Abstract
Background: Risperidone is a novel antipsychotic drug that has been tried in the treatment of several child psychiatric disorders. In an open clinical study, we evaluated the safety and efficacy of risperidone in children with developmental disorder and behavioral problems including attention-deficit/hyperactivity disorder (ADHD).
Method: Twelve patients aged 4 to 14 years who had a DSM-IV–diagnosed developmental disorder and ADHD in addition to other behavioral problems, in particular aggression, were treated with risperidone for a period of up to 2 years with daily doses ranging from 1 to 3 mg. Data were gathered from December 2002 to December 2004.
Results: A positive clinical response was noted in 9 of the 12 patients within 3 months of study recruitment according to the Clinical Global Impressions-Improvement scale. Risperidone was well tolerated by all 12 patients. The most commonly reported side effect was sedation, which necessitated dosage reduction in 2 patients, but not discontinuation.
Conclusions: Our findings suggest that risperidone may be an effective and safe treatment for children and adolescents with developmental disorder and disruptive behaviors.
Behavioral problems often coexist with developmental and learning disorders, and in these instances, irritability, aggression, hyperactivity, insomnia, and self-injurious behaviors are the main features. Reports have been published indicating that risperidone is effective in treating behavioral problems in patients with mental retardation1,2 and developmental disabilities3,4 and in children with autism and other pervasive developmental disorders.5–8 A large double-blind, placebo-controlled study6 of risperidone for the treatment of autism and serious behavioral disorders published recently showed the efficacy of the drug in the treatment of tantrums, aggression, and self-injurious behavior in children with these disorders. A few other reports have suggested its usefulness in children with a wide range of behavioral problems, including aggression and self-injurious behaviors,1 aberrant behaviors,9 pathologic aggression,10 explosive aggressive autism,11 and comorbid attention-deficit/ hyperactivity disorder (ADHD) and conduct disorder,12 as well as aggressive behavior in the context of mood disorders.13 This article reports on the open clinical experience of treating with risperidone 12 children who had a developmental disorder and comorbid ADHD.
PATIENTS AND METHODS
In this series of 12 patients, the subjects' ages ranged from 4 to 14 years (mean = 7.5 years, SD = 3.513); all patients were male with the exception of 1. All of the patients referred to the child psychiatry and neurodevelopmental clinics from the primary health and school health clinics for the Al Ain Medical District of United Arab Emirates over a period of 1 year were included in the study. Informed consent was obtained from the patients' guardians, and Declaration of Helsinki guidelines were followed. Assessments were made at the University Teaching Hospital clinics. Data were gathered from December 2002 to December 2004.
All of the children were evaluated by a child psychiatrist, and diagnoses were made according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).14 A checklist based on the DSM-IV criteria was used, and corroborative information was obtained using the Arabic version of the Conners' Parent Rating Scale or Conners' Teacher Rating Scale15 during the initial evaluation. ADHD was present in all of the patients (8 had the “combined” subtype and 4 had the “hyperactive” subtype of ADHD) in addition to other disruptive behaviors (Table 1). The children reported here were clinically heterogeneous, with different DSM-IV diagnoses (see Table 1), but they all had a developmental disorder (6 had mental retardation, 4 had pervasive developmental disorder, and 2 had communication disorder).
Table 1.
Characteristics of Children With Developmental Disorders and Disruptive Behaviors Treated With Risperidone
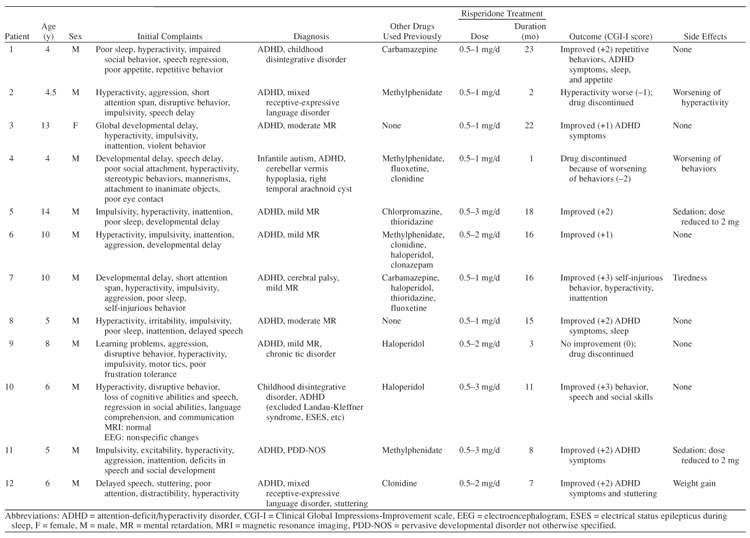
Risperidone treatment was started in doses of 0.5 mg once daily and increased gradually (in increments of 0.5 mg per month) to a maximum of 3 mg per day. Clinical efficacy was assessed on a monthly basis using the Clinical Global Impressions-Improvement scale (CGI-I),16 and parents were prompted to report commonly observed side effects such as sedation, weight or appetite changes, tiredness, and extrapyramidal side effects.
RESULTS
Clinical improvement was noted in 9 patients within 3 months of recruitment into the study (Table 1): scores on the CGI-I indicated mild improvement (score of +1) in 2 patients (cases 3, 6), moderate improvement (score of +2) in 5 (cases 1, 5, 8, 11, 12), significant improvement (score of +3) in 2 (cases 7, 10), and no change (score of 0) in 1 (case 9). Symptoms were reported to be worse (score of −1) in 1 patient (case 2) and much worse (score of −2) in another (case 4). Mean CGI-I score was 1.25 (SD = 1.545). Risperidone was effective in improving ADHD, aggression, and self-injurious behaviors. Sedation was reported in 2 patients, necessitating reduction in dosage but not discontinuation. The other side effects reported included tiredness and weight gain. No patients experienced extrapyramidal symptoms. Those who showed a positive clinical response were followed up for periods ranging from 12 to 23 months.
DISCUSSION
Although psychostimulants remain the mainstay of pharmacologic treatment for ADHD, they may not be tolerated by some children, may be ineffective in some, and in yet others may exacerbate a comorbid medical condition such as seizures or tics. Furthermore, most of the conventional antipsychotics used in these situations do not combat persistent irritability, extreme aggression, and other maladaptive behaviors, which often coexist in children with developmental disorders. In our cohort, risperidone was also used for different reasons including concern about the epileptogenic potential of certain drugs in the context of nonspecific electroencephalographic changes, precipitation of tics with methylphenidate, and undesirable side effects or poor response to alternative pharmacologic interventions.
Risperidone has been noted to be useful for insomnia in pervasive developmental disorder17 and for behavioral problems in developmentally disabled children18 and in adults with profound retardation and autism.19 Our findings that risperidone was effective in improving ADHD, aggression, and self-injurious behaviors suggest that risperidone has promise for the treatment of children with developmental disorders and disruptive behaviors. Furthermore, there was improvement in coexisting sleep and appetite problems. The drug was well tolerated by the children in the dosages used (1–3 mg). The most common side effect reported was sedation in 2 patients, followed by tiredness and weight gain in 1 patient each. Earlier studies have reported weight gain,20 chorea and dyskinesia,21 reversible withdrawal dyskinesia,22 and facial dystonia and amenorrhea23 with the use of risperidone. However, in a study by Simon and colleagues,24 traditional antipsychotics were substituted with risperidone in 10 individuals with mental retardation, and all participants evidenced improvement or resolution in side effects attributed to previous antipsychotic medication, with no worsening in behavioral or psychiatric status. Furthermore, Zuddas et al.23 and Croonenberghs et al.25 observed in their 1-year follow-up studies that risperidone is relatively safe for long-term treatment of behavioral problems.
In this study, risperidone was used for periods of up to 2 years with no undesirable effects. Because it is the usual clinical practice that children who show short-term benefit from a drug will be maintained on treatment with the medication indefinitely, it is important to evaluate the longer-term effectiveness and safety of risperidone in this population.
Drug names: carbamazepine (Carbatrol, Equetro, and others), chlor-promazine (Thorazine, Sonazine, and others), clonazepam (Klonopin and others), clonidine (Catapres, Duraclon, and others), fluoxetine (Prozac and others), haloperidol (Haldol and others), methylphenidate (Ritalin, Metadate, and others), risperidone (Risperdal).
Footnotes
Drs. Eapen and Gururaj report no financial relationship or affiliation relevant to the subject of this article.
REFERENCES
- Cohen SA, Ihrig K, and Lott RS. et al. Risperidone for aggression and self-injurious behavior in adults with mental retardation. J Autism Dev Disord. 1998 28:229–233. [DOI] [PubMed] [Google Scholar]
- Lott RS, Kerrick JM, Cohen SA. Clinical and economic aspects of risperidone treatment in adults with mental retardation and behavioral disturbance. Psychopharmacol Bull. 1996;32:721–729. [PubMed] [Google Scholar]
- Buitelaar JK, van der Gaag RJ, and Cohen-Kettenis P. et al. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry. 2001 62:239–248. [DOI] [PubMed] [Google Scholar]
- Hardan A, Johnson K, and Johnson C. et al. Risperidone treatment of children and adolescents with developmental disorders. J Am Acad Child Adolesc Psychiatry. 1996 35:1551–1556. [DOI] [PubMed] [Google Scholar]
- Shea S, Turgay A, and Carroll A. et al. Risperidone in the treatment of behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004 114:634–641. [DOI] [PubMed] [Google Scholar]
- McCracken JT, McGough J, and Shah B. et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002 347:314–321. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Holmes JP, and Carlson DC. et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998 55:633–641. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Scahill L, and McCracken JT. et al. Research Units on Pediatric Psychopharmacology (RUPP) Autism Network. Background and rationale for an initial controlled study of risperidone. Child Adolesc Psychiatr Clin North Am. 2000 9:201–224. [PubMed] [Google Scholar]
- Zarcone JR, Hellings JA, and Crandall K. et al. Effects of risperidone on aberrant behavior of persons with developmental disabilities, 1: a double-blind crossover study using multiple measures. Am J Ment Retard. 2001 106:525–538. [DOI] [PubMed] [Google Scholar]
- Fava M. Psychopharmacologic treatment of pathologic aggression. Psychiatr Clin North Am. 1997;20:427–451. doi: 10.1016/s0193-953x(05)70321-x. [DOI] [PubMed] [Google Scholar]
- Horrigan JP, Barnhill LJ. Risperidone and explosive aggressive autism. J Autism Dev Disord. 1997;27:313–323. doi: 10.1023/a:1025854532079. [DOI] [PubMed] [Google Scholar]
- Kewley GD. Risperidone in comorbid ADHD and ODD/CD [letter] J Am Acad Child Adolesc Psychiatry. 1999;38:1327–1328. doi: 10.1097/00004583-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Schreier HA. Risperidone for young children with mood disorders and aggressive behaviour. J Child Adolesc Psychopharmacol. 1998;8:49–59. doi: 10.1089/cap.1998.8.49. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- Daradkeh TK. Parent-teacher reliability in rating children on the 10-items Conners' Rating Scale. Egypt J Psychiatry. 1993;16:52–56. [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76-338. Rockville, Md: National Institute of Mental Health. 1976 218–222. [Google Scholar]
- Doan RJ. Risperidone for insomnia in PDDs [letter] Can J Psychiatry. 1998;43:1050–1051. [PubMed] [Google Scholar]
- Khan BU. Brief report: risperidone for severely disturbed behavior and tardive dyskinesia in developmentally disabled adults. J Autism Dev Disord. 1997;27:479–489. doi: 10.1023/a:1025813607005. [DOI] [PubMed] [Google Scholar]
- Dartnall NA, Holmes JP, and Morgan SN. et al. Brief report: two-year control of behavioral symptoms with risperidone in two profoundly retarded adults with autism. J Autism Dev Disord. 1999 29:87–91. [DOI] [PubMed] [Google Scholar]
- Hellings JA, Zarcone JR, and Crandall K. et al. Weight gain in a controlled study of risperidone in children, adolescents and adults with mental retardation and autism. J Child Adolesc Psychopharmacol. 2001 11:229–238. [DOI] [PubMed] [Google Scholar]
- Carroll NB, Boehm KE, Strickland RT. Chorea and tardive dyskinesia in a patient taking risperidone [letter] J Clin Psychiatry. 1999;60:485–487. doi: 10.4088/jcp.v60n0711b. [DOI] [PubMed] [Google Scholar]
- Malone RP, Maislin G, and Choudhury MS. et al. Risperidone treatment in children and adolescents with autism: short-and long-term safety and effectiveness. J Am Acad Child Adolesc Psychiatry. 2002 41:140–147. [DOI] [PubMed] [Google Scholar]
- Zuddas A, Di Martino A, and Muglia P. et al. Long-term risperidone for pervasive developmental disorder: efficacy, tolerability, and discontinuation. J Child Adolesc Psychopharmacol. 2000 10:79–90. [DOI] [PubMed] [Google Scholar]
- Simon EW, Blubaugh KM, Pippidis M. Substituting traditional antipsychotics with risperidone for individuals with mental retardation. Ment Retard. 1996;34:359–366. [PubMed] [Google Scholar]
- Croonenberghs J, Fegert JM, and Findling RL. et al. Risperidone in children with disruptive behavior disorders and subaverage intelligence: a 1-year, open-label study of 504 patients. J Am Acad Child Adolesc Psychiatry. 2005 44:64–72. [DOI] [PubMed] [Google Scholar]


