Abstract
Using highly purified proteins, we have identified intermediate reactions that lead to the assembly of molecular chaperone complexes with wild-type or mutant p53R175H protein. Hsp90 possesses higher affinity for wild-type p53 than for the conformational mutant p53R175H. The presence of Hsp90 in a complex with wild-type p53 inhibits the binding of Hsp40 and Hsc70 to p53, consequently preventing the formation of wild-type p53–multiple chaperone complexes. The conformational mutant p53R175H can form a stable heterocomplex with Hsp90 only in the presence of Hsc70, Hsp40, Hop and ATP. The anti-apoptotic factor Bag-1 can dissociate Hsp90 from a pre- assembled complex wild-type p53 protein, but it cannot dissociate a pre-assembled p53R175H–Hsp40– Hsc70–Hop–Hsp90 heterocomplex. The results presented here provide possible molecular mechanisms that can help to explain the observed in vivo role of molecular chaperones in the stabilization and cellular localization of wild-type and mutant p53 protein.
Keywords: apoptosis/cancer/degradation/localization
Introduction
Molecular chaperones take part in important cell metabolism and survival reactions through dynamic interactions with various components of these pathways. Chaperones belonging to the Hsp70 and Hsp90 families are key players in cellular events, such as DNA replication and transcription, protein folding and maturation, protein translocation through the endoplasmic reticulum and mitochondrial membranes, proteolysis, cell signaling and the immune response (for reviews see Mayer and Bukau, 1998; Ellis and Hartl, 1999; Jolly and Morimoto, 2000; Zylicz et al., 2001). Following exposure to heat and other environmental stresses, Hsp70-type chaperones participate in stabilizing native protein conformation as well as the refolding and disaggregation of partially unfolded protein (Skowyra et al., 1990). Hsp70 was also identified recently as a potent anti-apoptotic factor (Jaattela et al., 1998; Beere et al., 2000; Li et al. 2000; Mosser et al., 2000; Nylandsted et al., 2000).
The activities of Hsp70 and Hsp90 are modulated by co-chaperones. The Hsp70 co-chaperone Hip is a 50 kDa cytosolic protein that was found to interact with the ATPase domain of Hsc70 and enhance Hsc70–substrate interactions by stabilizing its ADP-bound form (Hohfeld et al., 1995). Hop is a unique co-chaperone that has the ability to interact with both Hsp70 and Hsp90 chaperones, thus providing a physical link between the Hsp70 and Hsp90 chaperone machinery in various systems, such as the glucocorticoid and progesterone hormone receptors (Dittmar et al., 1996; Chen and Smith, 1998). The ‘J’ domain, found in all Hsp40 proteins, is the primary factor that regulates Hsp70 ATPase activity (Wall et al., 1994). The simultaneous presence of Escherichia coli proteins DnaJ and the nucleotide exchange factor GrpE was shown to stimulate at least 50-fold the ATPase activity of the bacterial Hsp70 analog, DnaK (Liberek et al., 1991). The eukaryotic homolog of GrpE, Bag-1, was discovered originally as a Bcl-2-associated protein (Takayama et al., 1995), and it was shown to regulate Hsp70 nucleotide exchange and ATPase activity in a fashion similar to GrpE (Hohfeld et al., 1997; Sondermann et al., 2001). Members of the Bcl-2 family are regulators of apoptosis; Bcl-2, Bcl-XL and Mcl-1 inhibit apoptosis, whereas Bad, Bax, Bik and Bcl-XS promote apoptosis under various conditions (for a review see Hohfeld, 1998). It was shown that Bag-1 enhances the anti-apoptotic effect of Bcl-2 family members (Wang et al., 1996). Bag-1 also specifically interacts with and stimulates the activity of the protein kinase Raf-1, which is involved in signal transduction as well as modulating cell growth and differentiation (Wang et al., 1996). During stress conditions, increased levels of Hsp70 result in the formation of Bag-1–Hsp70 complexes that can compete against Bag-1–Raf-1 complex formation, thus down-regulating Raf-1 kinase activity (Song et al., 2001). Bag-1 can also modulate apoptosis by binding to the cellular domains of either the hepatocyte growth factor (HGF) receptor or the platelet-derived growth factor (PDGF) receptor (Bardelli et al., 1996).
During stress conditions, apoptosis can be induced by the p53 tumor suppressor protein. p53 is a transcription factor that initiates the transcription not only of sets of genes required for apoptosis but also of genes involved in cell growth arrest and DNA repair (for a review see Oren, 1999). Mutations in the p53 gene are among the most common genetic disorders in human cancer, including those of breast, colon, lung and liver origin (for review see Zylicz et al., 2001).
Several studies have found the constitutively expres sed Hsc70, and heat-induced Hsp70 or Hsp90 to be associated with mutant p53 but not with wild-type p53 protein (Pinhasi-Kimhi et al., 1986; Hinds et al., 1987; Sturzbecher et al., 1987; Ehrhart et al., 1988; Blagosklonny et al., 1996). Chaperones Hsc70, Hsp90, cyclophilin 40 and p23 have been co-immunoprecipitated exclusively with mutant p53 from an embryo fibroblast cell line that expresses a conformationally temperature- sensitive, murine p53A135V protein (Whitesell et al., 1998). Multiple chaperones were only found associated with p53 at high temperatures, in which p53A135V displayed a mutant conformation. Moreover, the addition of geldanamycin, which selectively inhibits Hsp90 substrate interactions (Panaretou et al., 1998; Schiebel et al., 1998), specifically released Hsp90 from multiple chaperone complexes with mutant p53 and stimulated the translocation of mutant p53 from the cytoplasm into the nucleus (Whitesell et al., 1998). A recent study demonstrated that Hsc70 plays an important role in masking the p53 nuclear localization signal (NLS) sequence while in a complex with the conformational mutant form of p53 (Akakura et al., 2001). Hsc70 in a complex with mutant p53 and other chaperones, such as Hsp90, prevented the mutant p53 NLS from accessing the nuclear import receptor, whereas nuclear import of p53 possessing the wild-type conformation was not affected. Cellular levels of wild-type p53 are regulated in part by ubiquitination and proteasome-mediated degradation (Maki et al., 1996). Hsp90-associated multiple chaperone complexes with mutant p53 protein have been linked to the impairment of mutant p53 ubiquitination. Treatment of cells that express mutant p53 protein with geldanamycin results in the increase of mutant p53 ubiquitination and proteosome-mediated degradation (Whitesell et al., 1997). Results from the above studies strongly suggest that multiple chaperone complexes are responsible for both the cytoplasmic sequestration and stabilization of conformational mutant p53 protein (for a review see Zylicz et al., 2001).
In this report, we utilize a reconstituted in vitro system that consists of highly purified, recombinant proteins to gain further insight into the mechanisms of molecular chaperone interactions with both wild-type and mutant p53 tumor suppressor proteins. We have discovered that wild-type and mutant p53 protein form different intermediate chaperone complexes in the presence of multiple chaperones, which can be distinguished by their ability to resist dissociation by Bag-1.
Results
Human recombinant wild-type p53 and the conformational mutant p53R175H tumor suppressor proteins were purified to near homogeneity using a new purification procedure (see Materials and methods). As previously described (Hupp et al., 1992), the recombinant wild-type p53 protein required activation by phosphorylation or protein interactions in order to bind DNA specifically, whereas the conformational mutant p53R175H displayed no detectable binding to the consensus DNA sequence, derived from the p21 promoter (data not shown). Conformational analysis of recombinant wild-type p53 and mutant p53R175H proteins by enzyme-linked immunosorbent assay (ELISA) showed that wild-type p53 is recognized by the wild-type conformational antibody Pab1620, whereas p53R175H was not detected. Under the same conditions, mutant p53R175H was detected by the mutant conformational antibody Pab240, while wild-type p53 displayed a weak response (data not shown).
Hsc70 can bind to both wild-type and mutant p53 in the presence of Hsp40 and ATP
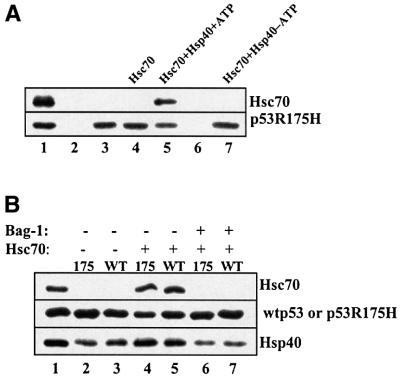
Immunoprecipitation was used to monitor interactions between Hsc70 and wild-type or mutant p53 protein. Hsc70 alone with ATP was unable to form a stable complex with mutant p53R175H (Figure 1A, lane 4). However, the addition of Hsp40 resulted in the co-immunoprecipitation of Hsc70 with mutant p53R175H in a reaction that required ATP (Figure 1A, compare lanes 4, 5 and 7). For non-specific binding controls, we show that p53 (lane 2) or Hsc70 and Hsp40 (lane 6) do not interact non-specifically with protein A–Sepharose under the conditions used for these experiments. Similar controls were performed for each immunoprecipitation experiment presented here, and non-specific binding to protein A–Sepharose or p53 monoclonal antibodies was not detected for any of the applied proteins (data not shown).
Fig. 1. Hsc70 co-immunoprecipitates with either wild-type or mutant p53 Arg175His in the presence of Hsp40 and ATP. (A) Mutant p53R175H (0.25 µM) was incubated alone (lane 3) or with 2 µM Hsc70 (lanes 4–7) or 1 µM Hsp40 (lanes 5–7) in the presence (lanes 2–6) or absence (lane 7) of 1 mM ATP for 1 h at 25°C. p53–chaperone complexes were immunoprecipitated with monoclonal antibody DO-1 and protein A–Sepharose as described in Materials and methods and resolved on a 9% SDS–polyacrylamide gel. Proteins were then transferred to nitrocellulose membrane and detected by the appropriate antibody as indicated (right side of panel). Lane 1 contains protein markers; lane 2 contains p53 and protein A–Sepharose but no DO-1 antibody; and lane 6 contains Hsc70 and Hsp40 but no p53. (B) Wild-type p53 (0.25 µM) (lanes 3, 5 and 7) or mutant p53R175H (lanes 2, 4 and 6) was incubated with 2 µM Hsc70 (lanes 4–7) or 1 µM Hsp40 (lanes 2–7) and ATP for 1 h at 25°C, followed by the addition of 6 µM Bag-1 (lanes 6 and 7) and a further incubation for 30 min at 25°C. p53–chaperone complexes were immunoprecipitated as above.
Surprisingly, in the presence of Hsp40 and ATP, Hsc70 was also detected in a stable complex with wild-type p53 (Figure 1B, lane 5). Hsp40 alone displayed the ability to bind to both wild-type and mutant p53R175H in the absence of Hsc70 and ATP (Figure 1B, lanes 2 and 3, and data not shown), which suggests that Hsp40 may be the driving factor for the formation of stable Hsc70–p53 complexes.
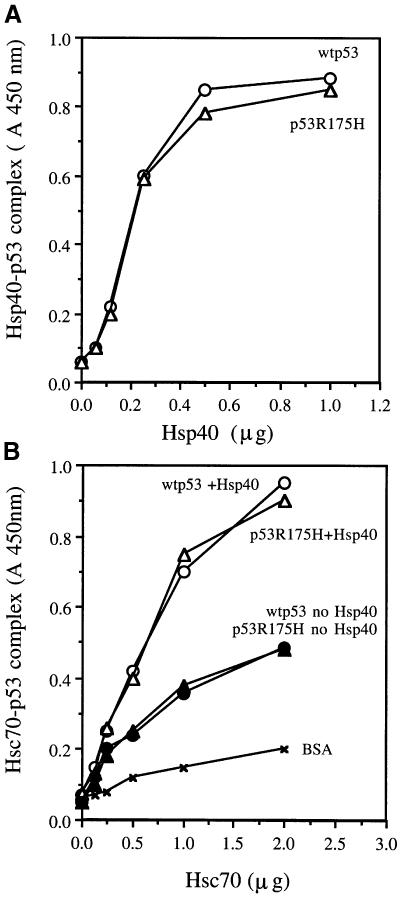
Hsc70 and Hsp40 interactions with p53 were also observed through experiments performed with ELISA. Hsp40 alone was able to bind to wild-type and mutant p53R175H proteins in the absence of ATP (Figure 2A). Unlike the relatively strong interactions between p53 and Hsp40, detection of complexes between Hsc70 alone and either wild-type p53 or mutant p53R175H protein required cross-linking with glutaraldehyde (Figure 2B), which indicates that Hsc70 interactions with p53 may be weak and transient. Similarly to immunoprecipitation experiments, the presence of both Hsp40 and ATP were required for the detection of a stable complex between Hsc70 and wild-type or mutant p53R175H (Figure 2B). The binding affinities of Hsc70 for wild-type p53 or mutant p53R175H protein are of similar magnitude, which suggests that purified mutant p53R175H protein is not a partially denatured protein. Previous studies by several laboratories have demonstrated that Hsp70 molecular chaperones bind more efficiently to denatured protein as compared with native protein structures (for reviews see Mayer and Bukau, 1998; Ellis and Hartl, 1999).
Fig. 2. Both Hsp40 and Hsc70 form a complex with p53 as detected by ELISA. (A) The indicated amounts of Hsp40 were coated onto an ELISA plate as described in Materials and methods, followed by the addition of 50 ng of wild-type p53 or p53R175H in 50 µl of ELISA reaction buffer and a 30 min incubation at 25°C. Hsp40–p53 complexes were detected by p53 antibody DO-1 as described in Materials and methods (no glutaraldehyde cross-linking was used). (B) Mutant p53R175H or wild-type p53 (200 ng) was coated onto an ELISA plate as described in Materials and methods. The indicated amounts of Hsc70 in the presence or absence of 1 mM ATP or 1 µg of Hsp40 were then added in 50 µl of ELISA reaction buffer for 1 h at room temperature, followed by cross-linking with the addition of glutaraldehyde for 1 min. Hsc70–p53 complexes were detected by anti-Hsc70 monoclonal antibodies as described in Materials and methods.
Results from the above experiments clearly demonstrate that Hsc70 can form a complex with both wild-type and mutant p53 with a minimal set of factors, Hsp40 and ATP. The fact that previous in vivo experiments show only mutant p53 co-immunoprecipitation with Hsc70 suggests that other cellular proteins may be involved in regulating chaperone interactions with wild-type p53 protein.
The anti-apoptotic factor, Bag-1, dissociates Hsc70 from pre-assembled complexes with p53
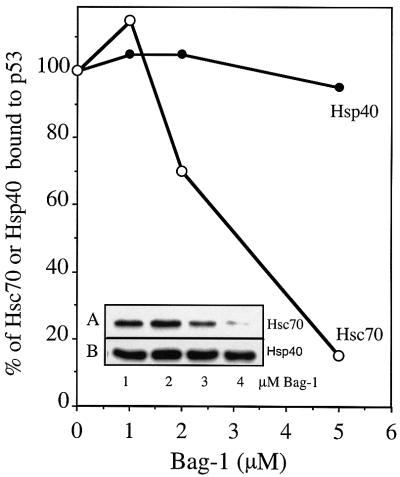
The anti-apoptotic factor Bag-1 was able to dissociate Hsc70 completely from a pre-assembled complex with either wild-type or mutant p53R175H (Figure 1B, lanes 6 and 7). To demonstrate the specificity of Bag-1 for Hsc70, we added increasing amounts of Bag-1 to reactions that contained mutant p53R175H in a pre-assembled complex with either Hsp40 alone or with both Hsp40 and Hsc70 (Figure 3). Bag-1 selectively displaced Hsc70 from the mutant p53 immunocomplex in a concentration-dependent manner, while Bag-1 was unable to release significant amounts of Hsp40 bound to mutant p53 protein. In addition, Bag-1 was not detected in an immunocomplex with either wild-type or mutant p53 in the absence or presence of Hsp40 and Hsc70 (data not shown).
Fig. 3. Bag-1 specifically dissociates Hsc70 from a pre-assembled complex with p53. Mutant p53R175H (0.25 µM) was incubated with 2 µM Hsc70 and 1 µM Hsp40 (lanes 1–4, A) or 1 µM Hsp40 (lanes 1–4, B) in the presence of 1 mM ATP for 1 h, followed by the addition of 1, 2 or 5 µM Bag-1 (lanes 2–4) and a further incubation for 30 min at 25°C. p53–chaperone complexes were immunoprecipitated with monoclonal antibody Pab421 as described in Figure 1. The presence of Hsc70 and/or Hsp40 in the complex with p53 was detected by densitometric analysis of western blots.
Hsp90 binds exclusively to p53 that possesses wild-type conformation
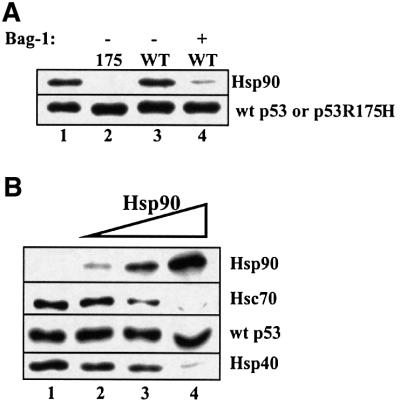
Interestingly, Hsp90 alone was observed to co-immunoprecipitate exclusively with wild-type p53, but not with the mutant p53R175H (Figure 4A, compare lanes 2 and 3). In addition, this complex between Hsp90 and wild-type p53 was vulnerable to dissociation by Bag-1 (Figure 4A, lane 4). To examine the influence of Hsp90 on Hsc70 interactions with wild-type p53, we added increasing amounts of Hsp90 to reactions that contained wild-type p53, Hsc70 and Hsp40. In a concentration-dependent manner, Hsp90 displayed the ability to bind to wild-type p53 and displace both Hsc70 and Hsp40 (Figure 4B). Since Hsp90 has not been shown previously to interact directly with either Hsc70 or Hsp40, then it is likely that Hsp90 may interact with the same p53 domains that are required for Hsc70 and Hsp40 binding. The specificity of Hsp90 for wild-type p53 protein was confirmed by using two additional independent methods: a modified ELISA assay (Wawrzynow and Zylicz, 1995) and the resonant mirror biosensor Iasys plus (Affinity Sensors) technique (Edwards et al., 1995).
Fig. 4. Hsp90 forms a stable complex with wild-type p53. (A) Wild-type p53 (0.25 µM) (lanes 3 and 4) or p53R175H (lane 2) was incubated with 3 µM Hsp90 and 1 mM ATP (lanes 2–4) for 1 h at 25°C, followed by the addition of 4 µM Bag-1 (lane 4) and a further incubation for 30 min at 25°C. p53–chaperone complexes were immunoprecipitated with monoclonal antibody Pab421 as described in Figure 1. (B) Wild-type p53 (0.25 µM) was incubated with 2 µM Hsc70, 1 µM Hsp40 and 1 mM ATP (lane 1–4) in the absence (lane 1) or presence of 2, 4 or 8 µM Hsp90 (lanes 2–4) for 1 h at 25°C. p53–chaperone complexes were immunoprecipitated as above.
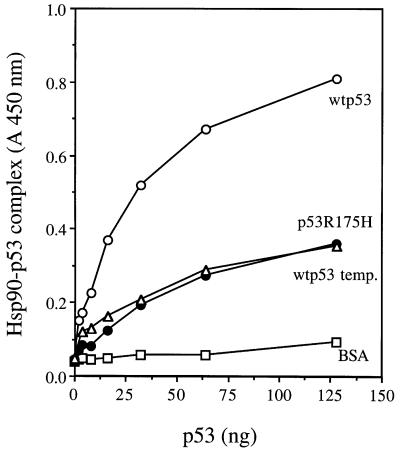
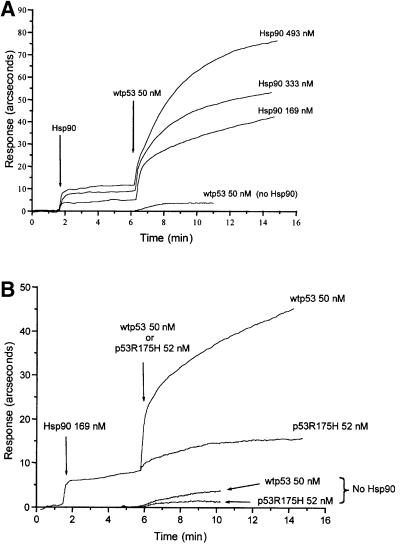
For ELISA, Hsp90 protein was first coated on to the wells of an ELISA plate, followed by the blocking of any remaining plate surface with excess bovine serum albumin (BSA). Wild-type p53 or mutant p53R175H was then added in the presence of ATP and a 1000 molar excess of BSA to prevent non-specific protein–protein interactions. Following washing, the amount of p53 bound to Hsp90 was detected by anti-p53 DO-1 antibodies as described in Materials and methods. As shown in Figure 5, Hsp90 forms a stable complex with wild-type p53 protein, whereas the mutant p53R175H displays a much lower affinity for Hsp90 (Figure 5). As described by others (for a review see Zylicz et al., 2001), the wild-type conformation of p53 is very unstable. Following a 90 min exposure at 37°C, wild-type p53 is not detected by the wild-type p53 conformational antibody, Pab1620 (data not shown), and it also has a drastically reduced affinity for the Hsp90 chaperone (Figure 5), which strongly suggests that Hsp90 specifically recognizes the wild-type conformation of the p53 tumor suppressor protein. Using the resonant mirror biosensor Iasys plus (Affinity Sensors) technique, we also demonstrate that a specific Hsp90–wtp53 complex is formed in the presence of Hop. The Hop protein was covalently linked to a carboxymethyl dextran (CMD) cuvette as described in Materials and methods. The addition of increasing amounts of Hsp90 resulted in formation of a Hop–Hsp90 complex (Figure 6A). After reaching a state of equilibrium between the formation and dissociation of a Hsp90–Hop–CMD complex, wild-type p53 protein was then added to the reaction. Each experiment was performed with the same concentration of p53 protein in the presence of increasing amounts of Hsp90. As Hsp90 concentrations were increased, the amount of wild-type p53 protein bound to Hsp90–Hop– CMD was also increased (Figure 6A), which suggests that p53 interacts with Hop through a Hsp90–p53 complex. In the absence of Hsp90, the affinity of wild-type p53 protein for Hop–CMD was reduced drastically (Figure 6A). Under the same conditions, interactions between the conformational mutant p53R175H and the Hsp90–Hop–CMD complex were not detected (Figure 6B), which indicates that the observed interactions between wild-type p53 protein and Hsp90–Hop are specific.
Fig. 5. Hsp90 binds to p53 that possesses wild-type conformation. Hsp90 (500 ng) or BSA (500 ng) was coated onto an ELISA plate as described in Materials and methods. The indicated amounts of wtp53, wtp53 pre-incubated for 90 min at 37°C or mutant p53R175H protein were then added in 50 µl of ELISA reaction buffer containing 1 mM ATP for 1 h at 25°C, followed by detection of p53 bound to Hsp90 as described in Materials and methods.
Fig. 6. Binding of wild-type or mutant p53R175H to Hop immobilized on a biosensor chip in the presence of Hsp90. (A) The indicated amount of Hsp90 was incubated in a Hop–CMD cuvette for 4 min, followed by the addition of wild-type p53 (50 nM). For control experiments, the same amount of wild-type p53 (50 nM) was incubated with a Hop–CMD cuvette in the absence of Hsp90. (B) The Hop–CMD cuvette was incubated with either wild-type p53 (50 nM) or mutant p53R175H (52 nM) in the presence or absence of Hsp90. Protein–protein binding reactions were carried out at 24°C.
In summary, the above experiments suggest that Hsp90 may be a cellular factor that can discriminate between wild-type and mutant p53 conformations.
Mutant p53, but not wild-type forms a stable complex with multiple chaperones that displays resistance to Bag-1 dissociation
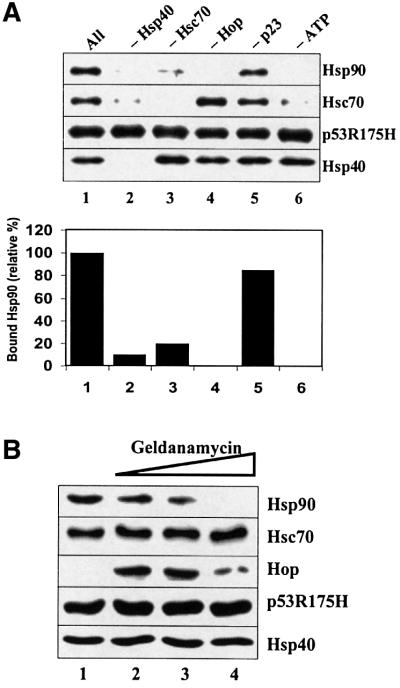
Further experiments were performed to examine the effects of multiple chaperones, including Hop and p23, on Hsp90 and Hsc70 association with either wild-type or mutant p53. Hsp90 was shown to co-immunoprecipitate with mutant p53R175H in the presence of Hsc70, Hsp40, Hop, p23 and ATP. The Hsp90 co-chaperone p23 stabilized bound Hsp90, but it was not required for Hsp90 association with mutant p53 immunocomplexes (Figure 7A). We show here that Hsc70, Hsp40, Hop and ATP are all critical factors required for Hsp90 binding to mutant p53R175H protein. Removal of any of these chaperones or ATP resulted in little or no Hsp90 co-immunoprecipitation with mutant p53 (Figure 7A). The removal of Hop from the reaction resulted in no Hsp90 being detected; however, both Hsc70 and Hsp40 remained associated with the p53 immunocomplex. Removal of ATP inhibited both Hsc70 and Hsp90 binding, whereas Hsp40 remained bound to mutant p53. If Hsp40 was removed from the reaction, then no chaperones were detected in a complex with p53R175H. These results suggest that Hsp40 may form intermediate complexes that act as the initial building blocks for the loading of other chaperones onto mutant p53R175H protein. Consistent with results from previous in vivo studies (Whitesell et al., 1998), we also did not detect Hop in a heterocomplex with mutant p53R175H. However, the addition of the antibiotic geldanamycin resulted in strong detection of Hop bound to mutant p53 immunocomplexes in the presence of multiple chaperones (Figure 7B). As the amount of geldanamycin was increased, Hsp90 was displaced selectively from the mutant p53 heterocomplex. However, the amount of bound Hsc70 and Hsp40 remained constant under the same conditions. Reconstituted glucocorticoid receptor heterocomplexes that were treated with geldanamycin also showed a displacement of Hsp90 (Morishima et al., 2000), which suggests that our in vitro reconstituted mutant p53R175H–multiple chaperone complex is assembled similarly to the glucocorticoid receptor–multiple chaperone complex.
Fig. 7. Chaperones required for the formation of a multiple chaperone complex with mutant p53. (A) Mutant p53R175H (0.25 µM) was incubated with 2 µM Hsc70, 1 µM Hsp40, 2 µM Hsp90, 0.4 µM Hop and 1 mM ATP for 1 h at 25°C, followed by the addition of 1 µM p23 and a further incubation for 15 min at 25°C. Lane 1 contains ‘All’ of the components listed above, whereas lanes 2–6 have the indicated component depleted from the reaction. p53–chaperone complexes were immunoprecipitated with monoclonal antibody Pab421 as described in Figure 1. (B) Mutant p53R175H (0.25 µM) was incubated with 2 µM Hsc70, 1 µM Hsp40, 2 µM Hsp90, 0.4 µM Hop and 1 mM ATP (lanes 1–4) in the absence (lane 1) or presence (lanes 2–4) of 5, 10 or 20 µM geldanamycin for 1 h at 25°C, followed by the addition of 1 µM p23 (lanes 1–4) and a further incubation for 15 min at 25°C. p53–chaperone complexes were immunoprecipitated as above.
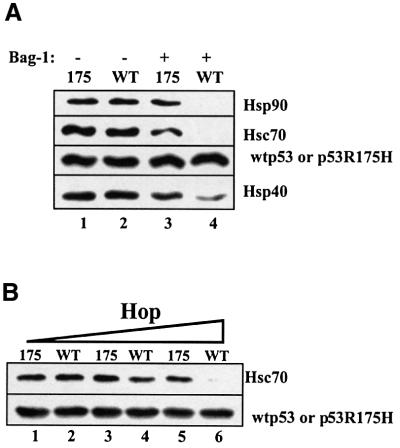
After establishing the optimal parameters for multiple chaperone complex formation with mutant p53R175H protein, we then examined the fate of multiple chaperones in a pre-assembled complex with either wild-type or mutant p53 protein, following the subsequent exposure to Bag-1 protein. Following the incubation of either wild-type p53 or mutant p53R175H with Hsc70, Hsp40, Hsp90 and Hop for 1 h at 25°C, the profile of chaperones bound to either the wild-type or mutant p53 immunocomplex are almost identical (Figure 8A, compare lanes 1 and 2). However, following the addition of Bag-1 and a further incubation for 30 min, apparent differences were observed in the amount of chaperones co-immunoprecipitated with wild-type or mutant p53R175H protein (Figure 8A, compare lanes 3 and 4). Both Hsc70 and Hsp90 were displaced from wild-type p53 by Bag-1, whereas substantial amounts of Hsc70 and Hsp90 remained bound to the mutant p53R175H heterocomplex. A marked reduction in the amount of Hsp40 bound to wild-type p53 was also observed. Next, wild-type or mutant p53 was pre-assembled with Hsc70, Hsp40, Hsp90 and increasing amounts of Hop, followed by the addition of Bag-1 (Figure 8B). As Hop concentrations were increased, the resistance of wild-type p53 heterocomplexes to Bag-1 dissociation was decreased.
Fig. 8. Mutant p53, but not wild-type 53 forms a complex with multiple chaperones that displays resistance to Bag-1 dissociation. (A) Wild-type p53 (0.25 µM) or mutant p53R175H was incubated with 2 µM Hsc70, 1 µM Hsp40, 2 µM Hsp90, 0.4 µM Hop and 1 mM ATP (lanes 1–4) for 1 h at 25°C, followed by the addition of 4 µM Bag-1 (lanes 3 and 4) and a further incubation for 30 min at 25°C. p53–chaperone complexes were immunoprecipitated with monoclonal antibody Pab421 as described in Figure 1. (B) Wild-type p53 (0.25 µM) or mutant p53R175H was incubated with 2 µM Hsc70, 1 µM Hsp40, 2 µM Hsp90, 1 mM ATP and 0.1 µM Hop (lanes 1 and 2), 0.2 µM Hop (lanes 3 and 4) or 0.4 µM Hop (lanes 5 and 6) for 1 h at 25°C, followed by the addition of 4 µM Bag-1 (lanes 1–6) and a further incubation for 30 min at 25°C. p53–chaperone complexes were immunoprecipitated as above.
Results from the above experiments indicate that the wild-type and mutant p53 form different types of heterocomplexes in the presence of multiple chaperones. Hop facilitates the formation of wild-type p53–chaperone complexes that are vulnerable to Bag-1 dissociation in the presence of Hsc70, Hsp40 and Hsp90, whereas mutant p53R175H–multiple chaperone complexes are more resistant to Bag-1-mediated dissociation.
Discussion
The existence of mutant p53–multiple chaperone complexes in vivo has been well documented (Pinhasi-Kimhi et al., 1986; Hinds et al., 1987; Sturzbecher et al., 1987; Ehrhart et al., 1988; Blagosklonny et al., 1996; Whitesell et al., 1998). Recent studies have shown that such heterocomplexes in vivo are responsible for the cytoplasmic sequestration of mutant p53 (Akakura et al., 2001) as well as the inhibition of proteasome-mediated proteolysis of mutant p53 (Whitesell et al., 1997). In this report, we have demonstrated for the first time that the co-chaperone Hsp40 binds directly to mutant p53R175H, forming an p53R175H–Hsp40 complex that is an essential precursor for the formation of an p53R175H–Hsp40–Hsc70–Hop– Hsp90 multiple chaperone complex, which displays resistance to Bag-1-mediated dissociation.
We have demonstrated that Bag-1 can selectively displace Hsc70, but not Hsp40 from pre-assembled complexes with wild-type or mutant p53 protein. Bag-1 has been shown previously to dissociate Hsc70 from pre-assembled complexes with denatured substrate, such as luciferase (Luders et al., 2000). The resistance of mutant p53–multiple chaperone complexes to Bag-1-mediated dissociation may be due to the inability of Bag-1 to release Hsp40 from a complex with mutant p53. Even if Bag-1 was able to dissociate some chaperones from a mutant p53 heterocomplex, the presence of Bag-1-resistant mutant p53–Hsp40 complexes could shift the equilibrium towards multiple chaperone complex formation.
However, in the case of wild-type p53 protein, Hsp90 is able to displace Hsp40 from a wild-type p53 complex, thus preventing the formation of wild-type p53 multiple chaperone complexes. The results from five different experimental methods suggest that Hsp90 selectively binds to wild-type but not mutant p53 protein: (i) Hsp90 co-immunoprecipitates with wild-type p53 but not with mutant p53R175H protein; (ii) Hsp90 inhibits Hsp40 and Hsc70 binding to wild-type p53 protein in a concentration-dependent manner; (iii) Hsp90 forms a stable complex with wild-type p53 as detected by ELISA, whereas p53R175H or heat-treated wild-type p53 have a much weaker affinity for Hsp90; (iv) the presence of Hsp90 increases the affinity of wild-type but not mutant p53R175H for a Hop–CMD complex as detected by the resonant mirror biosensor Iasys plus (Affinity Sensors) technique; and (v) Hsp90 stabilizes wild-type p53 sequence-specific DNA-binding activity as detected by the gel shift assay (our unpublished results).
As demonstrated in this report, Bag-1 can dissociate a pre-assembled wild-type p53–Hsp90 complex. This result may explain well documented in vivo results, which have shown that wild-type p53 is unable to form a stable complex with chaperones under conditions in which mutant p53–chaperone immunocomplexes are detected. The mechanism for Bag-1 dissociation of wild-type p53–Hsp90 complexes is not known. The ability of Bag-1 to regulate Hsp70 nucleotide exchange and ATPase activity may also apply to Hsp90.
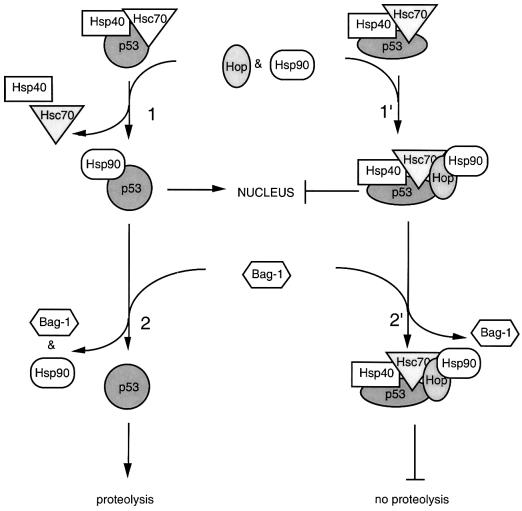
Based on the above combined results, we have constructed a hypothetical model that explains the different pathways taken by either wild-type or mutant p53 in the presence of multiple chaperones (Figure 9). Under non-stress conditions, both mutant and wild-type p53 proteins can be complexed with Hsc70 and Hsp40 chaperones. Such complexes may suppress the oligomerization of p53 protein in cytoplasm. Preliminary results have shown that Hsc70, Hsp40 and ATP are required for the in vitro dissociation of p53 oligomers (unpublished results). In the presence of Hsp90 and Hop, wild-type p53 forms a complex with Hsp90 (Figure 9, step 1). We propose that Hsp90 may assist wild-type p53 import into the nucleus (Zylicz et al., 2001). The wild-type p53–Hsp90 complex can be dissociated by Bag-1 (Figure 9, step 2), which results in free wild-type p53 that can be targeted for proteolysis. During stress conditions, increased Hsp70 levels could result in the formation of Bag-1–Hsp70 complexes (Song et al., 2001), which may in turn lead to the sequestration of Bag-1 and the subsequent import of wild-type p53–Hsp90 complexes into the nucleus.
Fig. 9. A hypothetical model for wild-type or mutant p53 interactions with multiple chaperones (see Discussion for details).
Conformational mutant p53 would follow a different pathway. In the presence of Hsp90 and Hop, mutant p53 can form a heterocomplex with Hsc70, Hsp40, Hsp90 and possibly Hop (Figure 9, step 1′). Since mutant p53 displayed little or no affinity for Hsp90, Hsp90 is not loaded directly onto mutant p53 (Figure 9, step 2′). Instead, mutant p53 remains in an intermediate heterocomplex with multiple chaperones. Chaperones bound to mutant p53 could facilitate both the stabilization and cytoplasmic sequestration of mutant p53 by masking p53 domains required for proteolysis and import into the nucleus.
As we have suggested in this report, chaperones Hsc70 and Hsp90 not only associate with mutant p53, but they also may play important roles in regulating wild-type p53 activity during normal cell growth and stress conditions. Understanding the mechanisms of chaperone interactions with p53 protein could help ultimately to explain how molecular chaperones can influence apoptosis, the dominant-negative effect of certain p53 mutants and other events that potentially can lead to cell transformation.
Materials and methods
Protein purification
Plasmids pET-HDJ-1, pETHsc70, pETp23 and pETHop, used for the expression of human recombinant Hsp40, Hsc70, p23 and Hop, respectively, were generous gifts from Dr Richard Morimoto, Northwestern University. Hsp40 (HDJ-1), p23 and Hop proteins were overexpressed in bacteria and purified as previously described (Deloche et al., 1997; Sullivan et al., 1997; Chen and Smith, 1998). Human Bag-1 was overexpressed in Sf9 insect cells and purified as previously described (Luders et al., 2000). Hsp90 was purified from bovine brain as previously described (Sullivan et al., 1997). Hsc70 was purified by a modified version of a previously published protocol (Freeman et al., 1995). Following the elution of Hsc70 from a DEAE-cellulose (Sigma) column, peak fractions that contained Hsc70 were pooled and precipitated with 0.35 g/ml ammonium sulfate. The precipitate was isolated by centrifugation, resuspended in a minimum volume of buffer A [10 mM imidazole pH 7.0, 10% sucrose, 0.1% β-mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluoride (PMSF) and 0.1 mM EDTA] and dialyzed in buffer A. Following dialysis, magnesium chloride was added to a final concentration of 10 mM, and protein was applied to an ATP–agarose column (Sigma: C8-linkage), equilibrated with buffer B (buffer A containing 10 mM MgCl2). The column was washed with 5 column volumes of buffer B containing 0.5 M NaCl, followed by 5 column volumes of buffer B, and protein was eluted with buffer B containing 5 mM ATP. Fractions that contained Hsc70 were pooled, dialyzed in buffer C (25 mM HEPES–KOH pH 7.6, 10% sucrose, 0.1% β-mercaptoethanol and 5 mM EDTA) and applied to a Q-Sepharose column. Protein was eluted with a 0–400 mM KCl linear gradient. Peak fractions containing Hsc70 were pooled, precipitated with ammonium sulfate (as above), dialyzed in buffer D [25 mM HEPES–KOH pH 7.6, 10% glycerol, 1 mM dithiothreitol (DTT), 0.1 mM EDTA and 50 mM KCl] and stored at –70°C. Casein kinase II was purified from rabbit muscle as previously described (Hupp and Lane, 1995).
Purification of p53 protein
BL21(DE3) strain E.coli cells containing the plasmid pT7-Hup53 (a generous gift from Dr Ted Hupp, Dundee University) were grown at 30°C in Luria broth to an OD600 = 0.6–0.7 and then induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 25°C. Approxim ately 25 g of induced E.coli cells were resuspended and incubated in 250 ml of lysis buffer (25 mM HEPES–KOH pH 7.3, 10% sucrose, 0.1% β-mercaptoethanol, 1 mM PMSF, 0.2 mg/ml lysozyme and 150 mM KCl) for 30 min on ice. Triton X-100 was added to a final concentration of 0.1%, and cells were incubated for an additional 15 min on ice. Cells were then disrupted by sonication and centrifuged for 1 h at 100 000 g. The supernatant was applied to a Q-Sepharose (Sigma) column (2.5 × 12 cm) that was equilibrated with buffer E (25 mM HEPES–KOH pH 7.3, 10% sucrose, 0.1% β-mercaptoethanol, 1 mM PMSF, 0.1% Triton X-100 and 150 mM KCl). Flowthrough fractions (non-bound) that contained p53 protein were pooled together and applied to a P11-Phosphocellulose (Whatman) column (2.5 × 8 cm), equilibrated with buffer E. The column was washed with buffer E containing 250 mM KCl, and protein was eluted by a step gradient with 1 column volume of buffer E containing 500 mM KCl, followed by buffer E containing 1.0 M KCl. All eluted fractions that contained p53 protein were pooled, dialyzed in buffer F (500 mM potassium phosphate pH 6.8, 10% sucrose, 0.1% β-mercaptoethanol, 1 mM PMSF, 0.1% Triton X-100 and 200 mM KCl) and applied to a phenyl-Sepharose (Pharmacia) column (2.5 × 6 cm). The column was washed with buffer F that contained 400 mM potassium phosphate, and p53 protein was eluted with buffer G (10 mM potassium phosphate pH 6.8, 10% sucrose, 0.1% β-mercaptoethanol, 1 mM PMSF, 0.1% Triton X-100 and 200 mM KCl). All fractions containing p53 protein were pooled, dialyzed in buffer G and applied to a hydroxyapatite (BioRad) column (1.5 × 6 cm). The column was washed with buffer G containing 80 mM potassium phosphate, and p53 was eluted with a 80–450 mM potassium phosphate linear gradient (100 ml). Peak fractions that contained p53 protein were dialyzed in buffer H (25 mM HEPES–KOH pH 7.6, 10% glycerol, 5 mM DTT, 0.1% Triton X-100 and 200 mM KCl) and applied to a Q-Sepharose column (1.5 × 3 cm). The column was washed with buffer H, and p53 protein was eluted by a step gradient with 1 column volume of buffer H containing 500 mM KCl, followed by buffer H containing 1.0 M KCl. All fractions containing p53 protein were pooled together, concentrated to 1–5 mg/ml with a Centricon YM-10 concentrator (Amicon) and stored at –80°C.
ELISA
Protein interactions were detected by a modified version of an ELISA that was described previously (Wawrzynow and Zylicz, 1995). The indicated amount of p53, Hsp90 or Hsp40 proteins was coated onto 96-well polystyrene plates (Costar) in 50 µl of coating buffer (25 mM HEPES–KOH pH 7.6, 5 mM DTT and 250 mM KCl) for 1 h at room temperature. The wells were blocked for 1 h at room temperature with 100 µl of blocking-wash buffer (25 mM HEPES–KOH pH 7.6, 5 mM DTT, 150 mM KCl and 2 mg/ml BSA). For the detection of Hsc70–p53 interactions, the indicated amounts of Hsc70 and/or Hsp40 were then added in 50 µl of ELISA reaction buffer (25 mM HEPES–KOH pH 7.6, 5% glycerol, 5 mM DTT, 5 mM MgCl2, 0.05% Triton X-100, 150 mM KCl, 2 mg/ml BSA and 1 mM ATP) for 1 h at 25°C, followed by cross-linking with the addition of glutaraldehyde (final concentration 0.1%) for 1 min. Hsc70–p53 complexes were detected by the addition of anti-Hsc70 rat monoclonal antibodies (SPA-815, StressGen Biotechnologies) in 50 µl of TBS + 2 mg/ml BSA for 1 h at room temperature, followed by anti-rat IgG–horseradish peroxidase (HRP) secondary antibodies (StressGen Biotechnologies). For the detection of Hsp90–p53 or Hsp40–p53 complexes, the indicated amounts of p53 were added in 50 µl of ELISA reaction buffer for 1 h at 25°C (no cross-linking). Complexes were detected by the addition of anti-p53 monoclonal antibody DO-1, followed by anti-mouse IgG–HRP secondary antibodies (Santa Cruz Biotechnology) as described above. For detection of p53 conformation, antibodies Pab240 or Pab1620 (Oncogene Science) were added to p53-coated wells in 50 µl of blocking-wash buffer for 1 h at room temperature, followed by anti-mouse IgG–HRP secondary antibodies (Santa Cruz Biotechnology). Analysis of bound antibodies was performed by colorimetric detection with the TMB peroxidase EIA substrate kit (BioRad), followed by absorbance measurements with a microplate reader (BioRad) at 450 nm. All ELISAs were repeated at least three times each, and the error of each point was never greater than 5%.
Immunoprecipitation
Wild-type p53 or p53R175H (250 nM) was incubated with the indicated amounts of chaperones in 60 µl of IP buffer (25 mM HEPES–KOH pH 7.6, 5% glycerol, 2 mM DTT, 5 mM MgCl2, 0.1% Triton X-100, 100 mM KCl, 1 mg/ml BSA, 1 mM ATP and ATP regeneration system) for 1 h at 25°C, followed by the addition of 0.6 µg of p53 monoclonal antibodies Pab421 or DO-1 (Oncogene Science), 120 µg of protein A–Sepharose (Pharmacia) and a further incubation for 15 min at room temperature. Immunocomplexes were washed three times with 1 ml of IP wash buffer (25 mM HEPES–KOH pH 7.6, 5% glycerol, 1 mM DTT, 1% Triton X-100 and 100 mM KCl) at 4°C. For reactions that contained Hsp90, 20 mM sodium molybdate was included in the IP wash buffer in order to stabilize pre-assembled Hsp90 complexes during the wash. Bound protein was released from the final immunopellet with SDS sample buffer at 100°C for 5 min and resolved on a 9% SDS–polyacrylamide gel. Proteins were transferred to nitrocellulose membrane (BioRad) and detected by the appropriate antibody as follows: p53 detected by DO-1, Hsp40 detected by polyclonal antibody SPA-400 (StressGen), Hsc70 detected by monoclonal antibody clone 3a3 (Affinity Bioreagents), Hop detected by monoclonal antibody clone DS14F5 (StressGen) and Hsp90 detected by polyclonal antibody SPA-771 (StressGen).
Protein–protein interactions monitored by the Affinity Sensors technique
Binding studies were carried out by using the resonant mirror biosensor Iasys plus (Affinity Sensors). CMD-type cuvettes with two wells were used. Immobilization of Hop onto a CMD cuvette was performed essentially as described (Davies et al., 1994) by activating the carboxyl groups of CMD with EDC/NHS for 7 min, and then incubating the protein with the activated CMD for a suitable time (7 min). After blocking the activated unreacted carboxyl groups with 1 M ethanolamine and washing with 25 mM HEPES–KOH pH 7.6, 100 mM KCl buffer several times, Hop (5.2 ng/mm2) was covalently linked to the CMD matrix (Hop–CMD). Hop was coupled in one well of the cuvette, whereas the second well was used as a control, which was subjected to the same treatment except for the protein immobilization step. All the experiments were carried out at 24°C. Binding studies were performed by first adding Hsp90 to the reaction, followed by the addition of wild-type p53 (wtp53) or p53R175H to Hop–CMD in the indicated volume of CMD buffer (25 mM HEPES–KOH pH 7.6, 150 mM KCl, 5% glycerol, 2 mM DTT, 3 mM ATP, 5 mM MgCl2 and 20 mM sodium molybdate). Hsp90–p53 complexes were removed from the Hop–CMD cuvette by washing with regeneration buffer (CMD buffer containing 3 M KCl).
Acknowledgments
Acknowledgements
We would like to thank Dr Richard Morimoto and Jaewhan Song for the pET-HDJ-1, pETHsc70, pETp23 and pETHop plasmids as well as their support and helpful discussions, Dr Ted Hupp for the pT7p53 plasmids, Drs Enrico Bertoli and Fabio Tanfani in whose laboratory the Affinity Sensor experiments were performed, and Aleksandra Helwak for her help in the purification of some proteins used in these experiments. This work was supported by: the State Committee for Scientific Research Grant number 6P04A4219, the Foundation for Polish Science and a UNESCO grant.
References
- Akakura S., Yoshida,M., Yoneda,Y. and Horinouchi,S. (2001) A role for Hsc70 in regulating nucleocytoplasmic transport of a temperature-sensitive p53 (p53Val-135). J. Biol. Chem., 276, 14649–14657. [DOI] [PubMed] [Google Scholar]
- Bardelli A., Longati ,P., Albero,D., Goruppi,S., Schneider,C., Ponzetto,C. and Comoglio,P,M. (1996) HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J., 15, 6205–6212. [PMC free article] [PubMed] [Google Scholar]
- Beere H.M. et al. (2000) Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biol., 2, 469–475. [DOI] [PubMed] [Google Scholar]
- Blagosklonny M.V., Toretsky,J., Bohen,S. and Neckers,L. (1996) Mutant conformation of p53 translated in vitro or in vivo requires functional Hsp90. Proc. Natl Acad. Sci. USA, 93, 8379–8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. and Smith,D.F. (1998) Hop as an adaptor in the heat shock protein 70 (Hsp70) and Hsp90 chaperone machinery. J. Biol. Chem., 273, 35194–35200. [DOI] [PubMed] [Google Scholar]
- Deloche O., Liberek,K., Zylicz,M. and Georgopoulos,C. (1997) Purification and biochemical properties of Saccharomyces cervisiae Mdj1p, the mitochondrial DnaJ homologue. J. Biol. Chem., 272, 28539–28544. [DOI] [PubMed] [Google Scholar]
- Dittmar K.D., Hutchison,K.A., Owens-Grillo,J.K. and Pratt,W.B. (1996) Reconstitution of the steroid receptor–hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J. Biol. Chem., 271, 12833–12839. [DOI] [PubMed] [Google Scholar]
- Edwards P.R., Gill,A., Pollard-Knight,D.V., Hoare,M., Buckle,P.E., Lowe,P.A. and Leatherbarrow,R.J. (1995) Kinetics of protein– protein interactions at the surface of an optical biosensor. Anal. Biochem., 231, 210–217. [DOI] [PubMed] [Google Scholar]
- Ehrhart J.C., Duthu,A., Ullrich,S., Appella,E. and May,P. (1988) Specific interaction between a subset of the p53 protein family and heat shock proteins hsp72/hsc73 in a human osteosarcoma cell line. Oncogene, 3, 595–603. [PubMed] [Google Scholar]
- Ellis R.J. and Hartl,F.U. (1999) Principles of protein folding in the cellular environment. Curr. Opin. Struct. Biol., 9, 102–110. [DOI] [PubMed] [Google Scholar]
- Freeman B.C., Myers,M.P., Schumacher,R. and Morimoto,R.I. (1995) Identification of a regulatory motif in Hsp70 that effects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J., 14, 2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P.W., Finlay,C.A., Frey,A.B. and Levine,A.J. (1987) Immunological evidence for the association of p53 with a heat shock protein, hsc70, in p53-plus-ras-transformed cell lines. Mol. Cell. Biol., 7, 2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohfeld J. (1998) Regulation of the heat shock conjugate Hsc70 in the mammalian cell: the characterization of the anti-apoptotic protein BAG-1 provides novel insights. Biol. Chem., 379, 269–274. [PubMed] [Google Scholar]
- Hohfeld J. and Jentsch,S. (1997) GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J., 16, 6209–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohfeld J., Minami,Y. and Hartl,F.U. (1995) Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell, 83, 589–598. [DOI] [PubMed] [Google Scholar]
- Hupp T.R. and Lane,D.P. (1995) Two distinct signaling pathways activate the latent DNA binding function of p53 in a casein kinase II-independent manner. J. Biol. Chem., 270, 18165–18174. [DOI] [PubMed] [Google Scholar]
- Hupp T.R., Meek,D.W., Midgley,C.A. and Lane,D.P. (1992) Regulation of the specific DNA binding function of p53. Cell, 71, 875–886. [DOI] [PubMed] [Google Scholar]
- Jaattela M., Wissing,D., Kokholm,K., Kallunki,T. and Egeblad,M. (1998) Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J., 17, 6124–6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C. and Morimoto,R.I. (2000) Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J. Natl Cancer Inst., 92, 1564–1572. [DOI] [PubMed] [Google Scholar]
- Li C.Y., Lee,J.S., Ko,Y.G., Kim,J.I. and Seo,J.S. (2000) Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J. Biol. Chem., 275, 25665–25671. [DOI] [PubMed] [Google Scholar]
- Liberek K., Marszalek,J., Ang,D., Georgopoulos,C. and Zylicz,M. (1991) Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc. Natl Acad. Sci. USA, 88, 2874–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders J., Demand,J., Papp,O. and Hohfeld,J. (2000) Distinct isoforms of the cofactor BAG-1 differentially affect Hsc70 chaperone function. J. Biol. Chem., 275, 14817–14823. [DOI] [PubMed] [Google Scholar]
- Maki C.G., Huibregtse,J.M. and Howley,P.M. (1996) In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res., 56, 2649–2654. [PubMed] [Google Scholar]
- Mayer M.P. and Bukau,B. (1998) Hsp70 chaperone systems: diversity of cellular functions and mechanism of action. Biol. Chem. 379, 261–268. [PubMed] [Google Scholar]
- Morishima Y., Kanelakis,K.C., Silverstein,A.M., Dittmar,K.D., Estrada,L. and Pratt,W.B. (2000) The Hsp organizer protein hop enhances the rate of but is not essential for glucocorticoid receptor folding by the multiprotein Hsp90-based chaperone system. J. Biol. Chem., 275, 6894–6900. [DOI] [PubMed] [Google Scholar]
- Mosser D.D., Caron,A.W., Bourget,L., Meriin,A.B., Sherman,M.Y., Morimoto,R.I. and Massie,B. (2000) The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol., 20, 7146–7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylandsted J., Rohde,M., Brand,K., Bastholm,L., Elling,F. and Jaattela,M. (2000) Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc. Natl Acad. Sci. USA, 97, 7871–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M. (1999) Regulation of the p53 tumor suppressor protein. J. Biol. Chem., 274, 36031–36034. [DOI] [PubMed] [Google Scholar]
- Panaretou B., Prodromou,C., Roe,S.M., O’Brien,R., Ladbury,J.E., Piper,P.W. and Pearl,L.H. (1998) ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J., 17, 4829–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhasi-Kimhi O., Michalovitz,D., Ben-Zeev,A. and Oren,M. (1986) Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature, 320, 182–184. [DOI] [PubMed] [Google Scholar]
- Schiebel T., Weikl,T. and Buchner,J. (1998) Two chaperone sites in Hsp90 differing in substrate specificity and ATP dependence. Proc. Natl Acad. Sci. USA, 95, 1495–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D., Georgopoulos,C. and Zylicz,M. (1990) The Escherichia coli dnaK gene product, the hsp70 homologue, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell, 62, 939–944. [DOI] [PubMed] [Google Scholar]
- Sondermann H., Scheufler,C., Schneider,C., Hohfeld,J., Hartl,F.U. and Moerefi,I. (2001) Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science, 291, 1553–1557. [DOI] [PubMed] [Google Scholar]
- Song J., Takeda,M. and Morimoto,R.I. (2001) Bag1-Hsp70 mediates a physiological stress signaling pathway that regulates Raf-1/ERK and cell growth. Nature Cell Biol., 3, 276–282. [DOI] [PubMed] [Google Scholar]
- Sturzbecher H.W., Chumakov,P., Welch,W.J. and Jenkins,J.R. (1987) Mutant p53 proteins bind hsp 72/73 cellular heat shock-related proteins in SV40-transformed monkey cells. Oncogene, 1, 201–211. [PubMed] [Google Scholar]
- Sullivan W., Stensgard,B., Caucutt,G., Bartha,B., McMahon,N., Alnemri,E., Litwack,G. and Toft,D. (1997) Nucleotides and two functional states of hsp90. J. Biol. Chem., 272, 8007–8012. [DOI] [PubMed] [Google Scholar]
- Takayama S., Sato,T., Krajewski,S., Kochel,K., Irie,S., Millan,J.A. and Reed,J.C. (1995) Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell, 80, 279–284. [DOI] [PubMed] [Google Scholar]
- Wall D., Zylicz,M. and Georgopoulos,C. (1994) The NH2-terminal 108 amino-acids of the Escherichia coli DnaJ protein stimulate the ATPase activity of DnaK and are sufficient for λ DNA replication. J. Biol. Chem., 269, 5446–5451. [PubMed] [Google Scholar]
- Wang H.G., Takayama,S., Rapp,U.R. and Reed,J.C. (1996) Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc. Natl Acad. Sci. USA, 93, 7063–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzynow A. and Zylicz,M. (1995) Divergent effects of ATP on the binding of the DnaK and DnaJ chaperones to each other, or to their various native and denatured protein substrates. J. Biol. Chem., 270, 19300–19306. [DOI] [PubMed] [Google Scholar]
- Whitesell L., Sutphin,P., An,W.G., Schulte,T., Blagosklonny,M.V. and Neckers,L. (1997) Geldanamycin-stimulated destabilization of mutated p53 is mediated by the proteasome in vivo. Oncogene, 14, 2809–2816. [DOI] [PubMed] [Google Scholar]
- Whitesell L., Sutphin,P.D., Pulcini,E.J., Martinez,J.D. and Cook,P.H. (1998) The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an hsp90-binding agent. Mol. Cell. Biol., 18, 1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., King,F.W. and Wawrzynow,A. (2001) The Hsp70 interactions with the p53 tumour suppressor protein. EMBO J., 20, 4634–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]