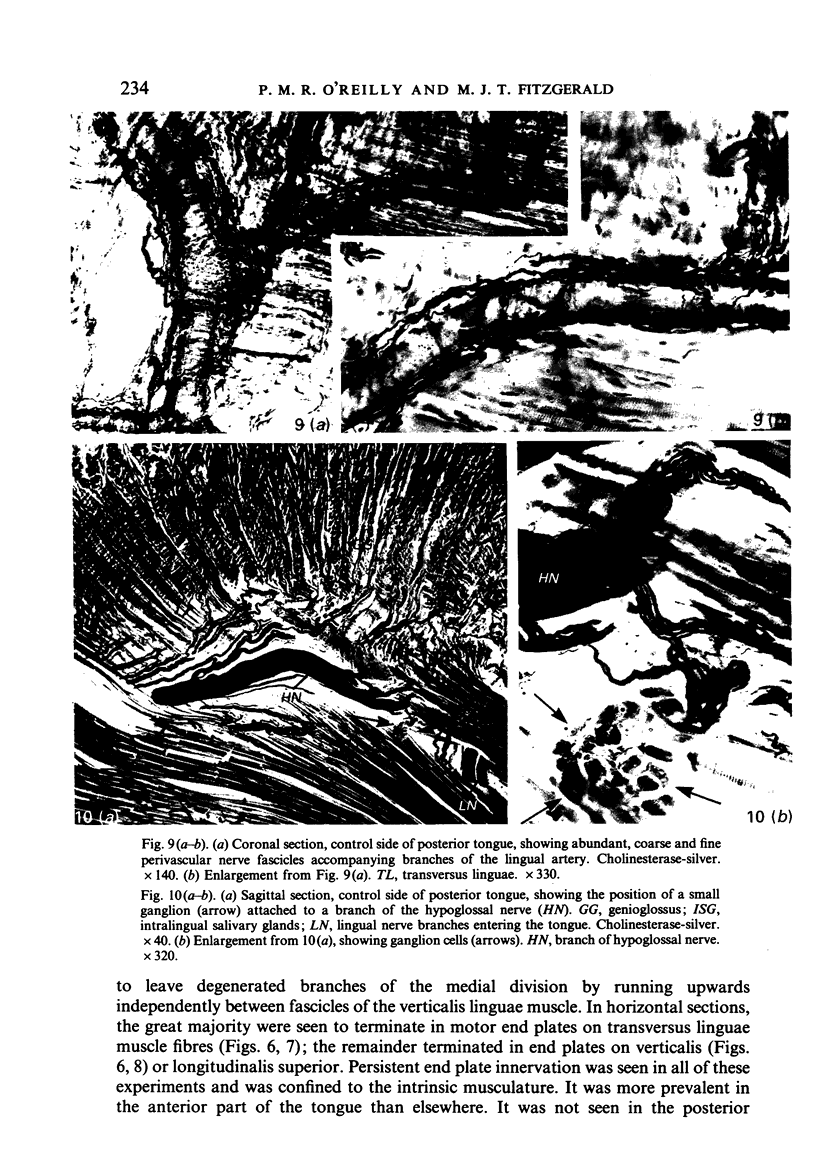
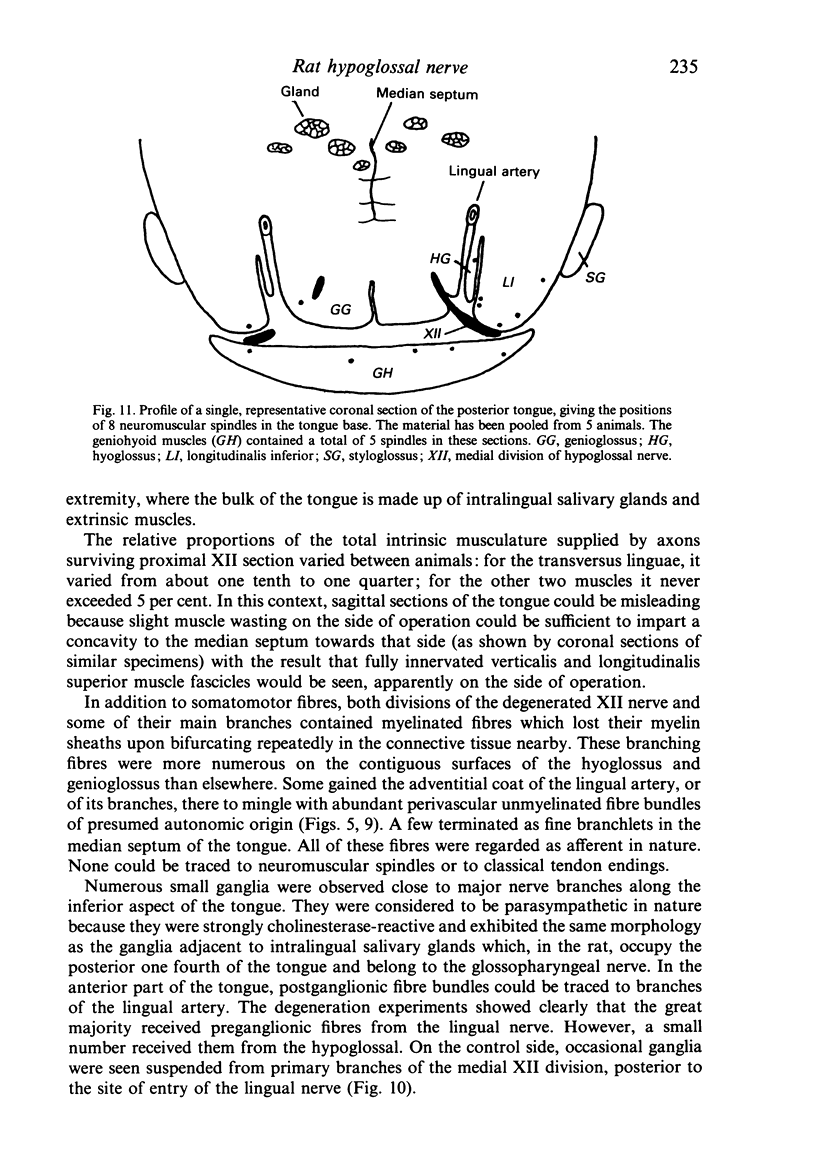
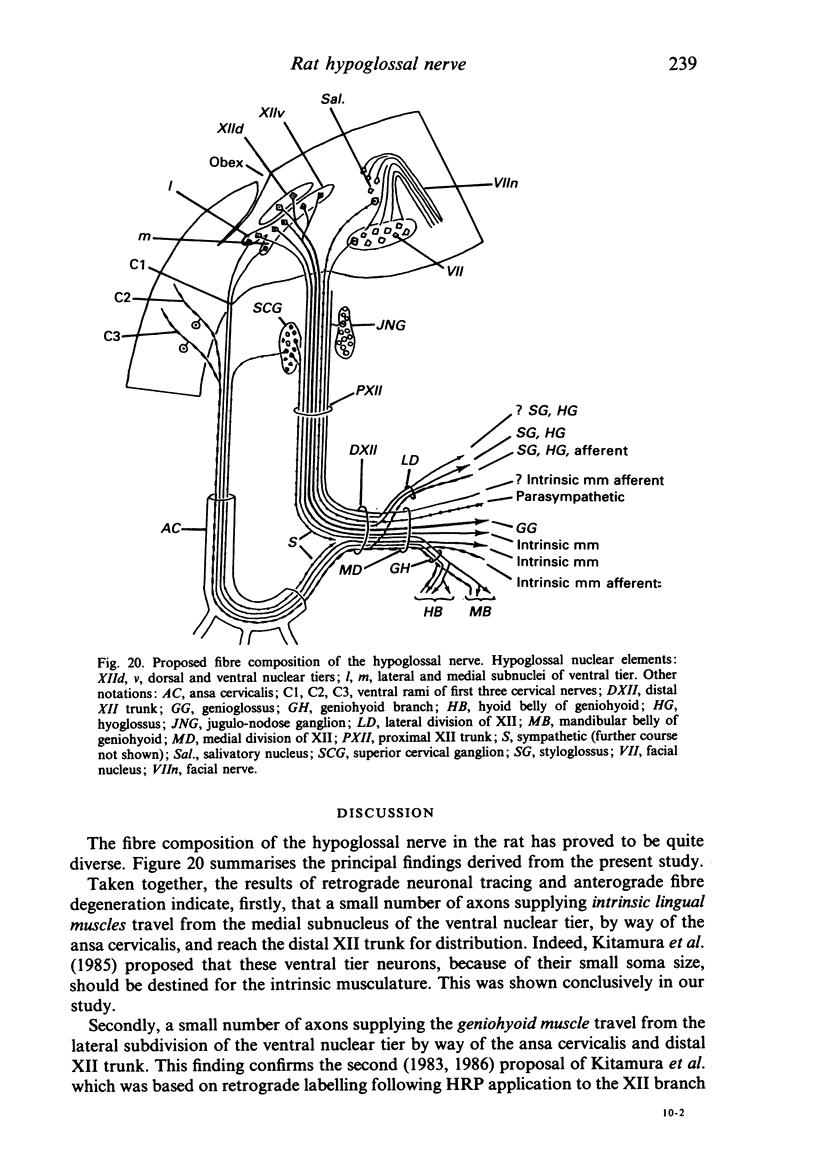
The fibre composition of the hypoglossal nerve of the rat has been investigated by means of (a) retrograde neuronal labelling following application of fluorescent dyes to the XII nerve and/or to the ansa cervicalis, lingual nerve and facial nerve; and (b) anterograde fibre degeneration in silver-impregnated sections of the tongue following interruption of one or more of these nerves. A search for neuromuscular spindles was carried out in coronal paraffin sections of eight control tongues. The ansa cervicalis was found to make three significant contributions to the distal XII trunk: one set of axons originated in the medial subnucleus of the ventral tier of the XII nucleus and contributed to the motor innervation of intrinsic lingual muscles: a second set originated in the lateral subnucleus of the ventral tier and contributed to the motor innervation of the posterior part of the geniohyoid muscle. A third set, having cell bodies in the two uppermost cervical ganglia, provided proprioceptive afferents to the tongue and geniohyoid. A small number of somata in the caudal end of the facial nucleus contributed axons to the XII nerve prior to its emergence from the medulla oblongata. The salivatory nucleus (pons) also contributed pre-emergence fibres, which supplied autonomic ganglia destined for the supply of small arteries in the tongue. The fibre composition of the XII trunk was completed by an afferent contribution from the jugulo-nodose ganglion complex of the vagus nerve, and by sympathetic fibres from the superior cervical ganglion. In control material, eight neuromuscular spindles were detected in five out of eight tongues. Six occupied the longitudinalis inferior, one the hyoglossus, and one the genioglossus. All eight were close to the surface of the tongue base.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baecker B., Yanaihara N., Forssmann W. G. VIP innervation of the tongue in vertebrates. Anat Embryol (Berl) 1983;167(2):173–189. doi: 10.1007/BF00298509. [DOI] [PubMed] [Google Scholar]
- Chibuzo G. A., Cummings J. F. An enzyme tracer study of the organization of the somatic motor center for the innervation of different muscles of the tongue: evidence for two sources. J Comp Neurol. 1982 Mar 1;205(3):273–281. doi: 10.1002/cne.902050307. [DOI] [PubMed] [Google Scholar]
- Contreras R. J., Gomez M. M., Norgren R. Central origins of cranial nerve parasympathetic neurons in the rat. J Comp Neurol. 1980 Mar 15;190(2):373–394. doi: 10.1002/cne.901900211. [DOI] [PubMed] [Google Scholar]
- FITZGERALD M. J. A GENERAL-PURPOSE SILVER TECHNIQUE FOR PERIPHERAL NERVE FIBERS IN FROZEN SECTIONS. Stain Technol. 1963 Nov;38:321–327. doi: 10.3109/10520296309061196. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. J., Alexander R. W. The intramscular ganglia of the cat's tongue. J Anat. 1969 Jul;105(Pt 1):27–46. [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. J., Sachithanandan S. R. The structure and source of lingual proprioceptors in the monkey. J Anat. 1979 May;128(Pt 3):523–552. [PMC free article] [PubMed] [Google Scholar]
- Friauf E. Morphology of motoneurons in different subdivisions of the rat facial nucleus stained intracellularly with horseradish peroxidase. J Comp Neurol. 1986 Nov 8;253(2):231–241. doi: 10.1002/cne.902530209. [DOI] [PubMed] [Google Scholar]
- Hedger J. H., Webber R. H. Anatomical study of the cervical sympathetic trunk and ganglia in the albino rat (Mus norvegicus albinus). Acta Anat (Basel) 1976;96(2):206–217. doi: 10.1159/000144674. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kitamura S., Nishiguchi T., Okubo J., Chen K. L., Sakai A. An HRP study of the motoneurons supplying the rat hypobranchial muscles: central localization, peripheral axon course and soma size. Anat Rec. 1986 Sep;216(1):73–81. doi: 10.1002/ar.1092160113. [DOI] [PubMed] [Google Scholar]
- Kitamura S., Nishiguchi T., Sakai A. A horseradish peroxidase study of rat lingual motoneurons with axons passing through the cervical nerve. Exp Neurol. 1985 Jan;87(1):20–34. doi: 10.1016/0014-4886(85)90130-x. [DOI] [PubMed] [Google Scholar]
- Kitamura S., Nishiguchi T., Sakai A. Location of cell somata and the peripheral course of axons of the geniohyoid and thyrohyoid motoneurons: a horseradish peroxidase study in the rat. Exp Neurol. 1983 Jan;79(1):87–96. doi: 10.1016/0014-4886(83)90380-1. [DOI] [PubMed] [Google Scholar]
- Klueber K. M., Ontell M. A new approach to intramuscular placement of horseradish peroxidase. Muscle Nerve. 1984 Feb;7(2):127–129. doi: 10.1002/mus.880070207. [DOI] [PubMed] [Google Scholar]
- Krammer E. B., Rath T., Lischka M. F. Somatotopic organization of the hypoglossal nucleus: a HRP study in the rat. Brain Res. 1979 Jul 20;170(3):533–537. doi: 10.1016/0006-8993(79)90970-3. [DOI] [PubMed] [Google Scholar]
- Lakars T. C., Herring S. W. Polymorphous geniohyoid muscles of mice, rats and hamsters. Arch Oral Biol. 1987;32(6):421–427. doi: 10.1016/0003-9969(87)90077-x. [DOI] [PubMed] [Google Scholar]
- Lodge D., Duggan A. W., Biscoe T. J., Caddy K. W. Concerning recurrent collaterals and afferent fibers in the hypoglossal nerve of the rat. Exp Neurol. 1973 Oct;41(1):63–75. doi: 10.1016/0014-4886(73)90181-7. [DOI] [PubMed] [Google Scholar]
- Maier A. Occurrence and distribution of muscle spindles in masticatory and suprahyoid muscles of the rat. Am J Anat. 1979 Aug;155(4):483–505. doi: 10.1002/aja.1001550406. [DOI] [PubMed] [Google Scholar]
- Müntener M., Gottschall J., Neuhuber W., Mysicka A., Zenker W. The ansa cervicalis and the infrahyoid muscles of the rat. I. Anatomy; distribution, number and diameter of fiber types; motor units. Anat Embryol (Berl) 1980;159(1):49–57. doi: 10.1007/BF00299254. [DOI] [PubMed] [Google Scholar]
- Neuhuber W., Mysicka A. Afferent neurons of the hypoglossal nerve of the rat as demonstrated by horseradish peroxidase tracing. Anat Embryol (Berl) 1980;158(3):349–360. doi: 10.1007/BF00301822. [DOI] [PubMed] [Google Scholar]
- Satomi H., Ninomiya T., Abe C., Takahashi K. Are cat lingual muscles dually innervated by the hypoglossal nerve and facial nerve? Exp Neurol. 1985 Mar;87(3):578–582. doi: 10.1016/0014-4886(85)90186-4. [DOI] [PubMed] [Google Scholar]
- Shohara E., Sakai A. Localization of motoneurons innervating deep and superficial facial muscles in the rat: a horseradish peroxidase and electrophysiologic study. Exp Neurol. 1983 Jul;81(1):14–33. doi: 10.1016/0014-4886(83)90154-1. [DOI] [PubMed] [Google Scholar]
- Smith K. K. Histological demonstration of muscle spindles in the tongue of the rat. Arch Oral Biol. 1989;34(7):529–534. doi: 10.1016/0003-9969(89)90091-5. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K., Wyss J. M. Two rapid methods of counterstaining fluorescent dye tracer containing sections without reducing the fluorescence. Brain Res. 1986 Nov 5;397(1):117–129. doi: 10.1016/0006-8993(86)91375-2. [DOI] [PubMed] [Google Scholar]
- Uemura-Sumi M., Itoh M., Mizuno N. The distribution of hypoglossal motoneurons in the dog, rabbit and rat. Anat Embryol (Berl) 1988;177(5):389–394. doi: 10.1007/BF00304735. [DOI] [PubMed] [Google Scholar]
- Yu W. H., Srinivasan R. Origin of the preganglionic visceral efferent fibers to the glands in the rat tongue as demonstrated by the horseradish peroxidase method. Neurosci Lett. 1980 Sep;19(2):143–148. doi: 10.1016/0304-3940(80)90185-8. [DOI] [PubMed] [Google Scholar]
- Zapata P., Torrealba G. Mechanosensory units in the hypoglossal nerve of the cat. Brain Res. 1971 Sep 24;32(2):349–367. doi: 10.1016/0006-8993(71)90329-5. [DOI] [PubMed] [Google Scholar]