Abstract
AP-4 is a member of the family of heterotetrameric adaptor protein (AP) complexes that mediate the sorting of integral membrane proteins in post-Golgi compartments. This complex consists of four subunits (ε, β4, µ4 and σ4) and localizes to the cytoplasmic face of the trans-Golgi network (TGN). Here, we show that the recruitment of endogenous AP-4 to the TGN in vivo is regulated by the small GTP-binding protein ARF1. In addition, we demonstrate a direct interaction of the ε and µ4 subunits of AP-4 with ARF1. ε binds only to ARF1·GTP and requires residues in the switch I and switch II regions of ARF1. In contrast, µ4 binds equally well to the GTP- and GDP-bound forms of ARF1 and is less dependent on switch I and switch II residues. These observations establish AP-4 as an ARF1 effector and suggest a novel mode of interaction between ARF1 and an AP complex involving both constitutive and regulated interactions.
Keywords: AP complex/ARF protein/brefeldin A/direct interaction/membrane recruitment
Introduction
AP-4 is the most recently described member of a family of adaptor protein (AP) complexes that also includes AP-1, AP-2 and AP-3 (reviewed by Kirchhausen, 1999; Robinson and Bonifacino, 2001). Like the other AP complexes, AP-4 is composed of four subunits: ε, β4, µ4 and σ4 (Dell’Angelica et al., 1999; Hirst et al., 1999), of which µ4 recognizes YXXØ-type tyrosine-based sorting signals involved in post-Golgi sorting of transmembrane proteins (Stephens and Banting, 1998; Hirst et al., 1999; Aguilar et al., 2001). To date, the existence of an AP-4 complex has only been demonstrated in mammalian cells (Dell’Angelica et al., 1999; Hirst et al., 1999), although homologs of some AP-4 subunits have also been identified in chicken (Wang and Kilimann, 1997), Dictyostelium discoideum (de Chassey et al., 2001) and Arabidopsis thaliana (reviewed by Boehm and Bonifacino, 2001). The mammalian AP-4 complex is associated with the cytoplasmic face of the trans-Golgi network (TGN), most likely as part of a non-clathrin coat (Dell’Angelica et al., 1999; Hirst et al., 1999). A cytosolic pool of AP-4 has also been described (Dell’Angelica et al., 1999), suggesting that this complex might be capable of cycling between TGN membranes and the cytosol. The regulation of this cycling, however, is poorly understood.
The recruitment of AP-1 and AP-3 to TGN and/or endosomal membranes is controlled by members of the ADP-ribosylation factor (ARF) family of guanine nucleotide-binding proteins (reviewed by Moss and Vaughan, 1998; Donaldson and Jackson, 2000). There are five human ARF proteins that are divided into three classes based on their degree of homology: class I (ARF1 and ARF3), class II (ARF4 and ARF5) and class III (ARF6) (Moss and Vaughan, 1998). Class I and class II ARFs appear to be the most active for AP-1 and AP-3 recruitment to membranes (Liang and Kornfeld, 1997; Ooi et al., 1998; Zhu et al., 1998; Drake et al., 2000). ARFs themselves also cycle between membranes and the cytosol in a process that is in turn regulated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). Exchange of GTP for GDP causes conformational changes on ARFs that are most pronounced in the effector-binding switch I and switch II regions (Goldberg, 1998; Roth, 1999; Menetrey et al., 2000; Pasqualato et al., 2001). In addition, nucleotide exchange results in the exposure of a myristoylated, amphipathic α-helix at the N-terminus of ARFs, which mediates stable association with membranes (Randazzo et al., 1995; Antonny et al., 1997). These changes thus allow for the ARF·GTP-dependent recruitment of AP-1 and AP-3 to membranes. ARFs also regulate the membrane association and/or activation of other coat proteins such as COPI (Donaldson et al., 1992; Palmer et al., 1993; Zhao et al., 1997) and the GGAs (Boman et al., 2000; Dell’Angelica et al., 2000; Puertollano et al., 2001; Zhdankina et al., 2001), lipid-modifying enzymes such as phospholipase D1 (Brown et al., 1993; Cockcroft et al., 1994; Kim et al., 1998) and phosphatidylinositol 4-phosphate 5-kinase (Honda et al., 1999; Jones et al., 2000), and several other effectors of unknown function including Arfaptin 1 (Kanoh et al., 1997), Arfaptin 2/POR1 (Van Aelst et al., 1996), MKLP1 (Boman et al., 1999), Arfophilin (Shin et al., 1999) and LTA (Stevens et al., 1999). AP-2 is the exception among AP complexes characterized to date in that its association with membranes is not regulated by ARFs (Robinson and Kreis, 1992). Rather, the transmembrane protein synaptotagmin appears to interact with AP-2 to facilitate the nucleation of endocytic clathrin-coated pits at the plasma membrane (J.Z.Zhang et al., 1994; Haucke and De Camilli, 1999; Haucke et al., 2000; reviewed by Kirchhausen, 1999; Slepnev and De Camilli, 2000). Thus far, it remains to be determined whether AP-4 is subject to regulation by ARFs.
In the present study, we show that the association of AP-4 with the TGN in vivo is indeed dependent on class I ARFs, more specifically ARF1. Moreover, we demonstrate the occurrence of direct interactions between the ε and µ4 subunits of AP-4 and ARF1. We map the interacting regions on the AP-4 subunits to the trunk region of ε and the signal-binding domain of µ4. The interaction between ε adaptin and ARF1 is dependent on the nucleotide status and involves the switch I and switch II regions of ARF1. The interaction of µ4 with ARF1, on the other hand, is nucleotide independent and less sensitive to mutations in both switch regions. These results suggest a model in which AP-4 and ARF1 form a low-affinity complex in the absence of GTP that is mediated by the µ4 subunit. Upon exchange of GTP for GDP on ARF1, AP-4 binds to the switch regions of ARF1 via its ε subunit, leading to the formation of a high-affinity complex between AP-4 and ARF1.
Results
Characterization of a new antibody to the σ4 subunit of AP-4
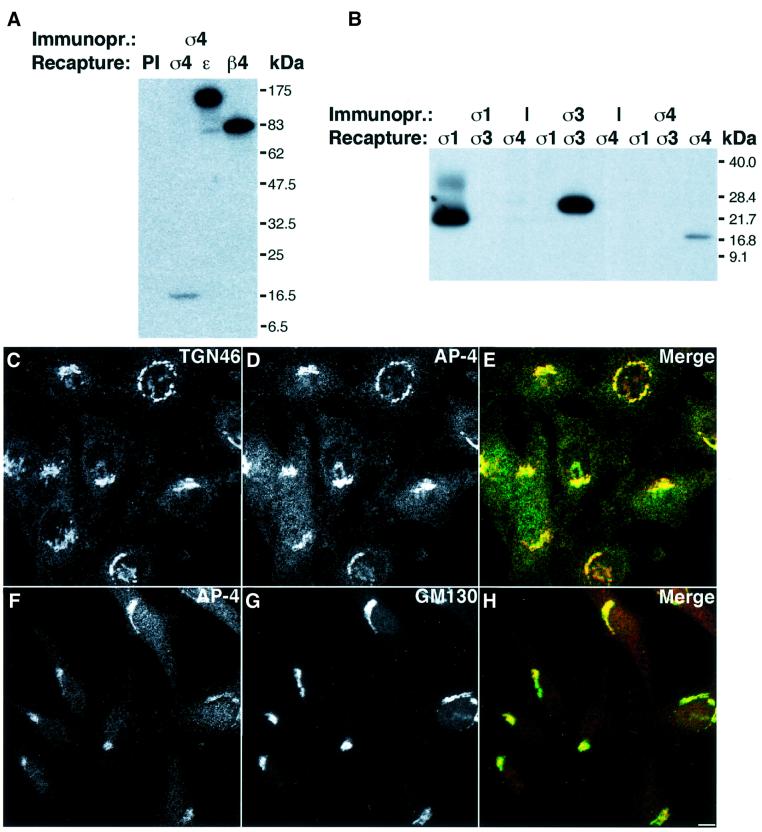
Since the available antibodies to AP-4 were not very sensitive for detection of the endogenous AP-4 complex, we prepared another antibody to recombinant σ4. To this end, the cDNA for σ4 was cloned into the expression vector pET28a-His10, and His10-σ4 was expressed in Escherichia coli. The purified protein was injected into rabbits and the sera purified over protein A–Sepharose. The affinity-purified antibody was then tested for specificity in immunoprecipitation–recapture experiments in which native immunoprecipitation using the anti-σ4 antibody was followed by a denaturing recapture step (Bonifacino and Dell’Angelica, 1998) (Figure 1A). The antibody specifically recognized σ4 both as part of the AP-4 complex and in its denatured form, as shown by the ability to recapture σ4, ε and β4 from the initial anti-σ4 immunoprecipitate (Figure 1A). Immunoprecipitation– recapture analysis also showed that the antibody to σ4 did not recognize the related σ1 and σ3 subunits of AP-1 and AP-3, respectively, in their native or denatured state (Figure 1B).
Fig. 1. The new anti-σ4 antibody specifically recognizes AP-4 in immunoprecipitation and immunofluorescence experiments and does not crossreact with σ1 or σ3. (A) Native immunoprecipitation (Immunopr.) of AP-4 by the anti-σ4 antibody followed by denaturing recapture using pre-immune serum (PI), the anti-σ4 antibody or previously described anti-ε and anti-β4 antibodies. (B) Native immunoprecipitation (Immunopr.) of AP-1, AP-3 and AP-4 using anti-σ1, anti-σ3 and anti-σ4 antibodies, respectively, followed by denaturing recapture using the anti-σ1, anti-σ3 or anti-σ4 antibody. (C–H) HeLa cells were grown in DMEM, 10% fetal calf serum (FCS) and fixed with 2% H2CO–PBS. Cells were labeled with rabbit anti-σ4 (D and F), sheep anti-TGN46 (C) or mouse anti-GM130 (G), followed by Cy3-conjugated anti-sheep Ig (C), Alexa 488-conjugated anti-rabbit Ig (D), Cy3-conjugated anti-rabbit Ig (F) and Alexa 488-conjugated anti-mouse Ig (G). Bar (C–H), 5 µm.
We then investigated the potential of the antibody for use in immunofluorescence microscopy. The anti-σ4 antibody showed specific staining of the Golgi complex that co-localized with the Golgi markers TGN46 (Figure 1C–E) and GM130 (Figure 1F–H), and could be competed by excess recombinant σ4 protein (data not shown).
The membrane localization of AP-4 is sensitive to brefeldin A
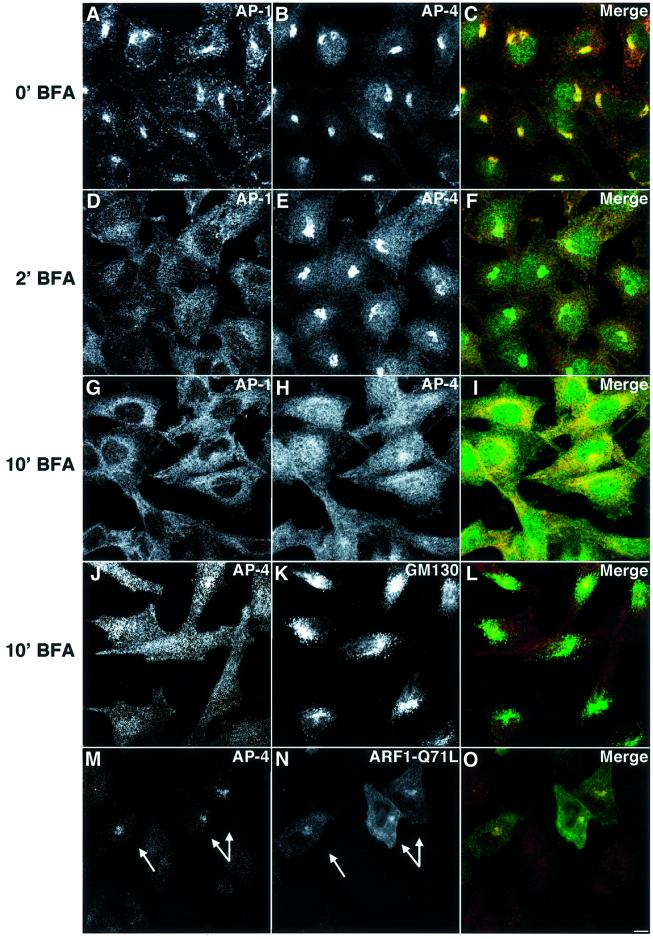
Dell’Angelica et al. (1999) and Hirst et al. (1999) had previously shown that the association of AP-4 with the TGN is sensitive to the fungal metabolite brefeldin A (BFA) that works by inactivating ARF-GEFs (Donaldson et al., 1992; Helms and Rothman, 1992; Peyroche et al., 1999). In agreement with these observations, treatment of HeLa cells with 5 µg/ml BFA for 10 min caused redistribution of AP-4 from the TGN to the cytosol (Figure 2G–I). The kinetics of AP-4 redistribution were slower than those observed for AP-1 in control experiments (Figure 2A–F). The distribution of the Golgi marker protein GM130 appeared more fragmented in the BFA-treated cells (Figure 2J–L), but, unlike AP-4, it was still mostly juxtanuclear. After removal of the drug and continued incubation at 37°C for 3 h, AP-4 was recruited back to the TGN (data not shown), thus demonstrating the reversibility of the BFA effects. These observations pointed to a possible regulation of AP-4 by ARFs. To further investigate this, we examined AP-4 dissociation by BFA in cells transiently over-expressing the constitutively activated ARF1-Q71L mutant, which fails to hydrolyze GTP to GDP (Dascher and Balch, 1994; Teal et al., 1994; C.J.Zhang et al., 1994). We observed that in cells over-expressing ARF1-Q71L, the AP-4 complex remained associated with the TGN even after incubation for 20 min with BFA (Figure 2M–O). The protection of AP-4 from the effects of BFA by the ARF1-Q71L mutant thus provided additional evidence for the regulation of AP-4 by ARFs.
Fig. 2. BFA redistributes AP-4 to the cytosol. ARF1-Q71L expression attenuates the BFA-induced effect. (A–L) HeLa cells were grown in DMEM, 10% FCS, and BFA was added to a final concentration of 5 µg/ml. Cells were fixed with 2% H2CO–PBS immediately (A–C), after 2 min (D–F) or after 10 min (G–L) of incubation at 37°C. Cells were stained with mouse anti-γ primary, Alexa 568-conjugated anti-mouse secondary antibody (A, D and G), rabbit anti-σ4 primary, Alexa 488-conjugated anti-rabbit secondary antibody (B, E and H), rabbit anti-σ4 primary, Cy3-conjugated anti-rabbit secondary antibody (J) or mouse anti-GM130 primary, Alexa 488-conjugated anti-mouse secondary antibody (K). (M–O) HeLa cells were transiently transfected with pXS-ARF1-Q71L-HA and after a 24 h incubation, BFA (5 µg/ml) was added to the cells. After 20 min, cells were fixed and stained using the anti-σ4 antibody (Cy3) for AP-4 and an anti-HA antibody (Alexa 488) for ARF1-Q71L. (M) and (N) Transfected cells are indicated by arrows. Bar, 5 µm.
ARF1 is particularly active in the regulation of AP-4 association with the TGN
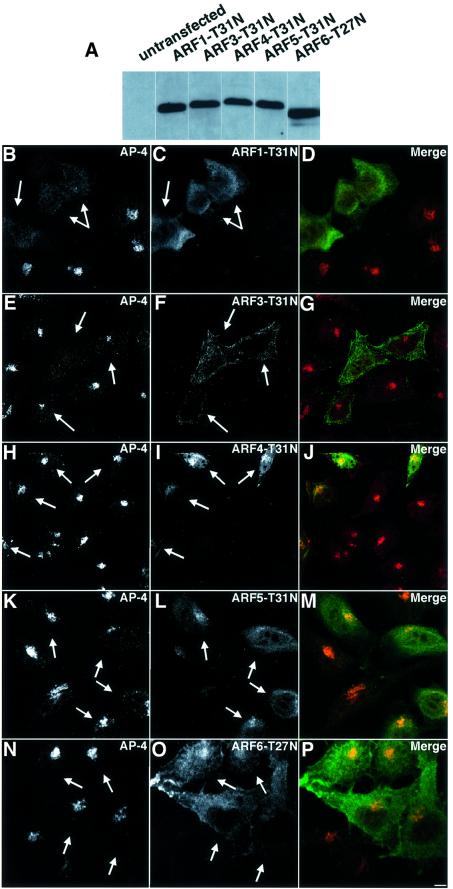
To compare the activity of different ARFs in the regulation of AP-4, we examined the effects of transiently over-expressing dominant-negative mutants of ARF1, ARF3, ARF4, ARF5 and ARF6 on the localization of AP-4. These mutants render the endogenous ARFs inactive, presumably by binding to and sequestering ARF-GEFs (Dascher and Balch, 1994; Peters et al., 1995). All constructs were expressed at similar levels, as assessed by immunoblotting on lysates of transfected cells (Figure 3A). We observed that the ARF1-T31N (Figure 3B–D) mutant caused redistribution of AP-4 to the cytosol, similarly to BFA. This was also observed for ARF3-T31N (Figure 3E–G), but only in a small subset of cells that were expressing very high levels of this construct. The ARF4-T31N (Figure 3H–J), ARF5-T31N (Figure 3K–M) and ARF6-T27N (Figure 3N–P) mutants, on the other hand, had no effect. Hence, the localization of AP-4 to the TGN appears to be mainly regulated by ARF1 and, to a lesser extent, ARF3. Class II and III ARFs appear to have no influence on recruitment of AP-4 to the TGN.
Fig. 3. Class I ARF proteins specifically affect the AP-4 TGN localization. HeLa cells were transiently transfected with pXS-ARF1-T31N-HA (B–D), pXS-ARF3-T31N-HA (E–G), pXS-ARF4-T31N-HA (H–J), pXS-ARF5-T31N-HA (K–M) or pXS-ARF6-T27N-HA (N–P) and incubated for 24 h. (A) Post-nuclear supernatants were prepared and immunoblotting was performed using an anti-HA antibody. (B–P) After 24 h, cells were fixed and double labeled for immunofluorescence microscopy using the anti-σ4 antibody (Cy3) for (B), (E), (H), (K) and (N) and an anti-HA (Alexa 488) antibody for (C), (F), (I), (L) and (O) to detect AP-4 localization and ARF expression, respectively. Transfected cells are indicated by arrows. Bar (B–P), 5 µm.
Effect on AP-4 localization of the over-expression of an ARF-GAP and an ARF effector domain
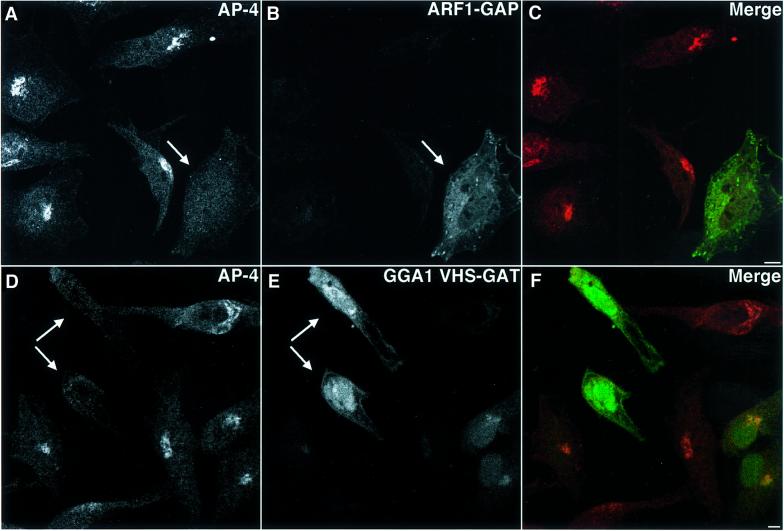
If the GTP-bound forms of ARF1 and ARF3 are required for the localization of AP-4 to the TGN membrane, then an increase in the rate of GTP hydrolysis induced by transient over-expression of an ARF-GAP should result in dissociation of AP-4 from the membrane, as has been shown for COPI (Aoe et al., 1997) and AP-3 (Ooi et al., 1998). Indeed, cells over-expressing ARF1-GAP (Cukierman et al., 1995) displayed redistribution of AP-4 to the cytosol (Figure 4A–C). In addition, the localization of AP-4 to the TGN could be abrogated by transient over-expression of the VHS-GAT domains of human GGA1, a well-characterized ARF effector (Figure 4D–F). Since the GAT domain of the GGAs has been shown to bind directly to ARF1 and ARF3 (Boman et al., 2000; Dell’Angelica et al., 2000; Puertollano et al., 2001), this observation suggested that AP-4 and the GGAs could be competing for interaction with the same effector-binding site on ARFs.
Fig. 4. AP-4 localization is affected by the expression of ARF1-GAP and GGA1 VHS-GAT. HeLa cells were transiently transfected with an ARF1-GAP expression plasmid (A–C) or a GGA1 YFP-VHS-GAT expression plasmid (D–F). After 24 h cells were fixed and stained using the anti-σ4 [Cy3 (A and D)] and anti-His6 antibodies [Alexa 488 (B)]. Transfected cells are indicated by arrows. Bars, 5 µm.
ARF1 binds directly to the ε and µ4 subunits of AP-4
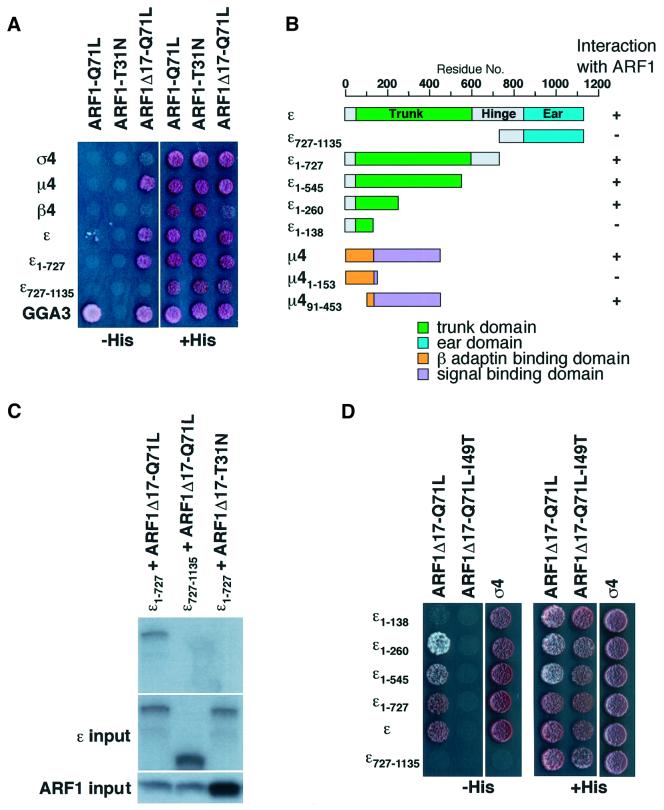
Two possible, although not mutually exclusive, mechanisms could explain the regulation of AP-4 by ARF1. First, ARF1 could act indirectly by affecting enzymes that control the lipid composition of membranes or by activation of a potential AP-4 docking protein. Secondly, ARF1 could interact directly with AP-4 at the TGN membrane. To investigate the possibility that AP-4 subunits interact directly with ARFs, we used the yeast two-hybrid system. We observed an interaction between the ε subunit of AP-4 and ARF1-Q71L but not ARF1-T31N (Figure 5A), consistent with GTP-dependent binding to ARF1. Under these conditions, no interactions between the ARF1 mutants and other AP-4 subunits could be detected. The interaction of ARF1-Q71L with ε was much weaker than that seen with GGA3 (Figure 5A).
Fig. 5. Interaction analyses between ARF1 and AP-4 subunits and truncation analyses of the ε–ARF1 interaction. (A and D) HF7C yeast strain was transfected with constructs expressing the indicated proteins and co-transformants were spotted on plates with (right) or without (left) histidine. Interaction of proteins was assessed by growth on the plate lacking histidine. (B) Bar diagram summarizing the results depicted in (A), (D) and Figure 6A. (C) In vitro transcribed/translated 35S-labeled ε and ARF-myc constructs were mixed, incubated, and ARF-myc was immunoprecipitated using an anti-myc antibody. Top, the co-precipitation of ε1–727 was detected by autoradiography. Middle and bottom, labeled ε and ARF1 constructs, respectively, which were used as input.
Recently, Eugster et al. (2000) reported that the sensitivity of the interaction between yeast ARF1 and various subunits of the COPI complex could be increased by removal of the 17 N-terminal residues of ARF1. The resulting ARF1Δ17 protein was soluble and fully active in exchange-factor assays (Paris et al., 1997); it interacted with COPI in vitro and competed with full-length myristoylated ARF1 for COPI recruitment to membranes (Goldberg, 1999). Indeed, we found that a truncated ARF1Δ17-Q71L interacted more strongly with the ε subunit relative to full-length ARF1 in our two-hybrid assays (Figure 5A). We also observed an interaction between ARF1Δ17-Q71L and µ4, but not with σ4 or β4, which was undetectable with the full-length ARF1-Q71L protein (Figure 5A).
ARF1 interacts specifically with the trunk domain of ε
To identify the regions of ε involved in interactions with ARF1, we conducted a deletion analysis, the results of which are summarized in Figure 5B. The large adaptins of the γ/α/δ/ε and β families contain three functional regions named ‘trunk’, ‘hinge’ and ‘ear’. The trunk comprises the 500–600 N-terminal residues. In the γ/α/δ/ε adaptins, this region is involved in binding to both the σ and the β adaptins (reviewed by Boehm and Bonifacino, 2001), as well as specific targeting of AP-1 and AP-2 to the TGN and the plasma membrane, respectively (Page and Robinson, 1995). The hinge region of β1, β2, β3 and γ is involved in binding to clathrin (reviewed by Kirchhausen, 2000). The ear region comprises the 150–300 C-terminal amino acids and binds to accessory proteins (Owen et al., 1999, 2000; Hirst et al., 2000; reviewed by Slepnev and De Camilli, 2000).
We found that two-thirds of the N-terminus of ε (residues 1–727), encompassing the entire trunk plus a portion of the hinge (Boehm and Bonifacino, 2001), was sufficient for interaction with ARF1Δ17-Q71L (Figure 5A), whereas the C-terminal ear domain of ε (residues 727–1135) did not interact with ARF1Δ17-Q71L (Figure 5A). These two-hybrid results were corroborated by binding assays using in vitro translated proteins. ARF1Δ17-Q71L-myc but not ARF1Δ17-T31N-myc was found to co-precipitate with ε1–727 (Figure 5C). In contrast, ε727–1135, comprising the complementary part of the hinge plus the ear, did not co-precipitate with the ARF1Δ17-Q71L-myc mutant (Figure 5C). Further truncation analyses revealed that a fragment of ε encompassing residues 1–138 was incapable of binding to ARF1Δ17-Q71L, whereas a longer fragment comprising residues 1–260 retained the ARF binding activity (Figure 5D). Func tionality of the ε1–138 construct was demonstrated by its interaction with σ4 adaptin (Figure 5D). Thus, the segment of the ε trunk, spanning residues 139–260, contains a determinant necessary for interactions with ARF1.
The ε subunit interacts with the switch I and switch II regions of ARF1
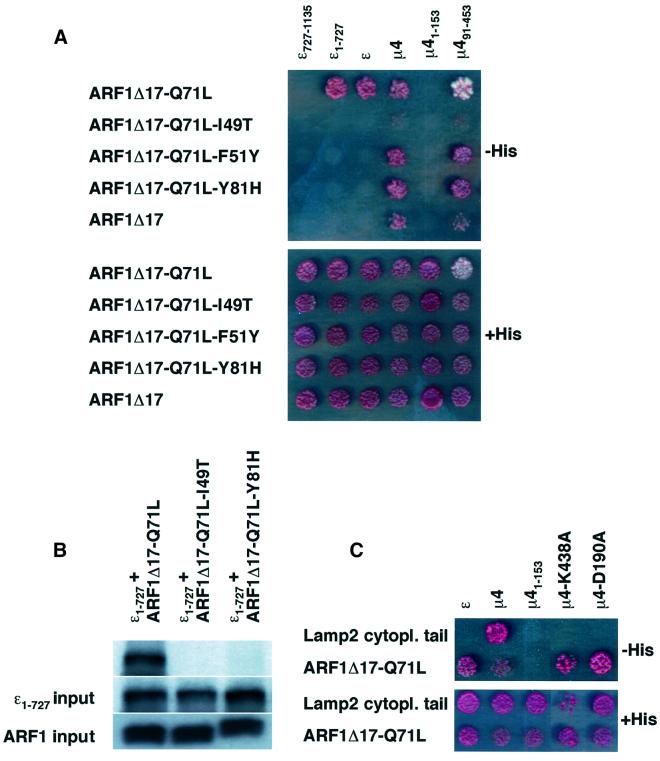
The most prominent structural changes upon GTP for GDP exchange on ARF1 take place in the switch I and switch II regions (Goldberg, 1998). These regions have been shown to be the main sites of interaction with effector molecules. ARF1 constructs with mutations in these regions, including ARF1-I49T (switch I), ARF1-F51Y (switch I) and ARF1-Y81H (switch II) are unable to interact with effectors (Kuai et al., 2000; Puertollano et al., 2001). We observed that placement of additional I49T, F51Y or Y81H mutations in the ARF1Δ17-Q71L protein abolished the interaction with both full-length ε and ε1–727 in two-hybrid experiments (Figure 6A). Similarly, in vitro translated ε1–727 failed to interact with in vitro trans lated ARF1Δ17-Q71L-I49T or ARF1Δ17-Q71L-Y81H (Figure 6B). ε, therefore, behaves as a true ARF1 effector, since the interaction is sensitive to the nucleotide status and involves the switch regions of ARF1.
Fig. 6. Interaction analyses of ε adaptin and µ4 adaptin with switch mutants of ARF1. (A and C) HF7C yeast strain was transfected with constructs expressing the indicated proteins and co-transformants were spotted on plates with (bottom) or without (top) histidine. Growth of colonies on the plate lacking histidine indicates an interaction of the proteins. (B) In vitro transcribed/translated 35S-labeled ε and ARF-myc constructs were mixed, incubated, and ARF-myc was immunoprecipitated using an anti-myc antibody. Top, the co-precipitation of ε1–727 was detected by autoradiography. Middle and bottom, labeled ε1–727 and ARF1 constructs, respectively, which were used as input.
ARF1 binds to the C-terminal signal-binding domain of µ4
Upon truncation of 17 N-terminal residues from ARF1-Q71L, we observed that it could also interact with the µ4 subunit of AP-4 (Figure 5A). Similarly to the homologous µ2 subunit (Aguilar et al., 1997), this protein consists of two structural domains (Aguilar et al., 2001; reviewed by Boehm and Bonifacino, 2001): an N-terminal domain that interacts with the β4 subunit (R.C.Aguilar and J.S.Bonifacino, unpublished observation) and a C-terminal domain that binds a specific subset of tyrosine-based sorting signals (Aguilar et al., 2001). We found that the ability to bind ARF1Δ17-Q71L resided within the C-terminal but not the N-terminal domain of µ4 (Figure 6A). Placement of the I49T mutation into ARF1Δ17-Q71L decreased the interaction while the F51Y and Y81H mutations were without effect (Figure 6A). This indicates that the requirements for µ4 binding to ARF1 are different from those of ε and that the binding may be less sensitive to the state of activation of ARF1. To test this, we used ARF1Δ17, which behaves as an inactive GDP-bound protein in COPI–ARF1 two-hybrid interaction studies (Eugster et al., 2000). In agreement with the previous observation, µ4 interacted with ARF1Δ17 protein while ε did not (Figure 6A). Mutations of µ4 residues potentially involved in recognition of tyrosine-based sorting signals, such as D190A and K438A (Aguilar et al., 1997, 2001; Owen and Evans, 1998), had no effect on interactions with ARF1 (Figure 6C), indicating that the process is not mediated by a cryptic tyrosine-based sorting signal in ARF1.
Discussion
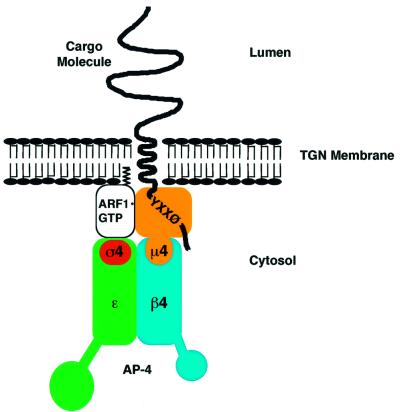
In this study, we present evidence that the association of AP-4 with the TGN in vivo is regulated by the GTP/GDP cycle of ARF1. In addition, we show that the ε and µ4 subunits of AP-4 interact directly with ARF1 both in the yeast two-hybrid system and in vitro. In the case of ε, these interactions occur with the GTP-bound form specifically and involve the effector-binding switch I and switch II regions of ARF1. Interactions with µ4, on the other hand, are independent of the nucleotide status of ARF1 and largely insensitive to mutations in the switch regions. These observations establish that AP-4 has the properties of an ARF effector and suggest a novel feature of AP complex–ARF interactions: the occurrence of GTP-independent interactions with a medium subunit. The model in Figure 7 depicts the bipartite interactions of AP-4 with ARF1 observed in our study.
Fig. 7. Model for the interaction of AP-4 with ARF1 and a trans membrane cargo molecule bearing a YXXØ-based tyrosine-based sorting motif in its cytosolic tail.
We have shown that expression of a constitutively activated GTP-locked mutant of ARF1 suppresses the BFA-induced dissociation of AP-4 from the TGN. Moreover, expression of inactive GDP-locked ARF1 and, to a lesser extent, ARF3 (although not ARF4, ARF5 or ARF6) mutants causes dissociation of AP-4 from the TGN. A similar effect is seen upon expression of an ARF-GAP that inactivates endogenous ARFs by inducing GTP hydrolysis. Finally, expression of an ARF-binding fragment from an ARF effector, GGA1, also causes displacement of AP-4 from membranes, probably due to saturation of all GTP-bound ARFs. All this evidence is consistent with regulation of AP-4 recruitment to membranes by ARF1 in vivo. In this regard, AP-4 behaves like AP-1, AP-3, the GGAs and COPI, all of which are regulated mainly by class I ARFs (Donaldson et al., 1992; Robinson and Kreis, 1992; Palmer et al., 1993; Ooi et al., 1998; Pavel et al., 1998; Zhu et al., 1998, 1999; Boman et al., 2000; Dell’Angelica et al., 2000; Drake et al., 2000).
As for other protein coats, the regulation of AP-4 localization by ARF1 could be indirect, for instance by alteration of the lipid composition of the target membranes. ARFs have in fact been shown to activate phospholipase D1, which catalyzes the conversion of phosphatidylcholine to phosphatidic acid (Brown et al., 1993; Cockcroft et al., 1994; Kim et al., 1998) and phosphatidylinositol 4-phosphate 5-kinase, which converts phosphatidylinositol 4-phosphate to phosphatidylinositol 4,5-bisphosphate (Honda et al., 1999; Jones et al., 2000). These lipids could in turn promote association of AP-4 and other protein coats with membranes, as has been proposed previously for COPI (Fleischer et al., 1994; Chaudhary et al., 1998). Also indirectly, ARFs could function to activate a putative docking protein for AP-4.
Another possibility is that ARFs themselves act as docking proteins (Zhao et al., 1997, 1999; Austin et al., 2000; Boman et al., 2000; Dell’Angelica et al., 2000; Eugster et al., 2000; Hirst et al., 2000). In support of this hypothesis, we find that ARF1 can interact with the ε and µ4 subunits of AP-4 but not with β4 or σ4. The AP-4 complex, therefore, behaves similarly to the coat complexes AP-1 and COPI in that more than one subunit interacts with ARF1. However, a more detailed investigation reveals mechanistic differences with respect to the interacting subunits. The two large AP-1 subunits γ and β1 (Austin et al., 2000) and the respective homologous COPI subunits β-COP and γ-COP (Zhao et al., 1997, 1999; Eugster et al., 2000) mediate binding to the switch I (all of the above) and switch II (β-COP only) regions of ARF1. In addition, the two-hybrid experiments revealed binding of ε-COP to ARF1 (Eugster et al., 2000). As the β4 chain of AP-4 does not interact with ARF, AP-4 represents the first case of a protein coat that binds without the involvement of a β-adaptin family protein. Instead, AP-4 employs its medium chain µ4 in the binding to ARF1, a feature that has not been observed so far for the homologous subunits from AP-1 and COPI, µ1 and δ-COP. While the dual interaction between AP-4 and ARF1 is different to AP-1 and COPI in these respects, it is reminiscent of the mode of synaptotagmin binding to AP-2. Synaptotagmin has been shown to interact directly with the α and µ2 subunits of AP-2 (Haucke et al., 2000), which are homologous to the ε and µ4 subunits, respectively, of AP-4. These interactions are thought to play a role in the recruitment of AP-2 to membranes (J.Z.Zhang et al., 1994; Haucke and De Camilli, 1999; Haucke et al., 2000), which is apparently not dependent on ARFs (Robinson and Kreis, 1992). No data are available yet on the interactions of AP-3 subunits with ARF1, and it will be interesting to see whether these interactions are more similar to those of AP-1 or AP-4.
The mode of interaction between AP-4 ε and ARF1 is similar to that of other ARF effectors. Like GGA1, POR1, MKLP1 and LTA1 (Kuai and Kahn, 2000; Kuai et al., 2000; Puertollano et al., 2001), AP-4 ε interacts with the GTP-bound but not the GDP-bound form of ARF1. In addition, interactions with ε involve residues in the effector-binding switch I and switch II regions of ARF1. Molecular dissection of ε revealed that the GTP-dependent ARF1-binding activity resides within the trunk domain, which by analogy with other AP complexes is expected to be part of the AP-4 core. A construct comprising only part of the hinge and the ear region of ε, on the other hand, failed to bind to ARF·GTP. A deletion analysis of the ε ‘trunk’ domain revealed that 260 N-terminal residues of ε were still capable of interacting with ARF, while a shorter construct comprising of 138 N-terminal residues was not. This is in contrast to the α-adaptin–synaptotagmin interaction that involves residues near the N-terminus of α (K55, K56 and K57; Haucke et al., 2000). This basic triad, which is also responsible for the IP6 binding activity of α-adaptin (Gaidarov and Keen, 1999), is not conserved in ε-adaptin.
ARF1 also binds to µ4, albeit weakly, as shown by binding to ARF1Δ17-Q71L, but not full-length ARF1-Q71L. The enhanced sensitivity of the truncated ARF1 might be due to its greater solubility. This finding is in agreement with that of Eugster et al. (2000), who could detect ε-COPI–ARF1 interactions only upon truncation of ARF1. The interaction of µ4 with ARF1 is independent of the ARF1 GTP/GDP cycle and does not involve residues F51 and Y81 located in the switch I and switch II regions, respectively, of ARF1. This indicates that ε and µ4 bind to different interfaces on the ARF1 molecule (Figure 7). While this observation may seem surprising, it might be a key element in ensuring specific recognition of ARF1 over other ARF proteins, and suggests that ARF–coat interactions are more complex than previously thought. The amino acid sequences of the ARF proteins are highly conserved in the switch I and switch II regions. In addition, the structures of the switch regions of the activated forms of ARF1 and ARF6 are virtually identical (Pasqualato et al., 2001). Hence, the switch regions are discriminatory in the inactive but not in the active state of ARF, and contacts outside the switch regions could specify the interaction between an AP complex and a particular ARF protein. Precedent for amino acids outside the switch I and switch II regions providing specificity for the interaction between an effector and a small GTPase comes from structural studies of the Rab3A–Rabphilin-3A complex. Rabphilin-3A contacts Rab3A at two distinct areas: the switch I and switch II regions, and another area known as Rab complementarity-determining region (RabCDR). The interaction between the effector and the RabCDR is thought to ensure the specific recognition of a particular Rab, while the interaction with the switch regions is sensitive to the activation state of the Rab protein (Ostermeier and Brünger, 1999). Hence, APs could have evolved to establish this dual interaction by means of two separate subunits; in the case of AP-4, ε and µ4 adaptin. Perhaps initial membrane recruitment occurs by a mechanism independent of ARF1·GTP and this initial interaction is stabilized when ARF1 becomes activated.
We mapped the ARF interaction site to the signal-binding domain of µ4. We ruled out the possibility that the interaction between µ4 and ARF1 was artificially induced by the exposure of a cryptic tyrosine-based sorting signal upon truncation of ARF1, by using two mutants of µ4 that are incapable of binding such motifs. Both mutants were able to bind ARF1 in ways similar to full-length µ4. The location of the ARF1- and signal-binding sites within the same domain of µ4 may allow for an interplay between membrane recruitment and cargo recognition of the AP-4 complex analogous to the enhanced membrane recruitment of AP-2 in the presence of cargo molecules (Haucke and De Camilli, 1999).
Materials and methods
DNA constructs
Constructs for two-hybrid and in vitro binding assays. The cDNA for the ε subunit of AP-4 was obtained by RT–PCR using gene-specific primers and total HeLa cell RNA. σ4, β4 (Dell’Angelica et al., 1999) and ε adaptin cDNAs were subcloned into pGADT7 and pGBT9 (σ4). pACTII-µ4, pACTII-µ4D190A, pACTII-µ4K438A and the construct containing the Lamp2 cytoplasmic tail fused to the Gal4-binding domain were described previously (Aguilar et al., 2001). pACTII-µ41–153 and pACTII-µ4271–453 were obtained by PCR amplification of the respective fragment and subcloning into pACTII. pGADT7-ε1–727 was obtained by introducing a stop codon at position 728 of the ε cDNA using the QuickChange mutagenesis kit (Stratagene, La Jolla, CA), whereas pGADT7-ε1–368 and pGADT7-ε727–1135 were cloned by PCR amplification of the corresponding ε fragment and ligation with pGADT7. During the process of preparing the pGADT7-ε constructs, an NdeI–BamHI piece of pET28a was used to replace the NdeI–BamHI sequence of pGADT7. cDNAs for ARF1-Q71L and ARF1-T31N as well as ARF1Δ17-Q71L and ARF1Δ17-T31N were cloned into pGBKT7. ARF1Δ17-Q71LI49T, ARF1Δ17-Q71LF51H and ARF1Δ17-Q71LY81H were obtained by PCR mutagenesis of the respective full-length constructs (Puertollano et al., 2001) and cloned into pGBKT7.
Constructs for immunofluorescence microscopy. pXS-ARF1-Q71L-HA, pXS-ARF1-T31N-HA, pXS-ARF5-T31N-HA and pXS-ARF6-T27N-HA as well as ARF1-GAP were as previously described (Ooi et al., 1998). The GGA1 YFP-VHS-GAT construct was described by Dell’Angelica et al. (2000). pXS-ARF3-HA and pXS-ARF4-HA were a gift from J.Donaldson (NIH, Bethesda, MD), and codons for amino acids T31 and Q71 were converted into codons for N31 and L71, respectively, using the QuickChange mutagenesis kit (Stratagene) on both constructs.
Antibodies
The construct pET28a-His10-σ4 was obtained by cloning the coding sequence of σ4 into pET28a (Invitrogen, Carlsbad, CA) following the procedure described for the generation of pET28a-His10-µ4156–435 (Aguilar et al., 2001). Escherichia coli BL21-DE3 was transformed with pET28a-His10-σ4. Expression and purification of σ4 were carried out according to the manufacturer’s instructions using Ni–NTA agarose resin (Qiagen, Valencia, CA). The recombinant protein was injected into rabbits and production bleeds were tested for suitability in immunoprecipitation–recapture and immunofluorescence experiments. Rabbit anti-σ1 antiserum was a kind gift from L.Traub (University of Pittsburgh). For immunoprecipitation experiments we used rabbit antibodies against σ3 (Dell’Angelica et al., 1997) and β4 (Dell’Angelica et al., 1999). Sheep anti-TGN46 was obtained from Serotec (Oxford, UK), mouse monoclonal anti-GM130 from Transduction Laboratories (http://www.bdbiosciences.com), mouse monoclonal anti-γ antibody (clone 100/3) from Sigma (St Louis, MO), anti-His6 monoclonal antibody from Novagen (Madison, WI), monoclonal anti-HA (HA.11) and monoclonal anti-myc (9E10) from Covance Research Products (http://www.babco.com) and Alexa 488- and Cy3-conjugated secondary antibodies from Jackson Immunoresearch (West Grove, PA).
Immunoprecipitation
Immunoprecipitation was carried out as described (Bonifacino and Dell’Angelica, 1998). In brief, HeLa cells were metabolically labeled overnight with Easytag™ expression protein labeling mix (DuPont NEN, Boston, MA), harvested and lysed in non-denaturing lysis buffer. AP-1, AP-3 and AP-4 were precipitated from the lysate by incubation with anti-σ1, anti-σ3 and anti-σ4 antibody, respectively, bound to protein A–Sepharose. The precipitate was denatured in elution buffer and a second immunoprecipitation was performed with antibodies as indicated in Figure 1. Samples were denatured in SDS sample buffer and analyzed by SDS–PAGE followed by autoradiography.
Immunofluorescence microscopy
HeLa cells (American Type Culture Collection, Manassas, VA) were trypsinized and plated on cover slips ∼24 h before transfection. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin (Biofluids, Rockville, MD). Transfection with expression constructs was carried out in 6-well plates (Costar, Corning, NY) using Fugene-6 (Roche, Indianapolis, IN) according to the manufacturer’s instructions. At 20–24 h after transfection, cells were fixed in 2% formaldehyde–phosphate-buffered saline (PBS) for 10 min followed by two washes with PBS. Fixed, permeabilized cells were incubated for 1 h with primary antibody, washed with PBS for 10 min, incubated for 30 min with secondary antibody, washed again in PBS for 10 min and mounted on slides using Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL).
BFA treatment
BFA (Epicentre Technologies, Madison, WI) at a concentration of 5 µg/ml in culture medium was added to HeLa cells on cover slips in 6-well plates. Cells were incubated at 37°C for the times indicated and fixed as described above. For recovery experiments, HeLa cells were incubated in BFA containing medium for 1 h at 37°C. Then, the medium was removed, cells were washed with PBS twice and fresh, pre-warmed medium was added. Cells were fixed at the indicated times.
Immunoblotting
Extracts of HeLa cells transiently transfected with ARF expression constructs were prepared as follows. After harvesting, 104 transfected cells were suspended in 1 ml of 25 mM Tris pH 7.5, 250 mM sucrose, 1 mM AEBSF, 10 µg/ml leupeptin, 5 µg/ml pepstatin and 100 µg/ml aprotinin. The suspension was passed 30 times through a 25″ gauge needle and centrifuged twice at 325 g (4°C, 10 min) and twice at 100 000 g (4°C, 1 h). The supernatant (10 µl) was loaded onto an SDS gel and, after electrophoresis, proteins were blotted onto PROTRAN® nitrocellulose membrane (Schleicher & Schuell, Keene, NH). Proteins were detected using a monoclonal anti-HA (HA.11) primary antibody and a secondary sheep anti-mouse Ig conjugated to horseradish peroxidase (Amersham Life Science).
Yeast two-hybrid assays
The Saccharomyces cerevisiae strain HF7c [MATa, ura3-52, HIS3-200, lys2-801, ade2-101, trp1-901, leu2-3,112, gal4-542, gal80-538, LYS2::GAL1-HIS3, URA3::(GAL4 17mers)3-CYC1-lacZ; Clontech, Palo Alto, CA] was maintained on YPD agar plates. Transformation was performed by the lithium acetate procedure as described in the instructions for the MATCHMAKER two-hybrid kit (Clontech). For colony growth assays, HF7c transformants were spotted on plates lacking leucine, tryptophan and histidine and allowed to grow at 30°C, usually for 3–4 days.
In vitro binding studies
35S-labeled myc-ARF1Δ17-Q71L, myc-ARF1Δ17-T31N, myc-ARF1Δ17-Q71LI49T, myc-ARF1Δ17-Q71LY81H, HA-ε1–727 and HA-ε727–1135 proteins were obtained from templates in pGBKT7 and pGADT7, respectively, by in vitro transcription/translation using TNT T7 quick coupled transcription/translation system (Promega, Madison, WI) and Easytag™ expression protein labeling mix (DuPont NEN, Boston, MA). After centrifugation (16 000 g, 5 min, 4°C) of the transcription/translation reactions, equal volumes of ε and ARF reaction supernatant were mixed and incubated for 4 h at 4°C. Incubations were diluted with 10 vols of 20 mM HEPES pH 7.4, 100 mM NaCl, 0.05% (w/v) bovine serum albumin (BSA) and centrifuged (16 000 g, 15 min, 4°C). After pre-clearing, immunoprecipitation of samples was carried out using anti-myc antibody (9E10) coupled to protein A–Sepharose for 3 h at 4°C. Beads were washed three times with ice-cold binding buffer with BSA at 4°C, once with ice-cold binding buffer without BSA at 4°C, boiled in Laemmli sample buffer and separated by SDS–PAGE. The SDS gel was soaked in sodium salicylate and subjected to autoradiography.
Acknowledgments
Acknowledgements
We thank C.Austin and S.Tooze for valuable discussions and S.Caplan, J.Donaldson, C.Jackson and C.Mullins for critical reading of the manuscript. M.B. was supported by a fellowship from the German Academic Exchange Service (DAAD).
References
- Aguilar R.C., Ohno,H., Roche,K.W. and Bonifacino,J.S. (1997) Functional domain mapping of the clathrin-associated adaptor medium chains µ1 and µ2. J. Biol. Chem., 272, 27160–27166. [DOI] [PubMed] [Google Scholar]
- Aguilar R.C., Boehm,M., Gorshkova,I., Crouch,R.J., Tomita,K., Saito,T., Ohno,H. and Bonifacino,J.S. (2001) Signal-binding specificity of the µ4 subunit of the adaptor protein complex AP-4. J. Biol. Chem., 276, 13145–13152. [DOI] [PubMed] [Google Scholar]
- Antonny B., Beraud-Dufour,S., Chardin,P. and Chabre,M. (1997) N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry, 36, 4675–4684. [DOI] [PubMed] [Google Scholar]
- Aoe T., Cukierman,E., Lee,A., Cassel,D., Peters,P.J. and Hsu,V.W. (1997) The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J., 16, 7305–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C., Hinners,I. and Tooze,S.A. (2000) Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem., 275, 21862–21869. [DOI] [PubMed] [Google Scholar]
- Boehm M. and Bonifacino,J.S. (2001) Adaptins: the final recount. Mol. Biol. Cell, 12, 2907–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman A.L., Kuai,J., Zhu,X., Chen,J., Kuriyama,R. and Kahn,R.A. (1999) Arf proteins bind to mitotic kinesin-like protein 1 (MKLP1) in a GTP-dependent fashion. Cell Motil. Cytoskeleton, 44, 119–132. [DOI] [PubMed] [Google Scholar]
- Boman A.L., Zhang,C., Zhu,X. and Kahn,R.A. (2000) A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol. Biol. Cell, 11, 1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S. and Dell’Angelica,E.C. (1998) Immunoprecipitation. In Bonifacino,J.S., Dasso,M., Harford,J.B., Lippincott-Schwartz,J. and Yamada,K.M. (eds), Current Protocols in Cell Biology. John Wiley & Sons, Inc., New York, NY, pp. 7.2.1–7.2.21.
- Brown H.A., Gutowski,S., Moomaw,C.R., Slaughter,C. and Sternweis,P.C. (1993) ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell, 75, 1137–1144. [DOI] [PubMed] [Google Scholar]
- Chaudhary A., Gu,Q.M., Thum,O., Profit,A.A., Qi,Y., Jeyakumar,L., Fleischer,S. and Prestwich,G.D. (1998) Specific interaction of Golgi coatomer protein α-COP with phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem., 273, 8344–8350. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. et al. (1994) Phospholipase D: a downstream effector of ARF in granulocytes. Science, 263, 523–526. [DOI] [PubMed] [Google Scholar]
- Cukierman E., Huber,I., Rotman,M. and Cassel,D. (1995) The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science, 270, 1999–2002. [DOI] [PubMed] [Google Scholar]
- Dascher C. and Balch,W.E. (1994) Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem., 269, 1437–1448. [PubMed] [Google Scholar]
- de Chassey B., Dubois,A., Lefkir,Y. and Letourneur,F. (2001) Identification of clathrin-adaptor medium chains in Dictyostelium discoideum: differential expression during development. Gene, 262, 115–122. [DOI] [PubMed] [Google Scholar]
- Dell’Angelica E.C., Ohno,H., Ooi,C.E., Rabinovich,E., Roche,K.W. and Bonifacino,J.S. (1997) AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J., 16, 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Angelica E.C., Mullins,C. and Bonifacino,J.S. (1999) AP-4, a novel protein complex related to clathrin adaptors. J. Biol. Chem., 274, 7278–7285. [DOI] [PubMed] [Google Scholar]
- Dell’Angelica E.C., Puertollano,R., Mullins,C., Aguilar,R.C., Vargas,J.D., Hartnell,L.M. and Bonifacino,J.S. (2000) GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol., 149, 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J.G. and Jackson,C.L. (2000) Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol., 12, 475–482. [DOI] [PubMed] [Google Scholar]
- Donaldson J.G., Cassel,D., Kahn,R.A. and Klausner,R.D. (1992) ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc. Natl Acad. Sci. USA, 89, 6408–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M.T., Zhu,Y. and Kornfeld,S. (2000) The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes. Mol. Biol. Cell, 11, 3723–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster A., Frigerio,G., Dale,M. and Duden,R. (2000) COP I domains required for coatomer integrity and novel interactions with ARF and ARF-GAP. EMBO J., 19, 3905–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Xie,J., Mayrleitner,M., Shears,S.B., Palmer,D.J. and Fleischer,S. (1994) Golgi coatomer binds and forms K+-selective channels gated by inositol polyphosphates. J. Biol. Chem., 269, 17826–17832. [PubMed] [Google Scholar]
- Gaidarov I. and Keen,J.H. (1999) Phosphoinositide–AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J. Cell Biol., 146, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. (1998) Structural basis for activation of ARF-GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell, 95, 237–248. [DOI] [PubMed] [Google Scholar]
- Goldberg J. (1999) Structural and functional analysis of the ARF1–ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell, 96, 893–902. [DOI] [PubMed] [Google Scholar]
- Haucke V. and De Camilli,P. (1999) AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science, 285, 1268–1271. [DOI] [PubMed] [Google Scholar]
- Haucke V., Wenk,M.R., Chapman,E.R., Farsad,K. and De Camilli,P. (2000) Dual interaction of synaptotagmin with µ2- and α-adaptin facilitates clathrin-coated pit nucleation. EMBO J., 19, 6011–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J.B. and Rothman,J.E. (1992) Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature, 360, 352–354. [DOI] [PubMed] [Google Scholar]
- Hirst J., Bright,N.A., Rous,B. and Robinson,M.S. (1999) Characteriz ation of a fourth adaptor-related protein complex. Mol. Biol. Cell, 10, 2787–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Lui,W.W., Bright,N.A., Totty,N., Seaman,M.N. and Robinson,M.S. (2000) A family of proteins with γ-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol., 149, 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A. et al. (1999) Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell, 99, 521–532. [DOI] [PubMed] [Google Scholar]
- Jones D.H., Morris,J.B., Morgan,C.P., Kondo,H., Irvine,R.F. and Cockcroft,S. (2000) Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the Golgi compartment. J. Biol. Chem., 275, 13962–13966. [DOI] [PubMed] [Google Scholar]
- Kanoh H., Williger,B.T. and Exton,J.H. (1997) Arfaptin 1, a putative cytosolic target protein of ADP-ribosylation factor, is recruited to Golgi membranes. J. Biol. Chem., 272, 5421–5429. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lee,S.D., Han,J.M., Lee,T.G., Kim,Y., Park,J.B., Lambeth,J.D., Suh,P.G. and Ryu,S.H. (1998) Activation of phospholipase D1 by direct interaction with ADP-ribosylation factor 1 and RalA. FEBS Lett., 430, 231–235. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. (1999) Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol., 15, 705–732. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. (2000) Clathrin. Annu. Rev. Biochem., 69, 699–727. [DOI] [PubMed] [Google Scholar]
- Kuai J. and Kahn,R.A. (2000) Residues forming a hydrophobic pocket in ARF3 are determinants of GDP dissociation and effector interactions. FEBS Lett., 487, 252–256. [DOI] [PubMed] [Google Scholar]
- Kuai J., Boman,A.L., Arnold,R.S., Zhu,X. and Kahn,R.A. (2000) Effects of activated ADP-ribosylation factors on Golgi morphology require neither activation of phospholipase D1 nor recruitment of coatomer. J. Biol. Chem., 275, 4022–4032. [DOI] [PubMed] [Google Scholar]
- Liang J.O. and Kornfeld,S. (1997) Comparative activity of ADP-ribosylation factor family members in the early steps of coated vesicle formation on rat liver Golgi membranes. J. Biol. Chem., 272, 4141–4148. [DOI] [PubMed] [Google Scholar]
- Menetrey J., Macia,E., Pasqualato,S., Franco,M. and Cherfils,J. (2000) Structure of Arf6-GDP suggests a basis for guanine nucleotide exchange factors specificity. Nature Struct. Biol., 7, 466–469. [DOI] [PubMed] [Google Scholar]
- Moss J. and Vaughan,M. (1998) Molecules in the ARF orbit. J. Biol. Chem., 273, 21431–21434. [DOI] [PubMed] [Google Scholar]
- Ooi C.E., Dell’Angelica,E.C. and Bonifacino,J.S. (1998) ADP-ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J. Cell Biol., 142, 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermeier C. and Brünger,A.T. (1999) Structural basis of Rab effector specificity: crystal structure of the small G protein Rab3A complexed with the effector domain of Rabphilin-3A. Cell, 96, 363–374. [DOI] [PubMed] [Google Scholar]
- Owen D.J. and Evans,P.R. (1998) A structural explanation for the recognition of tyrosine-based endocytotic signals. Science, 282, 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D.J., Vallis,Y., Noble,M.E., Hunter,J.B., Dafforn,T.R., Evans,P.R. and McMahon,H.T. (1999) A structural explanation for the binding of multiple ligands by the α-adaptin appendage domain. Cell, 97, 805–815. [DOI] [PubMed] [Google Scholar]
- Owen D.J., Vallis,Y., Pearse,B.M., McMahon,H.T. and Evans,P.R. (2000) The structure and function of the β2-adaptin appendage domain. EMBO J., 19, 4216–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page L.J. and Robinson,M.S. (1995) Targeting signals and subunit interactions in coated vesicle adaptor complexes. J. Cell Biol., 131, 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D.J., Helms,J.B., Beckers,C.J., Orci,L. and Rothman,J.E. (1993) Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J. Biol. Chem., 268, 12083–12089. [PubMed] [Google Scholar]
- Paris S., Beraud-Dufour,S., Robineau,S., Bigay,J., Antonny,B., Chabre,M. and Chardin,P. (1997) Role of protein–phospholipid interactions in the activation of ARF1 by the guanine nucleotide exchange factor Arno. J. Biol. Chem., 272, 22221–22226. [DOI] [PubMed] [Google Scholar]
- Pasqualato S., Menetrey,J., Franco,M. and Cherfils,J. (2001) The structural GDP/GTP cycle of human Arf6. EMBO Rep., 2, 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavel J., Harter,C. and Wieland,F.T. (1998) Reversible dissociation of coatomer: functional characterization of a β/δ–coat protein subcomplex. Proc. Natl Acad. Sci. USA, 95, 2140–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters P.J., Hsu,V.W., Ooi,C.E., Finazzi,D., Teal,S.B., Oorschot,V., Donaldson,J.G. and Klausner,R.D. (1995) Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J. Cell Biol., 128, 1003–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyroche A., Antonny,B., Robineau,S., Acker,J., Cherfils,J. and Jackson,C.L. (1999) Brefeldin A acts to stabilize an abortive ARF-GDP–Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol. Cell, 3, 275–285. [DOI] [PubMed] [Google Scholar]
- Puertollano R., Randazzo,P.A., Presley,J.F., Hartnell,L.M. and Bonifacino,J.S. (2001) The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell, 105, 93–102. [DOI] [PubMed] [Google Scholar]
- Randazzo P.A., Terui,T., Sturch,S., Fales,H.M., Ferrige,A.G. and Kahn,R.A. (1995) The myristoylated amino-terminus of ADP-ribosylation factor 1 is a phospholipid- and GTP-sensitive switch. J. Biol. Chem., 270, 14809–14815. [DOI] [PubMed] [Google Scholar]
- Robinson M.S. and Bonifacino,J.S. (2001) Adaptor-related proteins. Curr. Opin. Cell Biol., 13, 444–453. [DOI] [PubMed] [Google Scholar]
- Robinson M.S. and Kreis,T.E. (1992) Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell, 69, 129–138. [DOI] [PubMed] [Google Scholar]
- Roth M.G. (1999) Snapshots of ARF1: implications for mechanisms of activation and inactivation. Cell, 97, 149–152. [DOI] [PubMed] [Google Scholar]
- Shin O.H., Ross,A.H., Mihai,I. and Exton,J.H. (1999) Identification of arfophilin, a target protein for GTP-bound class II ADP-ribosylation factors. J. Biol. Chem., 274, 36609–36615. [DOI] [PubMed] [Google Scholar]
- Slepnev V.I. and De Camilli,P. (2000) Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nature Rev. Neurosci., 1, 161–172. [DOI] [PubMed] [Google Scholar]
- Stephens D.J. and Banting,G. (1998) Specificity of interaction between adaptor-complex medium chains and the tyrosine-based sorting motifs of TGN38 and lgp120. Biochem. J., 335, 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L.A., Moss,J., Vaughan,M., Pizza,M. and Rappuoli,R. (1999) Effects of site-directed mutagenesis of Escherichia coli heat-labile enterotoxin on ADP-ribosyltransferase activity and interaction with ADP-ribosylation factors. Infect. Immun., 67, 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal S.B., Hsu,V.W., Peters,P.J., Klausner,R.D. and Donaldson,J.G. (1994) An activating mutation in ARF1 stabilizes coatomer binding to Golgi membranes. J. Biol. Chem., 269, 3135–3138. [PubMed] [Google Scholar]
- Van Aelst L., Joneson,T. and Bar-Sagi,D. (1996) Identification of a novel Rac1-interacting protein involved in membrane ruffling. EMBO J., 15, 3778–3786. [PMC free article] [PubMed] [Google Scholar]
- Wang X. and Kilimann,M.W. (1997) Identification of two new µ-adaptin-related proteins, mu-ARP1 and mu-ARP2. FEBS Lett., 402, 57–61. [DOI] [PubMed] [Google Scholar]
- Zhang C.J., Rosenwald,A.G., Willingham,M.C., Skuntz,S., Clark,J. and Kahn,R.A. (1994) Expression of a dominant allele of human ARF1 inhibits membrane traffic in vivo. J. Cell Biol., 124, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Z., Davletov,B.A., Sudhof,T.C. and Anderson,R.G. (1994) Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell, 78, 751–760. [DOI] [PubMed] [Google Scholar]
- Zhao L., Helms,J.B., Brugger,B., Harter,C., Martoglio,B., Graf,R., Brunner,J. and Wieland,F.T. (1997) Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit β. Proc. Natl Acad. Sci. USA, 94, 4418–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Helms,J.B., Brunner,J. and Wieland,F.T. (1999) GTP-dependent binding of ADP-ribosylation factor to coatomer in close proximity to the binding site for dilysine retrieval motifs and p23. J. Biol. Chem., 274, 14198–14203. [DOI] [PubMed] [Google Scholar]
- Zhdankina O., Strand,N.L., Redmond,J.M. and Boman,A.L. (2001) Yeast GGA proteins interact with GTP-bound Arf and facilitate transport through the Golgi. Yeast, 18, 1–18. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Traub,L.M. and Kornfeld,S. (1998) ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol. Biol. Cell, 9, 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Drake,M.T. and Kornfeld,S. (1999) ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc. Natl Acad. Sci. USA, 96, 5013–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]