Abstract
A robust gonadotropin-releasing hormone (GnRH) surge is a prerequisite signal for the luteinizing hormone (LH) surge that triggers ovulation. In rodents, the GnRH surge is initiated by elevated estradiol and a diurnal switch in estrogen action from negative to positive feedback. The ability of constant estradiol treatment to induce daily LH surges was tested in adult mice that were ovariectomized (OVX) or OVX and treated with estradiol implants (OVX+E). LH in OVX mice showed no time-of-day difference. In contrast, OVX+E mice showed a large LH surge (8- to 124-fold relative to the a.m.) in p.m. samples on d 2–5 post-OVX+E. Targeted extracellular recordings were used to examine changes in firing activity of GnRH neurons in brain slices. There was no time-of-day difference in cells from OVX mice. In contrast, OVX+E cells recorded in the p.m. showed an increased mean firing rate and instantaneous firing frequency, which could increase GnRH release, and decreased duration of quiescence between bouts of firing, possibly reflecting increased pulse frequency, compared with cells recorded in the a.m. In the a.m., OVX+E cells showed changes in GnRH neuron firing reflecting negative feedback compared with OVX cells, whereas in the p.m., OVX+E cells exhibited changes suggesting positive feedback. These data indicate that differences in pattern and level of individual GnRH neuron firing may reflect the switch in estradiol action and underlie GnRH surge generation. The persistence of altered GnRH neuron activity in slices indicates that this approach can be used to study the neurobiological mechanisms of surge generation.
Keywords: luteinizing hormone, mouse model, surge, electrophysiology, neuroendocrinology
Gonadotropin-releasing hormone (GnRH) neurons of the preoptic area and hypothalamus are responsible for the production and secretion of GnRH and form the final common pathway in the central regulation of fertility. The ovarian steroid hormone estradiol feeds back to form both negative and positive loops to modulate the function of GnRH neurons and gonadotropes of the anterior pituitary (1–6). At the end of the follicular phase (proestrus in rodents), when estradiol levels are highest, the response to it switches from negative to positive feedback through mechanisms that are still not well understood. This switch, in turn, leads to a large, continuous increase in GnRH levels in pituitary portal blood, or a GnRH surge, which serves as the final, prerequisite signal for the pituitary luteinizing hormone (LH) surge, subsequently initiating ovulation. Preovulatory GnRH surges have been demonstrated in rats (7), sheep (8, 9), and monkeys (10).
An influence of the circadian timing system on reproduction has been shown in many mammals, including humans. The LH surge in most women begins in the early morning (11). Similarly, in nocturnal rodents, the LH surge begins immediately preceding their active period, around the time of lights off (12). The precise timing of the surge in rodents has been exploited in previous studies investigating surge regulation. When barbiturates are administered to temporarily block neural activity in the midafternoon of proestrus in rats, ovulation is delayed 24 h (13), indicating that a daily neural signal is required for ovulation. When animals are housed under different light–dark cycles, the LH surge occurs near the time of activity onset (14). Changing the environmental lighting schedule alters the time of the LH surge in the same manner as circadian locomotor activity, indicating a constant phase relationship between circadian rhythms and the timing of the LH surge (15). Mice and hamsters with altered circadian periods show parallel effects on LH surge timing and, in many cases, impaired fertility (16–18).
The use of mice in studies investigating the GnRH system has increased greatly in recent years (19–26). Previous studies in mice have used a regimen of ovariectomy followed by injections of estrogen and/or progesterone at specific times to induce a LH surge (12, 27). These experimental paradigms, however, are highly dependent on correct timing and dosage of multiple steroid administrations. The design of a one-variable mouse model would thus be highly advantageous for studying the endogenous generation and timing of GnRH surges from both an animal-handling and data-interpretation standpoint. Treatment of ovariectomized (OVX) rats and hamsters with estradiol implants (OVX+E) that mimic the sustained high levels of estradiol of proestrus induces repetitive daily LH surges that are appropriately timed to the late afternoon (28, 29).
Here, we created a mouse model exhibiting daily LH surges with OVX+E treatment using transgenic mice in which GnRH neurons are identifiable by GFP expression (22), allowing for direct electrophysiological examination of the surge mechanism. To test the hypothesis that the GnRH surge is generated by an increase in GnRH neuron activity, we made noninvasive single-unit extracellular recordings of GnRH neurons (30) to examine changes in individual GnRH neuron firing rates and patterns associated with both the LH surge and the switch from estradiol negative to positive feedback.
Materials and Methods
Animals. Adult (2–3 months old) transgenic female mice in which GFP is genetically targeted to GnRH neurons (22) were used. Mice were housed on a 14-h light:10-h dark cycle with lights off at 6:30 p.m. (all times are Eastern standard time) and maintained on Harlan 2916 chow and water ad libitum. At least 3 d before surgery, animals were transferred to a room in 14-h light:10-h dark with lights off at 4:30 p.m. to allow for acclimation to the advanced light cycle. For electrophysiology experiments, a subset of animals was not moved to the advanced light cycle before surgery; no differences in any recording parameters were observed. Mice were OVX (d 0) under isofluorane anesthesia (Abbott) and were either simultaneously implanted with a silastic capsule (Dow-Corning) containing 0.625 μg of estradiol suspended in sesame oil (OVX+E, n = 131 mice) or not treated further (OVX, n = 27 mice). LH levels measured in OVX animals implanted with a capsule containing only sesame oil vehicle were not different from levels in OVX alone (oil, 2.36 ± 1.2 ng/ml; no oil, 2.35 ± 0.5 ng/ml; P = 0.99). Estradiol levels on d 2 (33.6 ± 2.2 pg/ml) and 4 (35.2 ± 3.3 pg/ml) showed no difference from each other (P = 0.69) or our previous report on d 5–9 postimplantation (31) and were physiological (32). Estradiol was administered solely in vivo and was not present in any recording solutions. Postoperative analgesia was provided by a long-acting local anesthetic (0.25% bupivicaine, 7 μl per site, Abbott). For electrophysiology experiments, endocrine status was confirmed by measurements of uterine weight (OVX, 33.8 ± 1.3 mg; OVX+E, 120.4 ± 3.6 mg; P < 0.001). For LH level experiments, trunk blood was collected from each mouse after CO2 euthanasia. Serum LH concentration was determined by a modified, supersensitive two-site sandwich immunoassay described in refs. 33 and 34. All procedures were approved by the University of Virginia Animal Care and Use Committee.
Slice Preparation and Recordings. All reagents were purchased from Sigma; 200-μm coronal sections through the preoptic area and hypothalamus were prepared with slight modifications (35) of previous descriptions (30, 36). Normal saline contained the following (in mM): 135 NaCl/3.5 KCl/26 NaHCO3/1.25 NaH2PO4/2.5 CaCl2/1.2 MgSO4/10 d-glucose, pH 7.4. For a.m. recordings, mice were euthanized between 8:30 and 10:30 a.m.; for p.m. recordings, mice were euthanized between 2:30 and 3:30 p.m. Slices were incubated between 0.5 and 3.5 h before recording. For recording, individual slices were placed in a recording chamber mounted on the stage of a BX50WI upright fluorescent microscope (Olympus, Melville, NY). Slices were continuously superfused at 5–6 ml/min with oxygenated recording saline kept at 30–32°C with an inline-heating unit (Warner Instruments, Hamden, CT). Experiments were performed by using an EPC 8 amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany) with the Pulse Control XOP (Instrutech, Port Washington, NY) running in igor pro software (WaveMetrics, Lake Oswego, OR) on a G4 Macintosh computer to acquire data.
Targeted Extracellular Recordings. Targeted single-unit extracellular recordings were chosen as the approach for this study because this method allows recording from an identified neuron with minimal impact on the behavior of that neuron (30). Recording pipettes (1–3 MΩ) were filled with Hepes-buffered solution (30). Slight positive pressure was applied to the pipette before entering the bath solution. GFP-GnRH neurons were identified, pressure was released, and the pipette was moved next to the GnRH neuron. Seal resistance was measured at least every 30 min during recording. Initial seal resistances ranged from 5.2 to 26.1 MΩ and either remained stable or increased slowly over time to as high as 43.5 MΩ. If slice movement was noted during the recording, the pipette was repositioned slightly to compensate. Recordings were made in voltage-clamp mode with a pipette holding potential of 0 mV and filtering at 10 kHz and were digitized with an ITC-18 acquisition interface (Instrutech). Action currents (events), the membrane currents associated with action potential firing, were detected by using pulse control event tracker software (Instrutech). Recordings were performed from 10 a.m. to 1:30 p.m. (a.m. recordings) and 4–7:30 p.m. (p.m. recordings). Each recording lasted 60 min. If no firing was observed after 60 min, 15 mM KCl was added to the bath. If the cell fired in response to this depolarizing treatment, the cell was included in data analyses as a quiescent cell. If the cell did not respond, the data were discarded, because it was not possible to confirm recording integrity. No more than two cells were recorded per animal. The location of each GnRH neuron studied was mapped on figures of coronal sections obtained from a mouse brain atlas (37). The position of cells, however, did not affect the data.
Data Collection and Analysis. For each event, the time of the event and 10 ms centered on the event were digitized and stored to a data file. Events were detected offline by using custom programs in igor pro (36). Using excel (Microsoft) and instat (GraphPad, San Diego), binned data were evaluated and statistically analyzed for the following parameters: mean firing rate, median instantaneous firing frequency, percentage of quiescence, and duration of quiescence. Mean firing rate was determined by dividing the total number of events detected by the duration of the recording. The maximum instantaneous frequency (the interval between events converted to frequency in Hz) in each 1-min bin was determined, and the median value among all bins was used as representative for each cell. Quiescence was defined as 1-min bins containing one event or less. The percentage of total bins that were quiescent and the longest duration of consecutive quiescent bins were determined for each recording. For two-group studies, LH concentrations were compared by using Mann–Whitney tests (Fig. 1 A and B). For multiple-group studies, LH concentrations and recording parameters were compared by using Kruskal–Wallis tests followed by Dunn's multiple comparisons tests. Data are presented as means ± SEM. Statistical significance was set at P < 0.05.
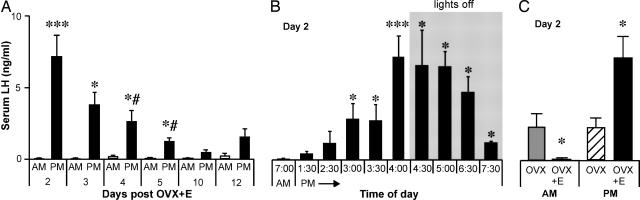
Fig. 1.
Induction of daily LH surges by estradiol in mice. (A) Bars represent serum LH concentrations (mean ± SEM). Open bars show samples obtained at 7 a.m., and filled bars show samples obtained at 4 p.m. (B) Serum LH levels (mean ± SEM) sampled in OVX+E mice at various times on d 2 after OVX+E. Gray shading indicates time during which lights were off. LH surge reliably begins ≈1.5 h before lights off (4:30 p.m.). (C) Serum LH levels show no diurnal difference in OVX mice, and estradiol induces negative feedback in the a.m. and positive feedback in the p.m. A, a.m. same-day control; B, 7 a.m. control; C, OVX control. *, P < 0.05; ***, P < 0.001; #, P < 0.01 vs. d 2 p.m.
Results
Constant in Vivo Estradiol Treatment Induces a Daily LH Surge in Mice. Daily LH surges can be induced by constant estradiol treatment in hamsters and rats (28, 29). We first tested whether estradiol treatment could induce daily surges in mice (n = 62). Trunk blood was collected and assayed for LH levels at 7 a.m. or 4 p.m. on d 2–5, 10, and 12 post-OVX+E. In all a.m. samples (n = 27), LH was low (0.14 ± 0.04 ng/ml), often near the level of detection for this assay. In marked contrast, a large surge in LH (8- to 124-fold) was consistently observed in the p.m. samples (n = 35) on d 2–5 (Fig. 1A)(P < 0.05). The LH levels measured in the p.m. on d 4–5 were significantly lower (P < 0.01) than the levels measured in the p.m. on d 2, indicating a possible damping phenomenon of the LH surge with persistent estradiol treatment. Within d 4 and 5, however, average p.m. levels were 8- to 29-fold greater than the average a.m. levels, suggesting that surges were occurring but with a lower amplitude. There was no significant a.m./p.m. change in LH levels on d 10 and 12, although the average p.m. value (n = 8) on both days was ≈6-fold higher than the average a.m. value, indicating that surges might still be occurring on those days.
Additional mice (n = 44) were sampled on d 2 from 1:30 to 7:30 p.m. to determine the onset and duration of the LH surge. Surge onset reliably occurred 1.5 h before lights off; elevated LH persisted through 3 h after lights off (Fig. 1B). OVX alone on d 2 (n = 8) showed no difference in LH levels between 7 a.m. and 4 p.m. (Fig. 1C)(P > 0.1). Comparison of OVX to OVX+E values on d 2 shows estradiol negative feedback in the a.m. (P < 0.05) and positive feedback in the p.m. (P < 0.05) (Fig. 1C). It should be noted that these values were obtained through averages of one sample per animal at different times, rather than many samples from the same animal, thus preventing a determination of exact surge onset time or duration for individual animals. These data indicate that estradiol is required for a daily LH surge and that this surge persists at least 5 d after OVX+E surgery.
Diurnal Changes in Firing Activity of GnRH Neurons From OVX vs. OVX+E Mice. The increase in LH levels in the p.m. could be due to an increase in responsiveness to GnRH, an increase in GnRH release, or both. Changes in GnRH release are likely reflected in changes in the action potential firing activity of these neurons. Using a targeted single-unit recording technique, the firing of GnRH neurons was monitored for 60 min by recording the changes in membrane current that produce action potentials. Although these currents are not action potentials per se, preliminary whole-cell current-clamp recordings in a small number of cells indicated the same trends as the extracellular data. For simplicity, we have used the phrase “firing rate” to refer to the frequency of events recorded extracellularly.
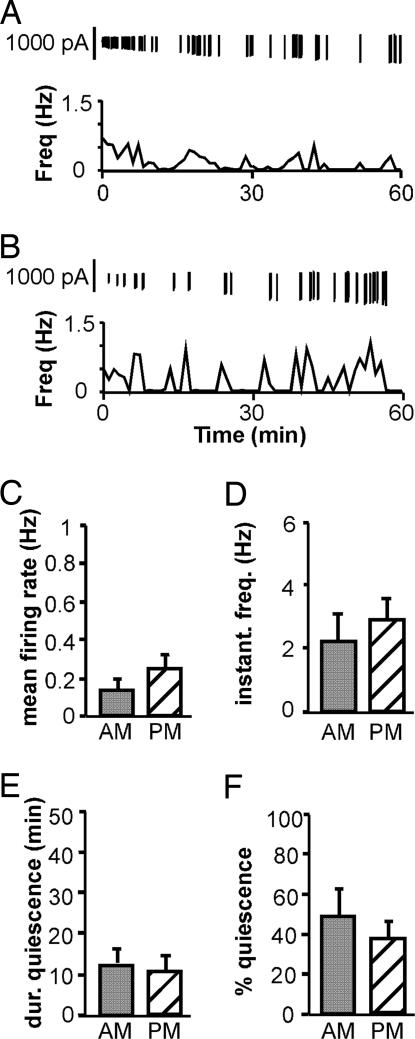
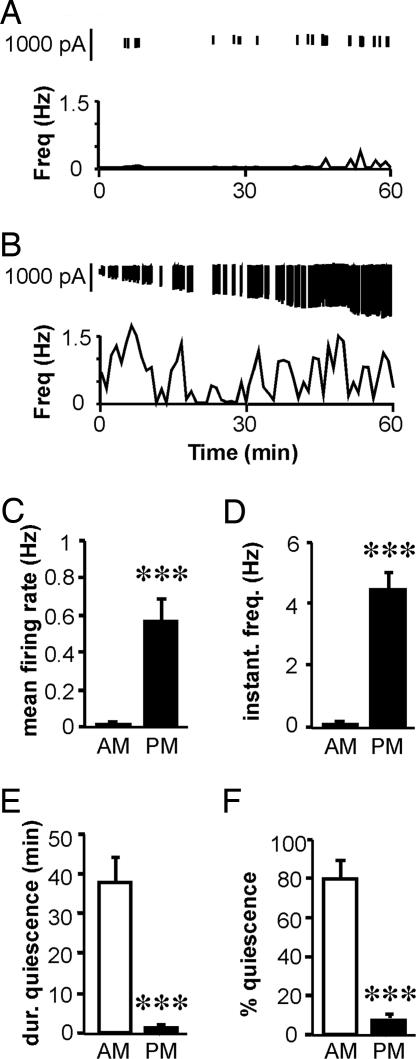
Fig. 2 A and B show representative examples of GnRH neuron firing in the a.m. and p.m., respectively. Group data for mean firing rate (a measure of overall activity), instantaneous frequency (a possible correlate of hormone release), and duration and percentage of time in quiescence are shown in Fig. 2 C–F. There was no time-of-day difference in any recording parameters in cells from OVX mice (a.m., n = 9 cells and seven mice; p.m., n = 13 cells and nine mice; P > 0.05; Fig. 2). Three of 9 OVX cells recorded in the a.m. and 2 of 13 OVX cells recorded in the p.m. were quiescent (≥90% time in quiescence). In marked contrast, OVX+E cells recorded in the p.m. (n = 11 cells and nine mice; Fig. 3B) showed increased mean firing rate (Fig. 3C; P < 0.001) and instantaneous firing frequency (Fig. 3D; P < 0.001) and decreased duration (Fig. 3E; P < 0.001) and percentage (Fig. 3F; P < 0.001) of time in quiescence compared with cells recorded in the a.m. (n = 8 cells and eight mice; Fig. 3A). Six of eight OVX+E cells recorded in the a.m., but no cells recorded in the p.m., were quiescent. Cells were recorded on d 2–4; although p.m. LH levels were lower on d 4 compared with d 2, there were no differences in any recording parameters within the OVX and OVX+E groups among animals recorded on different days in either the a.m. or p.m. Additionally, there were no differences within the OVX+E p.m. group among cells that were from slices prepared before or after surge onset. The increases in mean firing rate and instantaneous firing frequency, and the decrease in percentage of quiescent time, reflect an increase in overall GnRH neuron activity and would likely produce an associated increase in GnRH release. Alterations in the duration of quiescent time indicate an estradiol-dependent diurnal reorganization of GnRH neuron firing patterns. These data, together with the lack of any diurnal variation in GnRH neuron activity in the absence of estradiol, suggest that increased GnRH neuron activity in the p.m., as evidenced by changes in both firing rate and pattern, underlies the generation of the GnRH surge and that these changes are estradiol-dependent.
Fig. 2.
Time of day does not affect GnRH neuron firing pattern in OVX mice. (A and B) Representative examples of firing patterns are shown for GnRH neurons from OVX mice recorded in the a.m. (A) and in the p.m. (B). Firing rate is displayed at 1-min intervals. Vertical lines at the top of each graph illustrate the timing of the individual action currents detected. (C–F) Mean values for firing rate (C), median instantaneous frequency (D), maximum duration of quiescence (E), and percentage of time in quiescence (F). Gray bars represent values from cells recorded in the a.m., and hatched bars indicate values from cells recorded in the p.m.
Fig. 3.
Estradiol induces diurnal changes in GnRH neuron firing and pattern. (A and B) Representative examples of firing patterns are shown for GnRH neurons from OVX+E mice recorded in the a.m. (A) and in the p.m. (B). Data are plotted as mean firing rate at 1-min intervals. Vertical lines at the top of each graph illustrate the timing of the individual action currents detected. (C–F) Mean values for firing rate (C), median instantaneous frequency (D), maximum duration of quiescence (E), and percentage of time in quiescence (F). Open bars represent values from cells recorded in the a.m., and filled bars indicate values from cells recorded in the p.m. ***, P < 0.001.
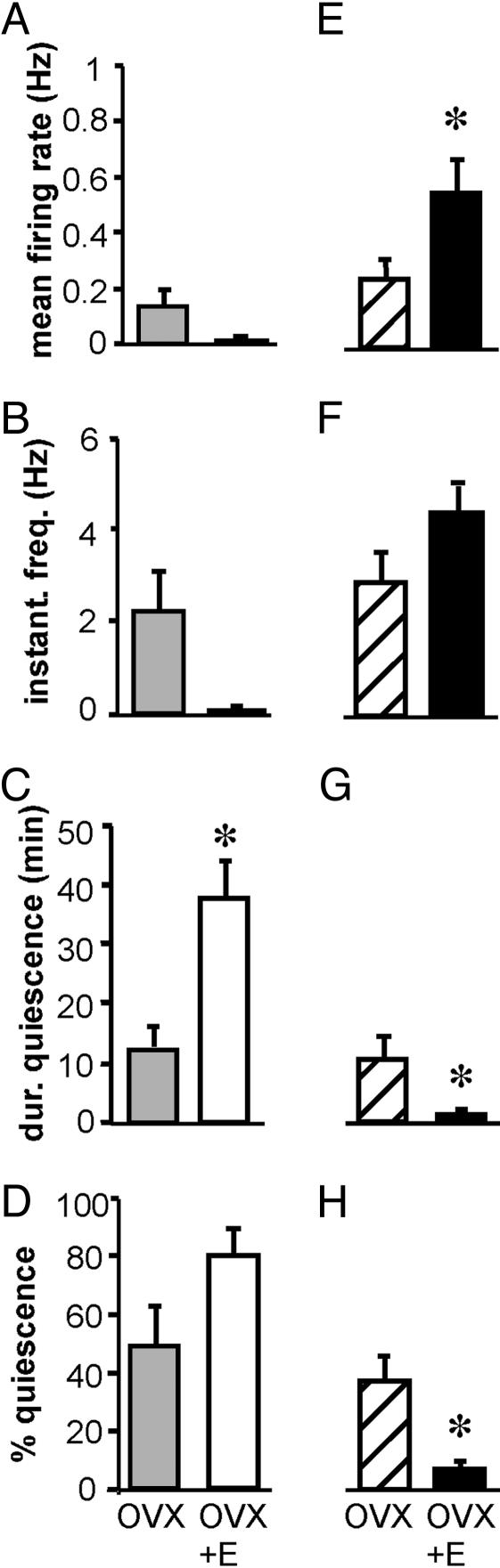
Firing Patterns in OVX vs. OVX+E Mice in the a.m. LH levels were lower in OVX+E animals than OVX animals in the a.m., indicative of estradiol negative feedback. Comparison of GnRH neuron firing patterns in the a.m. from OVX and OVX+E mice showed that estradiol treatment increased duration of quiescent time (Fig. 4C; P < 0.05). Mean firing rate, instantaneous firing frequency, and percentage of time in quiescence were not different. These data indicate that estradiol negative feedback may exert its effects through changes in the pattern of GnRH neuron firing, reflecting decreased GnRH neuron activity.
Fig. 4.
Estradiol induces negative feedback in the a.m. and positive feedback in the p.m. Shown are mean values for firing rate (A and E), median instantaneous frequency (B and F), maximum duration of quiescence (C and G), and percentage of time in quiescence (D and H) in the a.m. (A–D) and in the p.m. (E–H). Gray bars represent values from cells from OVX mice in the a.m., and hatched bars indicate values from cells from OVX mice in the p.m. Open bars represent values from cells from OVX+E mice in the a.m., and filled bars indicate values from cells from OVX+E mice in the p.m. *, P < 0.05.
Firing Patterns in OVX vs. OVX+E Mice in the p.m. LH levels were higher in OVX+E animals than OVX animals in the p.m., indicative of estradiol positive feedback. Comparison of GnRH neuron firing patterns in the p.m. from OVX and OVX+E mice showed that estradiol treatment increased mean firing rate (Fig. 4E; P < 0.05) and decreased duration (Fig. 4G; P < 0.05) and percentage (Fig. 4H; P < 0.05) of time in quiescence. Estradiol treatment did not significantly affect instantaneous firing frequency. These data indicate that changes in the rate and time of quiescence of GnRH neuron firing may underlie estradiol positive feedback, reflecting increased GnRH neuron activity.
Discussion
Although the preovulatory GnRH and LH surges are critically important to female reproductive function, their underlying mechanisms are only beginning to be understood. With the advent of transgenic mice allowing GnRH neuron identification in brain slices and the increased capability of genetic studies using the mouse system, the creation of a single-variable mouse model that reliably exhibits daily LH surges will greatly facilitate advancing our knowledge of the neurobiological changes responsible for surge regulation. Here, we demonstrated that ovariectomy and immediate constant physiological estradiol replacement through s.c. capsules can induce daily LH surges in mice. Importantly, these changes in LH in the whole animal are reflected in consistent changes in GnRH neuron firing activity in brain slices.
The ability to induce daily LH surges by ovariectomy and constant estradiol treatment in mice echoes findings using similar treatment regimens in rats and hamsters (28, 29). Previous reports indicated that constant estradiol treatment through s.c. capsules alone did not induce LH surges in mice (12). This discrepancy could be due to strain differences, although it is more likely a result of different estradiol dosages. In the previous study, capsules contained 5 μg, compared with 0.625 μg in the present experiments. The constant higher dosage in the previous study may have prevented the induction of LH surges through a damping or desensitizing phenomenon, such that a LH surge could only be induced by a secondary stimulus from a subsequent estradiol injection. The present findings, using an implant that produces a physiological level of estradiol and measuring LH levels on consecutive days soon after the day of surgery, indicate that, despite potential differences between rodent species in the degree of reproductive sensitivity to pheromonal and social cues, the underlying mechanisms of GnRH and LH surge generation and timing may largely be similar.
To investigate changes in GnRH neuron activity associated with the LH surge, the present experiments used targeted, single-unit extracellular recordings to examine changes in firing activity of GFP-identified GnRH neurons in brain slices. Estradiol treatment induced marked diurnal variations in GnRH neuron firing rates and patterns that were consistent with the change in LH levels characteristic of the LH surge. Additionally, comparison of OVX and OVX+E cells in the a.m. and the p.m. indicated changes in firing rates and patterns associated with estradiol negative and positive feedback, respectively, which were also consistent with the observed changes in LH levels. The relationship between electrical activity and hormone release has been demonstrated in magnocellular neurosecretory cells of the paraventricular (38, 39) and supraoptic nuclei (40), as well as the GnRH system (41). Thus, it is likely that the changes observed here are directly related to, and indicative of, the increased GnRH release that is characteristic of the GnRH surge.
The present studies are consistent with previous reports indicating that in vivo estradiol treatment produces changes in GnRH neuron function that can be measured in acutely prepared brain slices recorded in the absence of in vitro estradiol (31, 36). Furthermore, the results of the present experiments may help refine interpretation of these two previous studies, perhaps resolving some apparent discrepancies. Targeted extracellular recordings performed in the same manner as the present study but on d 5–9 post-OVX revealed that estradiol increased the interval between episodes of GnRH neuron firing, indicative of negative feedback, which was also reflected in LH levels in that model (36). In the same model, in vivo estradiol treatment was shown to affect the amplitude, duration, decay time, and voltage dependence of both activation and inactivation of A-type potassium currents (31). In contrast to what would be expected in a model of estrogen negative feedback, estradiol increased the excitability of GnRH neurons through a decrease in both the threshold and latency for action potential generation.
Although these results initially appear paradoxical, the time of day at which the above studies were performed helps place those data in concordance with the current observations. The previous extracellular recordings were done at different times of day, but largely at the time of negative feedback. This situation occurred because the single-recording setup then in the laboratory was in use for other studies (specifically, the recordings of potassium currents from 2–7 p.m., which largely overlaps with when positive feedback was observed in the present study). Thus, the changes in potassium currents and excitability may have, in fact, reflected changes involved in estradiol positive rather than negative feedback. Further studies on potassium current changes in GnRH neurons, using the surge model presented here, could ascertain whether the estradiol-induced changes in potassium channel function and neuronal excitability are associated solely with estradiol action or whether a diurnal component exists for these parameters as well.
Studies of rats and sheep have indicated that a GnRH surge accompanies and is required for the LH surge (7–9, 42), and more recent studies in rhesus monkeys have indicated that a GnRH surge is present and required in this species as well (10, 43). In rodents, the amplitude of the GnRH surge has been reported to be too small to trigger a LH surge without an accompanying increase in pituitary responsiveness to GnRH (44, 45). An increase in GnRH receptor expression in the pituitary before an estradiol-induced LH surge has been demonstrated in monkeys and sheep (46, 47). In rodents, the relationship of pituitary GnRH receptor expression to LH surge generation is unclear. Some studies in OVX rats indicated that pituitary GnRH receptor expression decreases before an estradiol-induced surge (48), suggesting that estradiol exerts a negative feedback effect at the pituitary before the LH surge, making a GnRH surge critical for LH surge generation. Other studies, however, have indicated an increase in GnRH receptor mRNA before both proestrus and estradiol-induced surges that persists throughout the LH surge in rats (49, 50). A self-priming effect of GnRH to increase GnRH receptor expression may provide another mechanism whereby a small-amplitude GnRH surge may trigger a LH surge (51). A decay in GnRH receptor expression or pituitary sensitivity may be a potential mechanism for the damping of LH surge amplitude observed with sustained estradiol treatment (29). The mouse model presented here could prove useful in dissecting out the roles of the neural and pituitary components responsible for surge regulation.
Previous studies on electrical activity in the hypothalamus during the surge, using radiotelemetric recordings of GnRH pulse generator activity, indicated a decrease in multiple unit activity (MUA) associated with LH surges in monkeys and goats (52, 53). This finding contrasts with the present data, which show dramatic increases in GnRH neuron activity associated with the LH surge in mice. A difference in experimental techniques likely accounts for these opposed findings. MUA recordings measure the neuronal activities of a pool of unidentified neurons, which may include both GnRH and non-GnRH neurons. In contrast, targeted extracellular recordings allow for measurement of individual GnRH neuron activity. Furthermore, because MUA represents firing activity of many cells, several of these cells must fire synchronously for a peak to be detected. Therefore, if there is general desynchronization among recorded cells during the GnRH surge, peaks in MUA might not be evident, even if individual cells had increased firing activity.
In this regard, it remains unclear whether the GnRH surge is generated through changes in individual GnRH neuron firing activity, a change in the coordination or synchronization of firing between cells, or a combination thereof. It is possible that the increase in GnRH release at the time of the surge is due to many cells firing at a higher rate simultaneously so that larger pulses of GnRH are secreted or cells alternating their firing so that GnRH levels in the portal pituitary blood are continuously elevated. Alternatively, the GnRH neuron population may be subdivided into “pulse” cells and “surge” cells, with the former primarily responsible for pulsatile GnRH release and the latter active only during the surge. The regularity of the observation of high firing frequency cells in the p.m. may indicate that many GnRH neurons participate in the surge response, as was suggested by the large percentage of GnRH neurons expressing cFos at that time in rats and sheep (54, 55).
The involvement of a daily neural signal required for ovulation was first indicated by classic studies in which barbiturate treatment of rats in the midafternoon of proestrus delayed ovulation by 24 h but had no effect when administered at other times (13), indicating that there is a “critical period” during which this signal is conveyed and after which ovulation becomes inevitable. The time of the critical period for daily neural signal transmission in mice is unknown. It is clear, however, that after a certain point in the day, brain slices can be prepared when LH levels are still low, yet the GnRH surge mechanism will persist in the brain slice, indicating that this approach can be used to study the underlying mechanisms of surge generation.
The studies presented here do not indicate whether the effects of estradiol feedback are due to direct or indirect actions of estradiol on GnRH neurons. Direct action of estradiol may be possible as GnRH neurons in rats and mice express mRNA of the estrogen receptor (ER) β-isoform (ERβ) (56, 57), although nuclear ERβ immunoreactivity has not been found in mouse GnRH neurons.§ Although the role of ERβ in LH surge regulation remains unclear, ERβ knockout mice show normal LH levels (58), and disruptions to fertility in ERβ knockouts, such as reduced litter size, appear to be due to effects on the ovary rather than at the level of the hypothalamus or pituitary (59). Female ERα knockouts, in contrast, have significantly higher levels of plasma estradiol, testosterone, and LH (20, 60) and are infertile, indicating that ERα is required for estradiol negative feedback and possibly for positive feedback as well. Thus, ERα-expressing inputs may be required for the GnRH surge, and the effects on GnRH neuron activity observed here are likely at least partly due to indirect action of estradiol through upstream neurons.
In summary, the present studies indicate that changes in the pattern and level of individual GnRH neuron firing activity may reflect the switch in estradiol action and underlie GnRH surge generation. The surge model presented here, and the demonstrated persistence of the surge in brain slices, could be used to examine the neurobiological mechanisms of GnRH surge generation. The requirement of GnRH and LH surges for ovulation underscores the importance of characterizing their physiology. Understanding the underlying mechanisms of the neural control of ovulation may be of therapeutic importance in treating infertility as well as designing different methods for contraception.
Acknowledgments
We thank Xu-Zhi Xu for expert technical assistance; Aleisha Schoenfelder and Emily Hurst for performing LH immunoassays; Gilbert Pitts for statistical advice; and Zhiguo Chu, Justyna Pielecka, Talent Shevchenko, Pei-San Tsai, and Heidi Walsh for editorial comments. This work was supported by National Institute of Child Health and Human Development/National Institutes of Health (NIH) Grant R01 HD41469 and cooperative agreement U54 HD28934 Ligand and Assay Core. C.A.C. was supported by the University of Virginia Neuroscience Graduate Program through NIH Grant T32 GM 008328.
Author contributions: C.A.C. and S.M.M. designed research; C.A.C. and J.L.M. performed research; C.A.C. and S.M.M. analyzed data; and C.A.C. and S.M.M. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; OVX, ovariectomized; OVX+E, ovariectomized and treated with estradiol; ER, estrogen receptor.
Footnotes
Hrabovszky, E., Barabas, K., Kallo, I., Merchenthaler, I., Moenter, S. M. & Liposits, Z., Fifth International Congress of Neuroendocrinology, Aug. 31–Sept. 4, 2002, Bristol, U.K., p. 336 (abstr.).
References
- 1.Döcke, F. & Dörner, G. (1965) J. Endocrinol. 33, 491–499. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar, D. K. & Fink, G. (1980) J. Endocrinol. 86, 511–524. [DOI] [PubMed] [Google Scholar]
- 3.Karsch, F. J., Cummins, J. T., Thomas, G. B. & Clarke, I. J. (1987) Biol. Reprod. 36, 1207–1218. [DOI] [PubMed] [Google Scholar]
- 4.Moenter, S. M., Caraty, A. & Karsch, F. J. (1990) Endocrinology 127, 1375–1384. [DOI] [PubMed] [Google Scholar]
- 5.Evans, N. P., Dahl, G. E., Glover, B. H. & Karsch, F. J. (1994) Endocrinology 134, 1806–1811. [DOI] [PubMed] [Google Scholar]
- 6.Herbison, A. E. (1998) Endocrine Rev. 19, 302–330. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar, D. K., Chiappa, S. A., Fink, G. & Sherwood, N. M. (1976) Nature 264, 461–463. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, I. J., Thomas, G. B., Yao, B. & Cummins, J. T. (1987) Neuroendocrinology 46, 82–88. [DOI] [PubMed] [Google Scholar]
- 9.Moenter, S. M., Caraty, A., Locatelli, A. & Karsch, F. J. (1991) Endocrinology 129, 1175–1182. [DOI] [PubMed] [Google Scholar]
- 10.Pau, K. Y., Berria, M., Hess, D. L. & Spies, H. G. (1993) Endocrinology 133, 1650–1656. [DOI] [PubMed] [Google Scholar]
- 11.Kerdelhue, B., Brown, S., Lenoir, V., Queenan, J. T., Jones, G. S., Scholler, R. & Jones, H. W. (2002) Neuroendocrinology 75, 158–163. [DOI] [PubMed] [Google Scholar]
- 12.Bronson, F. H. & Vom Saal, F. S. (1979) Endocrinology 104, 1247–1255. [DOI] [PubMed] [Google Scholar]
- 13.Everett, J. W. & Sawyer, C. H. (1950) Endocrinology 47, 198–218. [DOI] [PubMed] [Google Scholar]
- 14.Moline, M. L., Albers, H. E., Todd, R. B. & Moore-Ede, M. C. (1981) Horm. Behav. 15, 451–458. [DOI] [PubMed] [Google Scholar]
- 15.Moline, M. L. & Albers, H. E. (1988) Physiol. Behav. 43, 435–440. [DOI] [PubMed] [Google Scholar]
- 16.Swann, J. M. & Turek, F. W. (1985) Science 228, 898–900. [DOI] [PubMed] [Google Scholar]
- 17.Lucas, R. J., Stirland, J. A., Darrow, J. M., Menaker, M. & Loudon, A. S. (1999) Endocrinology 140, 758–764. [DOI] [PubMed] [Google Scholar]
- 18.Miller, B. H., Olson, S. L., Turek, F. W., Levine, J. E., Horton, T. H. & Takahashi, J. S. (2004) Curr. Biol. 14, 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chappell, P. E., Lydon, J. P., Conneely, O. M., O'Malley, B. W. & Levine, J. E. (1997) Endocrinology 138, 4147–4152. [DOI] [PubMed] [Google Scholar]
- 20.Rissman, E. F., Wersinger, S. R., Taylor, J. A. & Lubahn, D. B. (1997) Horm. Behav. 31, 232–243. [DOI] [PubMed] [Google Scholar]
- 21.Gore, A. C., Roberts, J. L. & Gibson, M. J. (1999) Endocrinology 140, 2280–2287. [DOI] [PubMed] [Google Scholar]
- 22.Suter, K. J., Song, W. J., Sampson, T. L., Wuarin, J. P., Saunders, J. T., Dudek, F. E. & Moenter, S. M. (2000) Endocrinology 141, 412–419. [DOI] [PubMed] [Google Scholar]
- 23.Herbison, A. E., Pape, J. R., Simonian, S. X., Skynner, M. J. & Sim, J. A. (2001) Mol. Cell. Endocrinol. 185, 185–194. [DOI] [PubMed] [Google Scholar]
- 24.Leupen, S. M., Tobet, S. A., Crowley, W. F., Jr., & Kaila, K. (2003) Endocrinology 144, 3031–3036. [DOI] [PubMed] [Google Scholar]
- 25.Temple, J. L., Laing, E., Sunder, A. & Wray, S. (2004) J. Neurosci. 24, 6326–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill, J. C., Moenter, S. M. & Tsai, P. S. (2004) Endocrinology 145, 3830–3839. [DOI] [PubMed] [Google Scholar]
- 27.Bronson, F. H. (1981) Endocrinology 108, 506–516. [DOI] [PubMed] [Google Scholar]
- 28.Norman, R. L., Blake, C. A. & Sawyer, C. H. (1973) Endocrinology 93, 965–970. [DOI] [PubMed] [Google Scholar]
- 29.Legan, S. J. & Karsch, F. J. (1975) Endocrinology 96, 57–62. [DOI] [PubMed] [Google Scholar]
- 30.Nunemaker, C. S., DeFazio, R. A. & Moenter, S. M. (2003) Biol. Proced. Online 5, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeFazio, R. A. & Moenter, S. M. (2002) Mol. Endocrinol. 16, 2255–2265. [DOI] [PubMed] [Google Scholar]
- 32.Nelson J. F., Felicio L. S., Osterburg H. H. & Finch C. E. (1992) Endocrinology 130, 805–810. [DOI] [PubMed] [Google Scholar]
- 33.Haavisto, A. M., Pettersson, K., Bergendahl, M., Perheentupa, A., Roser, J. F. & Huhtaniemi, I. (1993) Endocrinology 132, 1687–1691. [DOI] [PubMed] [Google Scholar]
- 34.Krieg, R. J., Tokieda, K., Chan, J. C. & Veldhuis, J. D. (2000) Kidney Int. 58, 569–574. [DOI] [PubMed] [Google Scholar]
- 35.Chu, Z. & Moenter, S. M. (2005) J. Neurosci. 25, 5740–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunemaker, C. S., DeFazio, R. A. & Moenter, S. M. (2002) Endocrinology 143, 2284–2292. [DOI] [PubMed] [Google Scholar]
- 37.Sidman, R. L., Angevine, J. B., Jr., & Pierce E. T. (1971) Atlas of the Mouse Brain and Spinal Cord (Harvard Univ. Press, Cambridge, MA).
- 38.Wakerley, J. B. & Lincoln, D. W. (1973) J. Endocrinol. 57, 477–493. [DOI] [PubMed] [Google Scholar]
- 39.Poulain, D. A., Wakerley, J. B. & Dyball, R. E. J. (1977) Proc. R. Soc. London B 196, 367–384. [DOI] [PubMed] [Google Scholar]
- 40.Dutton, A. & Dyball, R. E. J. (1979) J. Physiol. 290, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunemaker, C. S., DeFazio, R. A., Geusz, M. E., Herzog, E. D., Pitts, G. R. & Moenter, S. M. (2001) J. Neurophysiol. 86, 86–93. [DOI] [PubMed] [Google Scholar]
- 42.Kaynard, A. H., Malpaux, B., Robinson, J. E., Wayne, N. L. & Karsch, F. J. (1988) Neuroendocrinology 48, 296–303. [DOI] [PubMed] [Google Scholar]
- 43.Xia, L., Van Vugt, D., Alston, E. J., Luckhaus, J. & Ferin, M. (1992) Endocrinology 131, 2812–2820. [DOI] [PubMed] [Google Scholar]
- 44.Fink, G. (1979) Annu. Rev. Physiol. 41, 571–585. [DOI] [PubMed] [Google Scholar]
- 45.Fink, G. (1988) Q. J. Exp. Physiol. 73, 257–293. [DOI] [PubMed] [Google Scholar]
- 46.Adams, T. E., Norman, R. L. & Spies, H. G. (1981) Science 213, 1388–1390. [DOI] [PubMed] [Google Scholar]
- 47.Crowder, M. E. & Nett, T. M. (1984) Endocrinology 114, 234–239. [DOI] [PubMed] [Google Scholar]
- 48.Barkan, A. L., Regiani, S. R., Duncan, J. A. & Marshall, J. C. (1983) Endocrinology 112, 1042–1048. [DOI] [PubMed] [Google Scholar]
- 49.Bauer-Dantoin, A. C., Hollenberg, A. N. & Jameson, J. L. (1993) Endocrinology 133, 1911–1914. [DOI] [PubMed] [Google Scholar]
- 50.Bauer-Dantoin, A. C., Weiss, J. & Jameson, J. L. (1995) Endocrinology 136, 1014–1019. [DOI] [PubMed] [Google Scholar]
- 51.Fink, G. (1995) Front. Neuroendocrinol. 16, 183–190. [DOI] [PubMed] [Google Scholar]
- 52.O'Byrne, K. T., Thalabard, J. C., Grosser, P. M., Wilson, R. C., Williams, C. L., Chen, M. D., Ladendorf, D., Hotchkiss, J. & Knobil, E. (1991) Endocrinology 129, 1207–1214. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka, T., Mori, Y. & Hoshino, K. (1992) Neuroendocrinology 56, 641–645. [DOI] [PubMed] [Google Scholar]
- 54.Lee, W. S., Smith, M. S. & Hoffman, G. E. (1990) Proc. Natl. Acad. Sci. USA 87, 5163–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moenter, S. M., Karsch, F. J. & Lehman, M. N. (1993) Endocrinology 133, 896–903. [DOI] [PubMed] [Google Scholar]
- 56.Skynner, M. J., Sim, J. A. & Herbison, A. E. (1999) Endocrinology 140, 5195–5201. [DOI] [PubMed] [Google Scholar]
- 57.Hrabovszky, E., Steinhauser, A., Barabas, K., Shughrue, P. J., Petersen, S. L., Merchenthaler, I. & Liposits, Z. (2001) Endocrinology 142, 3261–3264. [DOI] [PubMed] [Google Scholar]
- 58.Couse, J. F., Yates, M. M., Walker, V. R. & Korach, K. S. (2003) Mol. Endocrinol. 17, 1039–1053. [DOI] [PubMed] [Google Scholar]
- 59.Krege, J. H., Hodgin, J. B., Couse, J. F., Enmark, E., Warner, M., Mahler, J. F., Sar, M., Korach, K. S., Gustafsson, J. A. & Smithies, O. (1998) Proc. Natl. Acad. Sci. USA 95, 15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Couse, J. F., Curtis, S. W., Washburn, T. F., Lindzey, J., Golding, T. S., Lubahn, D. B., Smithies, O. & Korach, K. S. (1995) Mol. Endocrinol. 9, 1441–1454. [DOI] [PubMed] [Google Scholar]