Abstract
Macular pigment optical density (MPOD) models enhance understanding of macular xanthophyll distribution, particularly relevant to age-related macular degeneration. This study investigates an existing model and introduces a novel, more accurate and biologically relevant approach. MPOD spatial profiles of 48 eyes were obtained using dual-wavelength autofluorescence imaging, with structural data from OCT and OCT-angiography. MPOD data were analyzed using (a) an existing sum of exponential and Gaussian model (MEG) and (b) a novel sum of three Gaussians model (M3G). Extracted parameters generated individualized MPOD models, from which gradients and volumes were derived. M3G-derived variables were analyzed against OCT/OCTA data using factor analysis and multiple regression. M3G demonstrated a superior fit to MPOD data (SSE = 2.60 × 10− 3) compared to MEG, (SSE = 35.7 × 10− 3) enabling automated fitting consistent over small and large datasets. M3G provided meaningful variables, including MPOD gradients, volumes and critical point eccentricities. Correlations included those between dependent variables of critical point eccentricities and central macular pigment volume with foveal avascular zone and foveal pit radii.
The excellent data fit of M3G enables automated extraction of physiologically relevant parameters. Its three-component configuration is consistent with the location of macular xanthophylls. M3G is similar to models of foveal structure, suggesting a fundamental relationship.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-21681-4.
Subject terms: Computational models, Retina, Retina
Introduction
The plant-derived yellow xanthophyll carotenoids, lutein and zeaxanthin, along with the metabolite mesozeaxanthin, are concentrated in the anatomically specialised region of the retina known as the macula lutea (Latin for ‘yellow spot’, commonly abbreviated as the ‘macula’)1,2. Collectively referred to as macular pigment (MP), these compounds are thought to optimise visual function, protect against short-wavelength visible light and scavenge free radicals3,4. It is now accepted that MP plays a protective role in reducing the risk of age-related macular degeneration (AMD)5: MP deficiency increases the risk of AMD6, while MP supplementation can mitigate this risk7.
Dual-wavelength autoflourescence8 has emerged as a reliable and accurate objective method for measuring in vivo macular pigment optical density (MPOD), a quantifier of macular pigment density9. MPOD data is typically plotted against circumferential eccentricity from a point centred on the foveola, creating a macular pigment spatial density profile9 .
Macular pigments are concentrated in the central fovea, declining to < 10% of peak value by 3° eccentricity (approximately 1 mm from the foveolar centre) and stabilizing at a low, constant level from about 7° eccentricity. Zeaxanthin and its isomer, meso-zeaxanthin, are confined to the fovea2. It is particularly concentrated in the inner plexiform layer, outer plexiform layer and outer nuclear layer of the fovea and foveola, most likely in association with the Mueller cell cone10,11. This supports the hypothesis that Mueller cells, rather than photoreceptor axons12, serve as the principal macular pigment reservoir10,11,13,14. Lutein, in contrast, is distributed more diffusely, located eccentrically to the fovea and at lower concentrations2,11,15.
There are considerable inter-individual variations in the spatial distribution of macular pigments, leading to qualitative spatial profile descriptions such as ‘central peak’, ‘ring-like’, ‘central dip’ and ‘intermediate’16. Some spatial density profiles exhibit a strictly monotonic decline with eccentricity from a central peak. Other profiles, while still declining monotonically, display gradient changes that form an eccentric shoulder. Non-monotonic profiles, on the other hand, feature eccentric peaks in the presence or absence of a central peak. The latter represent an annular or ‘ring-like’ pattern in the two-dimensional MPOD profile17.
Variations in MPOD spatial profiles are linked to morphological differences. For example, the presence of eccentric peaks in MPOD spatial profiles are associated with wider foveae18, and eyes with larger foveal avascular zones (FAZ) are more likely to exhibit an eccentric peak in their MPOD spatial profiles19.
Despite the variation in MP spatial profiles, these have been represented using computational models, including simple exponential20, Gaussian21,22, combined exponential-Gaussian23 and a two-dimensional Zernike polynomial model24. These models were selected to provide the ‘best fit’ to the data rather than being guided by physiological or anatomical principles. They have proven useful for classifying macular pigment profile variations23 and predicting central macular pigment optical density from noisy datasets22.
Analytic models enable complex forms, such as the variety of macular pigment spatial profiles, to be represented accurately using a small set of parameters. The parameters themselves can be analysed and used to reconstruct individual profiles with greater precision, allowing for more detailed analyses than may be possible with experimental data alone. Such models also serve as a foundation for the automatic extraction of quantitative features with potential clinical utility, such as peak values, volumes and rates of attenuation. Furthermore, accurate models can provide deeper insights than numeric data alone, as has shown to be the case for retinal morphology defined by optical coherence tomography (OCT), (see, for example25,26.
The present study aimed to develop and test an accurate analytic model of the macular pigment optical density (MPOD) spatial profile. Initially, the Berendschot and van Norren model23 was selected for evaluation using dual-wavelength autofluorescence data obtained through now-standard methodologies9. This model was chosen because it consistently fit MPOD data and describes the retinal spatial profile of macular pigment as a continuous function. Such a function enables the derivation of properties such as gradients and changes in gradients through differentiation and volumetric data through integration, although such analyses have not yet been reported in the literature for MPOD.
However, preliminary analyses revealed that the Berendschot and van Norren model did not fully capture the complexity of the experimental data. These findings indicated the need for a more nuanced approach, leading to the proposal of a three-component model. Each component of this novel model represents a specific region of the MPOD spatial profile, accounting for variations not addressed by the original two-component model.
The study then shifted its focus to developing, verifying and interrogating this three-component model. The model was rigorously tested against measured MPOD data and structural data obtained through optical coherence tomography (OCT) and OCT angiography (OCTA).
The new model successfully represents experimental data with high accuracy, correlates with structural data and generates novel parameters that represent the location and concentration of macular pigment in the human retina. These findings demonstrate the model’s potential for advancing both research and clinical applications related to macular pigment and retinal health.
Experimental methods and data collection
Data collection was conducted at the School of Optometry at Aston University, UK, between January and December 2021. The study adhered to the tenets of the Declaration of Helsinki and was approved by Aston University Local Ethics Committee (application #1566). All participants gave informed consent to participate in the study. Exclusion criteria were prior history or clinical evidence of ocular disease. Measurements were taken from 48 eyes, drawn from a participant pool of 13 males and 12 females (age range: 28 to 64 years; mean age ± standard deviation: 46.2 ± 11.7 years). All participants were white Europeans with the exception of two male participants who were British Asian. Whilst variations in macular pigment distribution occur in different ethnic groups9,27, the ethnicity of participants was not relevant to the aims and outcomes of the present study.
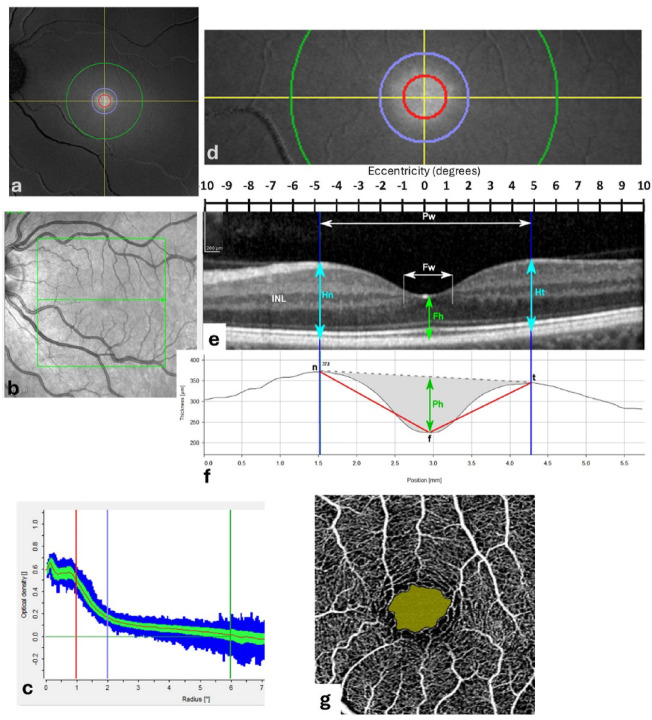
OCT and MPOD measurements (Table 1) were obtained using the Heidelberg Spectralis OCT/SLO (Heidelberg, Engineering, Heidelberg, Germany) following the manufacturer’s instructions and protocols detailed in previous studies9,14,28,29. The dual-wavelength autoflourescence module was used to extract macular pigment data (Fig. 1a, c, d), including averaged MPOD values and MPOD volume measures along and within circular paths with radii extending from the foveal centre of 0.2°, 1°, 2° and a reference radius of 6° (corresponding to machine calibrated values of 0.20°, 0.98°, 1.99° and 5.98°).
Table 1.
Data definitions and summary of macular pigment optical density (MPOD), OCT and OCTA measures for the 48 eyes. 95% CI is the 95% confidence Interval.
| Macular pigment optical density (arbitrary units) | Mean | Min | Max | sd | 95%CI | |
|---|---|---|---|---|---|---|
| MPOD | ||||||
| OD rad0.2 | At 0.2° eccentricity | 0.59 | 0.25 | 1.03 | 0.20 | 0.53–0.64 |
| OD rad 1 | At 1° eccentricity | 0.37 | 0.15 | 0.67 | 0.14 | 0.33–0.41 |
| OD rad 2 | At 2° eccentricity | 0.12 | 0.03 | 0.22 | 0.05 | 0.11–0.14 |
| OD rad 3 | At 3° eccentricity | 0.01 | 0.00 | 0.02 | 0.01 | 0.01–0.01 |
| sumV0.2 | Volume to 0.2° eccentricity | 57 | 25 | 101 | 19 | 52–63 |
| sumV 1 | Volume to 1° eccentricity | 924 | 408 | 1629 | 333 | 829 1018 |
| sumV 2 | Volume to 2° eccentricity | 2184 | 846 | 3843 | 828 | 1950–2418 |
| sumV 3 | Volume to 3° eccentricity | 5108 | 1581 | 9500 | 1999 | 4542–5674 |
| Peak | Central peak | 0.64 | 0.28 | 1.10 | 0.20 | 0.58–0.69 |
| OCT | ||||||
| Av CRT | Average central retinal thickness (µm) | 274 | 246 | 299 | 14 | 270–278 |
| Fr | Foveolar radius (degrees) | 0.81 | 0.51 | 1.13 | 0.16 | 0.77–0.86 |
| Pr | Foveal pit radius (degrees) | 3.96 | 2.80 | 5.09 | 0.44 | 3.83–4.08 |
| fov ang | Foveal pit angle (degrees) | 168 | 160 | 172 | 3 | 167–169 |
| fov th | Central foveal thickness (µm) | 231 | 206 | 272 | 14 | 227–235 |
| bwlht | Foveal bowl height (µm) | 123 | 68 | 166 | 22 | 117–129 |
| OCTA | ||||||
| FAZf | Foveal avascular zone maximum Feret’s diameter (degrees) | 2.27 | 1.3 | 2.88 | 0.41 | 2.15–2.39 |
| FAZm | Foveal avascular zone mean diameter (height + width)/2 (degrees) | 2.03 | 1.01 | 2.60 | 0.39 | 1.92–2.14 |
| FAZer | Foveal avascular zone equivalent radius  (degrees) (degrees) |
0.91 | 0.40 | 1.17 | 0.19 | 0.85–0.96 |
Fig. 1.
Fundal imagery and definitions: Case # 41. (a) Dual wavelength autoflourescence image of the left macular region, with circles marking eccentricities of 1° (red), 2° (blue) and 6° (green). (b) Infrared scanning laser ophthalmoscopy (SLO) fundus image of the same field as in (a), with 20°x 20° box (green) superimposed. (c) Machine-generated plot showing the mean and standard deviation of macular pigment optical density (MPOD) measured along circular paths at increasing eccentricities: red, blue and green vertical lines mark the 1°, 2° and 6° circles shown in (a). (d) Enlarged 20° x 6° dual-wavelength autoflourescence image across the fovea along the green arrow in (b). (e) Optical coherence tomography (OCT) image of the fovea along the green arrow in (b). (f) Machine-generated retinal thickness profile (distance between retinal pigment epithelium (RPE) and inner limiting membrane (ILM)) with machine calibration values. (g) 10° x 10° OCT angiography (OCTA) image showing machine-defined foveal avascular zone (FAZ). Annotations: INL, inner nuclear layer of retina; f, centre of foveola; Fh, foveolar height (light green); Fw, foveolar width (white); n/t, nasal/temporal peak macular thickness (light blue); Pw, foveal pit width; Ph, foveal pit height (dark green). The foveal angle is angle between lines drawn between n to f, and f to t (f).
Additional data extraction from the blue and green autofluorescence images was performed using ImageJ image analysis software30 and analysed following the methods of Kar et al.14 using their open source software (https://sites.imagej.net/CreativeComputation/).
Spectral-domain OCT macular images were presented as 20° x 20° blocks, with a single horizontal scan passing through the foveolar centre (Fig. 1b, e). Morphometry was performed using ImageJ. Morphometric landmarks, parameters and values are summarised in Table 1; Fig. 1e, f. The foveolar radius (Fr) was defined as half the distance between adjacent terminations of the inner nuclear layer (INL) in the horizontal plane of the OCT scan (INL and Fw in Fig. 1e). The foveal pit radius (Pr) was defined as half the distance between the maximum nasal and maximum temporal macular thicknesses in the horizontal plane OCT (Pw, n, t in Fig. 1e).
Degrees of visual angle were used for linear measurements in OCT and OCT angiography images to ensure consistency across different measurements and to avoid confounding errors due to magnification effects from inter-individual variations in ocular dimensions31. Machine-generated axial measures of retinal thicknesses were expressed in micrometers (µm).
OCT angiography was performed using the Cirrus 5000 HD-OCT (Carl Zeiss Meditec Inc., Dublin, CA, USA. software version 11.0.0.29946). Machine-generated values and foveal avascular zone (FAZ) delineation were obtained using the proprietary software with the 3 mm x 3 mm angiography setting (Fig. 1g). Images of machine delineated FAZ were further analysed and measured using ImageJ. Morphometric results were again expressed in degrees of visual angle, with the examination field assumed to be constant at 10° x 10°. Three FAZ metrics were recorded (Table 1): the maximum Feret’s (calliper) diameter (FAZf), the mean horizontal and vertical diameters (FAZm) and the equivalent radius (FAZer), calculated as the radius of a circle with the same area as the FAZ19.
Part 1: the existing model
Analysis and modelling
Mathematical manipulation, data processing and non-linear regression analyses were performed using Wolfram Mathematica (Wolfram Research, 2024; Version 11.1.1.0). Nonlinear regression was initially performed between MPOD data and the two-component model of Berendschot van Norren23. This model (MEG, Eq. 1) represents the sum of a declining exponential component and a Gaussian component: the exponential component accounts for the central MPOD peak and peripheral tail-off, while the Gaussian component accounts for any eccentric shoulders or eccentric peaks. Originally expressed in base 10, the Berendschot van Norren model is here expressed in base e for consistency and mathematical simplicity, and is defined as:
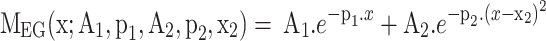 |
1 |
where the primary parameters A1 is the exponential amplitude, p1 the exponential decay constant, A2 the Gaussian amplitude, p2 the spread (‘peakedness’) of the Gaussian component, and x2 the eccentricity of the Gaussian component peak. Conversion to the base 10 model requires division of A1 and A2 by loge10, while all other parameters remain unchanged.
Results 1 MEG data fitting
The MEG model was fitted to DWAF-derived MPOD profile data from 0° to 2° and 0° to 5° eccentricity using Mathematica’s non-linear data fitting algorithms for each of the 48 eyes and for the mean of the 48 eye MPOD data set as a function of eccentricity (the MPOD grand mean). Data fitting was performed both automatically and with manual control of parameters. The deviation between model-predicted and experimental data was quantified as the sum of squared differences (Table 2).
Table 2.
Comparison of data fits for the MEG and M3G models. Summed squared of difference between data and model predicted values for the 48 eyes. The data fit is either to 2° or 5°. Parameters from the 2° data fit were then applied to the 2° and 5° data sets separately (MEG model: second and third results rows; MEG model: fifth and sixth rows; 95% CI is the 95% confidence Interval).
| Model | Fit to | Data to | Summed squares of difference between data and model x10− 3 | ||||
|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Sd | 95%CI | |||
| MEG | 2° | 2° | 11.0 | 0.8 | 61.8 | 16.5 | 6.3–15.6 |
| 2° | 5° | 118.8 | 8.7 | 333.6 | 83.7 | 95.1 -142.5 | |
| 5° | 5° | 35.7 | 1.6 | 109.3 | 24.8 | 28.6–42.7 | |
| M3G | 2° | 2° | 1.4 | 0.2 | 8.3 | 1.4 | 1.0 -1.8 |
| 2° | 5° | 641.4 | 2.4 | 13212.6 | 2083.3 | 52.0–1230.8 | |
| 5° | 5° | 2.6 | 0.5 | 8.3 | 1.7 | 2.1–3.1 | |
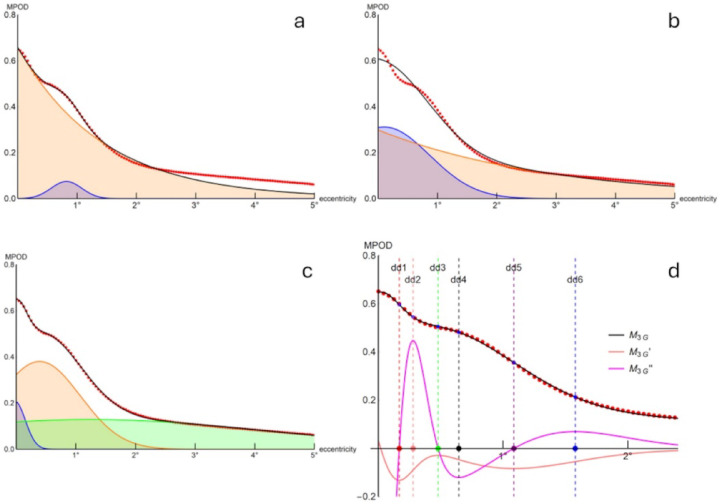
The MEG model fitted the 2° data set (Fig. 2a) with less deviation from the data than the 5° data set (Fig. 2b). When applied to the 5°data set, the model prioritized overall fit at the expense of finer details, such as the eccentric shoulder (Fig. 2b). In most cases, the 2° data set fit captured any eccentric peak or shoulder in the MPOD distribution (Fig. 2a) however did not offer an adequate fit when extended to the full 5° data set.
Fig. 2.
Grand mean of MPOD data (red dots) with model fit (black continuous line), model components (shaded areas and coloured continuous lines) and derivatives (coloured continuous lines in d). (a) MEG model with data fit from 0° to 2° and (b) from 0° to 5° eccentricity, with separation into exponential (orange line/shade) and Gaussian (blue line/shade) components. (c) M3G model with data fit from 0° to 5° eccentricity, with separation of Gaussian components (i = 1, blue; i = 2 orange; i = 3, light green). (d) as (c) for 0° – 2.5° eccentricity, showing first (orange line) and second (magenta line) derivatives with critical points dd1 – dd6 (coloured circles on x-axis with corresponding vertical dashed lines). See text for details.
Manual adjustment of primary parameters to optimize the fit for the central peak and tail-off came at the expense of accurately capturing the eccentric shoulder or peak. Similarly, manipulation of primary parameters to best fit the eccentric shoulder/peak was at the expense of the central peak and peripheral tail-off (compare Fig. 2a and b).
The incomplete fit led to the conclusion that the MEG model was not sufficiently accurate to represent the full extent of the macular pigment optical density profile, warranting the exploration of alternative models. Furthermore, the inability of the two-component model to simultaneously capture the central peak and peripheral tail-off while preserving any intermediate shoulder or peak suggests that a three-component model may provide a better fit.
Part 2: A novel three-component Gaussian (M3G) model
Following trials of several three-component models, a three-Gaussian-component model (M3G model; Eq. 2, Fig. 2c and d) provided the best fit to the data and was investigated further. The model,
 |
2 |
is defined by the primary parameters Ni, amplitude (scale parameter), gi peak location (eccentricity of component peak); qi the spread of components i = 1, 2, 3. Primary parameter ranges were constrained such that component i = 1 represented the central peak; i = 2 represented any intermediate eccentricity shoulders or eccentric; and i = 3 defined the peripheral tail-off.
For eccentricities x within physiological ranges, the function M3G (as is MEG) is differentiable, with its first and second derivatives providing gradients and changes in gradients. These derivatives indicate mathematically definable critical points (e.g., inflections, turning points) that will be used in further analysis (Fig. 2d, Fig. 4).
Fig. 4.
Summary graph showing the M3G model fitted to the grand mean MPOD data (black continuous curve) and its second derivative (M3G’’, magenta continuous curve) compared to structural landmarks (black annotations below x-axis). Eccentricities of points of interest dd1 – dd6 are identified by coloured dots and broken vertical lines. The grey shading is the area under the M3G curve between the centre point (eccentricity = 0°) and the eccentricity of the first local maximum of M3G’’ (dd2 = 0.28°); the orange shading is the area under the M3G curve from the eccentricity of the second local maximum of M3G’’ (dd6 = 1.46°) to the eccentricity limit of the graph (5°). Horizontal data bars are 95% confidence intervals.
The behaviour of the first derivative (MEG’, Fig. 2d) indicated whether MEG was monotonically declining (MEG’ everywhere negative for the interval of interest) or nonmonotonic (MEG’ not everywhere negative). The eccentricities of the zeros, local minima and local maxima of the second derivative (MEG’’), when present, defined up to six quantifiable points of interest (derived parameters) in the models (Fig. 2d, Fig. 4). Each of these points represents a critical change in the gradient of the MEG function. For example, the radius of the eccentric local minimum (dd4) represented the eccentricity of an eccentric peak or ‘shoulder’ of the MPOD spatial profile.
Five additional parameters of interest were derived from the MEG model (see Supplementary Material for derivations): the central peak amplitude at zero degrees eccentricity A03G = M3G(0); the amplitude of any eccentric peak, if present (Aecc); the areas under the curve at eccentricities of 0.2° and 5°; and the half central peak radius. The latter was calculated for comparison with similar measures in previous studies19.
Computational modelling and initial model comparison was performed with Mathematica. Subsequent statistical analysis was performed using Statistica software (Statsoft Inc., 2300 East 14th St, Tulsa, Ok, 74104, USA). Data redundancy of derived parameters was investigated by factor analysis. Parameters of interest selected by factor analysis were then investigated further with multiple regression analysis between macular pigment-related parameters and morphometric parameters.
Results 2: the M3G model
Data fit and comparison of models
The mean sum of squares of the difference between the data and model (SSE) for the M3G model was an order of magnitude smaller than that for the MEG model for both the 2° and 5° data sets (Table 2). As with the MEG model, M3G parameters derived from the 2° data set could not be applied to model the 5° data set, which required a different parameter set for an optimum fit. The M3G model fitted all data sets without manual parameter adjustments and did not exhibit the trade-off observed in the MEG model between fitting the central peak and the peripheral tail-off.
The nine primary parameters of the M3G model, determined from nonlinear regression of the 48 data sets with Eq. 2, are defined and presented in Table 3 together with the derived parameters. Parameter values for the model fitted to the data grand mean are presented in the final column of Table 3. The fit of the M3G model to data grand mean, with its separate components, is presented in Fig. 2c, where it can be compared to the MEG model (Fig. 2a, b). The first and second derivatives of the M3G model fitted to the data grand mean together with points of interest dd1 - dd6 are presented in Fig. 2d.
Table 3.
M3G model primary and derived parameters.
| Definition | Fit to MPOD data 0° − 5° | Fit to data grand mean | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | sd | 95%CI | n | |||
| Primary parameters | ||||||||
| N1 | 1st Gaussian component amplitude | 0.224 | 0.100 | 0.447 | 0.102 | 0.195–0.253 | 48 | 0.200 |
| N2 | 2nd Gaussian component amplitude | 0.296 | 0.095 | 0.598 | 0.125 | 0.261–0.331 | 48 | 0.250 |
| N3 | 3rd Gaussian component amplitude | 0.097 | 0.015 | 0.382 | 0.092 | 0.071–0.123 | 48 | 0.039 |
| p1 | 1st Gaussian component spread | 32.092 | 2.044 | 180.000 | 43.978 | 19.650–44.533 | 48 | 14.515 |
| p2 | 2nd Gaussian component spread | 1.938 | 0.859 | 4.838 | 0.792 | 1.714–2.162 | 48 | 1.700 |
| p3 | 3rd Gaussian component spread | -0.009 | -0.098 | 0.070 | 0.032 | -0.018–0.000 | 48 | -0.020 |
| g1 | 1st Gaussian component centre position | -0.027 | -0.100 | 0.100 | 0.067 | -0.046 - -0.008 | 48 | -0.017 |
| g2 | 2nd Gaussian component centre position | 0.547 | 0.000 | 0.960 | 0.265 | 0.472–0.622 | 48 | 0.562 |
| g3 | 3rd Gaussian component centre position | 5.345 | -2.000 | 10.000 | 5.243 | 3.861–6.828 | 48 | 10.000 |
| Derived parameters | ||||||||
| A03G | Central amplitude | 0.650 | 0.245 | 1.169 | 0.201 | 0.593–0.707 | 48 | 0.649 |
| Aecc | Amplitude of eccentric peak/convex inflection (dd4) | 0.502 | 0.228 | 0.899 | 0.198 | 0.437–0.568 | 35 | 0.475 |
| AUC02 | Area under the curve 0° − 0.2° | 0.125 | 0.044 | 0.230 | 0.041 | 0.113–0.136 | 48 | 0.125 |
| AUC5 | Area under the curve 0° − 5° | 1.048 | 0.393 | 1.860 | 0.394 | 0.937–1.159 | 48 | 1.048 |
| HHr | Radius of curve at half central amplitude (half height radius) | 1.170 | 0.337 | 1.851 | 0.359 | 1.067–1.272 | 47 | 1.177 |
| dd1 | Eccentricity of 1st zero of second derivative | 0.166 | 0.025 | 0.350 | 0.062 | 0.147–0.185 | 42 | 0.175 |
| dd2 | Eccentricity of 1st maximum of second derivative | 0.283 | 0.009 | 0.750 | 0.116 | 0.249–0.318 | 43 | 0.296 |
| dd3 | Eccentricity of 2nd zero of second derivative | 0.476 | 0.101 | 0.933 | 0.146 | 0.432–0.521 | 41 | 0.502 |
| dd4 | Eccentricity of 2nd minimum of second derivative | 0.647 | 0.300 | 0.943 | 0.118 | 0.608–0.686 | 35 | 0.687 |
| dd5 | Eccentricity of 3rd zero of second derivative | 1.117 | 0.474 | 1.574 | 0.218 | 1.051–1.183 | 42 | 1.086 |
| dd6 | Eccentricity of 2nd maximum of second derivative | 1.457 | 0.878 | 2.009 | 0.267 | 1.382–1.533 | 48 | 1.497 |
Factor analysis
Factor analysis was performed on the three data sets – MPOD, OCT and OCTA (independent, X-variables) – along with the M3G primary and derived parameters (dependent, Y-variables), to identify redundant variables that could be excluded in any subsequent analyses. Factor analysis using principal components32 identified which variables provided significant and independent information. Variables providing independent information were grouped into factors (F1, F2, etc.) that account for diminishing proportions of the total variance. Those variables providing significant information were quantified by a factor loading (FL). An absolute loading of |FL| > 0.7 was assumed to be significant. Detailed results are presented in the Supplementary Material and are summarised here. The analysis indicates a considerable degree of redundancy among variables, allowing for a reduced set of independent and dependent variables to be further analysed (Table 4).
Table 4.
Summary factor analysis with multiple regression and Stepwise multiple regression of selected dependent (Y) and independent (X) variables.
| Factor analysis | Multiple regression | Stepwise multiple regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Grouping | % M3G variance | Significant Y | Loading (FL) | Significant X | β | R2 | Selected variables | R2 (%) |
| F1 | 28% | dd6 | − 0.83 | FAZer | 0.603 | 81% | FAZer | 65% |
| bwlht | 0.426 | bwlht | 13% | |||||
| Pr | 0.190 | Pr | 3% | |||||
| F2 | 25% | AUC02 | 0.87 | FAZer | − 0.540 | 24% | FAZer | 22% |
| Pr | 0.176 | |||||||
| F3 | 20% | dd4 | − 0.84 | None | None | |||
| F4 | 11% | p1 | 0.71 | bwlht | 0.237 | 7% | bwlht | 7% |
Two MPOD X-variable factors accounted for 91% of the total variance (F1: 78%; F2 13%) with all variables except ODrad3 significantly loaded onto F1. No variable loaded significantly onto F2. This suggests that all variables loading onto F1 provide similar predictions for the dependent (Y) variables, with sum V1 having the greatest absolute factor loading (-0.98). This result is expected, as all values tested are from the data set of measured and inter-related macular pigment variables.
Two factors accounted for 79% of the total OCT X-variable variance (F1: 47%; F2: 32%). Among the three significant F1 loadings, foveal bowl height had the greatest factor loading (bwlht, FL = 0.96) with foveal angle (fov ang, FL = -0.87) and foveal thickness (fov th, FL = -0.79) also being significant. For F2, average central retinal thickness (AvCRT, FL = -0.86) and foveal pit radius (Pr, FL = 0.85) had nearly equally loadings. Foveal bowl height (F1) and foveal pit radius (F2) were used in further analysis.
A single factor accounted for 97.6% of total OCTA/FAZ variable variance, with each variable (FAZ ferret, FAZ mean, FAZ equivalent radius) having loadings of approximately FL = -0.99. This indicates that any of the three variables would be sufficient as a dependent variable for subsequent analysis. FAZ equivalent radius (FAZer) was used in further analysis in keeping with data published elsewhere.
Five factors – F1 (28%), F2 (25%), F3 (20%), F4 (11%) and F5 (6%) – accounted for 90% of the total variance in the Y-variables (M3G derived parameters), with F1 – F4 variable groupings having significant loadings (Supplementary Table S2). The variable with the greatest loading in each of the four groupings was used in further analysis (F1: dd6, FL = -0.83; F2: AUC02, FL = 0.87; F3: dd4, FL = -0.84; F4: p1, FL = 0.71). Notably, the F1 factor loading of half-height radius (HHr; FL = -0.74) ranked third, following dd5 (FL = -0.76); both were considered redundant and excluded from further analysis.
Multiple regression analysis
Multiple regression analysis was conducted between the X-variables and Y-variables selected through factor analysis. Two methods were used: (1) multiple regression (MR), which tested the significance of the regression slopes (β) and provided an equation expressing Y in relation to all tested X-variables33, and (2) stepwise multiple regression using the ‘forward’ method (SMR), which identified X-variables that were significantly related to Y and ranked them by importance34. A multiple regression coefficient of determination (R2) accounting for less than 50% of the total variance in Y was considered unlikely to be useful for prediction.
Of all M3G parameters selected by factor analysis (Table 4), dd6 had the greatest coefficient of determination (81%) being related to FAZ equivalent radius, foveal bowl height and foveal pit radius by the model: dd6 = 0.605*FAZer + 0.426*bwlht + 0.19*Pr. The univariate relationship between foveal avascular zone equivalent radius, foveal bowl height and dd6 is demonstrated in Fig. 3.
Fig. 3.
Relationship, with univariate linear regression model and coefficients of determination, between (a) foveal avascular zone equivalent radius (FAZer) and (b) foveal bowl height (bwlht) with M3G derived parameter dd6.
The three other selected M3G variables combined accounted for less of the total M3G variance than dd6 alone. The volume of macular pigment within the central 0.2° of eccentricity (AOC02, R2 = 24%) was significantly related to FAZ equivalent radius (β = -0.54) and foveal pit radius (Pr, β = 0.176). The spread of the first Gaussian component of M3G (p1) was significantly related to foveal bowl height (bwlht, β = 0.237). No specific variables were significantly correlated with the eccentricity of eccentric peak/shoulder (dd4), although this parameter was selected by factor analysis into F3, which accounted for 20% of the M3G parameter variance.
Discussion: interpretation
The initial aim of this study was to determine how an existing model of MPOD relates to experimental data of a wide range of MPOD spatial profiles. A further aim was to explore the biological and clinical significance, if any, of parameters derived from the model or its mathematical interrogation. The existing two-component exponential + Gaussian sum model provided a reasonable data fit for most data sets within the first 2° of eccentricity but was less effective for data sets extending to 5° eccentricity: fitting the model to account for shoulders and eccentric profile peaks was compatible with fitting either the central peak or peripheral tail-off but not both (Fig. 2a and b).
These preliminary findings led to the development of a novel three-component sum-of-Gaussians model, M3G, that provided a significantly better fit to more extended data sets, and MPOD profile details, than the best-fit version of the published model. The parameters of the first, second and third components of the M3G model corresponded respectively to the central peak, any eccentric shoulder or eccentric peak, and the peripheral tail-off of the MPOD spatial profile (Fig. 2c).
The M3G model was at least twice differentiable over assumed physiological ranges, allowing the shape of the M3G curve to be interrogated, analysed and quantitatively compared with morphometric data. Whilst derivatives have been used in the analysis of macular pigment spectrophotometric data35, in macular spatial analysis25,26 and in fundus reflection spectroscopy36, such analysis appears not to have been performed on previously published macular pigment distribution models defined by continuous functions.
The first derivative of the M3G function (M3G‘) describes the gradient of the M3G curve, providing insight into the monotonicity and variability of the macular pigment optical density profile. The second derivative (M3G‘’) describes the rate of change this gradient with respect to eccentricity and is used to identify up to six critical points or points of interest (inflection and turning points) in the M3G - modelled MPOD profile. For example, the radius of the fourth point of interest (dd4, the second minimum of the second derivative) precisely defines the eccentricity of the eccentric peak or shoulder of the profile. Areas under the M3G curve are indices of macular pigment volume and can be obtained by integration between eccentricities of interest, such as from the centre (0°) to 0.2° and 5°.
Inspection of Figs. 2c and 3 shows that the MPOD spatial profile can be quantitatively separated into three sections by the first and second maxima of M3G’’. It is reasonable to hypothesise that these three sections correspond to the anatomical partitioning of the macular pigments. The central peak, between zero eccentricity and dd2, defined primarily by the first Gaussian component (i = 1) of M3G, relates to the central MPOD peak/Mueller cell cone/ zeaxanthin/mesozeaxanthin locus. The intermediate section, between dd2 and dd6, defined primarily by the second Gaussian component (i = 2) of M3G, incorporating any shoulder or eccentric peak, relates to the more eccentric IPL/OPL/Henle fibre/zeaxanthin/mesozeaxanthin locus: the eccentricities of shoulders, secondary peaks being quantified by the eccentricity of the local minimum of M3G’’ between these values (dd4). The third section from dd6 to the extent of measurable retina, defined primarily by the third Gaussian component (i = 3) of M3G, relates to the low background lutein-binding layers and centrifugal tail-off of macular pigment optical density.
This hypothesis requires further study using high-resolution morphometry, particularly of the Henle fibre layer. The Henle fibre layer is difficult to image quantitatively with available instrumentation and was not measured in the present study. Quantification of the Henle fibre layer, for example, using polarization sensitive OCT37 or OCT methods based on the manual misalignment of OCT instrumentation38,39, could provide valuable insights into the relationship between this layer and macular pigment distribution, the foveal avascular zone and foveal morphology in general.
Data fitting of the M3G model to individual eyes allowed extraction of variables for correlation with morphometric data. The relationship of the M3G curve and its specific features with key macular morphological landmarks is shown in Fig. 4. The greatest proportion of macular pigment lies within the first half of the radius of the foveal pit and central to the peak thickness of the Henle fibre layer, which extends beyond the 5° boundary of the graph. The inflection point of any MPOD eccentric peak or shoulder, identified by the eccentricity of the local minimum of M3G’’ (dd4), is located just within the boundary of the foveal avascular zone and foveolar radius.
Quantitative assessment of the relationship between the distribution of macular pigment and morphology was preceded by factor analysis, which demonstrated significant data redundancy in both M3G variables and morphometric variables. The variable with greatest factor loading in each factor was used in this study to focus subsequent multiple regression analysis. Significant variables within each factor with lesser loadings are not invalidated by the factor analysis and might benefit from further investigation.
Multiple regression analysis of the selected variables demonstrated a strong correlation between the eccentricity of the second maximum of M3G ‘’ (dd6) and both foveal avascular zone equivalent radius and foveal bowl height. The dd6 variable is a constant feature in all eyes examined and may be taken as a definable boundary of the radial extent of macular pigment. In other words, the area of macular pigment correlates with the depth of the foveal pit and the area of the foveal avascular zone. This conclusion is compatible with known association between larger foveal avascular zones and deeper, broader foveal pits40.
Furthermore, the positive correlation between the radial extent of macular pigment and the foveal avascular zone equivalent radius is consistent with the positive correlation between the latter and the eccentricity of the half-height of the peak central macular pigment density (half-height radius)19. Whilst the half-height radius metric is mathematically meaningful as an index of the ‘spread’ of macular pigment if the profile were purely exponentially declining (being analogous to the half-life constant of exponential decay), it is less meaningful in a non-exponential decline and is potentially ambiguous in a non-monotonic decline, particularly where there is a shoulder or eccentric peak with values near to the half-central peak height value.
There is no such ambiguity with the eccentricities of either the primary or derived parameters of the M3G model, and the strong structural correlations of the dd6 eccentricity support their value in defining the radial extent of macular pigment. Furthermore, the area under the MPOD profile curve from zero to an eccentric point of interest, such as dd6, might be a clearly defined and useful individualised metric of total macular pigment volume.
Other potentially useful metrics of macular pigment highlighted by the factor analysis include the central macular pigment volume, quantified by AOC02, and the radius of the eccentric peak/shoulder, quantified by dd4. Amplitude values of M3G at the dd4 and dd6 eccentricities, whilst not investigated in the present study, require further evaluation, as do their clinical applications. Additional metrics of interest can be derived from the M3G model depending on the intended application. For example, the eccentricity at which the volume of macular pigment is 50% (or another fraction) of a predefined limiting eccentricity can be readily calculated.
The precise fit of the M3G model when applied to experimental data has potential clinical utility in data interpretation and analysis. The function can be used to effectively smooth otherwise noisy clinical data, with the primary and derived parameters providing potentially clinically useful information of macular pigment densities, volumes and the shape of the macular pigment distribution in the retina.
In summary, this study demonstrates that a new model of the macular pigment optical density profile accurately represents measured data to retinal eccentricities of 5° and beyond. Useful and consistent data can be obtained particularly from second derivative critical points, which correlate with OCT-derived morphological features. Whilst there is redundancy between variables, factor analysis identified four parameters that strongly correlate with morphometric landmarks, particularly the extent of the foveal avascular zone. The macular pigment density profile, as modelled by M3G, can be subdivided naturally into three components, which, it is hypothesised, correspond to the observed anatomical segregation of the macular pigments.
A sum of three Gaussian model is flexible in that it can, with careful parameter selection, be used to define a large variety of different profiles. It is noteworthy that a similar model has been used to accurately represent central macular retinal thickness and topography25,26. The similarity of these models suggests a possible integration of macular pigment and structural models, and raises a deeper question as to what common processes, if any, are involved in the formation of macular topography and the deposition of macular pigment.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
GPM: conception, design, data acquisition, data analysis, data interpretation, manuscript draft, macuscript edit SJA: design, data analysis, data interpretation, manuscript edit RAA: data analysis, data interpretation RH: data acquisition, manuscript edit.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Further detailed analysis is available in the Supplementary Information.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Snodderly, D. M., Brown, P. K., Delori, F. C. & Auran, J. D. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest. Ophthalmol. Vis. Sci.25, 660–673 (1984). [PubMed] [Google Scholar]
- 2.Bone, R. A. et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res.64, 211–218. 10.1006/exer.1996.0210 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Arunkumar, R., Calvo, C. M., Conrady, C. D. & Bernstein, P. S. What do we know about the macular pigment in AMD: the past, the present, and the future. Eye32, 992–1004. 10.1038/s41433-018-0044-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson, E. J., Avendano, E. E., Mohn, E. S. & Raman, G. The association between macular pigment optical density and visual function outcomes: a systematic review and meta-analysis. Eye (London England). 35, 1620–1628. 10.1038/s41433-020-01124-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyers, K. J. et al. Genetic evidence for role of carotenoids in age-related macular degeneration in the carotenoids in age-related eye disease study (CAREDS). Investig. Ophthalmol. Vis. Sci.55, 587–599 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatty, S. et al. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest. Ophthalmol. Vis. Sci.42, 439–446 (2001). [PubMed] [Google Scholar]
- 7.Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. Jama309, 2005–2015. 10.1001/jama.2013.4997 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Delori, F. C. Autofluorescence method to measure macular pigment optical densities fluorometry and autofluorescence imaging. Arch. Biochem. Biophys.430, 156–162. 10.1016/j.abb.2004.05.016 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Green-Gomez, M. et al. Standardizing the assessment of macular pigment using a dual-wavelength autofluorescence technique. Translational Vis. Sci. Technol.8, 41–41. 10.1167/tvst.8.6.41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bringmann, A. et al. The primate fovea: Structure, function and development. Prog Retin Eye Res.66, 49–84. 10.1016/j.preteyeres.2018.03.006 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Li, B. et al. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proc. Natl. Acad. Sci.117, 12352–12358. 10.1073/pnas.1922793117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snodderly, D. M., Auran, J. D. & Delori, F. C. The macular pigment. II. Spatial distribution in primate retinas. Invest. Ophthalmol. Vis. Sci.25, 674–685 (1984). [PubMed] [Google Scholar]
- 13.Gass, J. D. M. Müller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacular retinoschisis. Arch. Ophthalmol.117, 821–823. 10.1001/archopht.117.6.821 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Kar, D. et al. Local abundance of macular xanthophyll pigment is associated with rod- and cone-mediated vision in aging and age-related macular degeneration. Investig. Ophthalmol. Vis. Sci.61, 46–46. 10.1167/iovs.61.8.46 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grudzinski, W. et al. Physiological significance of the heterogeneous distribution of zeaxanthin and lutein in the retina of the human eye. Int. J. Mol. Sci.24, 10702 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obana, A. et al. Spatial distribution of macular pigment estimated by autofluorescence imaging in elderly Japanese individuals. Jpn J. Ophthalmol.64, 160–170. 10.1007/s10384-020-00716-5 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Sharifzadeh, M., Bernstein, P. S. & Gellermann, W. Nonmydriatic fluorescence-based quantitative imaging of human macular pigment distributions. J. Opt. Soc. Am. Opt. Image Sci. Vis.23, 2373–2387 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirby, M. L. et al. Foveal anatomic associations with the secondary peak and the slope of the macular pigment spatial profile. Investig. Ophthalmol. Vis. Sci.50, 1383–1391. 10.1167/iovs.08-2494 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Balaratnasingam, C. et al. The spatial profile of macular pigments is related to the topological characteristics of the foveal avascular zone. Investig. Ophthalmol. Vis. Sci.56, 7859–7865. 10.1167/iovs.15-17532 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Hammond, J. B. R., Wooten, B. R. & Snodderly, D. M. Individual variations in the spatial profile of human macular pigment. J. Opt. Soc. Am. A. 14, 1187–1196 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Chen, S. F., Chang, Y. & Wu, J. C. The spatial distribution of macular pigment in humans. Curr. Eye Res.23, 422–434. 10.1076/ceyr.23.6.422.6963 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Wenzel, A. J., Fuld, K., Stringham, J. M. & Curran-Celentano, J. Macular pigment optical density and photophobia light threshold. Vision. Res.46, 4615–4622. 10.1016/j.visres.2006.09.019 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Berendschot, T. T. & van Norren, D. Macular pigment shows ringlike structures. Invest. Ophthalmol. Vis. Sci.47, 709–714. 10.1167/iovs.05-0663 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Allen, P., Calcagni, A., Robson, A. G. & Claridge, E. Investigating the potential of Zernike polynomials to characterise spatial distribution of macular pigment. PLOS ONE. 14, e0217265. 10.1371/journal.pone.0217265 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheibe, P. et al. Parametric model for the 3D reconstruction of individual fovea shape from OCT data. Exp. Eye Res.119, 19–26 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Breher, K., Agarwala, R., Leube, A. & Wahl, S. Direct modeling of foveal pit morphology from distortion-corrected OCT images. Biomedical Opt. Express. 10, 4815–4824. 10.1364/BOE.10.004815 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ctori, I. & Huntjens, B. The association between foveal morphology and macular pigment Spatial distribution: an ethnicity study. PLOS ONE. 12, e0169520. 10.1371/journal.pone.0169520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolan, J. M., Stringham, J. M., Beatty, S. & Snodderly, D. M. Spatial profile of macular pigment and its relationship to foveal architecture. Invest. Ophthalmol. Vis. Sci.49, 2134–2142. 10.1167/iovs.07-0933 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Conrady, C. D. et al. Correlations between macular, skin, and serum carotenoids. Investig. Ophthalmol. Vis. Sci.58, 3616–3627. 10.1167/iovs.17-21818 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ImageJ v. 1.48s (U. S. National Institutes of Health, 1997–2018).
- 31.Ctori, I., Gruppetta, S. & Huntjens, B. The effects of ocular magnification on spectralis spectral domain optical coherence tomography scan length. Graefes Arch. Clin. Exp. Ophthalmol.253, 733–738. 10.1007/s00417-014-2915-9 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Hilton, A. & Armstrong, R. A. Statnote 27: principal components analysis (PCA). Microbiologist12, 37–40 (2011). [Google Scholar]
- 33.Hilton, A. & Armstrong, R. A. Statnote 24: multiple regression. Microbiologist12, 40–43 (2011). [Google Scholar]
- 34.Hilton, A. & Armstrong, R. A. Statnote 25: Stepwise multiple regression. Microbiologist12, 38–39 (2011). [Google Scholar]
- 35.Victor, P. & Camarena-Bernard, C. Lutein, violaxanthin, and Zeaxanthin spectrophotometric quantification: A machine learning approach. J. Appl. Phycol.35, 73–84. 10.1007/s10811-022-02855-3 (2023). [Google Scholar]
- 36.Martin, H. & Dietrich, S. Quantitative reflection spectroscopy at the human ocular fundus. Phys. Med. Biol.47, 179. 10.1088/0031-9155/47/2/301 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Motschi, A. R.et al. Characteristics of Henle’s fiber layer in healthy and glaucoma eyes assessed by polarization-sensitive optical coherence tomography. Biomed. Opt. Express.14, 2709–2725 10.1364/BOE.485327 (2023). [DOI] [PMC free article] [PubMed]
- 38.Lujan, B. J., Roorda, A., Knighton, R. W. & Carroll, J. Revealing Henle’s fiber layer using spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci.52, 1486–1492. 10.1167/iovs.10-5946 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kesim, C. et al. Henle fibre layer mapping with directional optical coherence tomography. Retina42, 1780–1787. 10.1097/IAE.0000000000003514 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Dubis, A. M. et al. Relationship between the foveal avascular zone and foveal pit morphology. Investig. Ophthalmol. Vis. Sci.53, 1628–1636 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Further detailed analysis is available in the Supplementary Information.