Abstract
Background: Depression is a common condition associated with hepatitis C and may be induced by interferon alfa, the primary treatment for hepatitis C. Depression is also a major barrier to the initiation of such treatment. This study examined the effect of escitalopram on measures of depression, quality of life, and tests of liver function in subjects with comorbid hepatitis C and depression.
Method: Subjects with DSM-IV major depressive disorder and hepatitis C were included in this open-label study. The recruitment period was from October 2002 through February 2004. Treatment status with regard to interferon therapy was neither an inclusion nor an exclusion criterion. Subjects received escitalopram for 8 weeks starting at 10 mg/day. Dosage adjustments up to 20 mg/day were made after week 4, as deemed clinically necessary. Scores on the 17-item Hamilton Rating Scale for Depression (HAM-D-17) and the Clinical Global Impressions-Severity of Illness scale (CGI-S) and results of liver function tests (AST, ALT, GGT) were obtained at baseline, 2 weeks, 4 weeks, and 8 weeks. Medical Outcomes Study Short Form Health Survey (SF-36) ratings and Hopkins Symptom Checklist-90-Revised (SCL-90-R) scores were obtained at baseline and week 8.
Results: Eighteen subjects (12 female, 6 male) participated in this study. The mean daily dose of escitalopram at endpoint was 12.78 mg. Mean HAM-D-17 scores decreased significantly with treatment (t = 8.535, df = 17, p < .0001). Statistically significant improvement was also demonstrated on many subscales of the SF-36, the SCL-90-R, and the CGI-S. Tests of liver function showed no significant changes.
Conclusion: These results suggest that depression in patients with hepatitis C may be effectively and safely treated with escitalopram.
Hepatitis C is a common and potentially fatal illness that affects an estimated 170 million people worldwide.1 Alter et al.,2 examining 21,241 serum samples from the third National Health and Nutrition Examination Survey, found a 1.8% overall prevalence of serum antibodies to hepatitis C, correlating to an estimated 3.9 million people infected in the United States. Hepatitis C is currently the primary indication for liver transplantation in adults in the United States.3
In addition, patients with hepatitis C appear to have elevated rates of psychiatric illness, with depression and substance abuse being the most frequent and clinically important.4–11 El-Serag et al.,4 in a retrospective chart review of 33,824 veterans, found that 86% of patients with hepatitis C had at least 1 past or present psychiatric illness or substance abuse problem. Depression was found in 49.5% of this sample, and alcohol and drug use disorders were identified in 77.6% and 69.4% of the sample, respectively. Rates of depression have been reported in the range of 22% to 49% of patients with hepatitis C.4–11 Dwight et al.5 found a 28% prevalence of depressive disorders among 50 patients with hepatitis C who were evaluated with structured interviews and standardized rating scales. Lee et al.,6 in a retrospective chart review of 359 patients who had not received interferon therapy, found that 24% had depression. A prospective study conducted by Gohier and colleagues7 in France examined 71 patients referred for interferon therapy and found 24% to have a prior history of depression. Kraus and colleagues8 examined 113 patients with chronic hepatitis C who were without evidence of de-compensated liver disease and found that 22.4% of patients demonstrated positive depression scores using the Hospital Anxiety and Depression Scale (German version). Hunt et al.9 prospectively evaluated 28 subjects with hepatitis C and found a 30% prevalence of depression using the Beck Depression Inventory. Yovtcheva and colleagues10 studied 306 veterans untreated for hepatitis C and found 28% to have depression. These rates are higher than the 16.6% lifetime prevalence of major depressive disorder (MDD) reported in the general population.12
Depression is also a common side effect of interferon alfa, the primary treatment for hepatitis C.13–18 Dieperink et al.14 found that 23% of patients treated for hepatitis C with interferon developed MDD, compared to a 33% prevalence identified by Hauser et al.15 and a 23% prevalence found by Horikawa et al.16 Kraus et al.17 reported that 35% of patients developed depression during interferon therapy. Hence, due to concerns of exacerbating a preexisting depression, patients with hepatitis C are often excluded from therapy, and depression is a common reason for discontinuation of interferon.19–21 Prophylactic treatment of interferon-induced depression has been recommended,14 but remains controversial since this approach could result in overtreatment of up to 65% of patients who are unlikely to develop depression.22
The safety of using selective serotonin reuptake inhibitors (SSRIs) in patients with hepatitis C has been questioned, particularly with regard to the potential for increased risk of bleeding and retinopathy in patients with hepatitis C who are receiving SSRIs.23,24 Weinrieb et al.23 reviewed the literature on bleeding associated with the use of SSRIs. Of the studies and case reports reviewed, the authors found that bleeding events in 19 (79%) of 24 individuals were closely associated with the administration of SSRIs. In the same article, the authors reported a case of a fatal gastrointestinal bleed in a patient with hepatitis C who had recently been started on paroxetine. Hejny et al.24 described 7 patients who developed retinopathy while receiving interferon alfa-2b therapy for melanoma. Six of these patients were also receiving the SSRI paroxetine. Although this was not a hepatitis C population, interferon alfa is used to treat hepatitis C, underscoring the importance of this observation.
Non-SSRI antidepressants present safety concerns as well. Mirtazapine is associated with an increased risk of bone marrow suppression and agranulocytosis,25 potentially placing the hepatitis C patient treated with interferon, which commonly suppresses bone marrow, at increased risk. Nefazodone has been shown to induce hepatotoxicity and is contraindicated in patients with liver disease.26 Finally, all currently available antidepressants are hepatically metabolized.
The use of the SSRIs has become widespread, primarily due to their relatively mild side effect profile, multiple psychiatric indications, and ease of administration. However, there is a lack of randomized, controlled trials of SSRIs or other antidepressants in hepatitis C–positive patients. The majority of the research in this field has focused on the treatment or prevention of interferon-induced depression. However, this approach omits a substantial number of patients with hepatitis C, up to 72%, who are not candidates for interferon therapy.21 Falck-Ytter et al.21 retrospectively reviewed the charts of 327 patients referred to a liver clinic with a hepatitis C–antibody-positive result and found that only 28% were treated.
There is growing evidence of the usefulness of antidepressants in the treatment of interferon-induced depression.15,27–32 However, we are aware of only 1 prior published study32 that has examined the usefulness of anti-depressant therapy in the broader hepatitis C population, without regard to interferon treatment status. In that study,32 we reported on the safety and efficacy of the anti-depressant citalopram for depression in hepatitis C. More empirical research is needed to guide health care providers in the safe and effective treatment of depression in patients with hepatitis C. Psychiatrists and primary care physicians need to be aware of the associations between hepatitis C and depression and should be knowledgeable regarding potential treatment options for these patients.
The current study focused on the broader hepatitis C population, patients with comorbid MDD, but without regard to interferon status. The a priori hypothesis of this 8-week open-label study was that escitalopram, the S-isomer of citalopram, would safely and effectively treat MDD in patients with comorbid hepatitis C. Escitalopram was chosen because of its minimal cytochrome P450 enzyme activity, few drug-drug interactions, linear pharmacokinetics, and no known hepatotoxicity. However, after this study was completed, a case of hepatotoxicity related to citalopram has been reported.33 The fact that escitalopram is the S-isomer of citalopram further underscores the need for research into the safety of antidepressant use in patients with liver disease.
METHOD
Subjects
Adults aged 18 to 65 years with hepatitis C and MDD were recruited by advertisement and from local physicians from October 2002 through February 2004. Interested subjects were instructed to call for a phone screening for preliminary eligibility. Subjects with documented hepatitis C and DSM-IV MDD were eligible for study participation after being informed of the potential risks and benefits of study participation, including possible medication side effects, and giving informed consent. This study was reviewed and approved by the Institutional Review Board at the University of Oklahoma College of Medicine.
Exclusion criteria included ongoing antidepressant or anxiolytic therapy, use of herbals marketed for behavioral effects (e.g., St. John's wort), evidence of cirrhosis (Child-Pugh stages B or C), evidence of liver failure, liver enzyme elevations greater than 2.5 times the upper limit of normal, and evidence of cognitive impairment demonstrated by Mini-Mental State Examination34 scores of less than 23 or pathologic time on the Trails A and B tests.35 Current or past treatment with interferon were neither inclusion nor exclusion criteria for this study. Due to concerns about the potential for interferon-induced depression in those subjects that were concurrently being treated with interferon, concurrent use of interferon and baseline 17-item Hamilton Rating Scale for Depression (HAM-D-17) scores greater than 25 was an exclusion criterion. However, only 1 subject currently taking interferon was screened for study involvement, and that individual's HAM-D-17 score was below 25. Subjects identified as having bipolar disorder, active substance use disorders, or active suicidal ideation were excluded from study participation.
Subjects participated in the study for 8 weeks. The study included a baseline visit and 3 office visits at weeks 2, 4, and 8. Data were collected in a research chart and then transferred to a computer database that did not include identifying information to protect patient confidentiality.
Clinical Measures
The Mini-International Neuropsychiatric Interview (MINI)36 was administered at baseline to confirm the presence of MDD and to evaluate for other comorbid psychiatric conditions. Other baseline measures included the HAM-D-17,37 the Hopkins Symptom Checklist-90-Revised (SCL-90-R),38 the Medical Outcomes Study Short Form Health Survey (SF-36),39 and the Clinical Global Impressions-Severity of Illness scale (CGI-S).40 The HAM-D-17 and CGI-S rating scales were readministered at 2, 4, and 8 weeks. Subjects were administered Mini-Mental State Examination and Trails A and B tests at baseline to screen for hepatic encephalopathy or other evidence of cognitive impairment. Tests of liver function (aspartate aminotransferase [AST], alanine aminotransferase [ALT], γ–glutamyltransferase [GGT]) were measured at all visits. Serum albumin and protime values were obtained at baseline for Child-Pugh staging.
Statistical Analysis
Changes in HAM-D-17 scores were analyzed using a repeated-measures analysis of variance based on measurements at baseline, 2 weeks, 4 weeks, and 8 weeks. The SCL-90-R and the mental and physical component summaries from the SF-36 were also analyzed using a paired t test, based on measurements at baseline and 8 weeks. For nonnormal distribution of any of the continuous measures, the nonparametric method (Friedman's test) was used. The continuous measure analysis was performed on a last-observation-carried-forward basis. All determinations of statistical significance were made using an α of .05. A secondary analysis compared the effects of escitalopram on hepatitis activity using the AST, ALT, and GGT values as dependent variables.
Subjects were grouped into a responder category by demonstrating at least a 50% reduction in the HAM-D-17 score from baseline to week 8. An estimate of the response rate was made by dividing the total number of responders by the total number of completers. A 95% confidence interval for the response rate was calculated using the method outlined by Fleiss.41
RESULTS
Study Group
Twenty-four subjects were initially enrolled in the study. Four subjects were excluded at the baseline visit due to elevations in tests of liver function that were greater than 2.5 times the upper limit of normal. One was excluded at the baseline visit after administration of the MINI,36 due to identification of a diagnosis of bipolar disorder rather than MDD. One subject was withdrawn from the study at week 4 due to a liver biopsy report from a biopsy that was done prior to study entry, indicating the presence of cirrhosis, a study exclusion criterion. Of the 18 subjects maintained in the study, 1 subject withdrew after 4 weeks due to adverse effects (primarily sexual dysfunction) and no significant improvement on escitalopram treatment. One subject withdrew after 1 week due to headache, diarrhea, and tremor. One subject was lost to follow-up after week 2.
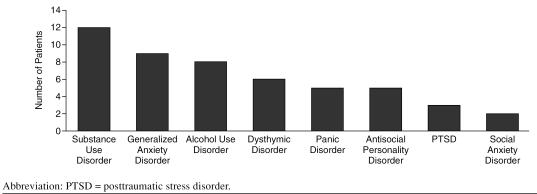
The study group consisted of 18 subjects, 12 women and 6 men. The mean ± SD age was 44.7 ± 8.1 years. The mean education level was 11.7 years, just under high school graduate. Five subjects had been on interferon therapy at some time in their lives, 1 of whom was receiving interferon alfa and ribavirin therapy during the course of this study. Thirteen subjects had never been treated with interferon. Psychiatric comorbidity, in addition to MDD, was high in this group (Figure 1). Prior history of substance use disorders was common. Substance use disorders other than alcohol were identified in 67% of study subjects, and alcohol use disorders were identified in 44% of the subjects. Half of the subjects met DSM-IV diagnostic criteria for generalized anxiety disorder.
Figure 1.
Number of Patients With Comorbid Psychiatric Diagnoses (in addition to major depressive disorder) as Identified by the Mini-International Neuropsychiatric Interview (N = 18)
Subjects were treated with standard doses of escitalopram. The starting dose of escitalopram was 10 mg by mouth daily. Escitalopram was increased to 15 mg in 2 subjects and to 20 mg in 4 subjects by week 8. Mean daily dose of escitalopram at endpoint was 12.78 mg.
Hamilton Rating Scale for Depression Scores
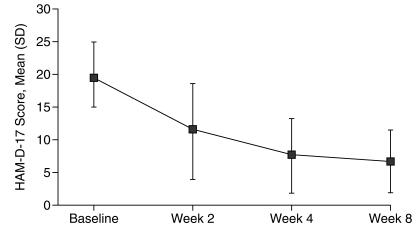
HAM-D-17 scores decreased significantly over the 8-week period of treatment with escitalopram (t = 8.535, df = 17, p < .0001) as shown in Figure 2. Mean HAM-D-17 scores were 19.5 (range, 12–30) at the beginning of the study and 6.61 (range, 1–22) at study completion. Fifteen (88.2%) of the 17 subjects who completed at least visit 2 demonstrated a clinical response, defined as a 50% or greater reduction in HAM-D-17 score. The 1 patient who was receiving interferon therapy during study enrollment was also a clinical responder, with a reduction in the HAM-D-17 score from 18 at baseline to 5 at week 8. Three of the 4 subjects who had previously received interferon therapy were clinical responders. Statistically significant reductions (p < .0001) in mean HAM-D-17 scores were seen by week 4.
Figure 2.
17-Item Hamilton Rating Scale for Depression (HAM-D-17) Scores in 18 Patients During Treatment With Escitalopram
Quality of Life Measures
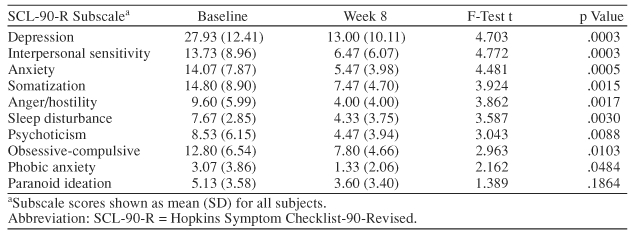
The SF-36 and the SCL-90-R are patient-rated scales that assess quality of life and psychiatric symptoms, respectively. Statistically significant improvements were seen on most of the subscales of these tests (Tables 1 and 2). The most significant improvements were demonstrated on SCL-90-R subscales measuring depression, interpersonal sensitivity, and anxiety and on the SF-36 measuring emotional well-being (p ≤ .0005). Hepatitis C is often associated with significant reductions in physical energy and fatigue. These difficulties can lead to job loss and other social and relationship problems. Depression has been found to have a more significant impact on fatigue and functional impairment than does severity of liver disease.5 Subjects in this study demonstrated statistically significant reductions in fatigue and pain, as measured by the SF-36.
Table 1.
Effect of Escitalopram on Quality of Life (SF-36 scores) (N = 18)
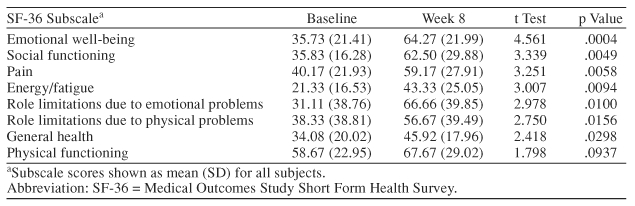
Table 2.
Effect of Escitalopram on Psychiatric Symptoms (SCL-90-R scores) (N = 18)
Clinical Global Impressions-Severity of Illness Scale Scores
The CGI-S is a physician-rated scale that assesses a patient's global severity of illness. This is rated on a 7-point scale (1 = normal, 7 = among the most extremely ill patients). The mean ± SD CGI-S scores at baseline and at visit 4 were 4.39 ± 0.91 and 2.17 ± 1.04, respectively, demonstrating statistically significant improvement, globally, in the study subjects (p < .0001).
Liver Function Tests
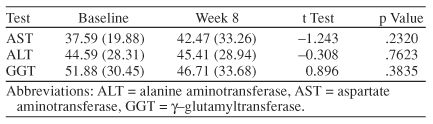
To assess the effect, if any, that escitalopram would have on tests of liver function, levels of AST, ALT, and GGT were obtained at baseline and weeks 2, 4, and 8. No statistically significant changes were seen during the 8-week trial (Table 3).
Table 3.
Effect of Escitalopram on Liver Enzyme Levels (N= 18)
DISCUSSION
The results of this study add to our current knowledge regarding the safety and efficacy of antidepressants in the hepatitis C population and provide additional information about the psychiatric comorbidity among a community sample of patients with hepatitis C. Subjects in this study demonstrated significant reductions in depressive symptoms as measured by the HAM-D-17 and a broader range of psychiatric symptoms (interpersonal sensitivity, anxiety, somatization, anger, sleep disturbance, etc.) as measured by the SCL-90-R. Furthermore, use of escitalopram was associated with improved quality of life, as demonstrated by improved scores on subscales of the SF-36 measuring emotional well-being, social functioning, pain, energy/fatigue, and, importantly, reductions in perceived role limitations due to physical and emotional problems. Excessive fatigue and lack of energy are common complaints among patients with hepatitis C, particularly those with comorbid depression. These symptoms can interfere with an individual's professional and personal productivity. The use of escitalopram, at standard doses, did not result in any significant elevations in hepatic enzymes in this group of patients with mild-to-moderate liver disease. The drug was also well tolerated, with only 2 of 18 subjects withdrawing due to adverse effects.
As in other studies, our subjects had high rates of prior substance use disorders, including use of non-alcohol substances (67%) and alcohol use disorders (44%). Additionally, high rates of anxiety were identified, with 50% of subjects meeting criteria for generalized anxiety disorder.
The treatment of interferon-induced depression was not specifically evaluated during this trial; however, the 1 subject who received interferon during the study responded to the escitalopram with a greater than 50% reduction in HAM-D-17 scores. Additionally, 3 of the 4 subjects who had been previously treated with interferon (prior to study entry) were also treatment responders.
As 1 of only 2 studies32 examining the usefulness of antidepressants in the broader hepatitis C population (without regard to interferon status), this study provided a unique opportunity to evaluate the effect of escitalopram on MDD, psychosocial functioning, and liver function. This study evaluated measures of quality of life using patient-rated scales, thereby limiting investigator bias, and changes in liver enzymes were objectively evaluated. Limitations of this study include the use of an open-label design, lack of a placebo control group, and a relatively small sample size. Placebo effects from various psychosocial interventions that may be associated with psychopharmacologic trials could result in falsely elevated response rates. This study utilized the HAM-D-17, a clinician-rated scale, which could increase the likelihood of investigator bias, particularly in an open-label study.42,43 However, patient-rated scales were also utilized, including the SCL-90-R, on which statistically significant reductions in the depression subscale were measured.
Effective management of depression among patients with hepatitis C is important given the higher prevalence of depression in this group compared to the general population, the potential barrier to medical treatment for hepatitis C posed by the presence of comorbid depression, and the potential serious complication of interferon-induced depression. Furthermore, all currently available antidepressants are hepatically metabolized, making the evaluation of the safety of antidepressant therapy in hepatitis C important. The results of this study suggest that escitalopram was safe, effective, and well-tolerated in the treatment of depression in this relatively small and heterogeneous sample of patients with comorbid mild-to-moderate hepatitis C. Larger, double-blind, placebo-controlled studies would be useful to confirm the results of this study.
Drug names: citalopram (Celexa and others), escitalopram (Lexapro), mirtazapine (Remeron and others), paroxetine (Paxil, Pexeva, and others).
Footnotes
This investigator-initiated study was funded by Forest Laboratories, Inc., New York, N.Y.
Dr. Gleason has received grant/research support and honoraria from and has served on the speakers bureau of Forest Pharmaceuticals. Dr. Yates has served as a consultant for Eli Lilly, Otsuka, Wyeth-Ayerst, and Forest and has received grant/research support from Eli Lilly, Pfizer, Pherin, and Forest. Ms. Philipsen reports no other financial affiliation relative to the subject of this article.
REFERENCES
- World Health Organization. Available at: http://www.who.int/csr/disease/hepatitis/en/. Accessed Aug 29, 2005. [Google Scholar]
- Alter MJ, Kruszon-Moran D, and Nainain OV. et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999 341:556–562. [DOI] [PubMed] [Google Scholar]
- Everhart JE, Wei Y, and Eng H. et al. Recurrent and new hepatitis C virus infection after liver transplantation. Hepatology. 1999 4:1220–1226. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Kunik M, and Richardson P. et al. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002 123:476–482. [DOI] [PubMed] [Google Scholar]
- Dwight MM, Kowdley KV, and Russo JE. et al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000 49:311–317. [DOI] [PubMed] [Google Scholar]
- Lee DH, Jamal H, and Regenstein FG. et al. Morbidity of chronic hepatitis C as seen in a tertiary care medical center. Dig Dis Sci. 1997 42:186–191. [DOI] [PubMed] [Google Scholar]
- Gohier B, Goeb JL, and Rannou-Dubas K. et al. Hepatitis C, alpha interferon, anxiety and depression disorders: a prospective study of 71 patients. World J Biol Psychiatry. 2003 4:115–118. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Schafer HC, and Scheurlen M. et al. Emotional state, coping styles, and somatic variables in patients with chronic hepatitis C. Psychosomatics. 2000 41:377–384.11015623 [Google Scholar]
- Hunt CM, Dominitz JA, and Philips Bute B. et al. Effect of interferon-alfa treatment of chronic hepatitis C on health-related quality of life. Dig Dis Sci. 1997 42:2482–2486. [DOI] [PubMed] [Google Scholar]
- Yovtcheva SP, Rifai MA, and Moles JK. et al. Psychiatric comorbidity among hepatitis C-positive patients. Psychosomatics. 2001 42:411–415. [DOI] [PubMed] [Google Scholar]
- Lehman CL, Cheung RC. Depression, anxiety, post-traumatic stress, and alcohol-related problems among veterans with chronic hepatitis C. Am J Gastroenterol. 2002;97:2640–2646. doi: 10.1111/j.1572-0241.2002.06042.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Zdilar D, Franco-Bronson K, and Buchler N. et al. Hepatitis C, interferon alfa, and depression. Hepatology. 2000 31:1207–1211. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Ho SB, and Thuras P. et al. A prospective study of neuropsychiatric symptoms associated with interferon-alfa-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003 44:104–112. [DOI] [PubMed] [Google Scholar]
- Hauser P, Khosla J, and Aurora H. et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002 7:942–947. [DOI] [PubMed] [Google Scholar]
- Horikawa N, Yamazaki T, Izumi N. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: a prospective study. Gen Hosp Psychiatry. 2003;25:34–38. doi: 10.1016/s0163-8343(02)00239-6. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Schäfer A, and Faller H. et al. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. J Clin Psychiatry. 2003 64:708–714. [DOI] [PubMed] [Google Scholar]
- Pariante C, Orru MG, and Baita A. et al. Treatment with interferon-alfa in patients with chronic hepatitis and mood or anxiety disorders [letter]. Lancet. 1999 354:131–132. [DOI] [PubMed] [Google Scholar]
- Rowan PJ, Tabasi S, and Abdul-Latif M. et al. Psychosocial factors are the most common contraindications for antiviral therapy at initial evaluation in veterans with chronic hepatitis C. J Clin Gastroenterol. 2004 38:530–534. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Willenbring E, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am J Psychiatry. 2000;157:867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter Y, Kale H, and Mullen KD. et al. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002 136:288–292. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Hauser P. Safety of the treatment of interferon-alpha-induced depression [letter] Psychosomatics. 2003;44:524–526. doi: 10.1176/appi.psy.44.6.524. [DOI] [PubMed] [Google Scholar]
- Weinrieb RM, Auriacombe M, and Lynch KG. et al. A critical review of selective serotonin reuptake inhibitor-associated bleeding: balancing the risk of treating hepatitis C-infected patients. J Clin Psychiatry. 2003 64:1502–1510. [DOI] [PubMed] [Google Scholar]
- Hejny C, Sternberg P, and Lawson DH. et al. Retinopathy associated with high-dose interferon alfa-2b therapy. Am J Ophthalmol. 2001 131:782–787. [DOI] [PubMed] [Google Scholar]
- Ozcanli T, Unsalver B, and Ozdemir S. et al. Sertraline- and mirtazapine-induced severe neutropenia [letter]. Am J Psychiatry. 2005 162:1386. [DOI] [PubMed] [Google Scholar]
- Choi S. Nefazodone (Serzone) withdrawn because of hepatotoxicity. CMAJ. 2003;169:1187. [PMC free article] [PubMed] [Google Scholar]
- Gleason OC, Yates WR. Five cases of interferon-alfa-induced depression treated with antidepressant therapy. Psychosomatics. 1999;40:510–512. doi: 10.1016/S0033-3182(99)71190-4. [DOI] [PubMed] [Google Scholar]
- Levenson JL, Fallon HJ. Fluoxetine treatment of depression caused by interferon-alfa. Am J Gastroenterol. 1993;88:760–761. [PubMed] [Google Scholar]
- Schramm TM, Lawford BR, and Macdonald GA. et al. Sertraline treatment of interferon-alfa-induced depressive disorder. Med J Aust. 2000 173:359–361. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Schäfer A, and Faller H. et al. Paroxetine for the treatment of interferon-alfa-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002 16:1091–1099. [DOI] [PubMed] [Google Scholar]
- Goldman LS. Successful treatment of interferon alfa-induced mood disorder with nortriptyline [letter] Psychosomatics. 1994;35:412–413. doi: 10.1016/S0033-3182(94)71769-2. [DOI] [PubMed] [Google Scholar]
- Gleason OC, Yates WR, and Isbell MD. et al. An open-label trial of citalopram for major depression in patients with hepatitis C. J Clin Psychiatry. 2002 63:194–198. [DOI] [PubMed] [Google Scholar]
- Lopez-Torres E. Hepatotoxicity related to citalopram [letter] Am J Psychiatry. 2004;161:923–924. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Berg R, Franzen M, and Wedding D. Screening for Brain Impairment: A Manual for Mental Health Practice. New York, NY: Springer Publishing Company, Inc. 1987 [Google Scholar]
- Sheehan DV, Lecrubier Y, and Harnett-Sheehan K. et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview. J Clin Psychiatry. 1998 59suppl 20. 22–33. [PubMed] [Google Scholar]
- Hamilton M. Hamilton Depression Scale. In: Guy W, ed. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76-338. Rockville, Md: National Institute of Mental Health. 1976 179–192. [Google Scholar]
- Derogatis LR. Symptom Checklist-90-Revised (SCL-90-R). In: Task Force for the Handbook of Psychiatric Measures. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association. 2000 81–84. [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), 1: conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- National Institute of Mental Health. CGI: Clinical Global Impressions. In: Guy W, Bonato RR, eds. Manual for the ECDEU Assessment Battery, 2, Revised Edition. Chevy Chase, Md: National Institute of Mental Health. 1970 12-1–12-6. [Google Scholar]
- Fleiss J. Statistical Methods for Rates and Proportions. New York, NY: Wiley & Sons. 1981 [DOI] [PubMed] [Google Scholar]
- Hoyt WT. Rater bias in psychological research: when is it a problem and what can we do about it? Psychol Methods. 2000;5:64–86. doi: 10.1037/1082-989x.5.1.64. [DOI] [PubMed] [Google Scholar]
- Petkova E, Quitkin FM, and McGrath PJ. et al. A method to quantify rater bias in antidepressant trials. Neuropsychopharmacology. 2000 22:559–565. [DOI] [PubMed] [Google Scholar]