Abstract
This study sought to evaluate the levels of mRNA expression and protein synthesis of MMP-13, cathepsin K, aggrecanase-1 (ADAMTS-4), aggrecanase-2 (ADAMTS-5) and 5-lipoxygenase (5-LOX) in cartilage in the experimental anterior cruciate ligament (ACL) dog model of osteoarthritis (OA), and to examine the effects of treatment with licofelone, a 5-lipoxygenase (LOX)/cyclooxygenase (COX) inhibitor, on the levels of these catabolic factors. Sectioning of the ACL of the right knee was performed in three experimental groups: group 1 received no active treatment (placebo group); and groups 2 and 3 received therapeutic concentrations of licofelone (2.5 or 5.0 mg/kg/day orally, respectively) for 8 weeks, beginning the day following surgery. A fourth group consisted of untreated dogs that were used as normal controls. Specimens of cartilage were selected from lesional areas of OA femoral condyles and tibial plateaus, and were processed for real-time quantitative PCR and immunohistochemical analyses. The levels of MMP-13, cathepsin K, ADAMTS-4, ADAMTS-5 and 5-LOX were found to be significantly increased in OA cartilage. Licofelone treatment decreased the levels of both mRNA expression and protein synthesis of the factors studied. Of note was the marked reduction in the level of 5-LOX gene expression. The effects of the drug were about the same at both tested dosages. In vivo treatment with therapeutic dosages of licofelone has been found to reduce the degradation of OA cartilage in experimental OA. This, coupled with the results of the present study, indicates that the effects of licofelone are mediated by the inhibition of the major cartilage catabolic pathways involved in the destruction of cartilage matrix macromolecules. Moreover, our findings also indicate the possible auto-regulation of 5-LOX gene expression by licofelone in OA cartilage.
Introduction
Along with the graying of the world's population, osteoarthritis (OA), the most common form of arthritis, is becoming an increasingly significant medical and financial burden. In this context, the clear need for a better understanding of the disease process has rendered undeniable the importance of finding drugs that can reduce or stop its progression.
Recent studies have revealed new and interesting information regarding the role played by eicosanoids in the pathophysiology of arthritic diseases, including OA [1-6]. For instance, leukotriene-B4 (LTB4) has proven to be an important regulating factor in the synthesis of IL-1β by OA synovium [6-8]. Both in vitro and in vivo studies have demonstrated that the excess production of IL-1β in OA tissue is a key factor in its destruction and in the progression of the disease itself [1,9]. The endogenous production of LTB4 in OA synovium is a crucial element in the upregulation of IL-1β synthesis in this tissue [8]. The synthesis of LTB4, and subsequently of IL-1β, can be significantly increased by non-steroidal anti-inflammatory drugs (NSAIDs) [10,11]. It has been hypothesized that this could be related to a 'shunt' of the arachidonic acid cascade from the cyclooxygenase (COX) to the lipoxygenase (LOX) pathway [2]. These findings could help explain how some NSAIDs accelerate the progression of clinical OA [12]. A recent study from our laboratory has demonstrated that, in in vivo experimental OA, licofelone, a drug that can inhibit both the COX and 5-LOX pathways, was capable of reducing the development of OA structural changes while simultaneously reducing the synthesis of LTB4 and IL-1β by the OA synovium [6]. These findings are in strong support of the in situ role played by LTB4 in the structural changes that occur in OA.
The progression of the structural changes that occur during the course of the disease is related to a number of complex pathways and mechanisms, among which the excess production of proteolytic enzymes that can degrade the cartilage matrix and soft tissues surrounding the joint is believed to be of particular importance [1]. The degradation of the OA cartilage matrix has been shown to be related to the excess synthesis of a large number of proteases and, more particularly, to that of the matrix metalloproteinases (MMPs) and thiol-dependent families. Among the MMPs, two collagenases, MMP-1 and MMP-13, have been the subject of extensive investigation and were found likely to be the primary enzymes involved in the breakdown of type II collagen in OA cartilage [13]. Cathepsin K, a thiol-dependent enzyme that works preferentially under acidic pH conditions, has also been demonstrated to be synthesized by OA chondrocytes and is likewise believed to play an important role in the breakdown of the OA cartilage collagen network [14] as well as the aggrecans, and thus likely involved in degrading the cartilage extracellular matrix. The mechanisms involved in the degradation of the aggrecans in OA cartilage have also been extensively explored and studied, which has led to the identification of a number of proteolytic enzymes that can specifically degrade aggrecans [15]. Comprehensive investigation has indicated that the MMPs, including MMP-13, aggrecanase-1 (a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4) and aggrecanase-2 (ADAMTS-5), are the proteolytic enzymes that seem the most likely to be involved in the degradation of aggrecans in OA cartilage [16,17].
The present study is an extension of previous ones that investigated the mechanisms by which licofelone, a dual inhibitor of 5-LOX and COXs, can reduce the development of experimental OA. This study focuses on the in situ effect of licofelone on the gene expression and protein synthesis of the major collagenolytic enzymes (MMP-13 and cathepsin K) and aggrecan-degrading proteases (ADAMTS-4 and ADAMTS-5) in OA cartilage using the experimental anterior cruciate ligament (ACL) model in dogs. The level of 5-LOX in OA cartilage as well as the drug treatment effects were also explored.
Materials and methods
Experimental groups
Specimens were obtained from different experimental groups, including some that had been included in previous studies [6,18]. Adult crossbred dogs of 2 to 3 years of age, weighing 20 to 25 kg each, were used in the study. The surgical sectioning of the ACL of the right knee was performed through a stab wound, as previously described [6]. Prior to surgery, the animals were intravenously anesthetized with pentobarbital sodium (25 mg/kg) and intubated. After surgery, the dogs were kept in animal care facilities for one week, and were then sent to a housing farm. Dogs were housed in a large pen in which they could exercise ad libitum under supervision to ensure that they were bearing weight on the operated knee. The University of Montreal Hospital Centre Research Ethics Committee at the Notre-Dame Hospital approved the protocol.
The dogs were separated into four experimental groups: group 1 (n = 7) consisted of OA operated dogs that received the placebo (encapsulated methylcellulose); group 2 (n = 7) of OA operated dogs that received encapsulated licofelone (2.5 mg/kg/day orally) (Merckle GmbH, Ulm, Germany); group 3 (n = 7) of OA operated dogs that received encapsulated licofelone (5.0 mg/kg/day orally); and group 4 (n = 6) of normal unoperated dogs (n = 6) that received no treatment. All treatments began the day after surgery. The dosages were selected on the basis of those given to patients for the treatment of symptomatic OA [6]. Licofelone was administered twice daily (at 8 a.m. and 4 p.m.) with food to a total dosage of 2.5 or 5.0 mg/kg. All dogs were sacrificed 8 weeks after surgery, including group 4, which was used as a control group. Morphologic changes in OA dogs have already been reported [6].
Specimen selection and preparation
As previously described [6,19], a full-thickness section of articular cartilage was removed from the lesional areas of the femoral condyles and tibial plateaus of the placebo-treated OA dogs, and from the OA dogs treated with 2.5 mg/kg/day or 5.0 mg/kg/day of licofelone. Specimens were also obtained from equivalent anatomical sites in the normal dogs. The specimens were embedded in paraffin and processed for immunohistological studies.
Histologic grading
Histologic evaluation was performed on sagittal sections of cartilage from the lesional areas of femoral condyles and tibial plateaus as described [6]. Specimens were fixed in TissuFix #2 (Chaptec Inc., Montreal, QC, Canada) for 24 h, then embedded in paraffin. Serial sections (5 μm) of paraffin-embedded specimens were stained with safranin-O. The severity of the OA lesions was graded on a scale of 0–14 by two independent observers using the histologic/histochemical scale of Mankin et al. [20]. The scale evaluates the loss of safranin-O staining (scale 0–4), cellular changes (scale 0–3), invasion of the tide mark by blood vessels (scale 0–1) and structural changes (scale 0–6, where 0 = normal cartilage structure and 6 = erosion of the cartilage down to the subchondral bone). Scoring was based on the most severe histologic changes within each cartilage section.
Immunohistochemistry
Cartilage specimens from femoral condyles and tibial plateaus (n = 5 per group) were processed for immunohistochemical analysis, as previously described [6,18,19]. Specimens were fixed in TissuFix #2 (Chaptec Inc.) for 24 h, then embedded in paraffin. Sections (5 μm) of paraffin-embedded specimens were placed on Superfrost Plus slides (Fisher Scientific, Nepean, ON, Canada), deparaffinized in xylene, rehydrated in a reverse-graded series of ethanol, and preincubated with chondroitinase ABC 0.25 units/ml (Sigma-Aldrich Canada, Oakville, ON, Canada) in PBS pH 8.0 for 60 minutes at 37°C. The specimens were subsequently washed in PBS, incubated in 0.3% Triton X-100/PBS for 30 minutes, and then placed in 3% hydrogen peroxide/PBS for 15 minutes. Slides were further incubated with a blocking serum (Vectastain ABC kit; Vector Laboratories Inc., Burlingame, CA, USA) for 60 minutes, after which they were blotted and then overlaid with the primary polyclonal goat antibody against collagenase-3 (MMP-13) (15 μg/ml; R&D Systems, Minneapolis, MN, USA); polyclonal goat antibody against cathepsin K (1 μg/ml; Santa Cruz, Santa Cruz, CA, USA); polyclonal rabbit antibody against ADAMTS-4 (RP1ADAMTS-4) or ADAMTS-5 (RP1ADAMTS-5) (10 μg/ml; Triple Point Biologics Inc., Forest Grove, OR, USA); or rabbit antiserum against 5-LOX (dilution 1:50; Cayman Chemical, Ann Arbor, MI, USA) for 18 h at 4°C in a humidified chamber. The antibodies against MMP-13, ADAMTS-4 and ADAMTS-5 recognized both the pro- and active forms of the enzyme. Each slide was washed three times in PBS (pH 7.4) and stained using the avidin-biotin complex method (Vectastain ABC kit), which entails incubation in the presence of the biotin-conjugated secondary antibody for 45 minutes at room temperature, followed by the addition of the avidin-biotin-peroxidase complex for 45 minutes. All incubations were carried out in a humidified chamber at room temperature and the colour was developed with 3,3'-diaminobenzidine (Vector Laboratories, Inc.) containing hydrogen peroxide. Slides were counterstained with eosin.
To determine the specificity of staining, different control procedures were employed according to the same experimental protocol: first, the use of adsorbed immune serum (1 h, 37°C) with a 20-fold excess of human recombinant for MMP-13 protein (R&D Systems) and for 5-LOX protein (Cayman Chemical), or human blocking peptide for cathepsin K (Santa Cruz) and ADAMTS-4 (Triple Point Biologics Inc.) (the peptide for ADAMTS-5 was not commercially available); second, omission of the primary antibody; and third, substitution of the primary antibody with an autologous pre-immune serum. The results of control experiments for MMP-13 and cathepsin K have already been published [18] and showed only background staining.
Immunohistomorphometric analysis
Several sections were made from each block of cartilage, and three non-consecutive representative sections from each specimen were processed for immunohistochemical analysis. Each section was examined under a light microscope (Leitz Orthoplan; Wild Leitz, St. Laurent, QC, Canada) and photographed with a CoolSNAP cf Photometrics camera (Roper Scientific, Rochester, NY, USA). The different antigen levels were quantified using a method modified from our previously published studies [6,21]. by determining the number (percentage) of chondrocytes that stained positive. Each section was divided into six macroscopic fields (three in superficial and three in the deep zones of cartilage) (×40; Leitz Diaplan). The superficial zone of cartilage corresponds to the superficial and to the upper intermediate layers. The deep zone of cartilage corresponds to the lower intermediate and the deep layers. The results from the six fields were averaged for each section. The total number of cells and the number of cells that stained positive for the specific antigen were determined. The results were expressed as the percentage of cells that stained positive for the antigen (cell score), with the maximum score being 100%. Each slide was subjected to a double-blind evaluation, which resulted in a variation of less than 5%. For the purposes of statistical analysis, the data obtained for each specimen (mean score of three sections) were considered independent.
Real-time quantitative PCR analysis
Extraction of total RNA from cartilage
Total RNA was extracted directly from the cartilage. The cartilage from the condyles and the plateaus (0.5–1.0 g) was pooled to allow for the processing of a sufficient amount of tissue for RNA extraction. Cartilage was suspended in a TRIzol buffer (Invitrogen; Life Technologies, Burlington, ON, Canada) and processed as previously described [22]. The purified RNA was quantified by spectrophotometry.
PCR analysis
The quantification of gene expression for MMP-13, cathepsin K, 5-LOX, ADAMTS-4, and ADAMTS-5 was determined by real-time quantitative PCR with the GeneAmp® 5700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using the Quantitect Sybr Green PCR kit (Qiagen Inc., Mississauga, ON, Canada), as previously described [23].
The oligonucleotides used for PCR studies are described in Table 1. The data were collected and processed with GeneAmp® 5700 SDS software and given as a threshold cycle (Ct). Plasmid DNA containing the target gene sequences was used to generate standard curves. A DNA standard curve for each gene was prepared and used in quantitative PCR reactions. The Ct was then converted to a number of molecules, and the value for each sample was calculated as the ratio of the number of molecules of the target gene to the number of molecules of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. The primer efficiencies for the test genes were the same as those for the GAPDH gene.
Table 1.
Primer design for quantitative RT-PCR analysis
| mRNA | Primersa |
| MMP-13 | Fw: 5'-TTGGTCAGATGTGACACCTC Rv: 5'-ATCGGGAAGCATAAAGTGGC |
| Cathepsin K | Fw: 5'-AGGTGGATGAAATCTCTCGG Rv: 5'-TTCTTGAGTTGGCCCTCCAG |
| 5-LOX | Fw: 5'-TGCGTTCCAGTGACTTCCAC Rv: 5'-CTCTGCACCATCTGCACGTG |
| ADAMTS-4 | Fw: 5'-TACTACTATGTGCTGGAGCC Rv: 5'-AGTGACCACATTGTTGTATCC |
| ADAMTS-5 | Fw: 5'-GGCATCATTCATGTGACAC Rv: 5'-GCATCGTAGGTCTGTCCTG |
| GAPDH | Fw: 5'-AGGCTGTGGGCAAGGTCATC Rv: 5'-AAGGTGGAAGAGTGGGTGTC |
aFw, forward; Rv, reverse. GAPDH, glyseraldehyde-3-phosphate dehydrogenase; MMP, matrix metalloproteinase.
Statistical analysis
Unless otherwise specified, values are expressed as the median with the range in parentheses. Statistical analysis was performed using the Mann-Whitney U test. Correlations between the histologic grade and the cell score were analyzed using a linear regression test. Statistical analysis was performed using the parametric (Pearson) linear correlation test. P-values ≤ 0.05 were considered significant.
Results
Histologic analysis
Cartilage from normal controls had normal microscopic appearance. Specimens from the OA group presented typical OA changes with a Mankin score of 5.1 (3–11) and a safranin-O score of 1 (0–4). Specimens from licofelone-treated groups had a Mankin score of 3.5 (0–10) and a safranin-O score of 0.4 (0–3) with the 2.5 mg dosage and a Mankin score of 4.2 (1.5–6.5) and a safranin-O score of 0.3 (0–1.5) with the 5.0 mg dosage.
MMP-13 gene expression and protein synthesis
PCR analysis found a marked and significant increase in the expression of mRNA for MMP-13 in OA cartilage compared to normal (Fig. 1). Immunohistochemical analysis revealed that the increased synthesis of MMP-13 was mainly found throughout the tissue, as previously reported [18]; the controls were negative (data not shown). A good correlation exists between the mRNA and protein levels. At the two dosages tested, the licofelone treatment significantly reduced the levels of both MMP-13 mRNA expression and the protein to an approximately similar extent.
Figure 1.
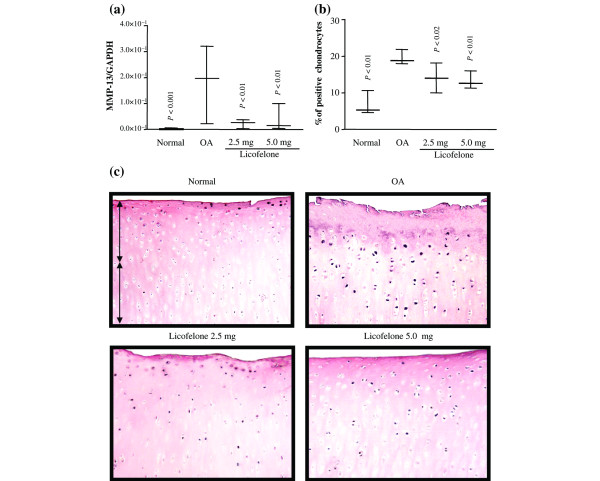
MMP-13 gene expression and protein synthesis. (a) mRNA levels, as determined by real-time quantitative PCR analysis as described in Materials and methods. (b) Morphometric analysis of MMP-13 immunostaining. (a, b) Data are expressed as median and range and are presented as box plots, where the boxes represent the 1st and 3rd quartiles, the line within the box represents the median, and the lines outside the box represent the spread of values. P-values were compared to the placebo group (OA) using the Mann-Whitney U test. (c) Representative MMP-13 immunohistochemical sections of tibial plateaus. Superficial (superfical and upper intermediate layers) and deep (lower intermediate and deep layers) zones of cartilage are indicated on the picture with arrows. No specific staining was detected in the OA cartilage with immunoabsorbed serum (data not shown) (original magnification × 250). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MMP, matrix metalloproteinase.
Cathepsin K gene expression and protein synthesis
The levels of both the gene expression and the protein of cathepsin K were significantly increased in OA cartilage, compared to normal cartilage (Fig. 2). These two levels were also well correlated. Immunohistochemical staining showed that the enzyme was found to be preferentially located in the superficial zone of the OA cartilage, as previously reported [18]. The controls were found to be negative (data not shown). Treatment with licofelone at both concentrations reduced the levels of mRNA expression and protein synthesis of cathepsin K. The effect was similar at both of the tested dosages for gene expression and more pronounced at the highest dosage tested for the level of the enzyme per se.
Figure 2.
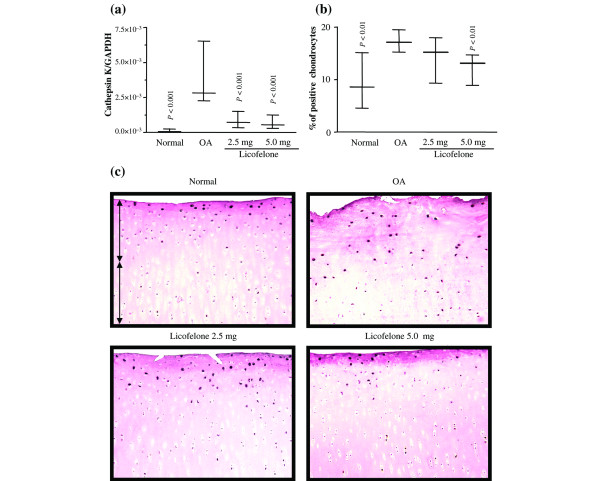
Cathepsin K gene expression and protein synthesis. (a) mRNA levels, as determined by real-time quantitative PCR analysis as described in Materials and methods. (b) Morphometric analysis of cathepsin K immunostaining. (a, b) Data are expressed as median and range and are presented as box plots, where the boxes represent the 1st and 3rd quartiles, the line within the box represents the median, and the lines outside the box represent the spread of values. P-values were compared to the placebo group (OA) using the Mann-Whitney U test. (c) Representative cathepsin K immunohistochemical sections of tibial plateaus. Superficial (superfical and upper intermediate layers) and deep (lower intermediate and deep layers) zones of cartilage are indicated on the picture with arrows. No specific staining was detected in the OA cartilage with immunoabsorbed serum (data not shown) (original magnification × 250). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
ADAMTS-4 and ADAMTS-5 gene expression and protein synthesis
The level of gene expression of ADAMTS-5 in OA cartilage determined by PCR analysis was highly variable and, although sometimes higher than that in normal cartilage, the differences did not reach statistical significance (Fig. 3). The results were somewhat similar with regards to the immunohistochemical analysis. The staining showed that the enzyme was in the chondrocytes mainly located in the superficial zone; some matrix staining was also observed. The protein level of ADAMTS-5 in OA cartilage was found to be significantly higher than normal; the controls were found to be negative and showed only background staining. Treatment with licofelone had little effect on the level of its gene expression or on the level of protein. In contrast, the level of expression of mRNA for ADAMTS-4 was found to be significantly increased in OA cartilage compared to normal (Fig. 4). This was also reflected in the immunohistochemical analysis, in which an increased level of the enzyme was found more particularly in the superficial layers. The level of the enzyme was found to be significantly decreased by licofelone treatment at both the tested dosages.
Figure 3.
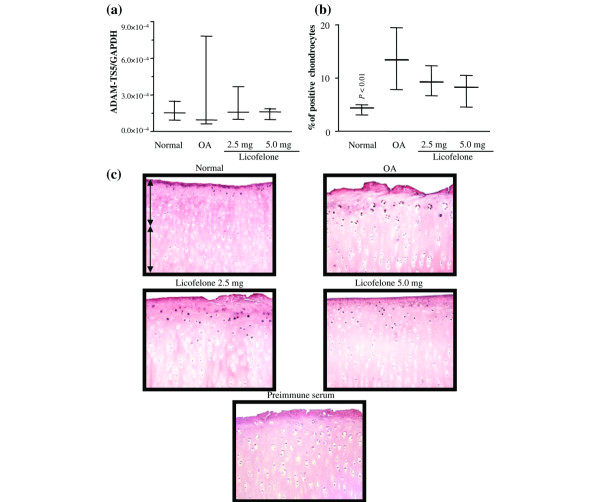
ADAMTS-5 gene expression and protein synthesis. (a) mRNA levels, as determined by real-time quantitative PCR analysis as described in Materials and methods. (b) Morphometric analysis of ADAMTS-5 immunostaining. (a, b) Data are expressed as median and range and are presented as box plots, where the boxes represent the 1st and 3rd quartiles, the line within the box represents the median, and the lines outside the box represent the spread of values. P-values were compared to the placebo group (OA) using the Mann-Whitney U test. (c) Representative ADAMTS-5 immunohistochemical sections of tibial plateaus. Superficial (superfical and upper intermediate layers) and deep (lower intermediate and deep layers) zones of cartilage are indicated on the picture with arrows. No specific staining was detected in the OA cartilage with immunoabsorbed serum (data not shown) (original magnification × 250). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 4.
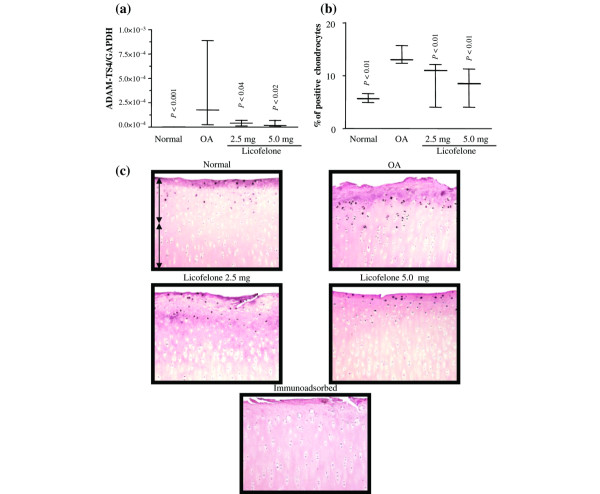
ADAMTS-4 gene expression and protein synthesis. (a) mRNA levels, as determined by real-time quantitative PCR analysis as described in Materials and methods. (b) Morphometric analysis of ADAMTS-4 immunostaining. (a, b) Data are expressed as median and range and are presented as box plots, where the boxes represent the 1st and 3rd quartiles, the line within the box represents the median, and the lines outside the box represent the spread of values. P-values were compared to the placebo group (OA) using the Mann-Whitney U test. (c) Representative ADAMTS-4 immunohistochemical sections of tibial plateaus. Superficial (superfical and upper intermediate layers) and deep (lower intermediate and deep layers) zones of cartilage are indicated on the picture with arrows. No specific staining was detected in the OA cartilage with immunoabsorbed serum (data not shown) (original magnification × 250). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
5-LOX gene expression and protein synthesis
Although the level of gene expression of 5-LOX in normal cartilage was very low, as demonstrated by quantitative PCR analysis (Fig. 5), it showed a marked and significant increase in OA cartilage. There was a good correlation between these results and those from immunohistochemistry, which also showed a marked and significant increase in the level of the enzyme that was mainly located in the superficial zone of OA cartilage. The controls were negative. At both of the tested dosages, licofelone treatment significantly reduced the level of gene expression and protein synthesis of the enzyme to a similar extent. There was also a correlation between the reduction in the mRNA and protein levels.
Figure 5.
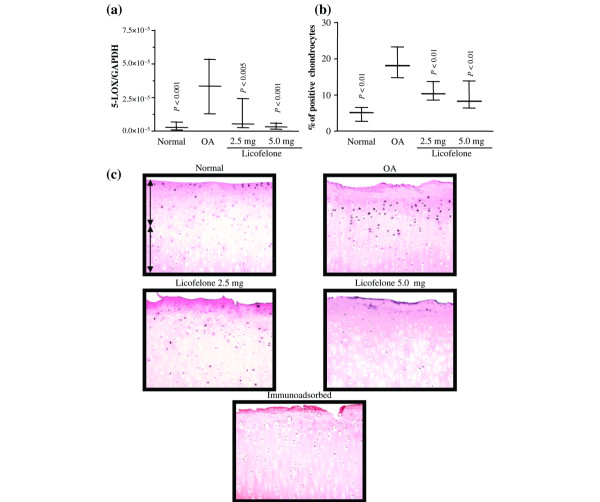
5-LOX gene expression and protein synthesis. (a) mRNA levels, as determined by real-time quantitative PCR analysis as described in Materials and methods. (b) Morphometric analysis of 5-LOX immunostaining. (a, b) Data are expressed as median and range and are presented as box plots, where the boxes represent the 1st and 3rd quartiles, the line within the box represents the median, and the lines outside the box represent the spread of values. P-values were compared to the placebo group (OA) using the Mann-Whitney U test. (c) Representative 5-LOX immunohistochemical sections of tibial plateaus. Superficial (superfical and upper intermediate layers) and deep (lower intermediate and deep layers) zones of cartilage are indicated on the picture with arrows. No specific staining was detected in the OA cartilage with immunoabsorbed serum (data not shown) (original magnification × 250). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Correlation analysis: Mankin score, safranin-O and cell score
In specimens from OA dogs, a positive and significant correlation was found between the Mankin score or the safranin-O staining score and the chondrocyte cell score for ADAMTS-4 (r = 0.50, p = 0.005 for the Mankin score, and r = 0.59, p = 0.001 with safranin-O) and ADAMTS-5 (r = 0.52, p = 0.005 for the Mankin score, and r = 0.47, p = 0.019 with safranin-O) staining. A positive and significant correlation was also found between the Mankin score or the safranin-O staining score and the chondrocyte cell score for 5-LOX (r = 0.45, p = 0.01 for the Mankin score, and r = 0.44, p = 0.02 with safranin-O). No significant correlation was found between the histologic score and MMP-13 or cathepsin-K cell score.
Discussion
This study brings to light some new and interesting information about the mechanisms by which licofelone could reduce the progression of the structural changes caused by OA. A previous study demonstrated that licofelone, a dual inhibitor of both COXs and 5-LOX, had a protective effect on the structural changes in experimental dog OA [6]; the results of this study indicated that the drug works through the inhibition of the synthesis of IL-1β and MMP-1. The present study aimed to expand our knowledge about the effect of licofelone on other major factors involved in the in situ degradation of OA cartilage macromolecules, including aggrecans and type II collagen. Moreover, we sought to gain new insight into the effect of the drug on the regulation of 5-LOX, one of the main enzymes involved in the synthesis of LTB4 by chondrocytes [4,24].
The results of the study demonstrate that licofelone was very effective at reducing the synthesis of cathepsin K and MMP-13, two highly potent enzymes involved in the in situ degradation of type II collagen in OA cartilage. The role of cathepsin K in OA pathophysiology has been previously well documented [14,18,25]. This enzyme has been found not only to be involved in hyalin cartilage degradation, but also likely to be responsible for the resorption of the calcified cartilage and subchondral bone in the early phase of the disease [18]. Licofelone treatment was shown to reduce the level of synthesis of cathepsin K in both of these tissues in experimental dog OA, which may at least partially explain the effects of the drug. The exact mechanism(s) by which licofelone reduces the mRNA expression level of cathepsin K is not fully understood; it is under exploration. Because the synthesis of cathepsin K has been demonstrated to be upregulated by proinflammatory cytokines such as IL-1β and tumor necrosis factor-α [26], however, the capacity of licofelone to inhibit the synthesis of IL-1β [6-8] may explain, at least in part, the effect of the drug on the synthesis of cathepsin K.
Licofelone treatment was also shown in vitro to reduce the mRNA expression and protein synthesis of MMP-13 in OA chondrocytes [18,23]. A previous study demonstrated that it also reduces in situ the level of this enzyme in OA calcified cartilage and subchondral bone [18], which might explain its effect of inhibiting the remodeling of these tissues. The exact mechanisms by which licofelone could reduce MMP-13 expression in OA chondrocytes have been extensively explored, giving rise to a number of interesting hypotheses. The inhibitory effect of licofelone on the synthesis of IL-1β by the OA synovium is likely to be an important contributing factor [6-8]. In addition, a recent study from our laboratory has demonstrated that the drug's most likely mode of action on MMP synthesis is the inhibition of major intercellular signaling pathways. Experiments have shown that licofelone can inhibit the mRNA expression of IL-1β induced MMP-13 in OA chondrocytes, and that this effect is mediated by the selective inhibition of the activation of the p38 pathway and the downstream transcription factor cyclic-AMP responsive element binding protein (CREB) [23]. This effect is of prime importance for better understanding the mechanisms by which this drug can exert its positive effect on the progression of OA structural changes. MMP-13, or collagenase-3, can cleave both type I and type II collagen; however, the enzyme has a higher degrading activity on type II collagen and can also degrade the aggrecan core protein. Therefore, the inhibition of MMP-13 synthesis in OA chondrocytes by licofelone could explain the drug's positive effect of protecting the cartilage matrix macromolecules that contain predominantly type II collagen and aggrecan. Similarly, the inhibition of MMP-13 synthesis by bone cells and osteoclasts could also exert a positive effect by reducing the extent of the degradation of type I collagen in the subchondral bone matrix [18].
Aggrecans are large aggregating proteoglycans that fill the interstices of the collagen meshwork and give the cartilage its ability to resist compressive loads. MMPs are considered among the main enzymes involved in the degradation of aggrecans in cartilage [15-17,27,28]. Many MMPs that can degrade aggrecans have been demonstrated to be overexpressed in OA cartilage. Of most interest is the recent discovery of a group of enzymes of the ADAMTS family named aggrecanases, which cleave the aggrecan core protein and are believed to play a determinant role in arthritis in the breakdown of aggrecan [15]. Three aggrecanases have thus far been identified: ADAMTS-1, ADAMTS-4, and ADAMTS-5. ADAMTS-1 and ADAMTS-5 are two enzymes that are constitutively expressed in both normal and OA cartilage. There are conflicting reports about the factors regulating the synthesis of these enzymes [15,29,30]. Moreover, the synthesis of these two enzymes in chondrocytes does appear to be variably regulated by IL-1β; in fact, this cytokine might, under certain conditions, downregulate ADAMTS-1 synthesis [31]. The results of a number of studies indicate that the regulatory mechanisms of ADAMTS-4 and ADAMTS-5 gene expression and protein synthesis are complex and may vary based on species and culture conditions [15]. The present study found the level of expression of ADAMTS-5 to be detectable in both normal and OA cartilage, with a somewhat higher, yet variable, level in OA cartilage; however, its level of synthesis in OA cartilage was demonstrated to be significantly increased. Moreover, a diffusion of the enzyme in the OA matrix as shown by immunostaining is in support of this enzyme being involved in situ in cartilage matrix macromolecule degradation. Additional support for this hypothesis is also provided by the positive correlation between the immunohistological score of ADAMTS-5 and safranin-O staining in OA cartilage. Dogs treated with licofelone showed a decrease in this level that did not, however, reach statistical significance. Nevertheless, based on recent studies demonstrating the predominant role of ADAMTS-5 in OA cartilage degradation [32,33], it is likely that the latter finding has real significance. Our data are in line with previous studies that demonstrated great variability in the response of chondrocytes to the synthesis of ADAMTS-5 upon stimulation by cytokines and growth factors under actual conditions [15]. A regulation of the enzyme synthesis and activity at the post-transcriptional and/or post-translational level is possible; moreover, it is obvious that the synthesis of ADAMTS-5 in situ is likely the result of the combination of stimulation by multiple factors. These, in addition to IL-1β, may include such factors as oncostatin M and transforming growth factor-β, which have been demonstrated to strongly upregulate the genetic expression of aggrecanase in chondrocytes [34] and synovial fibroblasts [35].
Our data on ADAMTS-4 are in line with previous reports demonstrating that the level of this enzyme is very low in normal cartilage but that mRNA expression/protein synthesis of the enzyme is upregulated in OA cartilage [16,17,28,29,36], which supports its likely implication in the pathophysiology of the disease [15]. Similar to ADAMTS-5, our data on ADAMTS-4 demonstrate the presence of matrix staining and a positive correlation between the enzyme level and safranin-O staining, also strongly supporting the hypothesis of its role in OA. Licofelone treatment significantly inhibited the synthesis of the enzyme at the transcriptional level in a dose-dependent manner. The inhibition of both aggrecanases by licofelone also provides strong support for the hypothesis that the drug exerts its protective effect through the inhibition of major pathways involved in OA structural changes. The inhibition of aggrecanases could very well contribute to the net decrease in the loss of cartilage matrix and exert a positive effect on cartilage degradation. This result was confirmed and supported by the positive and significant correlation found with both safranin-O staining and the Mankin score.
The level of 5-LOX was found to be significantly increased in OA compared to normal cartilage. Previous studies have demonstrated that LTB4 is a potent factor responsible for upregulating the synthesis of IL-1β [7,8,10,24]. Moreover, both in OA chondrocytes and synovial membranes, the upregulation of IL-1β synthesis by LTB4 was responsible for inducing the synthesis of MMPs [8,10,24]. Therefore, it becomes obvious that the increased level of 5-LOX with the subsequent upregulation in IL-1β production in OA tissues could very well play a determining role in the degradation of OA cartilage, first by its local action on chondrocytes and the synthesis of catabolic factors and, second, by being an important upregulating factor in the synthesis of IL-1β by OA synovium. This concept is also supported by the positive correlation found between the 5-LOX cell score and both safranin-O staining and the Mankin score. Therefore, the downregulating effects of licofelone on the level of mRNA expression/protein synthesis of 5-LOX could provide an explanation of how the drug can reduce the synthesis of MMP-13 and ADAMTS-4 and ADAMTS-5 in cartilage [24] as well as the synthesis of IL-1β in synovium. These results also support the possible role of LTB4 itself in the autocrine regulation of 5-LOX gene expression.
Conclusion
This study provides evidence that licofelone treatment in the OA experimental dog model markedly reduces the mRNA expression/protein synthesis of key enzymes involved in the destruction of major cartilage matrix macromolecules, such as type II collagen and aggrecans. These findings provide additional information about the possible mechanisms of action of this drug on OA structural changes.
Abbreviations
ABC = avidin-biotin complex; ACL = anterior cruciate ligament; ADAMTS = a disintegrin and metalloproteinase with thrombospondin motifs; COX = cyclooxygenase; Ct = threshold cycle; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; IL = interleukin; LOX = lipoxygenase; LTB4 = leukotriene-B4; MMP = matrix metalloproteinase; NSAID = non-steroidal anti-inflammatory drug; OA = osteoarthritis; PBS = phosphate buffered saline.
Competing interests
JPP received support from Merckle GmbH, who manufactures Licofelone, and SL is a consultant for Merckle GmbH. JPP, SL, and JMP are co-authors of DMOAD patent applicate of Licofelone with Merckle GmbH.
Authors' contributions
JPP, CB, JB, PR, SL, DL and JMP contributed to the study design. JPP, CB, MB, JB, FM, CG and JMP performed the acquisition of data. JPP, CB, FM, PR, DL and JMP analyzed and interpreted the data. JPP, CB, PR, DL and JMP prepared the manuscript. JPP, CB, JB and JMP performed the statistical analysis. All authors read and approved the final manuscript. JPP and CB contributed equally to this manuscript.
Acknowledgments
Acknowledgements
The authors extend their appreciation to Merckle GmbH, Fonds de la recherche en santé du Québec, and Groupe de recherche des maladies rhumatismales du Québec, grants from which supported part of this work, as well as Martha Evans and Santa Fiori for their assistance in manuscript preparation.
References
- Martel-Pelletier J, Lajeunesse D, Pelletier JP. Etiopathogenesis of osteoarthritis. In: Koopman WJ, editor. Arthritis and Allied Conditions A Textbook of Rheumatology. Baltimore: Lippincott, Williams & Wilkins; 2004. pp. 2199–2226. [Google Scholar]
- Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62:501–509. doi: 10.1136/ard.62.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prete PE, Gurakar-Osborne A. The contribution of synovial fluid lipoproteins to the chronic synovitis of rheumatoid arthritis. Prostaglandins. 1997;54:689–698. doi: 10.1016/S0090-6980(97)00147-0. [DOI] [PubMed] [Google Scholar]
- Hansen ES, Fogh K, Hjortdal VE, Henriksen TB, Noer I, Ewald H, Herlin T, Kragballe K, Bunger C. Synovitis reduced by inhibition of leukotriene B4. Carrageenan-induced gonarthritis studied in dogs. Acta Orthop Scand. 1990;61:207–212. doi: 10.3109/17453679008993502. [DOI] [PubMed] [Google Scholar]
- Gay RE, Neidhart M, Pataky F, Tries S, Laufer S, Gay S. Dual inhibition of 5-lipoxygenase and cyclooxygenases 1 and 2 by ML3000 reduces joint destruction in adjuvant arthritis. J Rheumatol. 2001;28:2060–2065. [PubMed] [Google Scholar]
- Jovanovic DV, Fernandes JC, Martel-Pelletier J, Jolicoeur FC, Reboul P, Laufer S, Tries S, Pelletier JP. The in vivo dual inhibition of cyclooxygenase and lipoxygenase by ML-3000 reduces the progression of experimental osteoarthritis. Suppression of collagenase-1 and interleukin-1beta synthesis. Arthritis Rheum. 2001;44:2320–2330. doi: 10.1002/1529-0131(200110)44:10<2320::AID-ART394>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Rainsford KD, Ying C, Smith F. Effects of 5-lipoxygenase inhibitors on interleukin production by human synovial tissues in organ culture: comparison with interleukin-1-synthesis inhibitors. J Pharm Pharmacol. 1996;48:46–52. doi: 10.1111/j.2042-7158.1996.tb05875.x. [DOI] [PubMed] [Google Scholar]
- He W, Pelletier JP, Martel-Pelletier J, Laufer S, Di Battista JA. The synthesis of interleukin-1beta, tumour necrosis factor-a and interstitial collagenase (MMP-1) is eicosanoid dependent in human OA synovial membrane explants: Interactions with anti-inflammatory cytokines. J Rheumatol. 2002;29:546–553. [PubMed] [Google Scholar]
- Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Marcouiller P, Pelletier JP, Guévremont M, Martel-Pelletier J, Ranger P, Laufer S, Reboul P. Leukotriene and prostaglandin synthesis pathways in osteoarthritic synovial membranes: regulating factors for IL-1beta synthesis. J Rheumatol. 2005;32:704–712. [PubMed] [Google Scholar]
- Parades Y, Massicotte F, Pelletier JP, Martel-Pelletier J, Laufer S, Lajeunesse D. Study of role of leukotriene B4 in abnormal function of human subchondral osteoarthritis osteoblasts. Effects of cyclooxygenase and/or 5-lipoxygenase inhibition. Arthritis Rheum. 2002;46:1804–1812. doi: 10.1002/art.10357. [DOI] [PubMed] [Google Scholar]
- Huskisson EC, Berry H, Gishen P, Jubb RW, Whitehead J. Effects of antiinflammatory drugs on the progression of osteoarthritis of the knee. LINK Study Group. Longitudinal investigation of nonsteroidal antiinflammatory drugs in knee osteoarthritis. J Rheumatol. 1995;22:1941–1946. [PubMed] [Google Scholar]
- Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. In: Woolf AD, editor. Baillière's Best Practice and Research Clinical Rheumatology. East Sussex, United Kingdom: Baillière Tindall; 2001. pp. 805–829. [DOI] [PubMed] [Google Scholar]
- Konttinen YT, Mandelin J, Li TF, Salo J, Lassus J, Liljestrom M, Hukkanen M, Takagi M, Virtanen I, Santavirta S. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 2002;46:953–960. doi: 10.1002/art.10185. [DOI] [PubMed] [Google Scholar]
- Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5:94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, Singer II, Donatelli SA, Weidner JR, Williams HR, et al. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100:93–106. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MG, Cox L, Chong L, Suri N, Cover P, Bayliss MT, Mason RM. Matrix metalloproteinases and aggrecanases cleave aggrecan in different zones of normal cartilage but colocalize in the development of osteoarthritic lesions in STR/ort mice. Arthritis Rheum. 2001;44:1455–1465. doi: 10.1002/1529-0131(200106)44:6<1455::AID-ART241>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Pelletier JP, Boileau C, Brunet J, Boily M, Lajeunesse D, Reboul P, Laufer S, Martel-Pelletier J. The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone. 2004;34:527–538. doi: 10.1016/j.bone.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Fernandes JC, Caron JP, Martel-Pelletier J, Jovanovic D, Mineau F, Tardif G, Otterness IG, Pelletier JP. Effects of tenidap on the progression of osteoarthritic lesions in a canine experimental model: Suppression of metalloprotease and IL-1 activity. Arthritis Rheum. 1997;40:284–294. doi: 10.1002/art.1780400213. [DOI] [PubMed] [Google Scholar]
- Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- Moldovan F, Pelletier JP, Hambor J, Cloutier JM, Martel-Pelletier J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: In vitro mimicking effect by transforming growth factor beta. Arthritis Rheum. 1997;40:1653–1661. doi: 10.1002/art.1780400915. [DOI] [PubMed] [Google Scholar]
- Boileau C, Martel-Pelletier J, Brunet J, Tardif G, Schrier D, Flory C, El-Kattan A, Boily M, Pelletier JP. Oral treatment with PD-0200347, an α2δ ligand, reduces the development of experimental osteoarthritis by inhibiting metalloproteinases and inducible nitric oxide synthase gene expression and synthesis in cartilage chondrocytes. Arthritis Rheum. 2005;52:488–500. doi: 10.1002/art.20809. [DOI] [PubMed] [Google Scholar]
- Boileau C, Pelletier JP, Tardif G, Fahmi H, Laufer S, Lavigne M, Martel-Pelletier J. The regulation of human MMP-13 by licofelone, an inhibitor of cyclooxygenases and 5-lipoxygenase, in human osteoarthritic chondrocytes is mediated by the inhibition of the p38 MAP kinase signaling pathway. Ann Rheum Dis. 2005;64:891–898. doi: 10.1136/ard.2004.026906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J, Mineau F, Fahmi H, Laufer S, Reboul P, Boileau C, Lavigne M, Pelletier J-P. Regulation of the expression of FLAP/5-LOX and synthesis of LTB4 in osteoarthritic chondrocytes: role of the TGF-β and eicosanoids. Arthritis Rheum. 2004;50:3925–3933. doi: 10.1002/art.20632. [DOI] [PubMed] [Google Scholar]
- Dodds RA, Connor JR, Drake FH, Gowen M. Expression of cathepsin K messenger RNA in giant cells and their precursors in human osteoarthritic synovial tissues. Arthritis Rheum. 1999;42:1588–1593. doi: 10.1002/1529-0131(199908)42:8<1588::AID-ANR4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Huet G, Flipo RM, Colin C, Janin A, Hemon B, Collyn-d'Hooghe M, Lafyatis R, Duquesnoy B, Degand P. Stimulation of the secretion of latent cysteine proteinase activity by tumor necrosis factor alpha and interleukin-1. Arthritis Rheum. 1993;36:772–780. doi: 10.1002/art.1780360606. [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Neame PJ, Sandy JD. The structure of aggrecan fragments in human synovial fluid: Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993;36:1214–1222. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277:22201–22208. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, Flannery CR, Kanki K, Wang E, Peluso D, et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50:2547–2558. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- Wachsmuth L, Bau B, Fan Z, Pecht A, Gerwin N, Aigner T. ADAMTS-1, a gene product of articular chondrocytes in vivo and in vitro, is downregulated by interleukin 1beta. J Rheumatol. 2004;31:315–320. [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Koshy PJ, Lundy CJ, Rowan AD, Porter S, Edwards DR, Hogan A, Clark IM, Cawston TE. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum. 2002;46:961–967. doi: 10.1002/art.10212. [DOI] [PubMed] [Google Scholar]
- Yamanishi Y, Boyle DL, Clark M, Maki RA, Tortorella MD, Arner EC, Firestein GS. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J Immunol. 2002;168:1405–1412. doi: 10.4049/jimmunol.168.3.1405. [DOI] [PubMed] [Google Scholar]
- Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]


