Abstract
Sequential steps in the activation of the pro-apoptotic protein Bax are described for cells with different sensitivity to cytotoxins. SH-EP1 and SH-SY5Y human neuroblastoma cells, derived from a single precursor cell line, differed in their sensitivity to taxol but showed the same sensitivity to cisplatin. Both drugs, in both cell lines, induced exposure of a constitutively occluded N-terminal epitope of Bax. This was reversible and occurred before the translocation of cytosolic Bax to mitochondria. The N-terminal change in Bax, its subsequent movement to mitochondria and its dimerization/complex formation were insufficient for commitment to death, occurring in the same proportion of cells that either maintained (SH-SY5Y) or lost (SH-EP1) clonogenic survival after taxol treatment. Suppression of taxol-induced apoptosis occurred upstream of cytochrome c release from mitochondria in SH-SY5Y cells. The data suggest that a further drug damage-induced event occurs after Bax dimerization/complex formation but prior to cytochrome c release. This event was absent in the taxol-resistant cells.
Keywords: Bax/chemoresistance/mitochondria/neuroblastoma/taxol
Introduction
How cytotoxins induce mammalian cell death is unclear. Signals initiated by cellular damage may be integrated at a locus where molecules of the Bcl-2 family play a central role in orchestrating the subsequent fate of damaged cells (Adams and Cory, 1998). These damage signals activate pro-apoptotic Bcl-2 family proteins such as Bax and Bak, and one or other of these proteins is absolutely required for drug-induced apoptosis (Wei et al., 2001). Bax and Bak are constitutively expressed and do not always increase in amount after cell damage. Occluded epitopes at the N-termini of the pro-apoptotic proteins Bax or Bak become exposed after disparate apoptotic stimuli (Hsu and Youle, 1998; Desagher et al., 1999; Griffiths et al., 1999; Gilmore et al., 2000; Perez and White, 2000; Taylor et al., 2000). This suggests either that an intramolecular change occurs in Bax or Bak or that a protein bound to their N-terminal domains is released, exposing otherwise cryptic epitopes. The NMR solution structure of Bid suggested that the first α-helix of the N-terminus obscures its BH-3 domain (McDonnell et al., 1999), thought to be critical for pro-death function (Kelekar and Thompson, 1998). Caspase 8-mediated cleavage of the Bid N-terminus, downstream of death receptor signalling (Luo et al., 1998), is predicted to expose its killer BH-3 domain and appears to drive its association with mitochondria (Wei et al., 2000). However, despite suggestions that the structure of Bid may serve as a paradigm for that of Bax, the recent solution structure of monomeric Bax suggests that its N-terminus is highly mobile and does not occlude its BH-3 domain (Suzuki et al., 2000). This would suggest that a conformational change at the N-terminus of Bax, or the loss of an N-terminal domain-binding protein, would not necessarily induce exposure of the BH-3 domain (also thought to be essential for its killing activity; Wang et al., 1998), nor, therefore, would it lead to the engagement of apoptosis. It appears that exposure of an N-terminal epitope of Bax precedes its translocation from cytosol to mitochondria, where it inserts into the outer mitochondrial membrane after oligomerization (Gross et al., 1998; Eskes et al., 2000). How, exactly, Bax initiates apoptosis is unknown, although the opening of channels in the mitochondrial membrane to release apoptogenic factors is probably important (Desagher and Martinou, 2000).
Resistance to damage caused by cytotoxins such as anticancer drugs may arise as a consequence of a variety of damage limitation or repair mechanisms. It may also be determined by the modulation of mechanisms that control an intrinsic program of death (Dive and Hickman, 1991), such as that described for Bax. Drug resistance is a major obstacle to successful treatment of disseminated cancers and it is important to understand the underlying mechanisms. Here we describe how, in a drug-resistant neuroblastoma cell line, drug treatment leads to the exposure of an N-terminal epitope of Bax and its translocation to the mitochondria from the cytosol. Despite this relocation and the formation of Bax dimers/complexes, the majority of cells do not release cytochrome c and do not die. This suggests that event(s) additional to Bax oligomerization/complex formation at the mitochondrial surface are necessary for commitment to drug-induced cell death.
Results
SH-EP1 and SH-SY5Y cells have similar sensitivity to cisplatin but different sensitivity to taxol
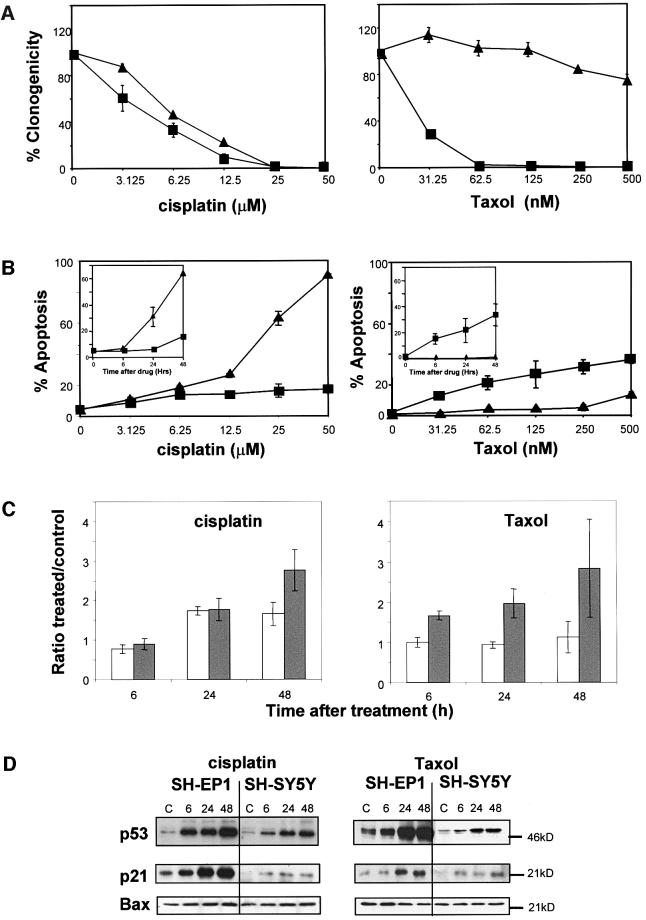
Two subclones of the human neuroblastoma cell line SK-N-SH, SH-EP1 (Schwann cell like) and SH-SY5Y (neuronal like) (Ross et al., 1983), were exposed to two anticancer agents with differing mechanisms of action. Cisplatin damages DNA directly and results in the formation of DNA double strand breaks, and taxol prevents tubulin depolymerization to cause mitotic arrest (Pratt et al., 1994). Both cell lines exhibited a similar concentration-dependent decrease in clonogenicity after exposure to cisplatin. The IC90 was 15 µM for SH-EP1 cells and 20 µM for SH-SY5Y cells (mean ± SE, n = 3, Figure 1A). Despite the similar response to cisplatin with respect to clonogenicity, there was a clear difference in the response of the two cell lines with respect to the percentage of cells exhibiting the morphology of apoptosis at 72 h (Figure 1B). After treatment with 25 µM cisplatin, the IC90 for clonogenicity, only 20% of SH-EP 1 cells were apoptotic, compared with 65% of SH-SY5Y cells. Both cell lines undergo apoptosis after 25 µM cisplatin, although the kinetics were much slower for SH-EP 1 cells (Figure 1B, inset). However, at this concentration of cisplatin, both cell lines underwent cell cycle arrest in G2/M, with similar kinetics (Figure 1C).
Fig. 1. Damage responses of SH-EP1 and SH-SY5Y cells to cisplatin and taxol. (A) Clonogenic survival at 10 days and (B) apoptotic response at 72 h following 1 h treatment with cisplatin or taxol. The kinetics of apoptosis after 25 µM cisplatin or 62.5 nM taxol are shown in (B) as inset graphs. SH-SY5Y, filled triangles; SH-EP1 filled squares. (C) The ratio between control or drug-treated cells as a percentage of cells in G2/M following a 1 h exposure to cisplatin (25 µM) or taxol (62.5 nM): grey bars, SH-EP1 cells; white bars, SH-SY5Y cells. The results shown in (A–C) are means of at least three independent experiments ± SEM. (D) Immunoblots for p53 protein, p21WAF1 and Bax after a 1 h exposure to cisplatin (25 µM) or taxol (62.5 nM). Representative blots of at least three independent experiments are shown.
Despite the similarities in loss of clonogenicity, cell cycle response and ultimate cell fate in response to cisplatin, the cell lines exhibited a very different response to taxol. SH-EP1 cells underwent rapid apoptosis, with 20% of cells having apoptotic morphology by 6 h after treatment with 62.5 nM taxol (mean ± SE, n =3, Figure 1B, inset). This rapid induction of apoptosis correlated with loss of clonogenicity (Figure 1A). In contrast, SH-SY5Y cells were resistant to taxol. Apoptosis was only induced in SH-SY5Y cells after treatment with 500 nM taxol and, even at this concentration, 80% of cells maintained clonogenicity (mean ± SE, n = 3, Figure 1A). In contrast to the situation after treatment with cisplatin where both cell lines undergo cell cycle arrest, taxol induced a rapid and sustained G2/M arrest in SH-EP1 cells, but failed to change the cell cycle phase distribution of SH-SY5Y cells (Figure 1C).
SH-EP1 and SH-SY5Y cells stabilize p53 and up-regulate p21WAF1 after treatment with either cisplatin or taxol, but do not alter their expression levels of Bax
The failure of SH-SY5Y cells to undergo apoptosis after taxol treatment is not due to their failure to ‘sense’ drug-induced damage. Protein levels of p53 are elevated above baseline in both cell lines by 6 h after treatment with either drug (Figure 1D). p53 is active as a transcriptional regulator in SH-EP1 and SH-SY5Y, as demonstrated by the up-regulation of the cyclin-dependent kinase inhibitor p21WAF1 that follows the same time course (Figure 1D). The basal levels of p21WAF1 were lower in SH-SY5Y than in SH-EP1 cells; however, the increases in both p53 and p21WAF1 were the same in SH-SY5Y cells after cisplatin (to which they are sensitive) and after taxol (to which they are resistant). Bax is also a transcriptional target of p53 in certain cell types (Miyashita and Reed, 1995) but neither cisplatin nor taxol altered the expression level of Bax in SH-EP1 or SH-SY5Y cells (Figure 1D).
Drug-induced damage signal(s) result in the exposure of an N-terminal epitope of Bax regardless of cell fate
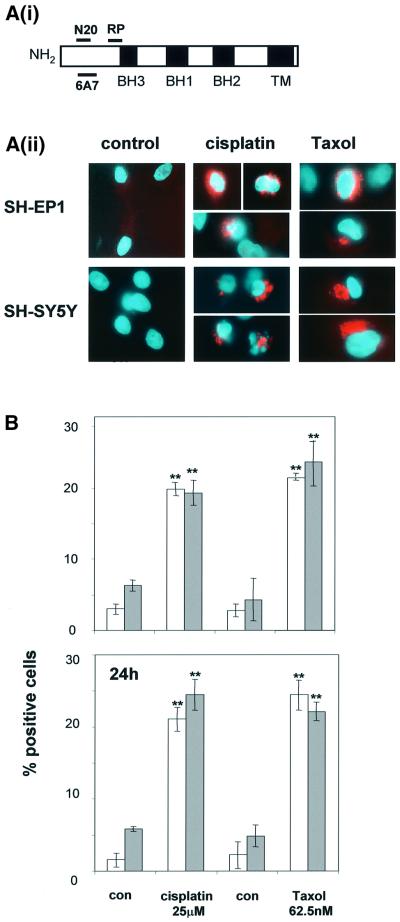
The N-terminus of Bax is occluded in unstressed intact cells and hence is not available for binding by Bax N-terminal epitope-specific antibodies (Desagher et al., 1999). When untreated SH-EP1 or SH-SY5Y cells were stained with an antibody raised against the N-terminus of Bax (N20, amino acids 11–30; see epitope map in Figure 2Ai), no Bax-associated immunofluorescence was detected (Figure 2Aii). However, the N-terminal epitope of Bax became accessible for N20 binding in both cell types after a 1 h exposure to cisplatin (25 µM) or to taxol (62.5 nM). The number of SH-EP1 or SH-SY5Y cells in which the Bax N-terminus was exposed following either cisplatin or taxol treatment is shown in Figure 2Aii. Similar results were obtained using the 6A7 Bax N-terminal-specific antibody (see Figure 2A and data not shown). After exposure of SH-SY5Y cells to 62.5 nM taxol, there was no loss of clonogenicity, no induction of apoptosis and no cell cycle arrest. This same concentration of taxol induced apoptosis and completely abrogated clonogenicity in SH-EP1 cells (Figure 1). In both cell lines treated with 62.5 nM taxol, there was rapid and sustained exposure of the N-terminus of Bax to the same extent (Figure 2B). From these data, we conclude that the N-terminus of Bax is exposed after damage-induced signals regardless of cellular fate.
Fig. 2. Drug-induced exposure of the Bax N-terminus occurs regardless of cell fate. (A) (i) Antibody epitope map of Bax. (ii) Immunostaining of the Bax N-terminus with Bax N20 antibody 24 h after a 1 h exposure to cisplatin (25 µM) or taxol (62.5 nM). Bax N20 staining is seen as red fluorescence. Representative cells shown at magnification ×400. (B) The numbers of cells showing positive staining for Bax N20 at 6 and 24 h after drug treatments (means of three independent experiments ± SEM, **P <0.01 for the comparison between treated and control cells). SH-EP1, open bars; SH-SY5Y, shaded bars.
The exposure of the N-terminus of Bax is a reversible event
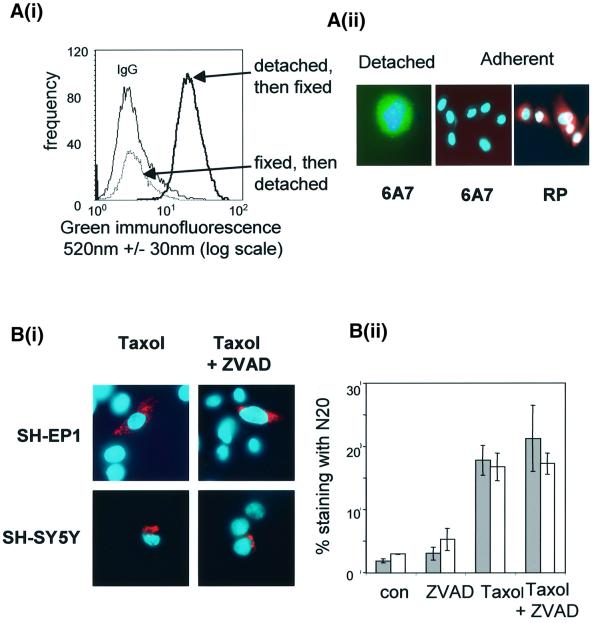
Taxol-treated SH-SY5Y cells maintain clonogenicity (Figure 1), thus the data above pose the question of whether or not the opening of the N-terminus is a reversible event. In attempting to recapitulate the N-terminal immunofluorescence data described above, we observed that when untreated cells that were detached, and then fixed and stained with the Bax N-terminal epitope-specific antibody 6A7 (amino acids 12–24, Figure 2), >90% cells had an exposed Bax N-terminus (Figure 3Ai). This is not merely a subpopulation of cells becoming positive for Bax 6A7, but a shift in the fluorescence intensity of the whole population. Less than 5 min elapsed between cell detachment and fixing, demonstrating that loss of cell–cell and/or cell–substrate interactions resulted in a rapid conformational change in Bax. However, if cells were first fixed and then physically detached from the plastic tissue culture flask prior to staining with 6A7, none of the cells exhibited fluorescence above that of the irrelevant antibody control cells. Furthermore, when a proportion of the detached cells were allowed to reform a monolayer, the fluorescence associated with the N-terminus of Bax was lost (compare the Bax 6A7 staining in the adherent cells with that in the detached cell in Figure 3Aii). The data show that exposure of the N-terminus of Bax is rapid and reversible and is not sufficient per se to determine cell fate.
Fig. 3. Drug-induced exposure of the N-terminus of Bax is reversible and is not blocked by caspase inhibition. (A) (i) Flow cytometric analysis of Bax 6A7 immunofluorescence (see epitope map in Figure 2) of SH-EP1 cells detached then fixed, compared with cells that have been fixed prior to detachment. IgG, histogram representing non-specific staining. These histograms are typical of at least three independent experiments. (ii) A typical cell that was detached then fixed and stained with Bax6A7 (green) (magnification ×500) is compared with Bax 6A7 and RP staining of cells that were allowed to re-attach and then examined 24 h later (magnification ×250). Nuclei were counterstained with Hoechst 33528. (B) (i) Bax N20 immunofluorescence (red) 24 h after taxol treatment (62.5 nM) in the presence or absence of Z-VAD-fmk (50 µM) (magnification ×400). (ii) Percentage of Bax N20-positive cells after taxol treatment in the presence and absence of Z-VAD-fmk. SH-EP1, grey bars; SH-SY5Y, white bars. Results represent the mean of three independent experiments ± SEM.
Drug-induced exposure of the N-terminus of Bax occurs independently of caspase activation
In order to determine whether the exposure of the N-terminus of Bax required drug-induced activation of a caspase cascade, cells were pre-treated for 1 h with the cell-permeable pan-caspase inhibitor Z-VAD-fmk (50 µM) before treatment with taxol (62.5 nM). Figure 3B demonstrates that taxol promoted Bax N20-associated immunofluorescence that was unaffected by caspase inhibition.
Drug treatment alters the subcellular distribution of Bax and this occurs regardless of cell fate
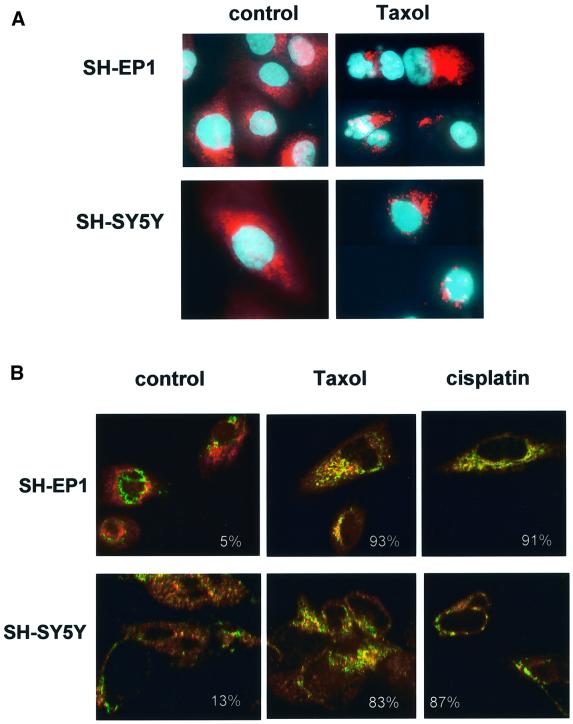
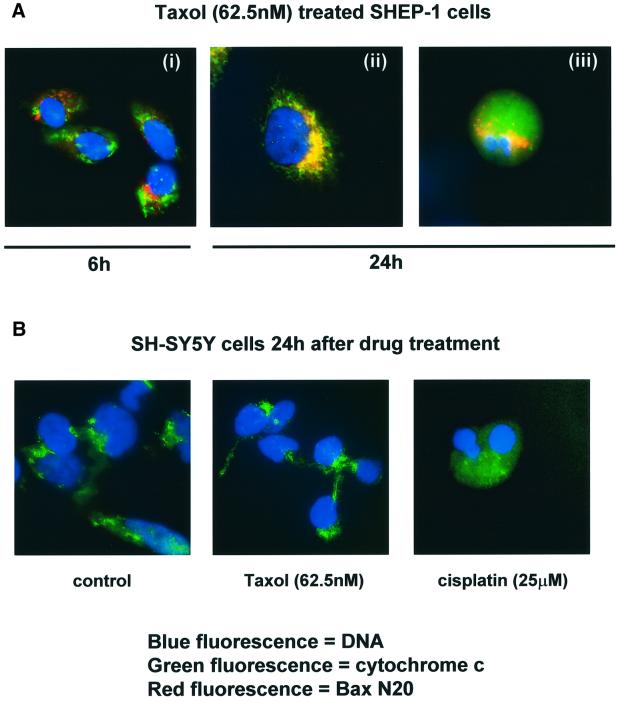
Previous studies showed that damage-induced signals promote the translocation of Bax from cytosol to mitochondrial membranes in certain cell types, whilst others reported that Bax is constitutively located in mitochondria (Wolter et al., 1997; Desagher et al., 1999). Untreated or taxol-treated SH-EP 1 and SH-SY5Y cells were stained with an antibody raised against an epitope proximal to the BH-3 domain of Bax (see Figure 2Ai, BaxRP, amino acids 43–61, red fluorescence). In untreated cells, Bax immunofluorescence was diffuse, whereas a punctate distribution was observed after taxol treatment of either cell line (Figure 4A). Confocal microscopy was used to examine the co-localization of cytochrome c (green fluorescence) and Bax (RP, red fluorescence) (Figure 4B). Minimal co-localization (yellow fluorescence) was seen in untreated cells, whereas in both cell lines, after either drug treatment, there was an increase in co-localization. Alternative studies that confirmed the translocation of Bax to mitochondria after drug damage were performed by assessing the degree of Bax co-localization with the mitochondrial dye Mitotracker Red™, or with an antibody to cytochrome oxidase subunit IV (Cox IV, data not shown). We then asked whether Bax moved from cytosol to mitochondria before or after the exposure of the N-terminus by damage-induced signal(s). Figure 5Ai shows that 6 h after treatment with taxol, a proportion of Bax molecules were detected with exposed N-terminal epitopes using Bax N20 (red fluorescence) and, although the staining pattern was punctate, this Bax was not co-localized with mitochondria (no coincidence with cytochrome c immunofluorescence). By 24 h after drug treatment, SH-EP1 cells were detected with the N-terminal epitope exposed and with co-localized cytochrome c (e.g. Figure 5Aii, yellow fluorescence). At this time, cells were also seen with diffuse cytochrome c and Bax N20 staining; such cells always had apoptotic nuclei (e.g. Figure 5Aiii). These data were recapitulated using Mitotracker Red™ or Cox IV as mitochondrial markers (data not shown).
Fig. 4. Translocation of Bax to mitochondria occurs regardless of cell fate. (A) Immunostaining of SH-EP1 and SH-SY5Y cells 24 h after a 1 h exposure to taxol (62.5 nM) with Bax RP (see Figure 2A), seen as red fluorescence. Nuclei are counterstained with Hoechst 33528 (blue). Magnification ×500. (B) Confocal microscopy of SH-EP1 and SH-SY5Y cells before and 24 h after a 1 h treatment with taxol (62.5 nM) or cisplatin (25 µM) stained with Bax RP (red) and cytochrome c (green). Co-localization is seen as yellow and the percentage of red fluorescence (Bax) associated with green fluorescence (cytochrome c) is shown for each image. Magnification ×500. Images are representative of at least three independent repeat experiments.
Fig. 5. (A) The exposure of the N-terminus of Bax occurs prior to translocation to mitochondria and the release of cytochrome c. Immunostaining of SH-EP1 cells 6 h (i) and 24 h (ii and iii) after a 1 h exposure to taxol (62.5 nM) with Bax N20 (red fluorescence) and cytochrome c (green fluorescence). Co-localization is seen as yellow. Nuclei are counterstained with Hoechst 33528 (blue fluorescence). Magnification at 6 h is ×500, and at 24 h is ×1000. (B) Abrogation of taxol-induced apoptosis occurs prior to cytochrome c release from mitochondria. Immunostaining of SH-SY5Y cells 24 h after a 1 h exposure to cisplatin (25 µM) or taxol (62.5 nM) with cytochrome c (green fluorescence). Nuclei are counterstained with Hoechst 33528 (blue fluorescence). Magnification for control and taxol-treated cells, ×500; for cisplatin-treated cells, ×1000. Data are representative of at least three repeat experiments in which >100 cells were examined.
Abrogation of drug-induced apoptosis occurs prior to cytochrome c release
Inhibitor of apoptosis proteins (IAPs) are able to prevent apoptosis after the release of mitochondrial cytochrome c (see Green, 2000). If the apoptotic pathway in taxol-treated drug-resistant SH-SY5Y cells was blocked after cytochrome c release, then cells with diffuse cytochrome c immunostaining, but non-apoptotic nuclei, would be seen. This was not the case. The punctate staining for cytochrome c 24 h after taxol treatment was identical to that observed in untreated control cells (Figure 5B). SH-SY5Y cells with diffuse cytochrome c immunostaining were seen frequently after cisplatin treatment (Figure 5B), and infrequently after taxol treatment (data not shown, in keeping with drug response data in Figure 1), but in both cases such cells always had apoptotic nuclear morphology.
Bax forms dimers/complexes in the mitochondrial fraction of taxol-resistant SH-SY5Y cells
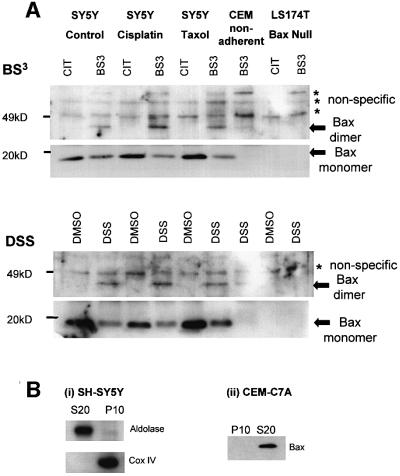
The oligomerization of Bax previously had been reported to occur only in apoptotic cells (Antonsson et al., 2001). The oligomerization state of Bax in SH-SY5Y cells was examined 24 h after a 1 h treatment with cisplatin (25 mM) or taxol (62.5 nM) (Figure 6A). In the presence of the soluble cross-linkers disuccinimidyl suberate (DSS) or bis[sulfosuccinimidyl]suberate (BS3), a Bax immunoreactive band of ∼46 kDa, previously reported as a Bax homodimer (Eskes et al., 2000), could be immunoprecipitated from the mitochondrial P10 fraction (Figure 6A). This 46 kDa Bax immunoreactive band was not detected in the Bax null colon carcinoma cell line LS174T (Carethers and Pham, 2000). In addition, the amount of monomeric Bax decreased when Bax dimers/complexes were formed. Together, these observations suggest that the 46 kDa band was Bax specific. Surprisingly, Bax dimers/complexes were observed in both drug-treated and untreated SH-SY5Y cells where in all cases Bax could be immunoprecipitated from the mitochondrial fraction. This conflicts with the confocal microscopy data in Figure 4 where Bax was not mitochondrial in untreated cells. When the T-lymphoma cell line CEMC7A (which grows in suspension and expresses Bax, see Figure 6B) was examined, Bax could not be immunoprecipitated from the mitochondrial fraction, and the 46 kDa Bax dimer/complex was barely detectable. Taken together, these data suggest that the 46 kDa band is a Bax dimer or complex, and that loss of cell–substrate interaction is sufficient stimulus for its formation. This additionally suggests that Bax dimerization/complex formation in mitochondria is insufficient for cell death as not only does this occur after taxol treatment, when clonogenicity is not lost, but it also occurs in untreated cells that do not lose viability after cell detachment.
Fig. 6. Formation of Bax dimers/complexes in the mitochondrial fraction of SH-SY5Y cells occurs regardless of cell fate. (A) At 24 h after a 1 h treatment of cells with cisplatin (25 µM) or taxol (62.5 nM), the multimerization of Bax was assessed by cross-linking the P10 fraction with DSS or BS3. Bax complexes were immunoprecipitated (using Bax 2D2) and western blotted using Bax RP. A Bax immunoreactive band at ∼46 kDa (marked with an arrow) was detected only in the presence of cross-linker, and not in the Bax null LS174T cells nor in the suspension cell line CEMC7A. Bax monomers were seen at 20 kDa (marked with an arrow) in SH-SY5Y cells but were not detectable in CEM cells despite their expression of Bax; see B(ii). Three bands noted between 50 and 70 kDa occurred in the presence or absence of the cross-linker and in the Bax null cell line (asterisks). All the data for each cross-linker are from the same gel although the middle section of the gel has been cut to exclude immunoglobulin light chain. CIT, citrate buffer alone; DMSO, dimethylsulfoxide alone. (B) The purity of SH-SY5Y P10 and S20 fractions was assessed using aldolase as a cytosolic marker and cytochrome oxidase IV (Cox IV) as a mitochondrial marker (i) and Bax was detected in the cytosolic but not the mitochondrial fractions of CEM C7A cells (ii). Results shown are typical of three independent experiments.
Discussion
Changes in the conformation of Bax, its relocation from cytosol to mitochondria and its oligomerization in the mitochondrial membrane are presumed to be important for the initiation of apoptosis after drug-induced damage (Wolter et al., 1997; Ghatan et al., 2000; Murphy et al., 2000; Antonsson et al., 2001). Here we have shown that none of these changes in Bax are sufficient to commit a taxol-resistant neuroblastoma cell line to apoptosis (Figures 1, 2, 4 and 6A). These events occurred to the same extent after drug treatment in two neuroblastoma cell lines derived from a single precursor that differed in their sensitivity to taxol but had the same sensitivity to cisplatin. We have also shown, quantitatively, that changes in the N-terminus of Bax were induced rapidly by loss of cell–cell or cell–substrate contacts, and that these were reversible (Figure 3), supporting similar observations using mouse mammary epithelial cells deprived of matrix attachment (Gilmore et al., 2000).
The abrogation of taxol-induced apoptosis in SH-SY5Y cells occurred upstream of cytochrome c release from mitochondria since cells with diffuse cytochrome c and non-condensed and fragmented nuclei were never observed. Our data therefore suggest that some signal, which is not required for cisplatin-induced apoptosis and probably emanates from a taxol-disrupted cytoskeleton, may be absent in the taxol-resistant SH-SY5Y cells. We presume that this signal is essential for a further activation step of Bax.
How does the exposure of the N-terminus of Bax serve to activate this pro-apoptotic protein? The three-dimensional structure of Bax has been elucidated recently and it suggests that the BH-3 domain of Bax is occluded not by the N-terminus, as had been implied previously (Nechustan et al., 1999), but by its C-terminus (Suzuki et al., 2000). The intramolecular contacts between the Bax C-terminus and its BH-3 domain are thought to prevent the docking of Bax at the mitochondrial surface and to prevent the binding via BH-3 interaction with other family members such as Bcl-xL (Suzuki et al., 2000). This new insight into the structure of soluble monomeric Bax provokes two assumptions with respect to the data presented here. First, if in the cytosol of an intact cell Bax adopts the three-dimensional structure observed in solution, a structural change in Bax to disengage its C-terminus from its BH-3 domain occurred after treatment of SH-EP1 and SH-SY5Y with cisplatin or taxol in order for Bax to translocate from cytosol to mitochondria. Secondly, since the N-terminal change in Bax that we observed in intact SHEP-1 and SH-SY5Y cells after both drug treatments occurs in the cytosol (e.g. Figure 5Ai), it most probably occurs before or concomitantly with C-terminal release of the BH-3 domain and Bax translocation to mitochondria. The structure of Bax suggests that the N-terminus is a flexible region (Suzuki et al., 2000) and it is possible that the exposure of N-terminal epitopes of cytosolic Bax may serve as a ‘reporter’ of the C-terminal change that promotes Bax movement to mitochondria. Whatever the precise relationship between the changes at the C- and N-termini of Bax, we can conclude from the data presented here (Figures 2, 3 and 4) that neither exposure of an occluded epitope in the N-terminus of Bax, nor translocation of Bax to mitochondria is sufficient to induce apoptosis in taxol-treated SH-SY5Y cells, but may instead prime the cell for apoptosis.
In our studies, Bax could be immunoprecipitated from the mitochondrial fraction of non-apoptotic, untreated SH-SY5Y cell lysates (Figure 6A). We suggest that this may reflect its translocation to the mitochondria subsequent to anoikis-mediated exposure of N-terminal epitopes (Figure 3A), in keeping with the rapid and reversible translocation of Bax following loss of cell–substrate interaction in the mouse mammary epithelial cell model (Gilmore et al., 2000). When cells were homogenized and S20 and P10 fractions (enriched for cytosol and mitochondria, respectively) were immunoblotted for Bax, it was present in both fractions in untreated cells (data not shown), contrasting with the subcellular localization observed by immunostaining of intact adherent cells. Bax dimers/complexes were present in the mitochondrial fraction of untreated SH-SY5Y cells, and after either cisplatin or taxol treatment regardless of eventual cell fate (Figure 6A). The majority of Bax in mitochondria remained monomeric after drug treatment, but this was reduced in the presence of the cross-linking agents (Figure 6A), reflecting the heterogeneity of the kinetics of drug-induced apoptosis. In taxol-resistant SH-SY5Y cells, cytochrome c release from mitochondria was abrogated, whilst in the same cells treated with cisplatin cytochrome c is released (Figure 5B). This suggests that in this cell background, cytochrome c release can be abrogated after Bax dimerization/complex formation.
What accounts for this resistance to taxol in the SH-SY5Y cells? The activation of p53 by taxol in both SH-EP1 (taxol sensitive) and SH-SY5Y cells (taxol resistant) suggests that both cell types have ‘sensed’ taxol-induced damage and that the resistance mechanism indeed lies downstream of damage. Bcl-2 overexpression in HeLa cells is able to prevent staurosporine-induced Bax translocation to mitochondria, its oligomerization and apoptosis (Desagher et al., 1999; Antonsson et al., 2001). The taxol-resistant SH-SY5Y cells express much higher levels of endogenous Bcl-2 than do SH-EP1 cells (Hanada et al., 1993; and data not shown), yet higher levels of Bcl-2 per se prevented neither the opening of the N-terminus the translocation of Bax to the mitochondria nor its dimerization/complex formation. Thus in SH-SY5Y cells, if Bcl-2 negates the pro-apoptotic function of Bax, it must do so downstream of these events and in some manner that is specific to taxol, since these same cells are sensitive to cisplatin. Enforced overexpression of Bcl-2 can suppress apoptosis after the translocation of Bax, either by inhibiting the release of cytochrome c (Antonsson et al., 1997; Finucane et al., 1999) or by preventing activation of caspases post-cytochrome c release (Rosse et al., 1998). We therefore suggest that some event, dependent upon taxol-induced damage (but not on cisplatin-induced damage), is absent in the SH-SY5Y cells. We propose that the reversible, structural change at the N-terminus of Bax seen after removal from substratum, or treatment with cisplatin or taxol may be ‘reporter’ of a C-terminal change that permits translocation of Bax to the mitochondria where it can be oligomerized/complexed. However, the release of cytochrome c only occurs on receipt of a further signal, and this signal is absent in SH-SY5Y but not SH-EP1 cells after taxol treatment. If a critical inhibitor of apoptosis, such as an IAP, functioned downstream of cytochrome c release, then SH-SY5Y cells would present with diffuse cytochrome c staining and nuclear morphology indicative of cell viability after taxol treatment. This was not observed. A clue to the possible identity of a second signal required for Bax activation post-oligomerization/complex formation comes from our observation that the resistant SH-SY5Y cells failed to undergo arrest in G2/M phase of the cell cycle (Figure 1C). Failure to arrest occurred despite having stabilized p53 in a transcriptionally active form (Figure 1D). It is therefore reasonable to suggest that this second Bax-activating signal might emanate from a G2/M checkpoint process that does not operate in taxol-treated SH-SY5Y cells. We currently are examining the role of G2/M-associated kinases in taxol-induced apoptosis. Bim is a strong candidate as the recipient of such a signal and might be expected to play a role in coupling taxol-induced damage to the engagement of apoptosis. Importantly, lymphocytes from bim–/– mice are 10- to 30-fold resistant to this drug (Bouillet et al., 1999) and Bim has been shown recently to be critical for neuronal apoptosis (Putcha et al., 2001).
The requirement for a ‘multidomain’ pro-apoptotic member Bax or Bak in drug-induced apoptosis has been demonstrated using bax–/–/bak–/– double knock out mice (Wei et al., 2001). These data do not exclude the possibility that in neuroblastoma cells, Bak, rather than Bax, is the more important death promoter after drug treatment. However, studies using bax–/– mice led to the conclusion that Bax is the key regulator of apoptosis in developing neurons (White et al., 1998). Furthermore, the lack of a phenotype in bak–/– mice (Lindsten et al., 2000) indicates that of these two key promoters of apoptosis, Bax is the more important, and indeed is required for neuronal apoptosis, at least during development. Neuroblastoma is the commonest extracranial solid neoplasm in childhood and when it regresses spontaneously it is suggested that this is due to the delayed activation of apoptosis (Pritchard and Hickman, 1994). By gaining a fuller understanding of the signalling events that couple drug-induced damage signals to the engagement of apoptosis, we hope to reveal how drug-damaged yet resistant neuroblastoma cells fail to execute the apoptotic pathway.
Materials and methods
Tissue culture
SH-EP1 and SH-SY5Y cells, derived from the parental cell line SK-N-SH, were the kind gift of Dr Robert Ross (Bronx University, NY). They were grown in Dulbecco’s modified Eagle’s medium/F12 nutrient mix supplemented with 15% heat-inactivated fetal calf serum (both from Life Technologies). Cells were used between passages 10 and 40 from the first subcloning. CEMC7A human T-lymphoma cells were cultured as described previously (Griffiths et al., 1999). LS174T Bax null human colon carcinoma cells were the kind gift of Professor Christos Paraskeva (Bristol University, UK) and were cultured as described previously (Carethers and Pham, 2000).
Assessment of clonogenicity and apoptosis
Standard methods were used to determine clonogenic survival (Wilson, 1986). Cells were plated at 500 viable cells/well and 24 h later cells were treated for 1 h with either taxol (31.25–500 nM) or cisplatin (3.125– 50 µM). Cells were washed with drug-free medium and cell colonies (>50 cells) counted at 10 (for SH-EP1) or 14 days (for SH-SY5Y). The absolute cloning efficiency was between 40 and 50%. Assays were performed in duplicate with at least three independent repeats per treatment. The percentage of apoptotic cell deaths was assessed 72 h after treating cells with varying concentrations of either taxol or cisplatin. Cells were fixed and stained with Hoechst 33528 (1 µg/ml) and the numbers of cells with nuclear morphology consistent with apoptosis were counted. Assays were performed in duplicate and, for each, two fields of 100 cells were counted.
Cell cycle analysis by flow cytometry
Cells were treated at subconfluence for 1 h with taxol (62.5 nM) or cisplatin (25 µM) and were collected at 6, 24 and 48 h after drug treatment. Cells were resuspended in glucose-modified saline (140 mM NaCl, 6 mM glucose, 50 mM KCl, 1 mM NaH2PO4·2H2O, 1 mM KH2PO4 and 700 µM EDTA) and then fixed with ice-cold ethanol [95% (v/v)]. DNA content was evaluated by staining cells with propidium iodide [10 µg/ml in phosphate-buffered saline (PBS) containing 100 µg/ml RNase; Molecular Probes]. Flow cytometric analysis was performed on a FACS Vantage flow cytometer equipped with an Enterprise laser (Innova Technology) set to excite at 250 mV using the 488 nm laser line. Red fluorescence (DNA-bound propidium) was detected at 630 ± 22 nm. Fluorescence was acquired using linear amplifiers. A total of 10 000 cells were analysed per sample at a flow rate of 300–500 cells/s. CellFit software (BD) was used to evaluate the data.
Western blotting
Cells were harvested by mechanical detachment into ice-cold versene (Life Technologies), centrifuged at 10 000 g for 2 min and then lysed by sonication. Protein content was determined using Bradford protein assay reagent (Bio-Rad). Proteins were separated by SDS–PAGE (Harlow and Lane, 1988) and transferred to PVDF membranes (Immobilon, Millipore). Membranes were blocked with 5% non-fat dried milk in 0.05% Tris-buffered saline-Tween (TBST), probed with primary antibody in 0.05% TBST at 4°C overnight, and then with secondary antibody conjugated to horseradish peroxidase (HRP) in 5% milk/0.05% TBST for 1 h at room temperature. Blots were visualized by ECL (NEN Life Sciences). Primary antibodies were from Oncogene Science, p53 mAb Ab6, p21WAF mAb Ab1; BD-PharMingen, Bax pAb RP (amino acids 43–61); Santa Cruz, Bax pAb N20 (amino acids 11–30); Trevigen, Bax mAb 6A7 (amino acids 12–24); and Sigma, actin mAb AC40. Secondary antibodies were either goat anti-mouse conjugated to HRP or goat anti-rabbit–HRP (both from Dako).
Subcellular fractionation and cross-linking studies
SH-SY5Y cells in exponential growth phase were treated with either taxol (62.5 nM) or cisplatin (25 µM) for 1 h. After 24 h, cells were mechanically detached on ice and pelleted. Pellets were resuspended in isolation buffer [0.1 M KCl, 0.12 M sucrose, 1 mM EDTA, 10 mM HEPES–KOH pH 7.4, 0.1% (w/v) bovine serum albumin], with protease inhibitors (Sigma) and disrupted using a Dounce homogenizer (100 strokes) until >90% cells no longer excluded trypan blue. The homogenate was spun at 2000 g for 5 min at 4°C, and pellets and supernatants were recombined and re-homogenized (using a further 50 strokes). After centrifugation at 2000 g for 10 min at 4°C, the supernatant was removed and spun at 10 300 g for 15 min at 4°C to generate the P10 pellet. The supernatant was spun again at 20 000 g for 20 min at 4°C to generate the S20 supernatant enriched in cytosol. A 0.25 mg aliquot of total protein of the P10 was spun at 10 000 g for 2 min, and resuspended in MB-EGTA (210 mM mannitol, 70 mM sucrose, 0.5 mM EGTA, 10 mM HEPES). The suspension was incubated with either DSS in dimethylsulfoxide (DMSO) or BS3 in 5 mM citrate buffer pH 5 (both cross-linkers were from Pierce) or the appropriate solvent control to a final concentration of 2 mM for 30 min at room temperature. The cross-linking reaction was quenched by the addition of Tris–HCl (pH 7.5) to a final concentration of 20 mM. Membranes were cleared by the addition of RIPA buffer and centrifuged at 12 000 g for 2 min. Bax was immunoprecipitated from the supernatant using the 2D2 monoclonal antibody (Trevigen), and immunoblotted using the Bax RP antibody.
Immunofluorescence microscopy
SH-EP1 (1 × 105 cells/well) and SH-SY5Y cells (7 × 105 cells/well) were seeded into 8-well tissue culture chamber slides (Falcon). After treatment with either taxol (62.5 nM) or cisplatin (25 µM), cells were fixed with formaldehyde [1% (v/v)] for 5 min and stained with primary antibody in 500 µg/ml digitonin (ICN) overnight at 4°C. Slides were then incubated with appropriate secondary antibody at room temperature for 1 h in darkness: goat anti-rabbit IgG conjugated to Cy3 (Jackson ImmunoResearch, Westgrove, PA) or goat anti-mouse IgG conjugated to fluorescein isothiocyanate (FITC; Dako). Nuclei were counterstained with Hoechst 33528 (1 µg/ml in PBS). Slides were examined with a Zeiss Axioplan 2 microscope fitted with the KS300 imaging system (Imaging Associates, Thame, UK). Images were colorized in Adobe PhotoShop. Confocal images were taken using an Ultraview confocal optical scanner with a Kr/Ar laser (Perkin-Elmer) mounted on an Olympus Ix70 microscope. Images were acquired with an Ulrapix digital camera and processed using the Ultraview software package.
Flow cytometry
Cells were collected either by 2 min incubation in trypsin followed by fixation in 1% formaldehyde in PBS for 5 min or by mechanical detachment of cells subsequent to fixation in situ. Cells were stained with primary antibody in digitonin (500 µg/ml in PBS) overnight at 4°C. Cells were then incubated with appropriate secondary antibody at room temperature for 1 h in the dark; goat anti-mouse IgG–FITC or donkey anti-rabbit IgG–FITC (Jackson ImmunoResearch). Mouse anti-Aspergillus niger glucose oxidase (Dako) was used as an irrelevant primary antibody control. Flow cytometric analysis was performed as above; green fluorescence (FITC) was detected at 530 ± 30 nm. Fluorescence was acquired using logarithmic amplifiers. A total of 10 000 cells were analysed per sample at a flow rate of 300–500 cells/s. CellFit software (BD) was used to evaluate the data.
Statistics
Means of data sets were compared for the significance of the difference between them using a two-tailed two-sample t-test. P-values <0.01 are shown with double asterisks over the relevant data sets.
Acknowledgments
Acknowledgements
The authors thank Dr Mauro Degli Esposti for his constructive comments on the manuscript and Katya Dohrendorf for technical assistance with flow cytometry. G.W.J.M. was funded by the Christie Hospital endowment fund, and B.M.C. and G.J.G. were funded by the Cancer Research Campaign. C.D. is a Fellow of the Lister Institute of Preventive Medicine.
References
- Adams J.M. and Cory,S. (1998) The Bcl-2 protein family: arbiters of cell survival. Science, 281, 1322–1326. [DOI] [PubMed] [Google Scholar]
- Antonsson B. et al. (1997) Inhibition of Bax channel-forming activity by Bcl-2. Science, 277, 370–372. [DOI] [PubMed] [Google Scholar]
- Antonsson B., Montessuit,S., Sanchez,B. and Martinou,J.-C. (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem., 276, 11615–11623. [DOI] [PubMed] [Google Scholar]
- Bouillet P., Metcalf,D., Huang,D.C., Tarlington,D.M., Kay,T.W., Kontgen,F., Adams,J.M. and Strasser,A. (1999) Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostatsis and to preclude autoimmunity. Science, 286, 1735–1738. [DOI] [PubMed] [Google Scholar]
- Carethers J.M. and Pham,T.T. (2000) Mutations of transforming growth factor β1 type II receptor, Bax and insulin like growth factor II receptor genes in microsatellite unstable cell lines. In Vivo, 14, 13–20. [PubMed] [Google Scholar]
- Desagher S. and Martinou,J.-C. (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol., 10, 369–377. [DOI] [PubMed] [Google Scholar]
- Desagher S., Osen-Sand,A., Nichols,A., Eskes,R., Montessuit,S., Lauper,S., Maundrell,K., Antonsson,B. and Martinou,J.C. (1999) Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol., 144, 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dive C. and Hickman,J.A. (1991) Drug–target interactions: only the first step in the commitment to programmed cell death? Br. J. Cancer, 64, 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R., Desagher,S., Antonsson,B. and Martinou,J.C. (2000) Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol., 20, 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane D.M., Bossy-Wetzel,E., Waterhouse,N.J., Cotter,T.G. and Green,D.R. (1999) Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-XL. J. Biol. Chem., 274, 2225–2233. [DOI] [PubMed] [Google Scholar]
- Ghatan S., Larner,S., Kinoshita,Y., Hetman,M., Patel,L., Xia,Z., Youle,R.J. and Morrison,R.S. (2000) p38 MAP kinase mediates Bax translocation in nitric oxide-induced apoptosis in neurons. J. Cell Biol., 150, 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A.P., Metcalfe,A.D., Romer,L.H. and Streuli,C.H. (2000) Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J. Cell Biol., 149, 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.R. (2000) Apoptotic pathways: paper wraps stone blunts scissors. Cell, 102, 2–4. [DOI] [PubMed] [Google Scholar]
- Griffiths G.J., Dubrez,L., Jones,N.H., Morgan,C.P., Whitehouse,J., Corfe,B.M., Dive,C. and Hickman,J.A. (1999) Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol., 144, 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A., Jockel,J., Wei,M.C. and Korsmeyer,S.J. (1998) Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J., 17, 3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada M., Krajewski,S., Tanaka,S., Cazalshatem,D., Spengler,B.A., Ross,R.A., Biedler,J.L. and Reed,J.C. (1993) Regulation of Bcl-2 oncoprotein levels with differentiation of human neuroblastoma cells. Cancer Res., 53, 4978–4986. [PubMed] [Google Scholar]
- Harlow E. and Lane,D. (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Hsu Y.T. and Youle,R.J. (1998) Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem., 273, 10777–10783. [DOI] [PubMed] [Google Scholar]
- Kelekar A. and Thompson,C.B. (1998) Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol., 8, 324–330. [DOI] [PubMed] [Google Scholar]
- Lindsten T. et al. (2000) The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell, 6, 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Budihardjo,I., Zou,H., Slaughter,C. and Wang,X. (1998) Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell, 94, 481–490. [DOI] [PubMed] [Google Scholar]
- McDonnell J.M., Fushman,D., Milliman,C.L., Korsmeyer,S.J. and Cowburn,D. (1999) Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell, 96, 625–634. [DOI] [PubMed] [Google Scholar]
- Miyashita T. and Reed,J.C. (1995) Tumor-suppressor p53 is a direct transcriptional activator of the human bax gene. Cell, 80, 293–299. [DOI] [PubMed] [Google Scholar]
- Murphy K.M., Streips,U.N. and Lock,R.B. (2000) Bcl-2 inhibits a Fas-induced conformational change in the Bax N terminus and Bax mitochondrial translocation. J. Biol. Chem., 275, 17225–17228. [DOI] [PubMed] [Google Scholar]
- Nechustan A., Smith,C.L., Hsu,Y.T. and Youle,R.J. (1999) Con formation of the Bax C-terminus regulates sub-cellular location and cell death. EMBO J., 18, 2330–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez D. and White,E. (2000) TNF-α signals apoptosis through a Bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol. Cell, 6, 53–63. [PubMed] [Google Scholar]
- Pratt W.B., Ruddon,R.W., Ensminger,W.D. and Maybaum,J. (1994) The Anticancer Drugs. Oxford University Press, Oxford, UK.
- Pritchard J. and Hickman,J.A. (1994) Why does stage 4s neuroblastoma regress spontaneously? Lancet, 344, 869–870. [DOI] [PubMed] [Google Scholar]
- Putcha G.V., Moulder,K.L., Golden,J.P., Bouillet,P., Adams,J.M., Strasser,A. and Johnson,E.M. (2001) Induction of Bim, a proapoptotic Bcl-2 family member, is critical for neuronal apoptosis. Neuron, 29, 615–628. [DOI] [PubMed] [Google Scholar]
- Ross R.A., Spengler,B.A. and Biedler,J.L. (1983) Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J. Natl Cancer Inst., 71, 741–749. [PubMed] [Google Scholar]
- Rosse T., Olivier,R., Monney,L., Rager,M., Conus,S., Fellay,I., Jansen,B. and Borner,C. (1998) Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature, 391, 496–499. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Youle,R.J. and Tjandra,N. (2000) Structure of Bax: co-regulation of dimer formation and intracellular localization. Cell, 103, 645–654. [DOI] [PubMed] [Google Scholar]
- Taylor S.T., Hickman,J.A. and Dive,C. (2000) Epigenetic determinants of resistance to etoposide regulation of Bcl-x(L) and Bax by tumor microenvironmental factors. J. Natl Cancer Inst., 92, 18–23. [DOI] [PubMed] [Google Scholar]
- Wang K., Gross,A., Waksman,G. and Korsmeyer,S.J. (1998) Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol. Cell. Biol., 18, 6083–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M.C., Lindsten,T., Mootha,V.K., Weiler,S., Gross,A., Ashiya,M., Thompson,C.B. and Korsmeyer,S.J. (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev., 14, 2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei M.C. et al. (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science, 292, 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F.A., Keller-Peck,C.R., Knudson,C.M., Korsmeyer,S.J. and Snider,W.D. (1998) Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J. Neurosci., 18, 1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.P. (1986) Cytotoxicity and viability assays. In Freshney,R.I. (ed.), Animal Cell Culture: A Practical Approach. IRL Press, Oxford, UK, pp. 183–216.
- Wolter K.G., Hsu,Y.-T., Smith,C.L., Nechushtan,A., Xi,X.-G. and Youle,R.J. (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol., 139, 1281–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]