Abstract
Fusel alcohols are natural products of amino acid catabolism in the yeast Saccharomyces cerevisiae that cause morphological changes similar to those seen during pseudohyphal growth. We have discovered that certain of these alcohols, including butanol and isoamyl alcohol, bring about a rapid inhibition of translation at the initiation step. This inhibition is strain specific and is not explained by previously described translational control pathways. Using genetic mapping, we have identified a proline to serine allelic variation at amino acid 180 of the GCD1 gene product as the genetic locus that allows translational regulation upon butanol addition. Gcd1p forms part of the eIF2B guanine nucleotide complex that is responsible for recycling eIF2-GDP to eIF2-GTP. This represents one of the key limiting steps of translation initiation and we provide evidence that fusel alcohols target eIF2B in order to bring about translational regulation.
Keywords: eIF2B/fusel alcohols/Gcd1p/translation
Introduction
Fusel oil is a complex mixture of alcohols obtained from yeast fermentations after most of the ethanol has been removed. Early studies suggested that these alcohols are produced via the catabolism of amino acids in yeast (reviewed by Webb and Ingraham, 1963). More recent analysis has confirmed that isoamyl alcohol and isobutyl alcohol are produced from leucine and valine catabolism, respectively (Dickinson et al., 1997, 1998). Therefore, the metabolism of branched chain amino acids is significantly different in yeast compared with other eukaryotes where amino acids are eventually broken down to components of the tricarboxylic acid (TCA) cycle. For example, yeast cannot use branched chain amino acids as a sole carbon source. However, they can be used as a nitrogen source under nitrogen-limiting conditions with the consequent production of fusel alcohols (Cooper, 1982). The addition of fusel alcohols to yeast has recently been shown to induce a range of strain-specific morphological effects, including filamentation (Dickinson, 1996; Lorenz et al., 2000). These effects are also seen if yeast are grown on leucine as the sole source of nitrogen. Hence, it has been suggested that these alcohols might somehow generate a signal that would normally indicate to the cell that nitrogen is scarce (Dickinson, 1996). It is also possible that these alcohols represent toxic by-products of metabolism.
The initiation of protein synthesis is a key regulatory step in the process of gene expression. As protein synthesis is intrinsically linked to amino acid pools, signalling pathways downstream of amino acid catabolites (such as fusel alcohols) might regulate translation. A major advantage of the translational control of gene expression is that levels of protein are affected immediately (Mathews et al., 2000). Two key steps in the translation initiation process are regulated: initiator methionyl-tRNA binding to the ribosome and ribosome recruitment of mRNAs.
The translation initiation factor eIF2, in the GTP-bound form, recruits the initiator methionyl-tRNA to the 40S small ribosomal subunit. Subsequent GTP hydrolysis facilitates joining of the 60S large ribosomal subunit at the initiator codon and consequently eIF2-GDP is released (Hershey and Merrick, 2000). Phosphorylation of the α-subunit of eIF2 competitively inhibits and titrates the guanine nucleotide exchange factor, eIF2B, which is responsible for recycling eIF2-GDP to eIF2-GTP (Hinnebusch, 2000). Several different kinases have been identified in higher eukaryotes which phosphorylate eIF2α and inhibit translation initiation (Dever, 1999). Gcn2p is the only eIF2α kinase found in yeast. Activation of Gcn2p by amino acid starvation not only inhibits general translation initiation but also activates the translation of GCN4 mRNA (Hinnebusch, 2000). This generates feedback regulation as Gcn4p is involved in the activation of amino acid biosynthesis at the level of gene transcription.
Control of protein synthesis also occurs via specific translation inhibitors known as eIF4E-binding proteins (4EBPs). These inhibitors bind to eIF4E and inhibit binding to the eIF4G subunit of eIF4F. This down-regulates the mRNA recruitment step of translation initiation. In higher eukaryotes, the ability of 4EBPs to interact with eIF4E is inhibited by phosphorylation in response to a number of hormones and growth factors. Although 4EBPs such as Caf20p and Eap1p have been identified in yeast, their involvement in the regulation of translation initiation is less well defined (Raught et al., 2000).
In addition to these mechanisms of translational control, other examples exist where the precise translational target of the regulation is unknown. For example, in yeast, the addition of the immunosuppressant drug rapamycin causes translational inhibition (Barbet et al., 1996). However, unlike higher eukaryotes, where this drug is thought to inhibit translation via activation of 4EBP1, the mechanism in yeast has yet to be defined fully. Similarly, the removal of glucose from yeast has recently been found to lead to a rapid inhibition of translation initiation (Ashe et al., 2000). In higher eukaryotes, glucose removal leads to endoplasmic reticulum stress, which activates an eIF2α kinase PERK/PEK (Harding et al., 1999). However, this mechanism is not conserved in yeast and, therefore, the mechanism of translational inhibition upon glucose starvation remains unknown (Ashe et al., 2000).
In this study, we have added to the relatively sparse list of translational controls described above by showing that fusel alcohols such as 1-butanol (butanol) or isoamyl alcohol can rapidly inhibit translation initiation in yeast. Rather than occurring via previously identified translational control mechanisms, we propose that fusel alcohols target the eIF2B guanine nucleotide exchange factor. Consistent with this interpretation, we show that a specific allele of the eIF2B γ-subunit is responsible for the genetic difference in translational sensitivity to butanol. Furthermore, butanol induces GCN4 by a Gcn2p- independent translational mechanism and overexpression of eIF2B titrates the inhibitory effects of butanol. It is intriguing that fusel alcohols should act via eIF2B, as studies on eIF2α kinase controls have identified this activity as a key regulatory step of translational initiation.
Results
Strain-specific inhibition of growth and translation by butanol
Fusel alcohols induce a range of strain-specific morphological changes in the yeast Saccharomyces cerevisiae. For example, addition of these alcohols causes pseudohyphal-like growth in Σ strains, hyphal-like extensions in W303-1A strains and has almost no effect in S288c strains (Dickinson, 1996; Lorenz et al., 2000). It has also been shown that butanol is a particularly potent inducer of these morphological phenotypes (Lorenz et al., 2000).
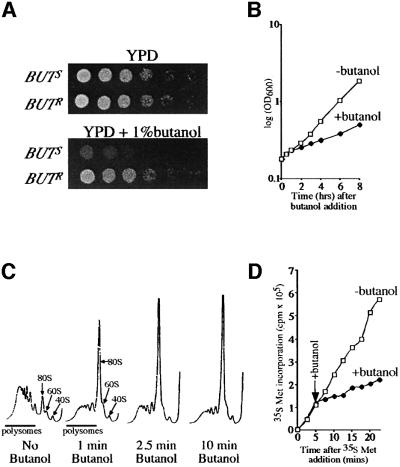
Given the reliance of translation on amino acid pools, we decided to test the effect of butanol (a potential signal for amino acid catabolism and nitrogen starvation) on the growth and translation of a variety of yeast strains. We identified two sources of the W303-1A strain that responded differently to the addition of butanol. In the absence of butanol, these strains are indistinguishable, whereas in its presence they grow at different rates. Approximately 1–1.5 h after butanol treatment, a 2- to 3-fold decreased growth rate was observed in the butanol-sensitive (BUTS) strain, whereas for the butanol-resistant (BUTR) strain little decrease was seen (Figure 1A and B). This butanol-dependent growth difference is confined to a single genetic locus, as sporulation of heterozygote BUTS/BUTR diploids always yield two BUTS and two BUTR haploid progeny (data not shown).
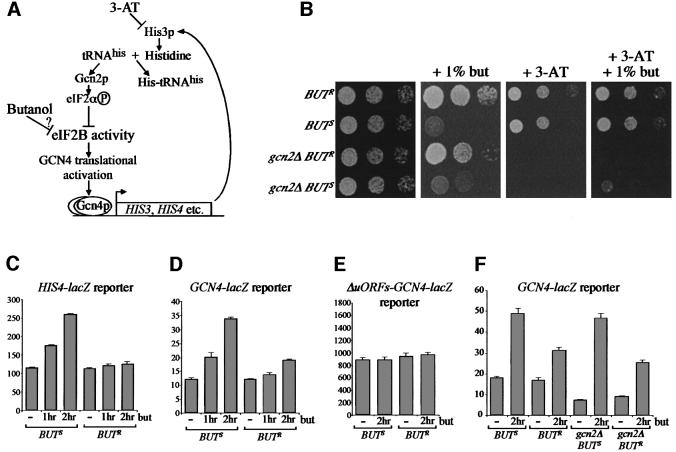
Fig. 1. Butanol inhibits growth and translation in specific genetic backgrounds. (A) Yeast strains yMK36 (BUTS) and yMK23 (BUTR) serially diluted on YPD (2 days at 30°C) or YPD + 1% butanol agar plates (3 days at 30°C). (B) Growth curves for yMK36 are shown either after the addition of 1% butanol at time zero or after no addition. (C) Polyribosome traces from yMK36. Yeast were grown in YPD and 1% butanol was added for the time periods indicated. Polyribosomes were analysed as described in Materials and methods. The 40S (small ribosomal subunit), 60S (large ribosomal subunit), 80S (monosome) and polysomes are labelled. (D) [35S]methionine incorporation into proteins over time in the presence or absence of 1% butanol. yMK36 was grown in synthetic complete media without methionine, split in two and at t0 [35S]methionine was added to each aliquot. After 5 min, 1% butanol was added to one of the aliquots. The level of [35S]methionine (c.p.m. × 105) incorporated into protein samples removed at the time points indicated was determined.
Another consequence of the addition of butanol to the W303-1A strain is the development of hyphal-like extensions (Dickinson, 1996; Lorenz et al., 2000). This phenotype was evident for both BUTS and BUTR strains after 6–8 h with butanol. However, for the BUTS strain, a larger proportion of cells had this phenotype compared with the BUTR strain (data not shown).
The difference in growth rate between these strains prompted us to examine the translational activity following butanol addition. An analysis of the distribution of polysomes across a sucrose gradient revealed that upon treatment of the BUTS strain with 1% (v/v) butanol, there was a dramatic shift of ribosomes from the polysomal region into the monosome or 80S peak (Figure 1C). The accumulation of ribosomes in the 80S peak of a sucrose gradient is indicative of decreased translation initiation. To confirm that protein synthesis is inhibited upon butanol treatment, yeast were labelled with [35S]methionine for 5 min prior to addition of butanol. Figure 1D shows that following butanol addition, there is an almost instantaneous 2- to 3-fold decrease in the rate of protein synthesis. This result confirms that there is a rapid decrease in translation initiation in the BUTS strain after butanol treatment. This inhibition is not observed in the BUTR strain (see Figure 3B). In addition, this inhibition of translation occurs almost instantaneously as there is a decrease in the rate of protein synthesis and an accumulation at the 80S peak of a polysome profile within 1 min (Figure 1C and D). Similar results were obtained using 0.5% isoamyl alcohol instead of 1% butanol (data not shown).
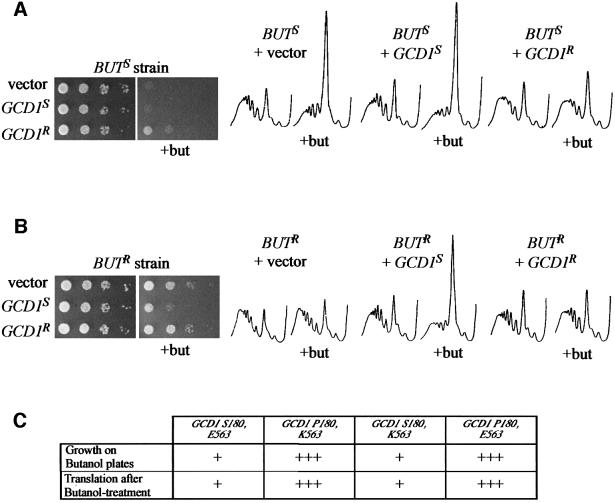
Fig. 3. Different alleles of the GCD1 gene account for the strain differences in butanol sensitivity/resistance. (A) A serial dilution plate assay and polyribosome traces from the butanol-sensitive background (BUTS strain) overexpressing either vector (yMK443), GCD1 isolated from the butanol-sensitive strain (GCD1S) (yMK444) or GCD1 isolated from the butanol-resistant strain (GCD1R) (yMK445). (B) A serial dilution plate assay and polyribosome traces from the butanol-resistant background (BUTR strain) overexpressing either vector (yMK446), GCD1 isolated from the butanol-sensitive strain (GCD1S) (yMK447) or GCD1 isolated from the butanol-resistant strain (GCD1R) (yMK448). For the serial dilution assay, SCD-Trp plates were incubated for 2 days at 30°C, whereas the butanol plates were incubated for 3–4 days at 30°C. For the polysome analyses, the strains were grown in SCD-Trp and there was either no addition or 1% butanol was added for 10 min (+but). (C) A summary of the growth and translational sensitivity to butanol for strains overexpressing GCD1 S180,E563 (yMK444, yMK447), GCD1 P180,K563 (yMK445, yMK448), GCD1 S180,K563 (yMK503, yMK505) and GCD1 P180,E563 (yMK504, yMK506).
Butanol-dependent translational regulation does not follow previously described translational control pathways
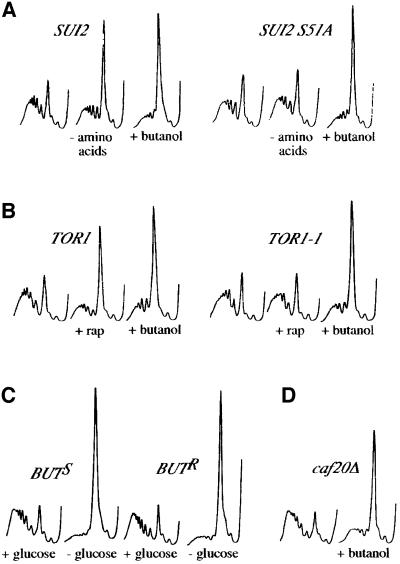
Probably the most significant translational regulatory pathway in yeast involves phosphorylation on Ser51 of the eIF2α subunit by the Gcn2p kinase. This kinase is activated in response to amino acid starvation and ultimately brings about the inhibition of translation initiation (Hinnebusch, 2000). Either substitution of Ser51 with alanine within the eIF2α gene (SUI2) or deletion of the GCN2 gene generates translational resistance to amino acid starvation (Dever et al., 1992). To test whether the butanol-dependent translational inhibition relied upon this pathway, we generated either wild-type or S51A versions of the SUI2 gene in the butanol-sensitive background. For the wild-type SUI2 BUTS strain, the polysome profiles following either severe amino acid starvation or butanol addition showed an accumulation of the 80S peak, indicative of translational inhibition (Figure 2A). Consistent with previous work (Dever et al., 1992), the SUI2 S51A strain is translationally resistant to severe amino acid starvation (Figure 2A). However, this strain is completely sensitive at the translational level to the addition of butanol (Figure 2A). A similar result was obtained using gcn2Δ strains (data not shown). Therefore, the addition of butanol to the BUTS strain does not lead to decreased translation via activation of the Gcn2p kinase and phosphorylation of eIF2α.
Fig. 2. Butanol inhibits translation in yeast by a previously unidentified mechanism. (A) Polyribosome traces from strains with wild-type SUI2 (yMK129) or mutant SUI2 S51A (yMK127). Yeast were grown in SCD-Leu and washed in SCD-Leu, SCD minus all amino acids (–amino acids) or SCD-Leu + 1% butanol (+butanol) for 10 min. (B) Polyribosome traces from strains with wild-type TOR1 (yMK449) or mutant TOR1 S1972I (yMK450). Strains were grown in SCD-Leu followed by the addition of either drug vehicle for 1 h, 0.2 µg/ml rapamycin (+rap) for 1 h or 1% butanol for 10 min (+butanol). (C) Polyribosome traces from butanol-sensitive (yMK36) and butanol-resistant (yMK23) strains. Yeast were grown in YPD and washed in either YPD (+glucose) or YP (–glucose) for 10 min. (D) Polyribosome traces from yMK441 (caf20Δ BUTS). Yeast were grown in YPD and 1% butanol was added to half the culture for 10 min (+butanol).
Yeast translation is also inhibited more slowly by the immunosuppressant drug rapamycin (Barbet et al., 1996). Rapamycin acts by inhibiting the activity of the Tor protein kinase homologues. A dominant mutant TOR1 S1972I has been described that is resistant to rapamycin treatment (Zheng et al., 1995). To test whether the pathways of translational inhibition for rapamycin and butanol treatment are the same, we transformed TOR1 S1972I into a butanol-sensitive strain. As opposed to the TOR1 control, where translation is inhibited after 1 h of rapamycin treatment, the TOR1 S1972I strain is almost completely resistant to rapamycin at the translational level. However, both rapamycin-resistant and -sensitive strains are equally sensitive to butanol at the translational level (Figure 2B). Furthermore, the kinetics of translational inhibition after butanol and rapamycin addition are different. Rapamycin inhibits translation relatively slowly after 30 min or more, whereas butanol affects translation almost instantly (data not shown). Taken together, these data suggest that the butanol and rapamycin pathways of translational control are not the same.
Recently, we have identified a translational inhibition mechanism with rapid kinetics similar to the butanol-dependent control of translation. This control occurs upon glucose removal from yeast media (Ashe et al., 2000). We tested both the butanol-sensitive and -resistant strains, and found that even though they were differentially sensitive to butanol, both strains were equally sensitive to the removal of glucose at the translational level (Figure 2C). Therefore, butanol addition and glucose starvation seem to elicit translational inhibition via different mechanisms.
Finally, in higher eukaryotes, translational controls can occur via eIF4E-binding proteins. Unlike the higher eukaryotic 4EBPs, the yeast 4EBP Caf20p has not been shown to regulate translation (Raught et al., 2000). However, it seems likely that, under specific conditions, this protein can regulate translation. Therefore, we deleted the gene for this protein in the butanol-sensitive background. Using this strain, translation is still inhibited upon butanol addition (Figure 2D). Therefore, Caf20p activation is not the mechanism of translational inhibition caused by butanol addition.
Genetic mapping of the locus responsible for butanol resistance and sensitivity
In the course of the studies using caf20Δ strains, we crossed the BUTR caf20Δ strain with the BUTS strain. After tetrad dissection of this diploid into four haploid progeny, we noticed that over many tetrads caf20::URA3 was linked to the BUTR phenotype. Table I shows the results from these dissection studies. Of 42 total tetrads, six gave a pattern indicative of a single meiotic crossover and we never saw a double crossover. Therefore, the gene responsible for the BUTR/BUTS phenotypic difference lies close to CAF20.
Table I. Results of meiotic mapping from a diploid strain constructed by crossing yMK442 (BUTS caf20::URA3 his3-11,15) and yMK430 (BUTR CAF20 HIS3).
|
BUTS/BUTR v caf20Δ/CAF20 |
his3/HIS3 v caf20Δ/CAF20 |
BUTS/BUTR v his3/HIS3 |
||||||
|---|---|---|---|---|---|---|---|---|
| PDa | TTb | NPDc | PDa | TTb | NPDc | PDa | TTb | NPDc |
| 36 | 6 | 0 | 13 | 29 | 0 | 15 | 27 | 0 |
aThe number of tetrads that are parental ditype (PD), i.e. if the parents are AB × ab then a PD tetrad pattern consists of two AB spores and two ab spores.
bThe number of tetrads that are tetratype (TT), i.e. if the parents are AB × ab then a TT tetrad pattern consists of AB, ab, Ab and aB spores.
cThe number of tetrads that are non-parental ditype (NPD), i.e. if the parents are AB × ab then a NPD tetrad pattern consists of two Ab spores and two aB spores.
CAF20 is on chromosome XV at position 843128. The HIS3 gene is on the same chromosome at position 721944. In order to map on which side of CAF20 the BUTR/BUTS gene lies, we crossed a HIS3 BUTR caf20 strain to a his3 BUTS CAF20 strain. As shown in Table I, HIS3 is also linked to the BUTR/BUTS gene. The tetrad numbers suggest that the BUTR/BUTS gene lies between HIS3 and CAF20, close to the CAF20 gene.
Gcd1p with serine at position 180 confers butanol sensitivity
A search through the list of genes on chromosome XV identified a prominent candidate gene, GCD1, at position 813980. GCD1 encodes the γ-subunit of eIF2B, a translation initiation factor involved in recycling eIF2-GDP to eIF2-GTP (Hinnebusch, 2000). To test whether allelic differences in this gene explain the differential butanol-dependent phenotypes, we PCR amplified the GCD1 gene from both the butanol-sensitive and -resistant strains. These fragments were subcloned into yeast overexpression vectors and transformed into both BUTS and BUTR strains. The BUTS/BUTR phenotype is semi-dominant (i.e. on butanol plates the heterozygote diploid grows faster than the BUTS homozygote diploid yet more slowly than the BUTR homozygote diploid; data not shown). On this basis, we anticipated that overexpression of a gene responsible for the butanol phenotype in a strain of the opposite phenotype might reverse the effects of butanol addition. Indeed, Figure 3A shows that when the BUTS strain was transformed with GCD1 isolated from the BUTR strain, the inhibition of both growth and translation upon exposure to butanol was reversed. Similarly, in the BUTR strain, transformation of GCD1 isolated from BUTS caused an inhibition of both growth and translation upon exposure to butanol (Figure 3B).
In order to establish whether the GCD1 locus explains these butanol-dependent phenotypic differences, we sequenced the two GCD1 variants. Compared with the S.cerevisiae Genome Database (SGD), resistant GCD1 carries several silent mutations and a mutation that changes glutamic acid to lysine at position 563 (E563K). The sensitive GCD1 version carries just one change compared with the SGD sequence: this is a mutation that changes Pro180 to serine (P180S). It was important to know whether these mutations explain the observed phenotypic differences upon butanol addition. Therefore, we replaced Pro180 with serine within the resistant form to give GCD1 S180,K563 and replaced Ser180 with proline in the sensitive form to give GCD1 P180,E563. Figure 3C summarizes the results where an S180P change within the sensitive GCD1 form (GCD1 P180,E563) generates resistance to butanol at the level of both translation and growth in both the BUTS and BUTR strains. In contrast, a switch of P180S within the resistant form of GCD1 (GCD1 S180,K563) generates sensitivity to butanol at the level of translation and growth in both BUTS and BUTR strains. This suggests that GCD1-S180/P180 is the critical allelic difference between these two supposedly identical W303-1A strains.
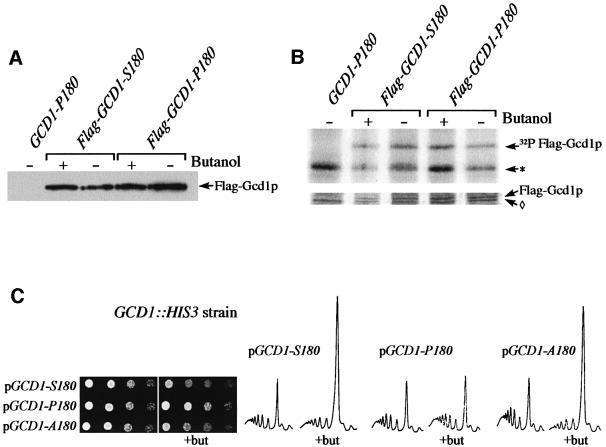
To confirm this, strains were generated where genomic GCD1 was disrupted and plasmid copies of either GCD1 S180 or GCD1 P180 were present to support yeast growth. We found that the GCD1-S180 strain was sensitive to butanol in terms of growth and translation, whereas the GCD1-P180 strain was resistant (see Figure 4C). The same analysis has now been performed in a total of three different genetic backgrounds (including S288c- and Σ1278b-derived strains) with identical results (data not shown). These data define the Pro180/Ser180 allelic variation between the butanol-sensitive and -resistant strains as the relevant change associated with the phenotypic difference upon butanol addition.
Fig. 4. The translational sensitivity to butanol does not require reversible phosphorylation at Ser180. (A) Gcd1p levels are unaffected by butanol treatment. Protein extracts from the yMK602 (GCD1-P180), yMK526 (Flag-GCD1-S180) and yMK527 (Flag-GCD1-P180) strains were blotted and probed with an antibody to the flag epitope. Strains were treated with 1% butanol where indicated. (B) Total Gcd1p phosphorylation is not changed measurably in response to butanol treatment. A [32P]orthophosphate metabolic labelling experiment using the same strains as in (A). [32P]orthophosphate was added to yeast 5 min prior to treatment with or without 1% butanol for 10 min. The lower panel shows Coomassie Blue-stained immunoprecipitated Flag-Gcd1p and a non-specific band (diamond). The upper panel shows an autoradiograph of the same gel where the predominant phosphate-labelled species are Flag-Gcd1p and a non-specific phosphoprotein (*). (C) Serial dilution plate assays and polyribosome traces from GCD1 disrupted strains harbouring either GCD1-S180 (yMK601), GCD1-P180 (yMK602) or GCD1-A180 (yMK603) plasmids. For serial dilutions, SCD-Trp plates were incubated at 30°C for 2 days, whereas butanol-containing plates were incubated for 3–4 days at 30°C. For the polysome analyses, the strains were grown in SCD-Trp and there was either no addition or 1% butanol was added for 10 min (+but).
Phosphorylation of Ser180 in butanol-sensitive Gcd1p is not required for the butanol-dependent inhibition of translation
To assess the effects of butanol on the Gcd1 protein, strains carrying Flag epitope-tagged GCD1 as the sole source of Gcd1 protein were made. The Flag-tagged GCD1-S180 and GCD1-P180 strains responded to butanol in the same way as non-tagged strains (data not shown): the Flag-GCD1-S180 strain specifically allowed the inhibition of growth and translation following butanol treatment. Western blot analysis using the Flag antibody did not show any major changes in the Gcd1 protein levels for either GCD1-P180 or GCD1-S180 strains after butanol addition (Figure 4A).
As the presence of a serine residue at position 180 allows the translational regulation, we analysed the phosphorylation status of Gcd1p following butanol treat ment. [32P]orthophosphate labelling–immunoprecipitation experiments showed that Gcd1p is labelled extensively over a 15 min period prior to extract preparation. However, no dramatic change in the level of 32P incorporation into Gcd1p was observed upon butanol treatment of either the GCD1-S180 or GCD1-P180 strains after normalization to the amount of Gcd1p in the immunoprecipitate (Figure 4B, top panel versus bottom). These data suggest that neither the abundance nor the gross phosphorylation status of Gcd1p changes upon butanol treatment for either the butanol-sensitive or -resistant alleles.
To assess directly whether reversible phosphorylation of Gcd1p at Ser180 is required for this regulation, we generated a mutant form of Gcd1p with alanine at position 180. Figure 4C shows that a strain carrying GCD1-A180 as the sole source of Gcd1p responds to butanol in the same manner as a GCD1-S180 strain. This demonstrates that Ser180 is not a requirement for the regulation of trans lation. Furthermore, this shows that potential reversible phosphorylation events at this site do not play a role in the inhibition of translation upon butanol treatment.
Butanol translationally induces Gcn4p in a GCN2-independent mechanism
As the GCD1 gene product forms part of the translation factor eIF2B, one possibility is that the butanol-dependent inhibition of translation is due to a decrease in activity of this factor. eIF2B is the guanine nucleotide exchange factor responsible for the essential recycling of eIF2-GDP to eIF2-GTP. However, the capacity to reduce eIF2B activity is essential for growth under amino acid starvation conditions. Amino acid starvation is commonly mimicked in yeast using 3-amino-triazole (3-AT) (see Figure 5A). This compound competitively inhibits the enzyme imidazoleglycerol-phosphate dehydratase, coded by the HIS3 gene. Addition of 3-AT to wild-type strains causes histidine starvation, which activates Gcn2p kinase, ultimately increasing GCN4 expression. The Gcn4p transcription factor then activates the HIS3 gene in order to overcome the histidine starvation. Mutations in this regulatory loop, such as deletion of GCN2 (gcn2Δ), prevent growth of strains under amino acid starvation conditions as they cannot inhibit eIF2B activity. Reduction of eIF2B activity in gcn2Δ strains allows growth on media containing 3-AT (e.g. Gomez and Pavitt, 2000). Therefore, growth of gcn2Δ strains on 3-AT can provide an in vivo measure of eIF2B activity.
Fig. 5. Gcn4p activity is increased upon butanol addition to the GCD1-S180 strain. (A) Diagram summarizing how 3-AT is thought to induce the general control pathway via Gcn4p activation at the translational level. (B) A GCD1-S180 gcn2 strain grows on 3-AT plates in the presence of 1% butanol. Serial dilution plate assays of butanol-resistant (BUTR) (yMK430), butanol-sensitive (BUTS) (yMK429), butanol-resistant gcn2 deleted (gcn2Δ BUTR) (yMK515) and butanol-sensitive gcn2 deleted (gcn2Δ BUTS) (yMK516) strains. The plates were grown at 30°C as follows: YPD (2 days); YPD + 1% butanol (3 days) (minor variations in the growth of butanol-sensitive strains occur on these plates due to slight differences in butanol concentration across plates); SCD-His + 10 mM 3-AT (4 days); SCD-His + 1% butanol + 10 mM 3-AT (4 days). (C–F) The GCD1-S180 allele permits increased HIS4-lacZ and GCN4-lacZ expression in response to butanol. β-galactosidase assays measured in Miller units from extracts prepared from the strains (C) yMK526 and yMK527 (HIS4-lacZ), (D) yMK597 and yMK598 (p[GCN4-lacZ]), (E) yMK599 and yMK600 (p[ΔuORFs-GCN4-lacZ]) and (F) yMK629–632 (<GCN4-lacZ>).
On this basis, we generated both BUTS (GCD1-S180) gcn2Δ and BUTR (GCD1-P180) gcn2Δ strains. As shown in Figure 5B, in rich media, these strains grew identically to the parental BUTS and BUTR strains, and were also sensitive and resistant to butanol where expected. In addition, both gcn2Δ strains were equally inhibited for growth on 3-AT plates. However, the inclusion of butanol in the 3-AT plate specifically allowed for limited growth of the BUTS gcn2Δ strain. This result is explained most easily if the butanol-dependent translation inhibition in the BUTS gcn2Δ strain overcomes the 3-AT-dependent amino acid starvation. Paradoxically, the parental BUTS and BUTR strains grew equally well on the 3-AT/butanol plates. Butanol inhibits the BUTS strain 2- to 3-fold for growth, whereas 3-AT inhibits either the BUTS or BUTR strain 3- to 4-fold for growth (Figure 1 and data not shown). Therefore, on the 3-AT/butanol plate, butanol is incapable of inhibiting growth of the BUTS strain beyond the inhibition caused by 3-AT.
The increased growth for the gcn2Δ BUTS strain on 3-AT/butanol must be due to increased expression or activity of His3p in the presence of butanol. One mechanism by which the 3-AT sensitivity of the gcn2Δ mutant can be overcome is via mutation of eIF2B genes and consequent induction of the Gcn4p transcription factor (Hinnebusch, 2000). In order to test whether Gcn4p is induced upon butanol treatment, we tested a series of reporter genes. The first contains the HIS4 promoter driving the lacZ gene. Expression from HIS4-lacZ is increased ∼2-fold after 2 h of amino acid starvation via translational activation of Gcn4p (Lucchini et al., 1984). Following butanol addition to a butanol-sensitive strain, there is a similar 2-fold increase in HIS4-lacZ expression after 2 h, whereas there is little increase from the butanol-resistant strain (Figure 5C). We also tested a GCN4-lacZ reporter containing the GCN4 promoter and 5′-untranslated region driving expression of the lacZ gene. This reporter has been widely used to assess the amino acid starvation-dependent inhibition of eIF2B activity. When expression from this GCN4-lacZ reporter was measured following butanol treatment for 2 h, ∼3-fold induction was seen for a butanol-sensitive strain (Figure 5D). Interest ingly, there is also an increase in GCN4-lacZ activity in the butanol-resistant strain, suggesting that butanol has less pronounced effects on this strain. Figure 5E shows that butanol does not increase the activity of a GCN4 reporter lacking the upstream open reading frames (uORFs). The requirement for these uORFs suggests that translational control is involved in the GCN4 activation by butanol. Finally, as described above, deletion of GCN2 inhibits the activation of GCN4 following amino acid starvation. However, following butanol treatment, GCN2 deletion strains show similar increases in GCN4-lacZ activity (Figure 5F). This confirms our previous result showing that neither Gcn2p nor phosphorylation of eIF2α is required for the effect of butanol upon translation.
Overall, these results show that GCN4 is activated by butanol addition via a translational mechanism requiring the uORFs which is independent of eIF2α phosphorylation. This suggests that butanol acts to increase the levels of the ternary complex (eIF2·GTP·Met-tRNAiMet).
Evidence to support a role for eIF2B as the target for butanol-dependent translation inhibition
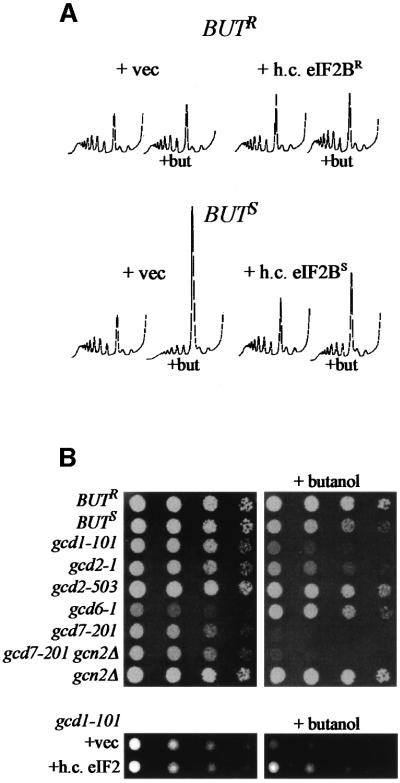
As butanol relies upon allelic changes within GCD1 to inhibit translation and activates GCN4 via a translational mechanism, it seems likely that the activity of eIF2B is decreased upon butanol treatment. To assess this more directly, we tested the effect of overexpression of the eIF2B subunits upon translation. Figure 6A shows that high copy eIF2B (with a sensitive version of GCD1) leads to a less pronounced change in the polysome profile following butanol treatment. Therefore, increasing the level of eIF2B can partially suppress the effects of butanol upon translation.
Fig. 6. Butanol is likely to inhibit translation via effects on eIF2B. (A) Polysome analyses from butanol-sensitive and -resistant strains carrying either the appropriate empty high copy vectors (pRS424 and pRS425) or the eIF2B high copy plasmids: sensitive pMK31 (p[GCD1S GCD6]); or resistant pMK32 (p[GCD1R GCD6 LEU2 2µ])] as well as pAV1492 p[GCD7 GCD2 GCN3 TRP1 2µ]. Strains were grown in SCD-Trp-Leu and 1% butanol was added to half the culture for 10 min (+but). (B) Serial dilution plate assays for butanol-resistant (BUTR) (yMK23), butanol-sensitive (BUTS) (yMK36) and various gcd mutant strains (yMK641–647). The plates were grown at 24°C (due to the temperature sensitivity of the gcd mutants) for 3 days (YPD) or 4 days (YPD + 1% butanol). The lower panels show the effects of a high copy eIF2 plasmid (p1780) or vector in the gcd1-101 strain background (yMK639 and yMK640). Growth was for 3 days (SCD-Ura) or 4 days (SCD-Ura + 1% butanol).
We also extended the analysis to look at a whole range of mutations in eIF2B subunits. Figure 6B shows that some of these mutants are sensitive to butanol (e.g. gcd1-101, gcd2-1 and gcd7-201) and some are relatively resistant to butanol (e.g. gcd2-503). One mutant, gcd6-1, appears completely unaffected by the presence of butanol in the growth medium (all other strains, even the butanol-resistant allele, are inhibited in terms of growth to some extent).
It has also been demonstrated that the phenotype of certain gcd mutants with reduced eIF2B activity can be suppressed by overexpression of the translation initiation factor eIF2 (Dever et al., 1995). Although overexpression of eIF2 did not affect the BUTS strain in the presence of butanol (data not shown), it did suppress the butanol sensitivity of the gcd1-101 strain (Figure 6B, lower panels). This result, in combination with the effects of high copy eIF2B on the BUTS strain and the activation of GCN4, suggests that butanol inhibits translation via an effect on the eIF2B guanine nucleotide exchange factor.
Discussion
The guanine nucleotide exchange reaction (eIF2-GDP to eIF2-GTP) catalysed by eIF2B is a major translational regulatory point in eukaryotic cells. This control has a conserved mechanism involving the conversion (via phosphorylation) of eIF2α from a substrate to a competitive inhibitor of eIF2B (Hinnebusch, 2000). This pathway is induced by a number of different eIF2 kinases, which are activated by a wide variety of conditions and stimuli in different organisms (Dever, 1999).
In addition, in higher eukaryotes, the ε-subunit of eIF2B is phosphorylated directly by several different protein kinases in vitro, including casein kinase (CK) I, CKII and glycogen synthase kinase-3 (GSK-3). Phosphorylation of eIF2Bε by CKI/CKII stimulates eIF2B activity whereas phosphorylation by GSK-3 is inhibitory (Kimball and Jefferson, 2000). In mammalian cells, insulin treatment inhibits GSK-3, thus activating eIF2B. This, combined with the insulin-dependent inhibition of 4EBPs, mediates the stimulation of protein synthesis upon insulin treatment (Proud and Denton, 1997).
We have identified a new translational control that is activated in specific yeast strains within minutes of butanol addition. In higher eukaryotic cells, amino alcohols inhibit translation indirectly via a competitive inhibition of aminoacyl-tRNA synthetases (Thomas and Mathews, 1984). This probably occurs due to accumulation of uncharged tRNAs, which results in the activation of Gcn2p kinase and a consequent increase in eIF2α phosphorylation (Kimball and Jefferson, 2000). Indeed, in yeast, we have found that leucinol-dependent inhibition of translation requires GCN2 (M.P.Ashe, unpublished data). This inhibition of aminoacyl-tRNA synthetases is therefore compensated by activation of GCN4. Initially we suspected that butanol might mimic an amino alcohol and therefore eIF2 phosphorylation would be necessary for the butanol-dependent inhibition of translation. However, as shown in Figure 2A, eIF2α Ser51 phosphorylation is not a component of the translational inhibition caused by butanol addition.
In higher eukaryotes, recent studies have highlighted a connection between amino acids and protein synthesis (Kimball and Jefferson, 2000). As fusel alcohols are amino acid metabolites in yeast, these observations in higher eukaryotes may be relevant. Indeed, one study has shown that GSK-3 is inactivated in L6 muscle cells in response to amino acid addition (Peyrollier et al., 2000). This is particularly pertinent since eIF2B dephosphorylation relies on the inactivation of this kinase in response to insulin (Proud and Denton, 1997). However, branched chain amino acids and their metabolites act as potential stimulators of protein synthesis for most of the effects described above, whereas fusel alcohols act as translational inhibitors in yeast. In addition, the effects of branched chain amino acids in higher eukaryotes are negated by rapamycin, whereas the use of a rapamycin-resistant mutant here suggests that the rapamycin-sensitive pathway is not involved in the butanol-dependent translational control. Branched chain amino acids in higher eukaryotes have also been demonstrated to increase the phosphorylation of 4EBP1 (Patti et al., 1998; Xu et al., 1998). We have found no evidence that one of the yeast 4EBPs, Caf20p, is involved in the regulation of translation by butanol. However, this control does rely on a specific allele of the GCD1 gene encoding the γ-subunit of eIF2B.
The specific GCD1 allelic change is Pro180 to serine, which generates the regulation of translation by butanol. The identified residue at position 180 lies within a domain towards the N-terminus of the protein that is conserved across eIF2B γ- and ε-subunits. This domain shares sequence similarity with prokaryotic nucleoside triphosphate-hexose pyrophosphorylases. On the basis of this similarity, it has been proposed that this domain may serve as a nucleotide-binding domain where the subdomain surrounding P180 would serve as the Mg2+-binding region (Koonin, 1995). However, recent mutagenesis of the catalytic ε-subunit of eIF2B has suggested that the C-terminus is involved in nucleotide exchange (Gomez and Pavitt, 2000). Further studies are required to assess the role of this domain in the eIF2B γ-subunit (Gcd1p) and whether it is involved in nucleotide binding or stabilization.
Butanol and other fusel alcohols that result from amino acid catabolism have been shown to lead to a variety of morphological changes in different strains of yeast. On the basis of this, it has been suggested that these alcohols represent a nitrogen starvation signal to the yeast cell (Dickinson, 1996; Lorenz et al., 2000). It is possible that the inhibition of translation to preserve amino acid pools under such stress conditions might be a logical extrapolation of this model. Furthermore, the activation of Gcn4p may allow a redistribution of the nitrogen obtained from the catabolism of specific amino acids. An alternative explanation is that the presence of proline at position 180 allows cells to continue growing in the presence of otherwise toxic levels of fusel alcohols. Further analysis of this phenotype will be required to distinguish between these hypotheses.
Given the kinetics, reversibility and amplification required for butanol to inhibit translational initiation, it is possible that a signal transduction cascade connects butanol to the translational machinery. However, Ser180 in the butanol-sensitive form of eIF2Bγ, which might have been involved via reversible phosphorylation in such a control mechanism, is not required. This does not necessarily rule out a signal transduction pathway in this control as the position 180 allelic difference may represent a modulatory mutation that has effects on other components within the eIF2B complex. Indeed, it is plausible that the inhibition of translation in response to butanol could result from the activation of the same signal transduction pathways that generate the morphological changes. It seems unlikely that translation inhibition is required for these morphological changes as strains bearing the two different alleles of GCD1 that are either translationally resistant or sensitive to butanol exhibited the same morphological responses, although with different kinetics (data not shown). A key objective of future work will be to assess the role of signal transduction pathways in the translation regulation in response to butanol.
It is difficult to speculate upon the evolutionary conservation of the butanol-dependent translational control pathway at this early stage in its characterization. However, protein kinases target the ε-subunit of eIF2B in mammalian cells, altering eIF2B activity, and may represent a similar control mechanism for regulating this key step of translation initiation. Once the details of the mechanism and pathway of butanol-dependent translational regulation become clearer, an ultimate goal would be the comparison of this regulation with that in other eukaryotic species. Obvious precedents exist in the translation field for the conservation of regulatory mechanisms responding to different upstream stimuli in different organisms.
Materials and methods
Strains and growth conditions
Table II lists the strains used in this study. Strains were grown either on standard yeast extract/peptone/glucose media (YPD) or synthetic complete yeast media (SCD) (Guthrie and Fink, 1991). Butanol was added at 1% (v/v) on plates or in liquid culture (generally for 10 min), whereas isoamyl alcohol was added at 0.5% (v/v) in liquid culture for 10 min. Rapamycin (a gift from Professor J.Thorner) in 90% ethanol, 10% Tween-20 was added to a final concentration of 0.2 µg/ml for 1 h (an equal volume of drug vehicle was used as a control). Severe amino acid starvation was brought about by the removal of all the amino acids for 10 min. Dilution series plate assays started with 2 µl of exponentially growing OD600 0.3 cultures and continued in 5-fold dilutions.
Table II. Yeast strains used in this study.
| Strain name | Genotype | Source |
|---|---|---|
| YMK36 (BUTS) | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 | J.Thorner W3031A |
| YMK37 (BUTS) | MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 | J.Thorner W3031A |
| YMK23 (BUTR) | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 | A.Sachs W3031A strain |
| yMK24 (BUTR) | MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 | A.Sachs W3031A strain |
| yMK75 | MATa ade2-1 his3-11,15 leu2-3 112 trp1-1 ura3-1 GCD1-S180,E563 gcn2::URA3 | Ashe et al. (2000) |
| yMK76 | MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 gcn2::URA3 | Ashe et al. (2000) |
| yMK129 | MATa ade2-1 his3-11,15 leu2-3,112 trp1 ura3 sui2Δ GCD1-S180,E563 p[SUI2 LEU2 CEN] | Ashe et al. (2000) |
| yMK127 | MATa ade2-1 his3-11,15 leu2-3,112 trp1 ura3 sui2Δ GCD1-S180,E563 p[SUI2 S51A LEU2 CEN] | Ashe et al. (2000) |
| yMK449 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 p[TOR1 LEU2 CEN] | this study |
| yMK450 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 p[TOR1 S1972I LEU2 CEN] | this study |
| yMK441 | MATa ade2-1 his3-11,15 leu2-3 112 trp1-1 ura3-1 GCD1-S180,E563 caf20::URA3 | this study |
| yMK442 | MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 caf20::URA3 | this study |
| yMK312 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 caf20::URA3 | Sachs strain collection |
| yMK429 | MATa ade2-1 HIS3 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 | this study |
| yMK430 | MATa ade2-1 HIS3 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 | this study |
| yMK443 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 p[TRP1 2µ] | this study |
| yMK444 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 p[GCD1-S180,E563 TRP1 2µ] | this study |
| yMK445 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 p[GCD1-P180,K563 TRP1 2µ] | this study |
| yMK446 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 p[TRP1 2µ] | this study |
| yMK447 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 p[GCD1-S180,E563 TRP1 2µ] | this study |
| yMK448 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 p[GCD1-P180,K563 TRP1 2µ] | this study |
| yMK503 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 p[GCD1-S180,K563 TRP1 2µ] | this study |
| yMK504 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 p[GCD1-P180,E563 TRP1 2µ] | this study |
| yMK505 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 p[GCD1-S180,K563 TRP1 2µ] | this study |
| yMK506 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 p[GCD1-P180,E563 TRP1 2µ] | this study |
| yMK507 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 gcd1::HIS3 p[GCD1-S180,E563 URA3 CEN] | this study |
| yMK516 | MATa ade2-1 HIS3 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 gcn2::URA3 | this study |
| yMK515 | MATa ade2-1 HIS3 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 gcn2::URA3 | this study |
| yMK537 | MATa leu2-3,112 trp1-Δ63 ura3-52 GAL2 <HIS4-lacZ@ura3-52> gcd1::LEU2 p[GCD1 URA3 CEN] | G.Pavitt (GP3063) |
| yMK526 | MATa leu2-3,112 trp1-Δ63 ura3-52 GAL2 <HIS4-lacZ@ura3-52> gcd1::LEU2p[Flag-GCD1-S180,E563 TRP1 2µ] | this study |
| yMK527 | MATa leu2-3,112 trp1-Δ63 ura3-52 GAL2 <HIS4-lacZ@ura3-52> gcd1::LEU2 p[Flag-GCD1-P180,E563 TRP1 2µ] | this study |
| yMK597 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 gcd1::HIS3 p[FlagGCD1-S180,E563 TRP1 2µ] p[GCN4-lacZ URA3 CEN] | this study |
| yMK598 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 gcd1::HIS3 p[FlagGCD1-P180,E563 TRP1 2µ]p[GCN4-lacZ URA3 CEN] | this study |
| yMK599 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 gcd1::HIS3 p[FlagGCD1-S180,E563 TRP1 2µ] p[ΔuORFs-GCN4-lacZ URA3 CEN] | this study |
| yMK600 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 gcd1::HIS3 p[FlagGCD1-P180,E563 TRP1 2µ] p[ΔuORFs-GCN4-lacZ URA3CEN] | this study |
| yMK601 | MATa leu2-3,112 trp1-Δ63 ura3-52 GAL2 <HIS4-lacZ@ura3-52> gcd1::LEU2 p[GCD1-S180,E563 TRP1 2µ] | this study |
| yMK602 | MATa leu2-3,112 trp1-Δ63 ura3-52 GAL2 <HIS4-lacZ@ura3-52> gcd1::LEU2 p[GCD1-P180,E563 TRP1 2µ] | this study |
| yMK603 | MATa leu2-3,112 trp1Δ63 ura3-52 GAL2 <HIS4-lacZ@ura3-52> gcd1::LEU2 p[GCD1-A180,E563 TRP1 2µ] | this study |
| yMK629 | MATa ade2-1 his3-11,15 leu2-3,112 <GCN4-lacZ@TRP1> ura3-1 gcn2::URA3 GCD1-S180,K563 | this study |
| yMK630 | MATa ade2-1 his3-11,15 leu2-3,112 <GCN4-lacZ@TRP1> ura3-1 gcn2::URA3 GCD1-P180,K563 | this study |
| yMK631 | MATa ade2-1 his3-11,15 leu2-3,112 <GCN4-lacZ@TRP1> ura3-1 GCD1-S180,E563 | this study |
| yMK632 | MATa ade2-1 his3-11,15 leu2-3 112 <GCN4-lacZ@TRP1> ura3-1 GCD1-P180,K563 | this study |
| yMK633 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 p[GCD1-P180 GCD6 LEU2 2µ] p[GCN3 GCD7 GCD2 TRP1 2µ] | this study |
| yMK635 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-P180,K563 p[LEU2 2µ] p[TRP1 2µ] | this study |
| yMK636 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 GCD1-S180,E563 p[GCD1-S180 GCD6 LEU2 2µ] p[GCN3 GCD7 GCD2 TRP1 2µ] | this study |
| yMK638 | MATa ade2-1 his3-11,15 leu2-3 112 trp1-1 ura3-1 GCD1-S180,E563 p[LEU2 2µ] p[TRP1 2µ] | this study |
| yMK639 | MATα ura3-52 gcd1-101 p[URA3 2µ] | this study |
| yMK640 | MATα ura3-52 gcd1-101 p[SUI2 SUI3 GCD11 URA3 2µ] | this study |
| yMK641 | MATα ura3-52 gcd1-101 | G.Pavitt (F98) |
| yMK642 | MATα ura3-52 leu2-3,112 gcd2-1 | G.Pavitt (H952) |
| yMK643 | MATa ura3-52 gcn3-101 gcd2-503 | G.Pavitt (H625) |
| yMK644 | MATα ura3-52 leu2-3,112 gcd6-1 | G.Pavitt (H1728) |
| yMK645 | MATα ura3-52 leu2-3,112 gcd7-201 | G.Pavitt (H1603) |
| yMK646 | MATα ura3-52 leu2-3,112 gcn2::LEU2 gcd7-201 | G.Pavitt (H1794) |
| yMK647 | MATα ura3-52 leu2-3,112 gcn2::LEU2 | G.Pavitt (H1795) |
The isogenic yMK36/37 strains are derived from a W303-1A strain (provided by Professor J.Thorner) by transformation with the HO gene, diploid selection for loss of HO followed by tetrad dissection (Guthrie and Fink, 1991). The yMK23/24 isogenic strains are derived from the Sachs strain collection W303-1A isolate. The generation of strains yMK75, 76, 127 and 129 has been described previously (Ashe et al., 2000). The GCD1::HIS3 disrupted strain (yMK507) was created by transforming a BamHI–ScaI fragment from pMK24 (see ‘Plasmid construction’) into yMK36 carrying a GCD1 URA3 CEN plasmid. GP3063 (kindly provided by Dr G.Pavitt) was derived from the strain MC1057 (Cigan et al., 1993) by plasmid shuffling. Strains with other GCD1-containing plasmids were obtained using standard plasmid shuffling techniques (Guthrie and Fink, 1991). yMK597 and 598 were derived from yMK507 by plasmid shuffling of pMK28 and 29 followed by transformation with p180 (pGCN4-lacZ URA3 CEN) (a gift from Dr A.Hinnebusch), yMK629– 633 were generated by transformation of yMK75, yMK515, yMK36 and yMK23, respectively, with a SnaBI-linearized TRP1-GCN4-lacZ integration plasmid (p1108) (kindly provided by Dr T.Dever) (Dever et al., 1992). Strains yMK641–647 were a gift from Dr G.Pavitt and were originally obtained from Dr A.Hinnebusch.
Plasmid construction
A –1335 to +2536 (where +1 is the A of the start codon) GCD1-containing fragment was amplified from yMK36 (BUTS) and yMK23 (BUTR) genomic DNA by PCR using EXPAND polymerase (Roche Diagnostics Ltd). These were ligated into EcoRV-digested pBluescrript SK+ to give pMK18 (GCD1S) and pMK19 (GCD1R) (GCD1 is orientated with the PstI site upstream and the Asp718 site downstream of the gene). The GCD1 ORFs were sequenced on both strands and, to ensure that the S180 and K563 mutations were not generated by PCR errors, subclones generated from three separate PCRs were sequenced for each mutation.
GCD1 fragments were subcloned from pMK18 and pMK19 into yEPlac112 to generate pMK20 (GCD1-S180,E563) and pMK21 (GCD1-P180,K563) using PstI and Asp718. pMK26 (GCD1-S180,K563) and pMK27 (GCD1-P180,E563) were generated by replacing a BamHI–BsmI fragment in pMK21 with that from pMK20, and vice versa. pMK30 (GCD1-A180,E563) was made using the megaprimer PCR mutagenesis technique (the mutant oligonucleotide changed CCA at position +538 to +540 to GCG). A 1.3 kb BglII fragment was then replaced in pMK27 and sequenced to confirm the mutagenesis. pMK24 (GCD1::HIS3 deletion construct) was generated by replacing a BstEII–AflII fragment in pMK18 with a BamHI–XhoI fragment containing HIS3. pMK22 (GCD1-S180,E563 URA3 CEN) was generated by subcloning the PstI–Asp718 fragment from pMK18 into yCPlac33. pMK28 (Flag-GCD1-S180,E563) and pMK29 (Flag-GCD1-P180,E563) were constructed by replacing a BsmI–AflII fragment with that from a C-terminal Flag epitope-tagged GCD1-containing plasmid pAV1431 (kindly provided by Dr G.Pavitt) (Gomez and Pavitt, 2000). pMK31 (GCD1-S180 GCD6 LEU2 2µ) and pMK32 (GCD1-P180 GCD6 LEU2 2µ) were constructed by replacing HindIII fragments pMK20 and pMK27, respectively, into pAV1355 (provided by Dr G.Pavitt). pAV1492 (GCD7 GCD2 GCN3 TRP1 2µ) and p1780 (GCD11 SUI2 SUI3 URA3 2µ) were kindly provided by Dr G.Pavitt.
Analysis of ribosome distribution on sucrose gradients
Yeast cultures were grown to an OD600 of 0.4 and treated with butanol, isoamyl alcohol and rapamycin as described above. Extracts were prepared in 100 µg/ml cycloheximide and these were layered onto 15–50% sucrose gradients. The gradients were sedimented via centrifugation at 40 000 r.p.m. for 2.5 h, and the A254 was measured continuously to give the traces shown in the figures (for a detailed description of the method see Ashe et al., 2000).
[35S]methionine incorporation assay
Yeast strains were grown to an OD600 of 0.4 in SCD medium lacking methionine. The culture was split into two flasks and methionine was added to a final concentration of 60 ng/ml, of which 0.5 ng/ml was [35S]methionine (cell-labelling grade, 1175 Ci/mmol; New England Nuclear, Boston, MA). Samples (1 ml) were taken and processed as described previously (Ashe et al., 2000). After 5 min of sampling, 1% (v/v) butanol was added to one of the cultures and sampling was continued.
Assays of HIS4 and GCN4 expression
Standard methods for measuring the β-galactosidase activity from strains bearing HIS4-lacZ and GCN4-lacZ fusions have been described previously (Lucchini et al., 1984). β-galactosidase is expressed as nanomoles of o-nitophenol-β-d-galactopyranoside (ONPG) hydrolysed per minute per microgram of total protein.
Immunoblotting of Flag-Gcd1p
Yeast strains yMK602, yMK526 and yMK527 were grown to an OD600 of 0.7 in YPD. For yMK602, one 15 ml aliquot and for yMK526/yMK527 two 15 ml aliquots were taken. Butanol (1%) was added to one of the yMK526/yMK527 aliquots. All the cells were pelleted at 30°C and rapidly frozen in liquid N2 such that the time in butanol was 10 min. The cells were lysed, and protein samples were prepared, electrophoretically separated and immunoblotted as described previously (Ashe et al., 2000). The M2 Flag antibody (Sigma-Aldrich, UK) was used to detect flag epitope-tagged Gcd1p.
[32P]orthophosphate labelling and immunoprecipitation
The yeast strains yMK602, yMK526 and yMK527 were grown to an OD600 of 0.7 in phosphate-free medium (Guthrie and Fink, 1991). For yMK602, one 15 ml aliquot and for yMK526/yMK527 two 15 ml aliquots were taken. Cells were pelleted and resuspended in 5 ml of phosphate-free medium. Then 250 µCi of [32P]orthophosphate (HCl free) (10 mCi/ml) (Amersham-Pharmacia Biotech UK Ltd) were added to each sample at t0; 1% butanol was added to one of the yMK526/527 samples after 5 min. The samples were pelleted and frozen in liquid N2 such that the time on freezing was 15 min after the [32P]orthophosphate addition and 10 min after butanol addition. A 200 µl aliquot of buffer A [30 mM HEPES pH 7.5, 100 mM KOAc, 2 mM Mg2OAc, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM dithiothreitol (DTT), complete™ protease inhibitor cocktail (Roche Diagnostics Ltd, UK)] was added to each sample and thawed at 4°C. The cells were lysed with 0.5 vol. of glass beads at 4°C by vortexing (six times for 20 s; 40 s intervals on ice). After 5 and 15 min spins at 10 000 r.p.m. in an Eppendorf microcentrifuge, the final supernatant was collected and the protein concentration determined using Bradford reagent (Bio-Rad Laboratories Ltd, UK). A 300 µg aliquot of protein was incubated with 15 µl (wet volume) of M2 Flag affinity gel (Sigma-Aldrich Company Ltd, UK) for 2 h at 4°C. The immunoprecipitates were washed six times with 1 ml of buffer A. A 30 µl aliquot of SDS loading buffer (without β-mercaptoethanol) was added, the samples were denatured at 95°C for 2 min and the supernatant transferred to a new Eppendorf tube containing β-mercaptoethanol. Samples were electrophoretically separated on an SDS–10% polyacrylamide gel, which was either stained using Coomassie Blue or exposed autoradiographically for 2 h.
Acknowledgments
Acknowledgements
We thank G.Pavitt, J.Thorner, T.Dever, A.Hinnebusch and G.Crabtree for their generous donations of strains and plasmids, G.Pavitt, M.Lorenz, J.Heitman and A.Hinnebusch for advice concerning this avenue of research, and C.Grant and L.Otero for their help with specific experiments. This work was supported in part by a National Institute of Health grant GM50308 to A.B.S., by a European Molecular Biology Organisation long-term fellowship to M.P.A. at UC Berkeley, and by a Wellcome Trust project grant 0-1867/Z/00/Z to M.P.A. since moving to UMIST.
References
- Ashe M.P., De Long,S.K. and Sachs,A.B. (2000) Glucose depletion rapidly inhibits translation initiation in yeast. Mol. Biol. Cell, 11, 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet N.C., Schreiber,U., Helliwell,S.B., Stansfield,I., Tuite,M.F. and Hall,M.N. (1996) TOR controls translation initiation and early G1 progression in yeast. Mol. Biol. Cell, 7, 25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan A.M., Bushman,J.L., Boal,T.R. and Hinnebusch,A.G. (1993) A protein complex of translational regulators of GCN4 mRNA is the guanine nucleotide-exchange factor for translation initiation factor 2 in yeast. Proc. Natl Acad. Sci. USA, 90, 5350–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T.G. (1982) Nitrogen metabolism in Saccharomyces cerevisiae. In Strathern,J.N., Jones,E.W. and Broach,J.R. (eds), The Molecular Biology of the Yeast Saccharomyces cerevisiae: Metabolism and Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 39–99.
- Dever T.E. (1999) Translation initiation: adept at adapting. Trends Biochem. Sci., 24, 398–403. [DOI] [PubMed] [Google Scholar]
- Dever T.E., Feng,L., Wek,R.C., Cigan,A.M., Donahue,T.F. and Hinnebusch,A.G. (1992) Phosphorylation of initiation factor 2α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell, 68, 585–596. [DOI] [PubMed] [Google Scholar]
- Dever T.E., Yang,W., Åstrom,S., Byström,A.S. and Hinnebusch,A.G. (1995) Modulation of tRNAiMet, eIF-2 and eIF-2B expression shows that GCN4 translation is inversely coupled to the level of eIF-2·GTP·Met-tRNAiMet ternary complexes. Mol. Cell. Biol., 15, 6351–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson J.R. (1996) ‘Fusel’ alcohols induce hyphal-like extensions and pseudohyphal formation in yeasts. Microbiology, 142, 1391–1397. [DOI] [PubMed] [Google Scholar]
- Dickinson J.R. Lanterman,M.M., Danner,D.J., Pearson,B.M., Sanz,P., Harrison,S.J. and Hewlins,M.J. (1997) A 13C nuclear magnetic resonance investigation of the metabolism of leucine to isoamyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem., 272, 26871–26878. [DOI] [PubMed] [Google Scholar]
- Dickinson J.R., Harrison,S.J. and Hewlins,M.J. (1998) An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem., 273, 25751–25756. [DOI] [PubMed] [Google Scholar]
- Gomez E. and Pavitt,G.D. (2000) Identification of domains and residues within the ε subunit of eukaryotic translation initiation factor 2B (eIF2Bε) required for guanine nucleotide exchange reveals a novel activation function promoted by eIF2B complex formation. Mol. Cell. Biol., 20, 3965–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C. and Fink,G.R. (eds) (1991) Guide to Yeast Genetics and Molecular Biology. Academic Press, San Diego, CA.
- Harding H.P., Zhang,Y. and Ron,D. (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature, 397, 271–274. [DOI] [PubMed] [Google Scholar]
- Hershey J.W.B. and Merrick,W.C. (2000) The pathway and mechanism of initiation of protein synthesis. In Sonenberg,N., Hershey J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 33–88.
- Hinnebusch A.G. (2000) Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In Sonenberg,N., Hershey,J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 185–244.
- Kimball S.R. and Jefferson,L.S. (2000) Regulation of translation initiation in mammalian cells by amino acids. In Sonenberg,N., Hershey,J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 561–579.
- Koonin E.V. (1995) Multidomain organization of eukaryotic guanine nucleotide exchange translation initiation factor eIF-2B subunits revealed by analysis of conserved sequence motifs. Protein Sci., 4, 1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M.C., Cutler,N.S. and Heitman,J. (2000) Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol. Biol. Cell, 11, 183–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini G., Hinnebusch,A.G., Chen,C. and Fink,G.R. (1984) Positive regulatory interactions of the HIS4 gene of Saccharomyces cerevisiae. Mol. Cell. Biol., 4, 1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M.B., Sonenberg,N. and Hershey,J.W.B. (2000) Origins and principles of translational control. In Sonenberg,N., Hershey,J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 1–32.
- Patti M.-E., Brambilla,E., Luzi,L., Landaker,E.J. and Kahn,C.R. (1998) Bidirectional modulation of insulin action by amino acids. J. Clin. Invest., 101, 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrollier K., Hajduch,E., Blair,A.S., Hyde,R. and Hundal,H.S. (2000) l-Leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the l-leucine-induced upregulation of system A amino acid transport. Biochem. J., 350, 361–368. [PMC free article] [PubMed] [Google Scholar]
- Proud C.G. and Denton,R.M. (1997) Molecular mechanisms for the control of translation by insulin. Biochem. J., 328, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B., Gingras,A.-C. and Sonenberg,N. (2000) Regulation of ribosome recruitment in eukaryotes. In Sonenberg,N., Hershey,J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 245–293.
- Thomas G.P. and Mathews,M.B. (1984) Alterations of transcription and translation in HeLa cells exposed to amino acid analogues. Mol. Cell. Biol., 4, 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A.D. and Ingraham,J.L. (1963) Fusel oil. Adv. Appl. Microbiol., 5, 317–353. [Google Scholar]
- Xu G., Kwon,G., Marshall,C.A., Lin,T.-A., Lawrence,J.C.,Jr and McDaniel,M.L. (1998) Branched-chain amino acids are essential in the regulation of PHAS-I and p70 S6 kinase by pancreatic β-cells. J. Biol. Chem., 273, 28178–28184. [DOI] [PubMed] [Google Scholar]
- Zheng X.-F., Fiorentino,D., Chen,J., Crabtree,G.R. and Schreiber,S.L. (1995) TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell, 82, 121–130. [DOI] [PubMed] [Google Scholar]