Abstract
ShcA and Grb2 are crucial components in signalling by most tyrosine kinase-associated receptors. How ever, it is not clear whether Grb2 bound directly to the receptor is equivalent to Grb2 associated via ShcA. We have used signalling stimulated by the middle T-antigen (MT) of polyoma virus to address this question. The two known Grb2-binding sites from murine ShcA, 313Y and 239/240YY, could functionally replace the MT ShcA-interacting region in transformation assays using Rat2 fibroblasts. This demonstrates that signal output from membrane-bound ShcA requires only these two sequences and the ShcA-binding site in MT does not recruit other signalling molecules. Two standard Grb2-interacting sequences, either from the EGF receptor or the ShcA 313Y region, could not replace the requirement for ShcA binding to MT, indicating an enhanced role for the ShcA 239/240YY motif. Sos1 and the docking protein Gab1 are brought into the MT complex through Grb2 association and this may be more effective using the 239/240YY sequence.
Keywords: polyoma virus MT/ShcA phosphorylation/signal transduction/transformation
Introduction
Signal transduction from tyrosine kinase-associated receptors (TKRs) takes place through a series of protein– protein or protein–lipid interactions involving a small number of binding modules. The phosphotyrosine-recognizing SH2 and PTB domains and the proline-containing motifs bound by SH3 domains typify these interactions (reviewed in Pawson, 1995). Tyrosine phosphorylation of the receptor itself or of a docking protein such as the insulin receptor substrates (IRS) 1 and 2, provides binding sites for SH2/PTB domain-containing polypeptides that assemble into a membrane-bound multi-protein complex, which activates a number of signalling pathways. Recent reports have added greatly to the number of proteins found in these complexes and this has made deciphering the precise role of each constituent extremely complex. This is illustrated by the activities of the ShcA family of polypeptides. The ShcA oncogene produces three proteins with molecular weights of approximately 66, 52 and 42 kDa (Pelicci et al., 1992). These polypeptides all share a C-terminal sequence but differ in their N-termini as a result of alternative mRNA splicing and translation initiation codon usage. Each ShcA polypeptide has an SH2 domain at its C-terminal end, an area with homology to collagen (the CH1 region) and a PTB domain. In addition, the p66 isoform contains an extra N-terminal collagen homology area, CH2. The PTB domain of ShcA binds to phosphorylated NPXY sequences in TKRs or docking proteins (Blaikie et al., 1994; van der Geer and Pawson, 1995; van der Geer et al., 1995) becomes tyrosine phosphorylated itself at two major sites within the CH1 region: the first containing the two tyrosines at positions 239 and 240 (239YY) (Blaikie et al., 1994; Gotoh et al., 1996; van der Geer et al., 1996) and the second consisting of the single tyrosine at position 317 (317Y in the human ShcA sequence, equivalent to 313Y in the mouse sequence) (Salcini et al., 1994). Both of these regions then bind the SH2 domain of Grb2. Through its SH3 domains, Grb2 is associated with the ras guanine nucleotide exchange factor Sos1 (reviewed in McCormick, 1993; Schlessinger, 1993). Consequently, ShcA binding to Grb2 brings Sos1 to a membrane site where it activates p21ras and the MAP kinase signalling cascade (Aronheim et al., 1994).
In this model, Grb2 is the only protein bound to ShcA that mediates downstream signalling events. It is not clear why Grb2 sometimes interacts with the receptor directly and at other times Grb2 associates via ShcA. One possible solution to this problem came with the discovery that a number of other proteins contain PTB domains that also bind NPXY motifs, suggesting that some of these species may interact with the same sites as ShcA and thus stimulate additional signalling pathways. In addition, there is evidence that in receptor signalling, ShcA and Grb2 have additional roles to this well-defined pathway. The 239YY and 317Y sites in ShcA are not equivalent in signalling terms, despite both being able to bind Grb2 (Gotoh et al., 1996; Blaikie et al., 1997). In particular, the 239YY sequence but not 317Y has been implicated in activating c-myc transcription (Gotoh et al., 1996, 1997); how this is achieved is unclear. The ShcA proteins are also known to interact with a number of other proteins, such as the adaptins (Okabayashi et al., 1996) and integrins via c-fyn (Wary et al., 1998), and any of these may be involved in downstream signalling. Similarly, Grb2 can also associate through its SH3 domains with proteins other than Sos1, including the Grb2-associated-binding (Gab) proteins 1 and 2 (Holgado Madruga et al., 1996; Bardelli et al., 1997; Fixman et al., 1997; Nguyen et al., 1997; Lock et al., 2000; Schaeper et al., 2000), which have homology to IRS1 and IRS2. This large number of interacting proteins has made it difficult to determine which associations are important for signal input or output from ShcA.
To replicate their genomes in the normally quiescent cells of a multicellular organism most DNA viruses encode a mitogenic protein. This is usually one of the first proteins expressed during infection and induces the cell to leave G0 and enter the cell cycle, thus creating a suitable cellular environment to support the high level of DNA synthesis required to replicate the viral genome. When expressed outside a lytic cycle, however, these effective mitogenic proteins are frequently tumorigenic. The middle T-antigen (MT) encoded by the murine polyomavirus is a potent oncogene that can fully transform established rodent fibroblasts in one step. It achieves this by interacting with a series of host polypeptides and altering their normal regulation (reviewed in Dilworth, 1995; Nicholson and Dilworth, 2001). In a series of interactions, MT binds first to the A and C subunits of protein phosphatase 2A (PP2A), and then associates with one of the src-family of tyrosine kinases, pp60c-src, pp59c-fyn or pp62c-yes, which stimulates tyrosine kinase activity. The pp60c-src then phosphorylates at least three tyrosines in the C-terminal half of MT, which act as binding sites for SH2 or PTB domain-containing proteins. Phosphorylated Tyr315 in MT binds to the SH2 domain of the 85 kDa component of phosphatidyl inositol 3′-OH kinase (PI3K), phosphotyrosine 322 to the SH2 domain of phospholipase Cγ-1 (PLCγ-1) and phosphotyrosine 250 to the PTB domain of ShcA (Campbell et al., 1994; Dilworth et al., 1994). The MT-associated PI3K, PLCγ-1 and ShcA are in turn tyrosine phosphorylated by pp60c-src, resulting in stimulation of PI3K and PLCγ-1 activity and interaction between ShcA and Grb2 (Campbell et al., 1994; Dilworth et al., 1994). This results in permanent activation of Erk1 and 2, an increase in the activity of the AP1 family of transcription factors (Srinivas et al., 1994; Urich et al., 1995), an increase in c-myc transcription (Rameh and Armelin, 1991) and activation of PKB/Akt (Meili et al., 1998). Thus, MT has used the normal signalling mechanisms initiated during mitogenic induction by growth factors to induce cell cycle entry for the virus and so can be considered as a permanently active analogue of a growth factor receptor.
To investigate directly the role of ShcA in signal transduction, we have made use of the ease with which MT can be manipulated and its transforming activity measured and hence the ability to activate all the relevant signalling pathways. By replacing the Y250 sequences in MT with different tyrosine-containing motifs, we have shown that only the 239YY and 313Y phosphorylation sites from murine ShcA are required to functionally replace the MT ShcA binding region during transformation of Rat2 fibroblasts. This demonstrates that these two regions are solely responsible for signal output from ShcA and eliminates any possibility that species other than ShcA are functionally bound to Y250 in MT. The ShcA 239YY region is more effective than a 313Y sequence and this may correlate not with its ability to bind Grb2 but with the associated Grb2’s capacity to associate with Sos1 and Gab1.
Results
ShcA interaction with MT can be functionally replaced by two different Grb2-binding sequences
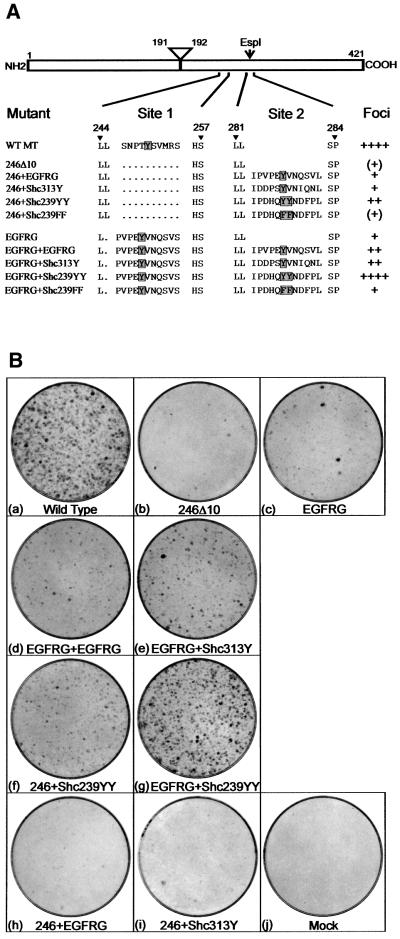
MT interacts with Grb2 via ShcA but it is not clear whether ShcA plays any additional role in inducing transformation. To establish whether any Grb2-binding site could substitute for the MT motif that interacts with ShcA, a deletion mutant that lacked this region was isolated first, mutant 246Δ10 (see Figure 1A). A DNA fragment that encodes the 10 amino acids from the mouse epidermal growth factor receptor (EGFR) that associates with Grb2 (Batzer et al., 1994) was then inserted into this area of MT (site 1), mutant EGFRG (Figure 1A). Both of these plasmids were transfected into the rat fibroblast cell line Rat2 and the number of foci induced scored after 14 days (Figure 1B). Removal of the ShcA-binding sequence reduced MT’s ability to form foci to a low (although not zero) level (Figure 1B, b). Addition of the Grb2-binding sequence from the mouse EGFR increased the number of foci formed by a small amount but still to <5% of the quantity induced by wild-type MT (Figure 1B, c).
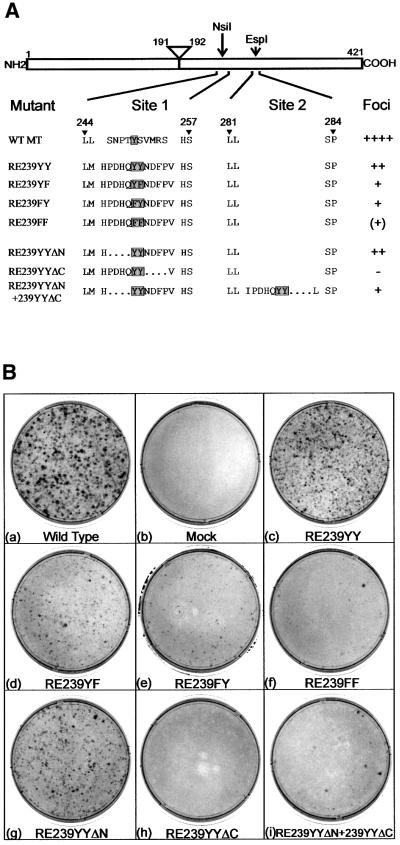
Fig. 1. (A) Sequences of mutations made to the 240–260 (site 1) and 282 (site 2) regions of MT. MT is represented schematically at the top, with the amino acid positions of the N- and C-terminal ends marked together with the locations of the intron removed to create the MT unique region and the EspI restriction site used to insert DNA sequences into site 2. Below, the amino acid sequences of the region between residues 244 and 257 and 281–284 are shown for wild-type MT and various mutants. A dot represents a deleted amino acid. The designation of each mutant is shown on the left. The position of the target phosphorylated tyrosine is shown by a shaded box. The amount of foci induced by each mutant plasmid after transfection into rat fibroblasts is indicated on the right. ++++ represents the amount of foci induced by similar amounts of wild-type MT DNA. (B) Foci induction by MT mutants. Plasmids containing each of the MT mutations were transfected by the calcium phosphate precipitation method into Rat2 fibroblasts. After 14 days, the medium was removed and the foci stained with Leishmann’s reagent. The MT mutant used is indicated beneath each dish. Dishes shown are representative of the results obtained in over 10 different experiments.
To determine whether the transforming defects in mutants 246Δ10 and EGFRG could be complemented by other Grb2-binding motifs, additional tyrosine-containing sequences were inserted between amino acid residues 282 and 283 of both mutants through the use of the unique EspI restriction site in the MT cDNA (site 2). Previously, this site has been used to show that addition of an extra NPTY coding sequence could complement a defect in the native ShcA-binding region (Druker et al., 1992), demonstrating that inserts placed here become tyrosine phosphorylated. First, an additional EGFR Grb2-binding sequence was introduced into mutant EGFRG to examine whether the Grb2-binding capacity of a single insert was limiting. EGFRG+EGFRG transformed with only a small increase in efficiency compared with EGFRG (Figure 1B, d). It is possible that the ShcA Grb2-binding sequences perform differently to those from the EGFR, so the 313Y region from murine ShcA (DDPSYVNIQN, equivalent to Y317 DDPSYVNVQN in the human sequence but with a single amino acid change) was inserted next into EGFRG. This created a mutant that showed slightly increased foci-inducing properties compared with the parental plasmid (EGFRG+Shc313Y; Figure 1B, e) but still <30% of the number induced by wild-type MT. Therefore, it was not an inability to bind sufficient Grb2 that was responsible for the defect in EGFRG. The sequences surrounding the double tyrosine at position 239/240 of ShcA (239YY) were then inserted into 246Δ10 and EGFRG. Insertion into 246Δ10 resulted in an increase in the transforming efficiency of the plasmid to nearly 30% that of wild-type MT (mutant 246+Shc239YY; Figure 1B, f), similar to the two Grb2-binding sequences in EGFRG+Shc313Y. Addition of the 239YY sequence together with the EGFR Grb2-binding region produced an MT mutant that transformed almost as well as the wild type (EGFR+Shc239YY; Figure 1B, g). Insertion of the same sequence but with the tyrosines mutated to phenylalanine did not increase the transformation efficiency of the parental 246Δ10 and EGFRG plasmids, suggesting that phosphorylation of the tyrosines is required (246+Shc239FF and EGFRG+Shc239FF; data not shown). Introduction of the EGFR or Shc313Y Grb2-binding sequences into site 2 of 246Δ10 only slightly increased foci induction (246+EGFRG and 246+Shc313Y; Figure 1B, h and i). In summary, a combination of the EGRF Grb2-binding motif (EGFRG) plus the 239YY sequence of ShcA can functionally replace the ShcA interaction region of MT in the transformation of fibroblasts, whereas two Grb2-binding sites containing a single tyrosine cannot.
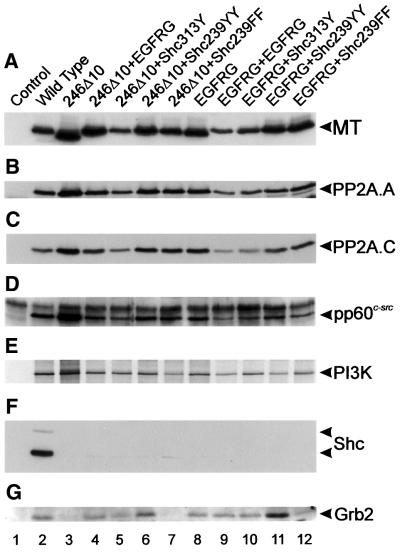
To ensure that the protein-binding properties of these MT mutants are as expected, stable cell lines expressing each species were isolated by co-transfection of the mutant DNA together with the plasmid pSV2neo and selection in G418-containing media. Lysate from each cell line was then immunoprecipitated with an anti-MT monoclonal antibody and the MT-associated proteins examined by western blotting or in vitro phosphorylation (Figure 2). All of the mutants interacted with the A and C subunits of PP2A, pp60c-src and the PI3K 85 kDa subunit exactly the same as wild-type MT (Figure 2B–E), so the mutations have not altered the ability of MT to bind other proteins. None of the MT species bound ShcA (Figure 2F) but all of those with a putative Grb2-binding insert, including the ShcA 239YY motif, associated with Grb2 (Figure 2G). This confirms that each of the tyrosine-containing sequences inserted into MT was phosphorylated in vivo, presumably by the MT-associated pp60c-src, and then interacted with Grb2. However, although these experiments are only semi-quantitative, we found little evidence for a correlation between the amount of Grb2 bound to each mutant MT and the transforming ability of each species. There was some variation in association, in particular the two species containing a 239YY motif bound slightly more Grb2 than the others (246Δ10+Shc239YY and EGFRG+Shc239YY; Figure 2G, lanes 6 and 11), but this did not parallel the transformation efficiency of each mutant. Thus, Grb2 association can be restored to an MT mutant lacking the ShcA-binding site without re-instating transforming ability to normal levels. Therefore, ShcA probably does more than just bind Grb2.
Fig. 2. Cellular polypeptides associated with MT mutants. Stable cell lines expressing each of the MT mutants shown in Figure 1 were lysed, the MT immunoprecipitated with PAb762, separated by SDS–PAGE and western blotted. MT-associated proteins were detected in duplicate blots by the use of specific antibodies. The probe used to detect each polypeptide is shown on the right together with the migration position. For detection of the 85 kDa subunit of PI3K, MT immunoprecipitates were incubated with [γ-33P]ATP, separated by SDS–PAGE and autoradiographed. The mutant used is indicated above each lane. Only the 52 and 66 kDa forms of ShcA were detected. The faint band migrating slightly faster than the 52 kDa form of ShcA is a non-specific reaction caused by the heavy chain of the immunoprecipitating PAb762. The experiments shown are representative of five different experiments performed with two different sets of cell lines.
Signalling downstream of ShcA requires only the two tyrosine phosphorylation sites
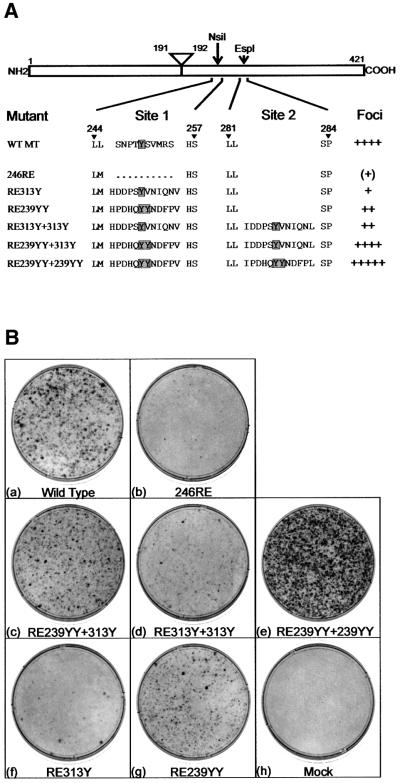
To establish whether the phosphorylation sites from ShcA alone are sufficient to replace ShcA binding to MT and to determine whether sequences function identically when placed in either insertion site, a procedure for introducing motifs into site 1 as easily as site 2 was required. To achieve this, we altered two nucleotides in the sequence of 246Δ10 to create a unique NsiI restriction site, mutant 246RE. This changes Leu245 to Met245 but enables simple insertion of any sequence into site 1. Transfection of 246RE into Rat2 fibroblasts showed that it had a severely impaired transforming efficiency similar to mutant 246Δ10 (Figure 3B, b). To determine whether the ShcA 313Y and 239YY motifs function together in the same way as EGFRG+Shc239YY shown above, both sequences were first inserted into the same MT molecule. RE239YY+313Y transformed with almost the same efficiency as wild-type MT (Figure 3B, c), although there were some differences in the morphology of the foci formed. The 313Y sequence from ShcA and the Grb2-binding motif from the EGFR are interchangeable, then, and it makes little difference whether sequences are inserted into site 1 or 2. Therefore, the ShcA-binding site in MT can be functionally replaced with just the two tyrosine phosphorylation sites from ShcA, indicating that signalling downstream of ShcA during MT transformation involves just these two regions.
Fig. 3. (A) Sequences of ShcA phosphorylation site insertion MT mutants. The names and sequences of a second series of MT deletion and insertions mutants are shown together with their relative transforming efficiencies, as in Figure 1A. (B) Foci assays on MT mutant. Plasmids containing the mutations shown in (A) were transfected into Rat2 fibroblasts and then stained after 14 days growth. The mutant used is indicated beneath each plate. The plates shown are representative of over 10 different experiments.
The results shown above demonstrate that the two Grb2-binding motifs from mouse ShcA can replace ShcA binding to MT. To determine whether there was any difference between these two sequences, two copies of each region were placed into the same MT. A mutant containing two ShcA 313Y motifs showed <30% transforming activity of wild-type MT (Figure 3B, d), similar to EGFR+Shc313Y. Therefore, two copies of 313Y were not sufficient for full transformation. Surprisingly, however, an MT mutant containing two copies of the 239YY sequence transformed better than wild-type MT (RE239YY+239YY, Figure 3B, e). A greater number of foci were formed and these appeared to be denser and faster growing than those expressing wild-type MT. Soft agar growth was also increased and the morphology of the cells isolated from these foci exhibited a more transformed phenotype (data not shown). Consequently, transformation by MT requires just two motifs from ShcA, 313Y and 239YY. 313Y cannot fully replace 239YY but the 239YY region is able to perform both functions. The data also suggest that both these sequences need to be on a single molecule, as a mutant with a single YY insert transforms less well than wild-type MT, which would not be the case if the two functions could effectively complement each other on separate molecules.
Insertion of a single ShcA 313Y-containing region improved transformation induction by 246RE to a small extent, but still below 10% of wild-type levels (RE313Y; Figure 3B, f). Insertion of a single 239YY ShcA motif had a more dramatic effect on transformation levels (RE239YY; Figure 3B, g), but this was still only ∼50% of the level of wild-type MT. These results are broadly comparable to the effect of inserting these sequences into site 2 (above). Therefore, the position at which these tyrosine-containing sequences are inserted into MT again has only a minor influence on their activity.
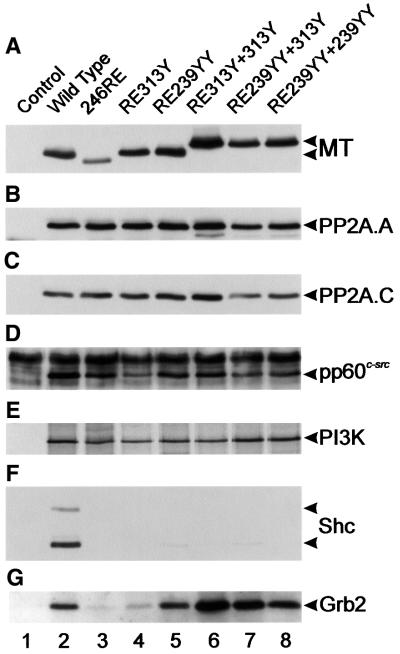
To ensure that these mutants were binding proteins in the cell as anticipated, cell lines expressing each species were isolated. Immunoprecipitation followed by western blotting studies showed that all the altered MTs bound PP2A, pp60c-src and the PI3K 85 kDa subunit in amounts similar to wild type (Figure 4), and all were defective in ShcA interaction, as expected. All of the insertion mutants associated with Grb2, although RE313Y bound less; however, once again the variation in the amount of Grb2 associated with MT did not parallel the wide difference in transforming ability of these mutants.
Fig. 4. Polypeptides associated with the MT mutants shown in Figure 3. Cell lines expressing the MT mutants shown in Figure 3 were lysed, the MT immunoprecipitated, blotted and probed with the antibodies shown on the right. Association with the PI3K 85 kDa subunit was detected by an in vitro kinase reaction. The mutant used is indicated above each lane. The experiment was repeated six times on two different sets of cell lines with similar results.
The ShcA 239YY motif binds SH2 domain-containing proteins
It is clear that the 239YY sequence of ShcA is able to bind Grb2 (van der Geer et al., 1996) (see Figures 2 and 4) but can do more than a motif containing a single Y. Therefore, it is feasible that 239YY binds other proteins in addition to Grb2. To investigate whether the function of this region could be separated from Grb2 binding, the residues required for activity were examined (Figure 5A). First, the role of the two tyrosines was studied. Altering either tyrosine (Y) to phenylalanine (F) decreased the foci-inducing activity of an MT containing a single 239YY motif (mutants RE239YY, RE239YF, RE239FY and RE239FF; Figure 5B, c–f). Both tyrosines are required for the function of this region, although mutation of the second has less effect than alteration of the first. SH2 domains recognize residues C-terminal to the phosphorylated tyrosine, whereas PTB domains bind to sequences on the N-terminal side. To determine which flanking region of the YY sequence is required for its activity, mutants lacking amino acids either N- or C-terminal to the YY motif were constructed next. Transfection into Rat2 fibroblasts showed that removal of the sequences N-terminal to the YY motif reduced foci-forming ability slightly (RE239YYΔN; Figure 5B, g), whereas removal of the C-terminal region destroyed activity completely (RE239YYΔC; Figure 5B, h). This suggests that it is recognition by an SH2 domain-containing protein that is critical to the activity of this region. Finally, it is apparent that two sequences in the same molecule are needed to induce full transformation. To determine whether a difference between these two activities could be found, both the N- and C-terminal deletions were inserted into the same mutant. Clearly, not only did these insertions not complement each other, but surprisingly the C-terminal mutation was dominant over the N-terminal one, resulting in less foci-inducing activity by the double insertion mutant than RE239YYΔN alone (RE239YYΔN+ 239YYΔC; Figure 5B, i). All of the MTs bind PP2A, pp60c-src and PI3K, but not ShcA, as expected (data not shown).
Fig. 5. (A) Sequence requirements for functioning of the ShcA 239YY motif. The names and amino acid sequences of mutations in the ShcA 239YY motif inserted into MT are shown, as in Figure 1A. (B) Foci assays on the mutants shown in (A). The transfections were repeated four times with essentially similar results.
Both ShcA tyrosine sequences bind Sos1 and Gab1 via Grb2
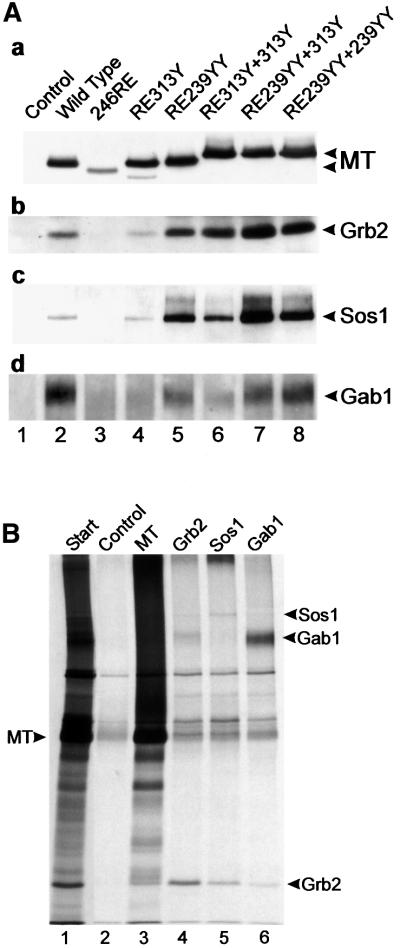
The data shown above demonstrate that the ShcA 239YY sequence plays a vital role in transformation induction by MT that cannot be achieved by the ShcA 313Y region. Both sequences bind the SH2 domain of Grb2, probably to similar levels, so the reason for this difference is not clear. One possible explanation for these observations could be that the Grb2 associated with each motif has an altered affinity for binding downstream signalling proteins. The SH3 domains of Grb2 have been reported to interact with a number of proteins. Sos1 associates mainly with the N-terminal domain (Raabe et al., 1995; Cheng et al., 1998) and the C-terminal domain can interact with the adapter molecule Gab1 (Lock et al., 2000). To determine whether Grb2 bound to an ShcA 313Y motif or ShcA 239YY sequence interacts with these proteins, MT from mutant-expressing cell lysates was immunoprecipitated, western blotted and then probed with antibodies directed against Sos1 and Gab1 (Figure 6A). Co-precipitation of both Sos1 and Gab1 is seen clearly with wild-type MT and any mutant with Grb2-binding activity. However, all of the mutants containing the ShcA 239YY motif seem to interact with more Sos1 and Gab1 than those expressing two 313Y regions and, in the case of Sos1, even more than wild-type MT. Therefore, both Sos1 and Gab1 associate with the MT complex via Grb2, and the 239YY motif may promote Grb2 association with Sos1 and Gab1.
Fig. 6. (A) Cellular proteins associated with Grb2 bound to MT mutants. The MT mutants shown in Figure 3 were immunoprecipitated, separated and then blotted. Duplicate blots were then probed with a series of antibodies to detect MT-bound Grb2 and Grb2-bound Sos1 and Gab1. The antibody used is indicated to the right, together with the migration position of the particular polypeptide. The mutant used is indicated above each lane. A 10% acrylamide gel (a and b), or an 8% gel (c and d) was used to aid in polypeptide separation. Similar results were obtained in three separate experiments using the same cell lines. (B) Grb2, Sos1 and Gab1 were phosphorylated in vitro in MT immunoprecipitates. Lysates containing wild-type MT were immunoprecipitated with PAb762 and then incubated with [γ-33P]ATP (Amersham Pharmacia). The phosphorylated polypeptides were eluted with 1% SDS, renatured and immunoprecipitated with anti-MT, anti-Grb2, anti-Sos1 or anti-Gab1, and then separated by SDS–PAGE and autoradiographed. The migration of MT is indicated on the left and the associated proteins on the right. The antibody used is indicated above each lane. No antibody was added to the control lane and the small amount of polypeptides observed here represents either non-specific precipitation or a small amount of renaturation of the original precipitating antibody. The start lane is shown at a lower exposure to aid identification of the MT-bound polypeptides. The experiment was performed twice with similar results.
Sos1 and Gab1 associated with MT migrate slower and more diffusely on SDS–PAGE than either polypeptide isolated from total cell lysates (data not shown). This suggests that both species become phosphorylated as a consequence of associating with MT. To determine whether this occurs in vitro, wild-type MT was immunoprecipitated, subjected to an in vitro kinase reaction and the phosphorylated polypeptides eluted by denaturation with SDS. Phosphoproteins were then re-precipitated with anti-Grb2, anti-Sos1 and anti-Gab1 antibodies (Figure 6B). As can be seen, Grb2, Gab1 and, to a lesser extent, Sos1 became phosphorylated in these reactions. As all of these phosphorylations are a consequence of src-family tyrosine kinase activity, and anti-phosphotyrosine antibodies recognize these proteins (data not shown), this was probably on tyrosine residues. A similar experiment using 246RE MT showed no phosphorylated Grb2, Sos1 or Gab1, but identical results to wild-type MT were obtained when mutants RE313Y+313Y and RE239YY+ 239YY were used (data not shown). Interestingly, anti-Grb2 immunoprecipitates also contained both Sos1 and Gab1, whereas anti-Sos1 or anti-Gab1 precipitates contained Grb2 but little of the other species. This probably indicates that renaturation of Grb2–Sos1 and Grb2–Gab1 complexes, but not of a Gab1–Grb2–Sos1 complex, has occurred. This suggests that, in vitro at least, Grb2 can only bind one species at a time and may explain why two Grb2-binding motifs are required to complement ShcA binding to MT.
Another possible explanation for the difference in activity observed for the ShcA 239YY and 313Y motifs may be that the 239YY sequence interacts with other proteins in addition to Grb2. A comparison of the total number of proteins phosphorylated in these reactions has not identified any species that is present in the RE239YY+239YY precipitate but not in a RE313Y+313Y mutant reaction (data not shown). Therefore, we can find no evidence so far for any additional species binding to the 239YY motif in fibroblasts. In summary, Grb2 bound to ShcA or MT containing an ShcA-derived tyrosine sequence associates with both Sos1 and Gab1. The Grb2 bound to the ShcA 239YY motif appears to interact with Sos1 and Gab1 more effectively than that associated with the 313Y sequence. As a consequence of binding to MT, Grb2, Sos1 and Gab1 become tyrosine phosphorylated, so, on the Gab1 at least, creating further potential SH2/PTB domain-binding sites.
Discussion
There are a number of ways that Grb2 can associate with a receptor, either directly via its SH2 domain or indirectly via a number of intermediate molecules, of which the best documented is the ShcA family. The MT oncogene from polyoma virus subverts normal growth factor receptor-stimulated signalling pathways to transform cells efficiently. MT contains no known direct Grb2-binding site but does contain an ShcA-interacting sequence, which is responsible for bringing Grb2 into the MT complex (Campbell et al., 1994; Dilworth et al., 1994). Here, we have used the similarities between MT and receptor signalling to investigate whether there is a difference between binding Grb2 through direct association or via ShcA.
To answer this question, we removed the ShcA-association sequence from MT and replaced it with either a Grb2-binding motif from the mouse EGFR or one containing a single tyrosine from ShcA itself. Both of these sequences were able to re-instate Grb2 binding to what appeared to be almost wild-type MT levels, but neither could restore full transforming capacity to the mutated MT, even when two motifs were inserted into the same molecule (Figures 1 and 3). Therefore, ShcA binding probably does more than just interact with Grb2. Insertion of both the main tyrosine phosphorylation motifs from murine ShcA (the YY sequence at positions 239–240 and the single Y at position 313) was sufficient to almost completely restore full transforming activity to a mutated MT (Figure 3). This demonstrates that signal output from the ShcA molecule during MT transformation of Rat2 fibroblasts involves only these two sites; no other regions of the protein are required. Consequently, it is likely that the other areas of ShcA (such as the SH2 domain and the remaining sequences in the CH region) are involved in receiving signals from upstream of ShcA, rather than signal output. In addition, the observation that these ShcA sequences can fully complement a 250Y MT deletion mutant indicates that this site functions only to bind ShcA and not other PTB domain-containing proteins, such as the IRS or fibroblast growth factor receptor substrate (FRS) polypeptides. Similar results to those reported here, but with some variability depending on cell type, were found using the same mutants inserted into retroviral vectors to transduce MT into a series of rat fibroblast lines (Ong et al., 2001).
To function effectively, the two tyrosine phosphorylation sites from ShcA have to be present on the same molecule. Both motifs bind Grb2 (Figure 4) but appear to perform different functions within the cell. A Grb2-binding sequence from the EGFR can functionally replace the murine ShcA 313Y motif but not the 239YY region (Figure 3). This supports the observation that the 239YY sequence is the significant motif required for MT-induced transformation (Blaikie et al., 1997) and the finding that the 239YY site is present in all ShcA species so far sequenced, whereas an equivalent to the murine 313Y site is not found in Drosophila ShcA (Lai et al., 1995; Cattaneo and Pelicci, 1998). Surprisingly, insertion of two copies of the 239YY motif generated a mutant that had increased transforming efficiency relative to wild-type MT (Figure 3). Cells transformed by RE239YY+239YY grow more rapidly and densely than wild-type MT-expressing cells, both in foci assays and when suspended in soft agar. Erk1 and 2 seem to be activated to a similar extent in all of the mutant MT-expressing cell lines, so the difference between these two motifs may reflect stimulation of additional pathways (data not shown).
It is clear, then, that the ShcA 239YY motif has additional activity compared with the 313Y region. The amount of Grb2 bound to ShcA 239YY and 313Y sequences did not appear to vary greatly in our experiments and certainly not to the same extent as the transforming efficiency of each of the mutants. However, more Sos1 and Gab1 were found associated with MT molecules containing an ShcA 239YY motif than those possessing only the 313Y sequences (Figure 6). It is not clear, however, that this was sufficient to account for the transforming deficiencies observed, as the level of Sos1, at least, associated with wild-type MT was not much greater than that associated with mutants containing 313Y sequences (Figures 3 and 6). However, as two Grb2-binding sites are required for MT transformation and two proteins have been shown to be associated with Grb2, it is tempting to speculate that both these proteins have to be bound to the same molecule for full transformation induction to occur. This implies that Grb2 cannot effectively bind both Sos1 and Gab1 at the same time, probably as a consequence of steric hindrance. Renaturation of in vitro phosphorylated polypeptides followed by re-precipitation experiments has shown that, at least in vitro, this may be correct (Figure 6B).
Enhanced binding of Sos1 and Gab1 to Grb2 as a consequence of binding to an ShcA 239YY motif may be caused by the interaction with the SH2 domain inducing a change in the SH3 domains that increases the amount of association with both polypeptides. The suggestion that phosphorylation of ShcA increases interaction between Grb2 and Sos1 has been made previously (Ravichandran et al., 1995) but without assigning this effect to association with any specific Grb2-binding site. Here, we have probably localized this effect to the 239YY motif. Although we have found a difference between the properties of the murine ShcA 239YY and 313Y motifs that may account for the variation in activity of each site, it is feasible that other disparities may also be involved. It is known that Grb2 associates with proteins other than Sos1 and Gab1, and one of these interactions may be regulated even more effectively by the phosphotyrosine-containing sequence associated with the Grb2 SH2 domain. Additionally, it is also possible that the 239YY sequence performs another function, such as interacting with a protein other than Grb2. However, we have not so far been able to identify any other polypeptide associated with this sequence. Should there be another protein bound to the 239YY motif it probably interacts via an SH2 domain, which limits the number of possible species, as sequences immediately C-terminal to the tyrosines are required for activity (Figure 5). The 239YY sequence has been implicated previously in suppressing apoptosis and stimulating c-myc activity (Gotoh et al., 1996, 1997) and this may be related to the effects on transformation observed here. However, so far we have not observed any difference in the steady-state levels of c-myc present in cell lines expressing these MT mutants (data not shown).
We have shown here for the first time that Gab1 associates with MT (Figure 6A) and becomes tyrosine phosphorylated as a consequence (Figure 6B). Phos phorylation of Gab1 provides binding sites for further SH2/PTB domain-containing proteins, including PI3K (Holgado Madruga et al., 1997; Takahashi Tezuka et al., 1998; Lecoq Lafon et al., 1999) and SHP-2 (Rocchi et al., 1998; Cunnick et al., 2000; Schaeper et al., 2000). Therefore, Gab1 association with MT may act to potentiate the PI3K signal already initiated by MT 315Y or initiate additional signals that are required to generate the full transformed phenotype. The observation that MT ShcA-binding site mutants are defective in inducing a sustained increase in the products of PI3K (Ling et al., 1992) supports a role for this region in PI3K activation, although this may be via p21ras (Rodriguez Viciana et al., 1994).
The demonstration that additional, alternative, tyrosine-containing sequences can be inserted into MT, become phosphorylated by the activated pp60c-src and function within the cell indicates that MT can be used as a recipient for studying other signalling pathways, including aspects of T-cell receptor activation (Kennedy et al., 1998). So far, three other tyrosine-containing sequences have been inserted into MT in these locations and each has become phosphorylated in cells and then associated with the appropriate SH2 domain-containing protein (our unpublished observations). MT, then, is potentially an important tool that may be used to analyse tyrosine kinase-regulated signalling pathways in a diversity of cell types.
Materials and methods
Mutant isolation
All mutations were made to the pUCMT plasmid, consisting of vector pUC19 containing the BamHI–EcoRI fragment of polyoma virus lacking the MT intron. All plasmid preparations were isolated by Qiagen columns (Qiagen). DNA manipulations were achieved by standard techniques (Sambrook et al., 1989).
The 246Δ10 deletion mutant was made using a PCR-based technique (Higuchi et al., 1988). Two PCR fragments were isolated using a primer 5′ to the MT region and an oligonucleotide containing the deletion first and a deletion-containing primer on the opposite strand and a 3′ oligonucleotide second. These DNA pieces were then combined by PCR using the two external primers. A SphI–CelII restriction enzyme fragment containing the deletion was then isolated from the resulting DNA and used to replace the same segment excised from pUCMT. The EGFRG mutant was made using oligonucleotides containing the sequences to be inserted and the ExSite kit (Stratagene). In both cases, the whole region was sequenced to confirm that the correct mutant had been generated. The point mutant 246RE was made from 246Δ10 using a mutation oligonucleotide and the Gene Editor kit (Promega).
Insertion mutants were produced by annealing two oligonucleotides containing the insertion sequences and appropriate sticky ends and ligation into 246Δ10, 246RE or EGFRG digested with either EspI or NsiI by conventional methods. Once again, all mutants were sequenced before use to confirm that the correct alterations had been isolated.
Antibodies
PAb762 (Dilworth et al., 1994) was used as the anti-MT monoclonal antibody and 327 (a kind gift from Dr Brugge) as the anti-pp60c-src antibody. Anti-ShcA polyclonal antibody and monoclonal anti-Sos1 were from Transduction Laboratories and anti-Grb2 polyclonal antibody was from Santa Cruz Antibodies. Anti-Gab1 was a kind gift from Dr D.Withers. Monoclonal antibodies directed against the A and C components of PP2A were isolated in our laboratory and will be described elsewhere.
Cell culture and foci assays
All cell culture was carried out by standard methods using Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum and incubated at 37°C and 10% CO2.
The ability to form foci was assayed on the Rat2 cell line. Just subconfluent cells on 90 mm Petri dishes were transfected with 10 µg of plasmid DNA using calcium phosphate precipitation. The cells were then kept in culture for 14 days, changing the culture medium every 3 days. After this time the medium was removed and the cells stained with Leishmann’s solution (Merck).
Cell lines expressing each mutant MT were isolated by co-transfecting 5 µg of mutant DNA into Rat2 cells on a 5 cm Petri dish together with 1 µg of the plasmid pSV2neo. The cells were subcultured into media containing 700 µg/ml G418 (Life Technologies) 24 h after transfection. After 7 days, individual colonies were isolated, checked by western blotting for MT synthesis and then re-cloned by limiting dilution.
Immunoprecipitations, western blotting and kinase assays
Mutant MT-expressing cells were washed with ice-cold phosphate-buffered saline (PBS), then lysed by incubation with 1 ml per 90 mm dish of lysis buffer [100 mM NaCl, 100 mM Tris–HCl pH 8.3, 0.5% NP-40 (Roche Biochemicals), 3 mM sodium orthovanadate and complete protease inhibitor tablets minus EDTA (Roche Biochemicals)] at 0°C for 20 min. The supernatant was then removed and cleared by centrifugation at 15 000 g for 5 min. Equivalent amounts of MT were immunoprecipitated by addition of PAb762 containing tissue culture fluid, incubation at 4°C for 60 min and collection on protein A–Sepharose beads (Amersham Pharmacia). The beads were then washed three times with Tris-buffered saline (TBS; 150 mM NaCl, 50 mM Tris–HCl pH 8.3 and 0.05% NP-40) and eluted with Laemmli sample buffer. Samples were boiled and separated by SDS–PAGE run as in Laemmli (1970).
For western blotting, the proteins were transferred onto 0.2 µm nitrocellulose (Merck) by electrophoresis. Unreacted sites on the membrane were blocked by incubation with 5% powdered skimmed milk in PBS for 1 h at room temperature (RT). Antigens were identified by incubation of the membrane with primary antibody diluted in 5% milk in PBS for 1 h, followed by washing and detection with HRP-labelled anti-mouse or anti-rabbit Fc secondary antibodies (Jackson Laboratories). MT was identified using biotinylated PAb762 and detected with HRP-labelled streptavidin (Amersham Pharmacia). The gel used for detecting Grb2 was run without reducing agent to separate the immunoprecipitating antibody light chain from the migration position of Grb2. In each case, binding was revealed by incubation of the blot with Supersignal Femto (Pierce) and exposure to X-ray film.
In vitro kinase assays were performed by immunoprecipitating MT as above. MT bound to protein A–Sepharose beads was then incubated for 20 min at 30°C with 2–20 µCi of [γ-33P]ATP in 25 mM Tris pH 7.5 and 5 mM manganese acetate. After washing twice, the MT was eluted from the beads by incubation with sample buffer for 5 min at RT, the supernatant boiled and separated by SDS–PAGE. For reprecipitation studies, after washing, the MT was eluted by incubation with 1% SDS, 80 mM Tris–HCl pH 6.8 for 10 min at RT. The supernatant was then removed, boiled, divided into aliquots and renatured by addition of 10 vols of TBS containing 1% NP-40. Phosphorylated polypeptides were then immunoprecipitated using specific antibodies as above.
Acknowledgments
Acknowledgements
We are grateful for the help and discussions provided by Dr Nick Dibb, Dr Nina Krausewicz and Dr Dominic Withers throughout this work. This work was supported by grants from the Cancer Research Campaign (UK) and the Biotechnology and Biological Sciences Research Council.
References
- Aronheim A., Engelberg,D., Li,N., Al-Alawi,N., Schlessinger,J. and Karin,M. (1994) Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the ras signaling pathway. Cell, 78, 949–961. [DOI] [PubMed] [Google Scholar]
- Bardelli A., Longati,P., Gramaglia,D., Stella,M.C. and Comoglio,P.M. (1997) Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene, 15, 3103–3111. [DOI] [PubMed] [Google Scholar]
- Batzer A.G., Rotin,D., Urena,J.M., Skolnik,E.Y. and Schlessinger,J. (1994) Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol., 14, 5192–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaikie P., Immanuel,D., Wu,J., Li,N., Yajnik,V. and Margolis,B. (1994) A region in Shc distinct from the SH2 domain can bind tyrosine-phosphorylated growth factor receptors. J. Biol. Chem., 269, 32031–32034. [PubMed] [Google Scholar]
- Blaikie P.A., Fournier,E., Dilworth,S.M., Birnbaum,D., Borg,J.P. and Margolis,B. (1997) The role of the Shc phosphotyrosine interaction/phosphotyrosine binding domain and tyrosine phosphorylation sites in polyoma middle T antigen-mediated cell transformation. J. Biol. Chem., 272, 20671–20677. [DOI] [PubMed] [Google Scholar]
- Campbell K.S., Ogris,E., Burke,B., Su,W., Auger,K.R., Druker,B.J., Schaffhausen,B.S., Roberts,T.M. and Pallas,D.C. (1994) Polyoma middle tumor antigen interacts with SHC protein via the NPTY (Asn-Pro-Thr-Tyr) motif in middle tumor antigen. Proc. Natl Acad. Sci. USA, 91, 6344–6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo E. and Pelicci,P.G. (1998) Emerging roles for SH2/PTB-containing Shc adaptor proteins in the developing mammalian brain. Trends Neurosci., 21, 476–481. [DOI] [PubMed] [Google Scholar]
- Cheng A.M. et al. (1998) Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell, 95, 793–803. [DOI] [PubMed] [Google Scholar]
- Cunnick J.M., Dorsey,J.F., Munoz Antonia,T., Mei,L. and Wu,J. (2000) Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J. Biol. Chem., 275, 13842–13848. [DOI] [PubMed] [Google Scholar]
- Dilworth S.M. (1995) Polyoma virus middle T antigen: meddler or mimic? Trends Microbiol., 3, 31–35. [DOI] [PubMed] [Google Scholar]
- Dilworth S.M., Brewster,C.E., Jones,M.D., Lanfrancone,L., Pelicci,G. and Pelicci,P.G. (1994) Transformation by polyoma virus middle T-antigen involves the binding and tyrosine phosphorylation of Shc. Nature, 367, 87–90. [DOI] [PubMed] [Google Scholar]
- Druker B.J., Sibert,L. and Roberts,T.M. (1992) Polyomavirus middle T-antigen NPTY mutants. J. Virol., 66, 5770–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixman E.D., Holgado Madruga,M., Nguyen,L., Kamikura,D.M., Fournier,T.M., Wong,A.J. and Park,M. (1997) Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins, Cbl and Gab1. J. Biol. Chem., 272, 20167–20172. [DOI] [PubMed] [Google Scholar]
- Gotoh N., Tojo,A. and Shibuya,M. (1996) A novel pathway from phosphorylation of tyrosine residues 239/240 of Shc, contributing to suppress apoptosis by IL-3. EMBO J., 15, 6197–6204. [PMC free article] [PubMed] [Google Scholar]
- Gotoh N., Toyoda,M. and Shibuya,M. (1997) Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol. Cell. Biol., 17, 1824–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel,B. and Saiki,R.K. (1988) A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res., 16, 7351–7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgado Madruga M., Emlet,D.R., Moscatello,D.K., Godwin,A.K. and Wong,A.J. (1996) A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature, 379, 560–564. [DOI] [PubMed] [Google Scholar]
- Holgado Madruga M., Moscatello,D.K., Emlet,D.R., Dieterich,R. and Wong,A.J. (1997) Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc. Natl Acad. Sci. USA, 94, 12419–12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A.P., Sekulic,A., Irvin,B.J., Nilson,A.E., Dilworth,S.M. and Abraham,R.T. (1998) Polyomavirus-derived middle-T antigen as a probe for T-cell antigen receptor-coupled signaling pathways. J. Biol. Chem., 273, 11505–11513. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lai K.M., Olivier,J.P., Gish,G.D., Henkemeyer,M., McGlade,J. and Pawson,T. (1995) A Drosophila shc gene product is implicated in signaling by the DER receptor tyrosine kinase. Mol. Cell. Biol., 15, 4810–4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoq Lafon C., Verdier,F., Fichelson,S., Chretien,S., Gisselbrecht,S., Lacombe,C. and Mayeux,P. (1999) Erythropoietin induces the tyrosine phosphorylation of GAB1 and its association with SHC, SHP2, SHIP and phosphatidylinositol 3-kinase. Blood, 93, 2578–2585. [PubMed] [Google Scholar]
- Ling L.E., Druker,B.J., Cantley,L.C. and Roberts,T.M. (1992) Transformation-defective mutants of polyomavirus middle T antigen associate with phosphatidylinositol 3-kinase (PI 3-kinase) but are unable to maintain wild-type levels of PI 3-kinase products in intact cells. J. Virol., 66, 1702–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L.S., Royal,I., Naujokas,M.A. and Park,M. (2000) Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J. Biol. Chem., 275, 31536–31545. [DOI] [PubMed] [Google Scholar]
- McCormick F. (1993) Signal transduction. How receptors turn Ras on. Nature, 363, 15–16. [DOI] [PubMed] [Google Scholar]
- Meili R., Cron,P., Hemmings,B.A. and Ballmer Hofer,K. (1998) Protein kinase B/Akt is activated by polyomavirus middle-T antigen via a phosphatidylinositol 3-kinase-dependent mechanism. Oncogene, 16, 903–907. [DOI] [PubMed] [Google Scholar]
- Nguyen L. et al. (1997) Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J. Biol. Chem., 272, 20811–20819. [DOI] [PubMed] [Google Scholar]
- Nicholson P.R. and Dilworth,S.M. (2001) Polyoma virus middle T-antigen: growth factor receptor mimic. In Grand,R.J.A. (ed.), Viruses, Cell Transformation and Cancer. Elsevier Science B.V., Amsterdam, The Netherlands, pp. 85–128.
- Okabayashi Y., Sugimoto,Y., Totty,N.F., Hsuan,J., Kido,Y., Sakaguchi,K., Gout,I., Waterfield,M.D. and Kasuga,M. (1996) Interaction of Shc with adaptor protein adaptins. J. Biol. Chem., 271, 5265–5269. [DOI] [PubMed] [Google Scholar]
- Ong S.H., Dilworth,S., Hauck-Schmalenberger,I., Pawson,T. and Kiefer,F. (2001) The ShcA and Grb2 adapter proteins mediate endothelial cell transformation and Gab1 tyrosine phosphorylation induced by polyomavirus middle T-antigen. EMBO J., 20, 6327–6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T. (1995) Protein modules and signalling networks. Nature, 373, 573–580. [DOI] [PubMed] [Google Scholar]
- Pelicci G. et al. (1992) A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell, 70, 93–104. [DOI] [PubMed] [Google Scholar]
- Raabe T., Olivier,J.P., Dickson,B., Liu,X., Gish,G.D., Pawson,T. and Hafen,E. (1995) Biochemical and genetic analysis of the Drk SH2/SH3 adaptor protein of Drosophila. EMBO J., 14, 2509–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh L.E. and Armelin,M.C. (1991) T antigens role in polyomavirus transformation: c-myc but not c-fos or c-jun expression is a target for middle T. Oncogene, 6, 1049–1056. [PubMed] [Google Scholar]
- Ravichandran K.S., Lorenz,U., Shoelson,S.E. and Burakoff,S.J. (1995) Interaction of Shc with Grb2 regulates association of Grb2 with mSOS. Mol. Cell. Biol., 15, 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi S., Tartare Deckert,S., Murdaca,J., Holgado Madruga,M., Wong,A.J. and Van Obberghen,E. (1998) Determination of Gab1 (Grb2-associated binder-1) interaction with insulin receptor-signaling molecules. Mol. Endocrinol., 12, 914–923. [DOI] [PubMed] [Google Scholar]
- Rodriguez Viciana P., Warne,P.H., Dhand,R., Vanhaesebroeck,B., Gout,I., Fry,M.J., Waterfield,M.D. and Downward,J. (1994) Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature, 370, 527–532. [DOI] [PubMed] [Google Scholar]
- Salcini A.E., McGlade,J., Pelicci,G., Nicoletti,I., Pawson,T. and Pelicci,P.G. (1994) Formation of Shc–Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene, 9, 2827–2836. [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Schaeper U., Gehring,N.H., Fuchs,K.P., Sachs,M., Kempkes,B. and Birchmeier,W. (2000) Coupling of Gab1 to c-Met, Grb2 and Shp2 mediates biological responses. J. Cell Biol., 149, 1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. (1993) How receptor tyrosine kinases activate Ras. Trends Biochem. Sci., 18, 273–275. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Schonthal,A. and Eckhart,W. (1994) Polyomavirus middle-sized tumor antigen modulates c-jun phosphorylation and transcriptional activity. Proc. Natl Acad. Sci. USA, 91, 10064–10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Tezuka M., Yoshida,Y., Fukada,T., Ohtani,T., Yamanaka,Y., Nishida,K., Nakajima,K., Hibi,M. and Hirano,T. (1998) Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol. Cell. Biol., 18, 4109–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urich M., el Shemerly,M.Y., Besser,D., Nagamine,Y. and Ballmer Hofer,K. (1995) Activation and nuclear translocation of mitogen-activated protein kinases by polyomavirus middle-T or serum depend on phosphatidylinositol 3-kinase. J. Biol. Chem., 270, 29286–29292. [DOI] [PubMed] [Google Scholar]
- van der Geer P. and Pawson,T. (1995) The PTB domain: a new protein module implicated in signal transduction. Trends Biochem. Sci., 20, 277–280. [DOI] [PubMed] [Google Scholar]
- van der Geer P., Wiley,S., Lai,V.K., Olivier,J.P., Gish,G.D., Stephens,R., Kaplan,D., Shoelson,S. and Pawson,T. (1995) A conserved amino-terminal Shc domain binds to phosphotyrosine motifs in activated receptors and phosphopeptides. Curr. Biol., 5, 404–412. [DOI] [PubMed] [Google Scholar]
- van der Geer P., Wiley,S., Gish,G.D. and Pawson,T. (1996) The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein–protein interactions. Curr. Biol., 6, 1435–1444. [DOI] [PubMed] [Google Scholar]
- Wary K.K., Mariotti,A., Zurzolo,C. and Giancotti,F.G. (1998) A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell, 94, 625–634. [DOI] [PubMed] [Google Scholar]