Abstract
To study the role of Rad50 in the DNA damage response, we cloned and deleted the Schizosaccharo myces pombe RAD50 homologue. The deletion is sensitive to a range of DNA-damaging agents and shows dynamic epistatic interactions with other recombin ation–repair genes. We show that Rad50 is necessary for recombinational repair of the DNA lesion at the mating-type locus and that rad50Δ shows slow DNA replication. We also find that Rad50 is not required for slowing down S phase in response to hydroxy urea or methyl methanesulfonate (MMS) treatment. Interestingly, in rad50Δ cells, the recombination frequency between two homologous chromosomes is increased at the expense of sister chromatid recombination. We propose that Rad50, an SMC-like protein, promotes the use of the sister chromatid as the template for homologous recombinational repair. In support of this, we found that Rad50 functions in the same pathway for the repair of MMS-induced damage as Rad21, the homologue of the Saccharomyces cerevisiae Scc1 cohesin protein. We speculate that Rad50 interacts with the cohesin complex during S phase to assist repair and possibly re-initiation of replication after replication fork collapse.
Keywords: DNA replication/Rad21/Rad50/recombinational DNA repair/sister chromatid cohesion
Introduction
During the cell cycle, DNA is likely to be damaged as a result of failures in DNA replication or by exposure to damage-inducing agents. In mammalian cells, different repair pathways have evolved to repair different types of DNA damage, and DNA repair defects can predispose organisms to cancer. One of the most genotoxic lesions is a DNA double strand break (DSB). A DSB can be repaired by homologous recombination (HR) or by DNA end joining. In the process of HR, a DSB is repaired by copying the missing information from the sister chromatid or homologous chromosome, resulting in the exact restoration of the DNA (for a review see Pâques and Haber, 1999). In the non-homologous DNA end joining (NHEJ) pathway, where the two DNA ends are connected without the need for longer stretches of homology, repair of a DSB is error prone and frequently leads to small deletions (for a review see Lewis and Resnick, 2000).
The RAD52 epistasis group of Saccharomyces cerevisiae consists of several genes which are involved in recombinational DSB repair. Mutants of these genes are sensitive to ionizing radiation and several other DNA-damaging agents, and have a role in mitotic and meiotic recombination. Based on the phenotypes of the mutated genes, two subgroups can be distinguished within the RAD52 epistasis group. The first group consists of the RAD51, -52, -54, -55 and -57 genes. They are involved in both mitotic and meiotic recombination; mutants of these genes are deficient in DSB repair and mating-type switching. They probably act in a protein complex and are thought to be responsible for DNA strand transfer during HR. Homologues of these genes have been found throughout the eukaryotes, suggesting a conserved function of these proteins (for a review see Petrini et al., 1997). The second group consists of S.cerevisiae RAD50, XRS2 and MRE11. When these genes are mutated, cells are deficient in DSB repair and meiotic recombination, but proficient in mitotic recombination. The three proteins are thought to act in a protein complex (reviewed in Petrini, 1999). In S.cerevisiae, Rad50, Mre11 and Xrs2 also have a role in NHEJ, together with Yku70, Yku80, Lig4 and others (reviewed in Lewis and Resnick, 2000). However, in Schizosaccharomyces pombe, Rad50 is not required for NHEJ (Manolis et al., 2001). In S.cerevisiae, Rad50 and interacting proteins have also been reported to function in telomere length maintenance (Boulton and Jackson, 1998), mating-type switching (Ivanov et al., 1994), replication or post-replicative repair (Merrill and Holm, 1998), chromatin configuration at meiotic DSB sites (Ohta et al., 1998) and the S-phase checkpoint response (Kironmai and Muniyappa, 1997).
Homologues of Rad50 and its interacting proteins have been found in higher eukaryotes, suggesting a conserved function of the protein complex. In human, the RAD50 gene is located in a region containing an acute myeloid leukaemia tumour suppressor gene, and hRad50 interacts with hMre11 (Dolganov et al., 1996). It was shown that a mutation in p95/Nbs1, which interacts with the human Mre11–Rad50 complex and is probably an Xrs2 homologue, is responsible for the Nijmegen breakage syndrome, which results in increased cancer incidence in human and failure to induce some DNA damage checkpoints (Carney et al., 1998; Varon et al., 1998; Petrini, 2000). Mutations in MRE11 have also been implicated in cancer predisposition (reviewed in Petrini, 2000). These findings significantly increase the importance of understanding the role of the Rad50–Mre11–Nbs1 protein complex. Disruptions of Mre11 (Xiao and Weaver, 1997) and Rad50 (Luo et al., 1999) in mouse are not viable, complicating the analysis of these genes in higher eukaryotes. At the moment, it is unknown what the precise role of Rad50 and its interacting proteins is in different processes which are essential for the stability, recombination and reliable replication of DNA. It is unknown how DSBs are detected, how it is decided which DSB repair pathway is utilized for repair and how the repair is coordinated with the checkpoint-dependent cell cycle delay.
To study the role of the Rad50 protein in the repair of DNA damage in S.pombe, we cloned and deleted the S.pombe RAD50 homologue. Our data suggest that Rad50 is involved in mating-type switching and DNA replication and has a role in choosing the sister chromatids for recombinational repair. Based on our genetic data, we speculate that, during S phase, the Rad50 complex interacts with the Rad21 cohesin complex to assist repair and possibly the re-initiation of collapsed replication forks.
Results
Sequence of the S.pombe RAD50 homologue
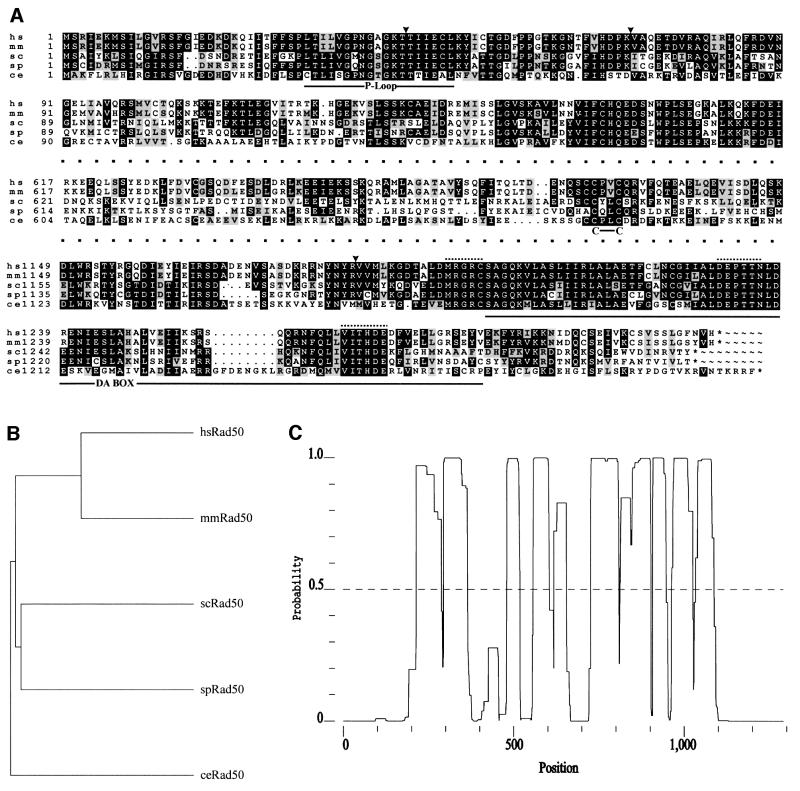
Based on sequence information available in the database of S.cerevisiae, Caenorhabditis elegans and human RAD50 homologues, we designed degenerate primers and were able to clone and sequence the S.pombe RAD50 homologue which we designated rad50+ (the gene has also been sequenced recently in the S.pombe genome sequencing project, and is located on cosmids SPAC1556.01C and SPAP4C9.01C). Sequence analysis of the rad50+ gene revealed an open reading frame (ORF) encoding 1291 amino acids. This ORF is interrupted by three putative introns (49, 43 and 37 bp, respectively), all containing 5′ and 3′ splice sites as well as the branch site as published by Prabhala et al. (1992). The putative introns are all located in regions with high amino acid sequence conservation (see Figure 1A) and would cause a frameshift if not excised, making it very likely that these introns are spliced as predicted. An alignment of the amino acid sequences of rad50+ and the RAD50 homologues of human, mouse, S.cerevisiae and C.elegans shows a very strong conservation at the N- and C-termini, where the ATP/GTP nucleotide-binding motif (Walker A; P-loop nucleotide-binding motif) and the DA box (Walker B, second half motif believed to be associated with the P-loop) are located (Walker et al., 1982; Figure 1A). In the middle section, the degree of conservation is low, and long stretches with a high probability of coiled-coil formation are found (Figure 1C). In the middle of the protein, a putative ‘half zinc finger’ is found which is remarkably conserved throughout the different homologues (Figure 1A). It was postulated that this site could co-ordinate binding of a zinc atom mediated by dimerization of the protein (Sharples and Leach, 1995). Phylogenetic ally, Rad50 is closest to its S.cerevisiae counterpart (Figure 1B), showing an overall identity of 35.0% (as determined with BestFit). Surprisingly, the conservation between the C.elegans Rad50 homologue and the mouse homologue (28.7% identity) is lower than between the S.pombe and mouse homologue (30.2% identity).
Fig. 1. (A) Alignment of the human (hs), mouse (mm), S.cerevisiae (sc), S.pombe (sp) and C.elegans (ce) RAD50 proteins. Putative sequence motifs are indicated in the figure. The inverted triangle above the alignment marks the position of a putative intron (see also Results). The dots above the alignment mark the positions of the degenerated primers used for PCR. P-loop = P-loop nucleotide-binding motif (ProfileScan); C–C = half zinc finger sequence; it was postulated that this site could co-ordinate protein dimerization (Sharples and Leach, 1995); DA BOX = second half motif for nucleotide binding, associated with the P-loop. (B) Dendrogram showing the relationship between the different RAD50 homologues. (C) Graph showing the probability of coiled-coil formation for the amino acid sequence of Rad50 (Paircoil, see Materials and methods).
Sensitivity of rad50Δ to DNA-damaging agents and epistasis analysis
We deleted the complete ORF of rad50+, replacing it with the kanMX6 cassette. We found that the deletion was sensitive to γ-irradiation, methyl methanesulfonate (MMS) and UV light (data not shown). In S.cerevisiae, RAD50, MRE11, RAD51, RAD55 and RAD52 have all been assigned to the same epistasis group (called the RAD52 epistasis group) and are thought to be involved in the recombinational repair of DNA DSBs (reviewed in Game, 1993). We studied the epistatic interactions between rad50Δ and selected S.pombe homologues of the S.cerevisiae RAD52 epistasis group genes in early stationary cells (see Table I for genotypes of the strains).
Table I. Strains used in this study.
| Strain | Genotype | Source and/or reference |
|---|---|---|
| EH6 | h– rad50::kanMX6 ura4-D18 | this study |
| EH65 | smt0 rad50::kanMX6 ura4-D18 | this study |
| EH68 | smt0 ura4-D18 | this study; Engelke et al. (1987) |
| 5-163 | h– ura4-D18 | strain collection Bern |
| EH62 | smt0 rad32::ura4+ ura4-D18 | this study; Tavassoli et al. (1995) |
| EH61 | smt0 rad50::kanMX6 rad32::ura4+ ura4-D18 | this study |
| EH69 | smt0 rhp51::his3+ his3-D1 | this study; R.Krähenbühl (Bern) |
| EH57 | smt0 rad50::kanMX6 rhp51::his3+ his3-D1 | this study |
| EH66 | smt0 rhp55::ura4+ ura4-D18 | this study; Khasanov et al. (1999) |
| EH63 | smt0 rad50::kanMX6 rhp55::ura4+ ura4-D18 | this study |
| EH60 | smt0 rad22::ura4+ ura4-D18 | this study; Ostermann et al. (1993) |
| 89-3531 | h+ rad2::ura4 ura4-D18 | strain collection Bern; Murray et al. (1994) |
| EH59 | smt0 rad22::ura4+ rad50::kanMX6 ura4-D18 | this study |
| EH44/EH45 | smt0/smt0 ade6-M210/ade6-M216ura4-D18/ura4+ | this study |
| EH42/EH46 | smt0/smt0 ade6-M210/ade6-M216 ura4-D18/ura4+ rad50::kanMX6/rad50::kanMX6 | this study |
| EH118 | smt0 ura4-D18 ade6-M375 int::pUC8/ura4+/ade6-469 | this study; Schuchert and Kohli (1988) |
| EH119 | smt0 ura4-D18 ade6-M375 int::pUC8/ura4+/ade6-469 rad50::kanMX6 | this study |
| EH85 | smt0 rad21-45 | this study; Birkenbihl and Subramani (1992) |
| EH80 | smt0 rad50::kanMX6 rad21-45 | this study |
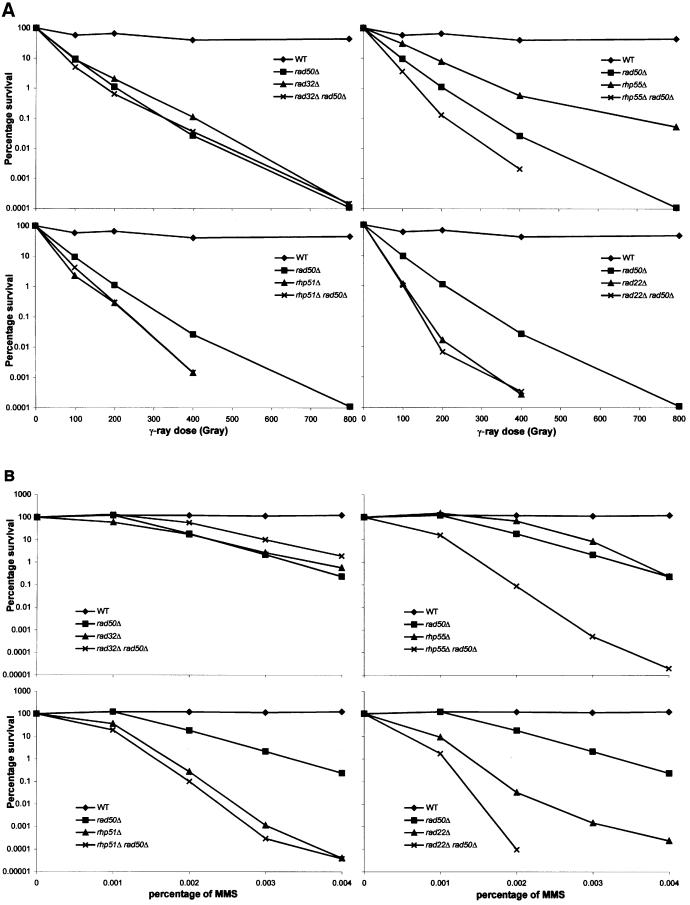
As can be seen in Figure 2A, for the repair of γ-ray-induced damage, rad32Δ (MRE11 homologue; Tavassoli et al., 1995), rhp51Δ (RAD51 homologue; Muris et al., 1993) and rad22Δ (RAD52 homologue; Ostermann et al., 1993) are epistatic with rad50Δ; the double mutants are not more sensitive than one or both of the single mutants. The rhp55 deletion (RAD55 homologue; Khasanov et al., 1999) is not epistatic with rad50; the double mutant is more sensitive than either single mutant.
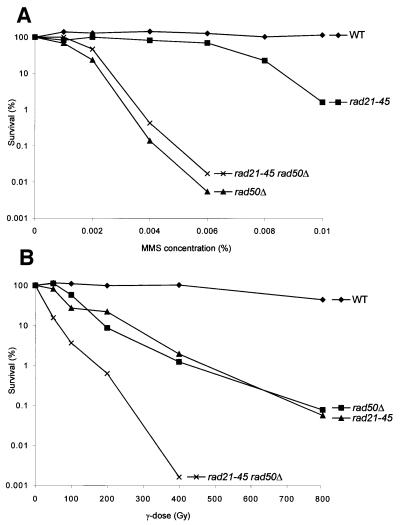
Fig. 2. Epistasis analysis for γ-irradiation (A) and MMS (B) sensitivity between rad50Δ and deletions of other S.pombe homologues of the RAD52 epistasis group. The survival (%; y-axis) of the different single mutants and double mutant combinations is plotted against the γ-ray dose (Gy; x-axis) and the MMS concentration (%; x-axis), respectively. For genotypes, see Table I.
We also performed an epistasis analysis with the same mutants for MMS-induced DNA damage. As can be seen in Figure 2B, rad50 is epistatic with rad32 and rhp51. As for γ-irradiation, rad50Δ is not epistatic with rhp55Δ. Surprisingly, the rad22Δ rad50Δ double mutant is not only much more sensitive than either of the single mutants, but also much more sensitive than any of the other mutants or double mutant combinations. Thus rad22 and rad50 are not epistatic for the repair of MMS-induced DNA damage, whereas they are epistatic for the repair of γ-ray-induced damaged.
Slow growth of a rad50 deletion is reduced in smt0 cells
Mating-type switching in S.pombe is initiated by a DNA lesion, probably a single strand break (SSB) or some other DNA modification, in the mat1 cassette (Arcangioli, 1998; Dalgaard and Klar, 1999). It has been proposed that mating-type switching is caused by a replication-stimulated recombination pathway, in which the ongoing replication fork is interrupted at the DNA lesion, resulting in the formation of a DSB which is repaired by a recombinational mechanism (Arcangioli, 1998; Arcangioli and de Lahondès, 2000).
We observed a strong slow growth phenotype in an h– rad50Δ strain (data not shown) and speculated that non- or mis-repair of the mating type lesion might be responsible for this phenotype. In an smt0 strain, no DNA lesion is present in the mating-type region (Engelke et al., 1987; Styrkársdóttir et al., 1993; Arcangioli and de Lahondès, 2000). To study if the absence of this lesion improved the growth rate of a rad50Δ strain, we determined the total number of cells and the fraction of dead and living cells at different time points of logarithmically growing liquid cultures of rad50Δ and rad50+ strains in h– and smt0 backgrounds. As can be seen in Table II, the doubling time and percentage of dead cells of smt0 rad50Δ indeed significantly decreased compared with h– rad50Δ, although they were not restored to wild-type levels. This suggested that the inability to repair the DNA lesion in the mating type region partially accounts for the slow growth phenotype in a h– rad50Δ strain and that the rad50+ gene is involved in the proper repair of this lesion.
Table II. Doubling time and percentage of dead cells in liquid cultures.
| Strain | Doubling time (min)a | Percentage dead cells |
|---|---|---|
| smt0 | 158.4 ± 6.1 | 2.8 ± 0.2 |
| h– | 156.8 ± 0.6 | 3.3 ± 0.4 |
| smt0 rad50Δ | 205.1 ± 13.4 | 24.0 ± 1.5 |
| h– rad50Δ | 242.9 ± 3.8 | 32.0 ± 0.7 |
aThe values are given ± SD.
Rad50 is involved in DNA replication
The initiation of the mating-type switching process in S.pombe (Arcangioli, 1998; Arcangioli and de Lahondès, 2000) is very similar to the postulated HR-dependent repair of a replication fork that is stalled due to the presence of damaged DNA in bacteria (for a review see Kowalczykowski, 2000). Therefore, we speculated that the residual slow growth phenotype in smt0 rad50Δ could be the result of problems during DNA replication, probably due to the inability to replicate spontaneously damaged DNA or to reinstate the replication fork after collapse.
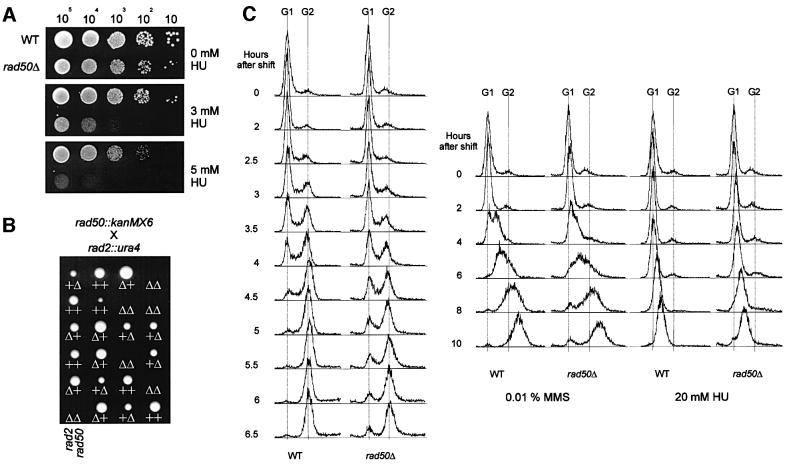
We tested the sensitivity of rad50+ and rad50Δ strains (EH68 and EH65; Table I) to hydroxyurea (HU), which is an inhibitor of ribonucleotide reductase and stalls the replication fork by depletion of nucleotides. As can be seen in Figure 3A, the rad50Δ strain is significantly more sensitive to HU than the wild-type, suggesting a role for Rad50 in S phase.
Fig. 3. Analysis of replication problems in rad50Δ. (A) Sensitivity of rad50Δ to different HU concentrations was determined in a spot test. Numbers above the figure indicate approximate number of cells spotted. (B) Tetrad analysis of a cross between rad50Δ and rad2Δ. The plus signs and triangles below the colonies show the presence of wild-type and deletion alleles of rad2 and rad50, respectively. Genotypes from non-growing colonies were deduced assuming a 2:2 segregation. (C) Analysis of replication progression using FACS analysis of cells released from G1 arrest (see Materials and methods). Progression through S phase is reflected by a transition from the G1 peak to the G2 peak.
Using fluorescence-activated cell sorting (FACS) analysis, we also looked at the kinetics of DNA replication in synchronized rad50+ and rad50Δ strains (EH45 and EH46, Table I). As can be seen in Figure 3B, in rad50Δ the progression through S phase is delayed compared with the wild-type and a significant fraction of the cells remains in G1 in the rad50 deletion. Using a method described by Cha et al. (2000), we estimated that the duration of S phase in the rad50+ strain is 33 min, whereas it is increased to 53 min in the rad50Δ strain. We also looked at the kinetics of DNA replication in the presence of MMS and HU. As can be seen in Figure 3B, replication in wild-type as well as in rad50Δ is significantly slowed down in the presence of 0.01% MMS. In the presence of 20 mM HU, replication is virtually blocked in the wild-type as well as in rad50Δ.
The S.pombe rad2+ gene is a homologue of the FEN1 gene (Murray et al., 1994), and is involved in Okazaki fragment processing during lagging strand DNA synthesis (Alleva and Doetsch, 1998). It has been reported previously that deletions of the S.pombe RAD51 and RAD54 homologues are synthetically lethal with rad2Δ (Muris et al., 1996). We crossed rad50Δ with rad2Δ, pulled 20 tetrads and found that rad50Δ is also synthetically lethal with rad2Δ (see Figure 3C), suggesting that Rad50 is necessary to retain cell viability when Okazaki fragment processing is defective.
Rad50 is involved in telomere length maintenance
In S.cerevisiae, Rad50 is involved in telomere length maintenance (Boulton and Jackson, 1998; Kironmai and Muniyappa, 1997). To check whether S.pombe Rad50 has a role in telomere length maintenance, we cultured wild-type and rad50Δ strains for at least 100 generations in liquid media. Genomic DNA was isolated, digested, separated on a gel, blotted and hybridized with a telomere-specific probe. As can be seen in Figure 4, the telomeres in rad50Δ are shorter than in rad50+ parental strains, suggesting that Rad50 plays a role in telomere length maintenance.
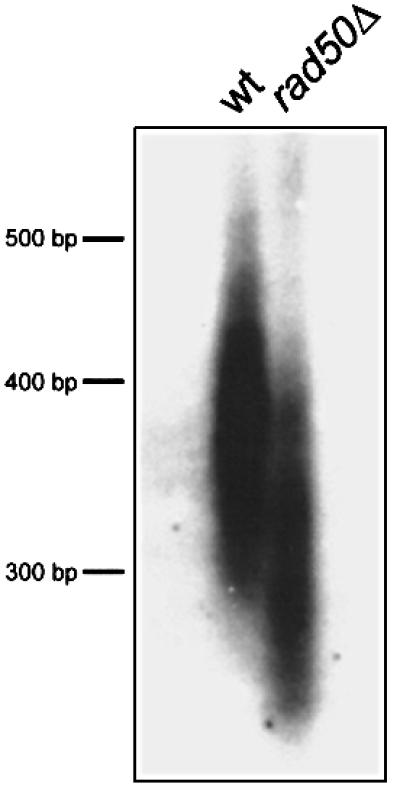
Fig. 4. Southern blot analysis of telomere length in wild-type and rad50Δ strains cultured for at least 100 generations in liquid media. Telomeres of the rad50Δ strain are shorter than those of the wild-type strain, showing that Rad50 is involved in telomere length maintenance.
Chromosome loss frequency is strongly increased in rad50Δ
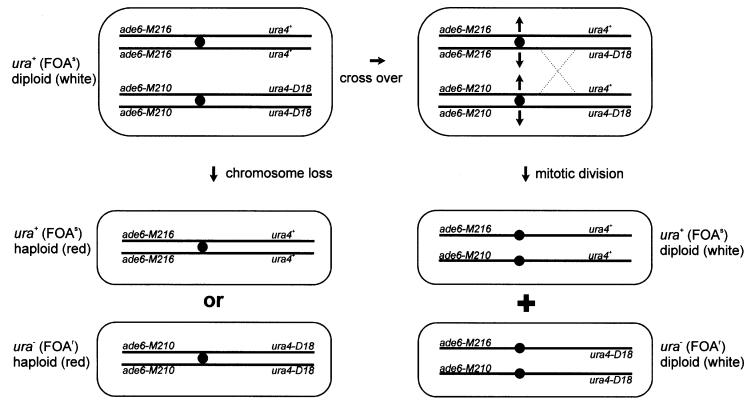
It has been reported previously that deletions of the S.pombe RAD51 and RAD54 homologues show an increased frequency of minichromosome loss (Muris et al., 1996). We developed a new chromosome loss/inter-homologue recombination assay, which is explained in Figure 5 (for explanation see figure legend). We determined the chromosome loss frequency in a fluctuation test (see Table III) and found that the frequency of chromosome loss is strongly increased in rad50Δ: the 705-fold increase in the average number of haploids per 1 × 106 plated cells is statistically significant (Mann– Whitney U-test) and translates to a 490-fold increase of chromosome loss per cell division (as determined with the Lea–Coulson method of the median).
Fig. 5. Diagram explaining the chromosome loss/inter-homologue recombination assay. A non-sporulating diploid (EH44/EH45 and EH42/EH46; see Table I for genotype) is grown under non-selective conditions in liquid medium until stationary. Cells are plated in appropriate dilutions on YEA medium containing FOA (selective for ura4– cells) with limiting amounts of adenine (ade6– cells turn red). The original diploid does not grow on this medium because it is ura4+. Red FOA-resistant colonies are the result of concomitant loss of the ura4+ gene (making them FOA resistant) and the ade6-M216 allele (making them red), due to loss of chromosome III. White diploid FOA-resistant colonies are the result of a crossover event between the centromere and ura4. Depending on the chromosome segregation pattern after this recombination event, two ura4-D18 alleles can be pulled to the same spindle pole, forming an FOA-resistant diploid cell which is still ade+ due to the interallelic complementation of the ade6-M216 and ade6-M210 alleles.
Table III. Frequency of chromosome III loss in wild-type and rad50Δ diploids.
| Average No. of haploids per 1 × 106 plated cellsa |
Haploidization frequency per divisionb |
||
|---|---|---|---|
| Wild-type | rad50Δ | Wild-type | rad50Δ |
| 1.7 × 102 | 1.2 × 105 (705×c; P <0.05d) | 1.36 × 10–5 | 6.66 × 10–3 (490×)c |
aBased on 10 independent cultures.
bCalculated with the Lea–Coulson method of the median (Lea and Coulson, 1949).
cFold increase compared with wild-type.
dProbability as determined with the Mann–Whitney U-test.
Recombination between homologous chromosomes is increased in rad50Δ
With the assay described in Figure 5, we were also able to determine the frequency of inter-homologue crossover recombination between the centromere and the ura4 locus. A crossover event between the centromere and the ura4 locus leads to fluoro-orotic acid (FOA)-resistant diploids (ura4-D18/ura4-D18) that are white on low adenine media. FOA-resistant colonies were not the result of a mutation in the ura4+ gene, as haploid strains EH45 and EH46 (Table I) did not give any FOA-resistant colonies using the same protocol plating the same numbers of cells (data not shown). White FOA-resistant colonies that were detected in the assay were all diploid, as confirmed with Coulter counter size analysis (data not shown). Therefore, they were not the result of a chromosome loss event (with concurrent gene conversion between the ade6 alleles to give an ade6+ gene) but of a mitotic crossover between the homologous chromosomes between ura4+ and the centromere. As can be seen in Table IV, the frequency of this inter-homologue recombination event per 1 × 106 plated cells is increased 31-fold in rad50Δ. This increase is statistically significant (Mann–Whitney U-test) and translates to a 28-fold increased recombination rate per cell division as determined with the Lea–Coulson method of the median.
Table IV. Inter-homologue crossover frequency between the centromere and the ura4 locus on chromosome III in wild-type and rad50Δ diploids.
| Average No. of recombinants per 1 × 106 plated cellsa |
Inter-homologue recombination frequency per divisionb |
||
|---|---|---|---|
| Wild-type | rad50Δ | Wild-type | rad50Δ |
| 1.8 × 103 | 5.5 × 104 (31×c; P <0.05d) | 1.40 × 10–4 | 3.85 × 10–3 (28×)c |
aBased on 10 independent cultures.
bCalculated with the Lea–Coulson method of the median (Lea and Coulson, 1949).
cFold increase compared with wild-type.
dProbability as determined with the Mann–Whitney U-test.
Frequency of sister chromatid recombination is decreased in rad50Δ
We wanted to know if the increase in recombination in rad50Δ was due to a general increase in recombination, or if it was specific for recombination between homologous chromosomes. Therefore, we used an assay to determine the sister chromatid recombination frequency as described by Schuchert et al. (1988). In the haploid strain used for this assay, a ura+ marker is flanked by the ade6-469 and ade6-M375 alleles. Homologous recombination between the ade– alleles of one sister chromatid (intra-sister recombination) or between alleles on different sister chromatids (inter-sister recombination) leads to a functional ade+ gene. As shown in Table V, we found that the frequency of sister chromatid recombination per 1 × 106 plated cells was decreased 11-fold. This decrease is statistically significant (Mann–Whitney U-test) and translates to a 15-fold decrease per cell division as determined with the Lea–Coulson method of the median.
Table V. Sister chromatid recombination frequency in wild-type and rad50Δ.
| Median No. of ade+ recombinants per 1 × 106 plated cellsa |
Inter/intra-sister recombination frequency per divisionb |
||
|---|---|---|---|
| Wild-type | rad50Δ | Wild-type | rad50Δ |
| 5.0 × 104 | 4.7 × 103 (11×c; P <0.05d) | 2.11 × 10–3 | 1.44 × 10–4 (15×)c |
aBased on 10 independent cultures.
bCalculated with the Lea–Coulson method of the median (Lea and Coulson, 1949).
cFold increase compared with wild-type.
dProbability as determined with the Mann–Whitney U-test.
rad50 is epistatic with the rad21 cohesin gene for the repair of MMS-induced DNA damage
We found that in rad50Δ, recombination between homologous chromosomes is increased whereas recombination between sister chromatids is reduced. Thus, Rad50 probably stimulates the use of the sister chromatid as a template for HR. The overall structure of Rad50 is reminiscent of that of SMC proteins, which are involved in sister chromatid cohesion. This suggested to us that Rad50 might provide a link between sister chromatid cohesion and repair. Interestingly, the rad21-45 allele of the sister chromatid cohesion gene rad21+, the S.pombe SCC1 homologue, is radiation sensitive and repair deficient (Birkenbihl and Subramani, 1992). To determine if rad50+ and rad21+ function in the same pathway for the repair of damaged DNA, we carried out an epistasis analysis with rad50Δ and rad21-45. As can be seen in Figure 6A, the rad21-45 rad50Δ double mutant is no more sensitive than rad50Δ, whereas rad21-45 is more sensitive than the wild-type for MMS-induced DNA damage. Three independent repetitions of this experiment also showed that the rad50Δ rad21-45 double mutant was no more sensitive than rad50Δ. Therefore, we conclude that rad21 and rad50 are epistatic and function in the same path way for the repair of MMS-induced DNA damage. Surprisingly, as can be seen in Figure 6B, rad21 and rad50 are not epistatic and function in different pathways for the repair of γ-ray-induced DNA damage.
Fig. 6. Epistasis analysis between rad50Δ and rad21-45 for MMS (A) and γ-ray (B) survival. The genes are epistatic for MMS-induced DNA damage, the rad50Δ rad21-45 double mutant is not more sensitive than the rad50Δ single mutant. However, the genes are not epistatic for γ-ray-induced damage, the double mutant being more sensitive than either of the single mutants.
Discussion
Sensitivity of rad50Δ to DNA-damaging agents and epistasis analysis
Different DNA-damaging agents cause different DNA lesions which might be repaired using different mechanisms. The main genotoxic lesions caused by γ-irradiation are DNA DSBs, which in S.pombe are repaired predominantly by HR in G2. MMS alkylates the DNA; alkylated DNA can block DNA synthesis and stall the replication fork. These alkylated sites can be excised enzymatically, leading to formation of SSBs (Laval, 1977). When S.cerevisiae is exposed to low (0.05%) MMS concentrations, SSBs can be detected in the DNA (Chlebowicz and Jachymczyk, 1979). The presence of SSBs is not necessarily an immediate threat to cell viability, but is likely to cause problems during DNA replication. Indeed, DNA synthesis in mammalian cells is inhibited when cells are exposed to low (1 mM ∼0.01%) concentrations of MMS (Dahle et al., 1978). In S.cerevisiae, DSBs are only detectable when high (0.5%) MMS concentrations are used (Chlebowicz and Jachymczyk, 1979). In this study, we showed that also in S.pombe, DNA replication is slowed down in the presence of 0.01% MMS, suggesting that the DNA damage caused by MMS causes problems during replication and is not repaired until S phase.
Whereas rad22+ and rad50+ function in the same pathway for repair of γ-ray-induced damage, they function in different pathways for the repair of MMS-induced damage. At first sight, this observation might be surprising because no differences have been found for epistatic interactions between rad22 and rhp55 for MMS and γ-irradiation (Khasanov et al., 1999). Our epistasis analysis probably reflects that the different fission yeast homologues of the S.cerevisiae ‘RAD52 epistasis group’ function in different inter-related pathways and that the epistatic interactions depend on the kind of DNA damage and phase of the cell cycle in which the damage is repaired. The synergistic contributions of rad50+ and rad22+ to the repair of MMS-induced damage therefore might reflect different requirements for these genes in S-phase repair. In our hands, the rad22Δ mutant is more sensitive to γ-irradiation than was shown by Muris et al. (1997). The sensitivities of other mutants used in this study are within the previously reported range, suggesting that the differences are not due to experimental differences. A possible explanation is that the rad22Δ strain used by Muris et al. (1997) might have contained a suppressor mutation. Strains deleted for rad22 have a strong slow growth phenotype and, therefore, suppressor mutations that improve the growth rate might occur and take over frequently. As we crossed the rad22 deletion into the smt0 background, we might have lost the suppressor.
What is the primary function of Rad50?
Because rad50 mutations in different organisms result in many pleiotropic phenotypes, it is challenging to find out what the primary function is of the Rad50 protein. In S.cerevisiae, Rad50 has been implicated in NHEJ (reviewed in Lewis and Resnick, 2000), S-phase checkpoint response (Kironmai and Muniyappa, 1997; D’Amours and Jackson, 2001; Usui et al., 2001) and HR (Bressan et al., 1999; for a review see Pâques and Haber, 1999). In S.pombe, however, Rad50 is not required for NHEJ (Manolis et al., 2001), suggesting that the protein might play a regulatory rather than a catalytic role in NHEJ in S.cerevisiae. In this study, we reported that S.pombe rad50Δ cells delay S-phase progression in response to MMS and HU treatment to a similar extent as wild-type cells. This suggests that in these circumstances, S.pombe Rad50 is not involved in establishing the intra S-phase checkpoint. Because of these differences, S.pombe is a useful organism to study the primary role of the Rad50 protein.
We showed that in rad50Δ, the spontaneous recombination frequency between homologous chromosomes is increased, whereas sister chromatid recombination is reduced. This suggested to us that the Rad50 protein might establish an interaction with the sister chromatid during repair, and thus promotes the use of the sister chromatid as a template for HR. Also in S.cerevisiae, the spontaneous inter-homologue recombination frequency is increased in rad50Δ strains (Malone et al., 1990). To our knowledge, sister chromatid recombination has not been measured in S.cerevisiae rad50Δ. Bressan et al. (1999) showed that in S.cerevisiae mre11Δ, the spontaneous sister chromatid recombination frequency is not decreased, whereas ionizing-radiation induced sister chromatid recombination is slightly decreased compared with the wild-type. However, S.cerevisiae spends more of its time in G1 than S.pombe, which is predominantly a G2 organism, and a defect in sister chromatid recombination might therefore be masked. The fact that haploid rad50Δ cells in G2 are much more sensitive than diploid G1 rad50Δ cells in S.cerevisiae has been interpreted to suggest that Rad50 and interacting proteins are involved in sister chromatid interactions (Pâques and Haber, 1999; and references cited therein).
The overall structure of the Rad50 protein is reminiscent of the SMC family of proteins. Members of this family have a role in chromatin condensation, sister chromatid cohesion and chromosome stability (for a review see Strunnikov and Jessberger, 1999). Also the results of a similarity search using Propsearch, which is based on the comparison of several protein properties other than amino acid sequence, showed a high similarity to SMC proteins (data not shown). Besides Rad18 (Lehmann et al., 1995), Rad50 is the second S.pombe protein which is involved in DNA repair and shows similarity to SMC proteins. Rad50 is proposed to be an antiparallel homodimer, with DNA-binding catalytic domains on both ends, therefore capable of connecting two DNA strands (Hopfner et al., 2000). Thus the protein would be ideally suited to make a connection between sister chromatids or homologous chromosomes to check the presence of a repair template and aid HR. HR is thought to be initiated with the 5′ to 3′ resection of the DNA strands, possibly by endo/exo nuclease activity of the Mre11 protein (Haber 1998). This resection can lead to loss of genetic information and is a potentially deleterious event if no sister chromatid is available for recombinational repair. Therefore, it is likely that the availability of a sister chromatid or homologous chromosome influences which repair pathway is chosen. In mammalian cells, Ku70, Rad50 and Mre11 physically interact with each other, and it has been suggested that this interaction is necessary to make a choice between HR and NHEJ (Goedecke et al., 1999). We propose that the primary role of S.pombe Rad50 is to check the presence of a sister chromatid and, if present, to promote HR using the sister as a template. We speculate that Rad50 regulates the nuclease activity of Rad32 (Mre11).
Role of Rad50 in mating-type switching, DNA replication and telomere maintenance
During the process of mating-type switching in S.pombe, the replication fork runs into a DNA lesion in the mating-type region which is then repaired by HR with one of the donor cassettes (Arcangioli and de Lahondès, 2000). This process is very similar to the postulated HR-dependent repair of a replication fork that is stalled due to the presence of damaged DNA in bacteria (for a review see Kowalczykowski, 2000). We showed that Rad50 plays a role in the recombinational repair of the lesion at the mating-type locus and that non- or mis-repair of this lesion in rad50Δ leads to cell death.
Muris et al. (1996) proposed that the S.pombe homologues of Rad51 and Rad54 play a role during DNA replication (see also Lehmann et al., 1995). Merrill and Holm (1998) found that in S.cerevisiae, several RAD52 group mutants, including rad50, are synthetically lethal with the pol30-104 mutation (Pol30 is the S.cerevisiae PCNA homologue), suggesting a function of these genes in DNA replication. We tested if the residual slow growth phenotype in smt0 rad50Δ could be the result of problems during DNA replication and found that replication in rad50Δ is delayed compared with wild-type. The rad50Δ strain is sensitive to HU, consistent with the idea that Rad50 is required for the recombination-dependent restart of collapsed replication forks. Rad2 is the S.pombe Fen1 homologue and is required for the removal of the lagging strand RNA primer (Murray et al., 1994; Alleva and Doetsch, 1998). In the absence of Fen1/Rad2, the last remaining ribonucleotide of the primer cannot be removed, and the Okazaki fragments cannot be ligated, leading to nicked DNA (Bambara et al., 1997). We found that rad50Δ is synthetically lethal with rad2Δ, suggesting that Rad50 functions in an alternative, presumably recombinational, pathway to remove the remaining RNA primer leftovers.
In rad50Δ, the telomeres are shortened. Saccharomyces cerevisiae Rad50 has also been shown to be involved in telomere length maintenance (Boulton and Jackson, 1998; Kironmai and Muniyappa, 1997). Nugent et al. (1998) found that S.cerevisiae rad50 is epistatic with the telomerase, and it was proposed that the exonuclease activity of Mre11 prepares the single strand substrate for the telomerase. In mammalian cells, telomeres end in a large duplex loop that is formed by invasion of the 3′ telomeric overhang into the duplex telomeric repeat array. Formation of this loop is catalysed by the Trf2 protein (Griffith et al., 1999). Zhu et al. (2000) showed that the Rad50–Mre11–Nbs1 complex is associated with the Trf2 protein and they proposed that the Rad50 protein complex is involved in formation and stabilization of this loop structure. Whereas Rad50 and Mre11 are found at the telomere throughout the cell cycle, Nbs1 localizes to the telomeres only in S phase, suggesting a role for Nbs1 in telomere replication. Possibly, Rad50 is involved in this process by signalling the presence of a sister chromatid (indicative of completed DNA replication) and subsequent initiation of telomere addition by the telomerase (see also Pâques and Haber, 1999). Recent data might suggest that components of the Rad50 protein complex provide accessory functions and recruit the telomerase to the telomeres (Ranganathan et al., 2001; Tsukamoto et al., 2001). In the absence of the telomerase, telomeres can be elongated in a recombination-dependent manner, which is dependent on RAD52 group genes in S.cerevisiae (reviewed in Kass-Eisler and Greider, 2000). In S.pombe telomerase-deficient mutants, survivors are formed that have circularized all their chromosomes, whereas a smaller population maintains their telomeres, presumably by a recombinational mechanism (Nakamura et al., 1998).
Mating-type switching, recombination-dependent replication and alternative recombination-dependent telomere lengthening require recombination with the sister chromatid. We therefore believe that defects in these processes in rad50Δ might well be explained by a role for Rad50 in initiating recombination using the sister chromatid as a template.
Rad50 functions in the same pathway as Rad21 for the repair of MMS-induced DNA damage
The S.pombe rad21+ gene, the homologue of the S.cerevisiae SCC1 sister chromatid cohesion gene, was characterized initially as a radiation-sensitive mutant, suggesting that sister chromatid cohesion plays a role in the repair of DSBs. Several lines of evidence suggest that in different organisms a link exists between sister chromatid cohesion and recombinational repair during meiosis (reviewed in Heemst and Heyting, 2000). We showed that for the repair of MMS-induced DNA damage (which leads to problems in S phase), rad50+ functions in the same pathway as rad21+, the S.pombe SCC1 homologue. Interestingly, rad50 and rad21 are not epistatic for the repair of γ-ray-induced damage. Because γ-ray-induced DNA damage in asynchronous S.pombe cells is repaired predominantly during G2, whereas MMS leads to problems in S phase (see beginning of Discus sion), we think it is likely that the genetic interaction between rad21 and rad50 for MMS-induced damage is S phase specific. At the moment, we can only speculate why a genetic interaction between rad21 and rad50 would be S phase specific. We proposed that Rad50 plays a role in preventing the initiation of homologous recombination when no sister chromatid is available for repair. Possibly, during G2, the cell cycle machinery is able to signal the presence of a sister chromatid to the repair machinery. However, during S phase, the DNA is only partially replicated, and an additional mechanism is needed to signal the availability of a sister. The S.cerevisiae cohesin complex, consisting of the Scc1, Scc3, Smc1 and Smc3 proteins, is loaded on the DNA after the passage of the replication fork (Uhlmann and Nasmyth, 1998) and the presence of the complex indicates that the DNA has been replicated. Possibly Rad21 supports Rad50 in the task of determining the presence of a sister chromatid during S phase, explaining the epistatic interaction between the two mutant genes for the repair of MMS damage. We can speculate that, during S phase, the Rad50 protein complex interacts with the cohesin complex to assist recombinational repair in regions that have already replicated. This interaction may be important for re-initiation of replication after replication fork collapse. The precise nature of this interaction remains unknown. Because Rad50 is an SMC-like protein, the interaction between Rad21 and Rad50 might be reminiscent of the interaction between Scc1 and the Smc1/3 proteins in the cohesin complex. We have not been able to co-immunoprecipitate Rad21 with Rad50, and vice versa, or see any significant Rad50-dependent change in Rad21 phosphorylation (Birkenbihl and Subramani, 1995). However, any possible Rad50–Rad21 complex is likely to be chromatin bound and would thus be insoluble and not detectable with our assays.
Materials and methods
Strains and media
For strain construction and propagation, standard genetic methods and media were used (Gutz et al., 1974). Strains used and constructed in this study are shown in Table I.
PCR, cloning, DNA sequencing and gene disruption of the S.pombe RAD50 homologue
Based on sequence information from the S.cerevisiae, human and C.elegans RAD50 homologues, conserved regions were chosen for primer synthesis. Degenerated primers derived from the amino acid sequences MRGRC [ATG(A/C)G(A/C/G/T)GG(A/C/G/T)(A/C)G(A/C/G/T)TG] and VITHDE [TC(A/G)TC(A/G)TG(A/C/G/T)GT(A/G/T)AT(A/C/G/T)AC] were used for PCR (1.5 µg of S.pombe genomic DNA as template, annealing temperature 39°C, 35 cycles). A fragment of the expected length was amplified weakly. PCR on the eluted fragment using internal forward and reverse primers derived from the amino acid sequence DEPTTN [forward: GA(C/T)GA(A/G)CC(A/C/G/T)AC(A/C/G/T)AC(A/C/G/T)AA] gave a product of the expected length in combination with both external primers, confirming that we indeed amplified a fragment of the S.pombe RAD50 homologue; we designated the gene rad50+. The PCR fragment was cloned into a T-vector (Promega). Using this fragment to hybridize the S.pombe cosmid library (Hoheisel et al., 1993), cosmid clone ICRFc60D1232D was identified. After restriction mapping and Southern analysis, a 5.6 kb EcoRV fragment containing the rad50+ fragment was identified, subcloned and partially sequenced by automated fluorescence sequencing (Microsynth GmbH, Balgach, Switzerland). As this fragment did not contain the 5′ part of the rad50+ gene, a partially overlapping 4.1 kb XhoI fragment containing the 5′ end was also subcloned and partially sequenced. For construction of a deletion, we precisely deleted the complete ORF of rad50+, and replaced it with the kanMX6 cassette as described by Bähler et al. (1998).
Protein sequence analysis
Sequence alignments and analysis were done using the Wisconsin sequence analysis package (GCG) and the ISREC BOXSHADE server (http://www.isrec.isb-sib.ch/software/BOX_form.html). Non-sequence-dependent protein similarity was detected using Propsearch (Hobohm and Sander, 1995; http://www.embl-heidelberg.de/prs.html).
Sensitivity to DNA-damaging agents
For γ-ray sensitivity, cells were irradiated in suspension in supplemented liquid yeast extract (YEL) with a 137Cs source (12.5 Gy/min) and immediately plated in duplicate on supplemented yeast extract (YEA). Cells were grown for 5 days at 30°C and colonies were counted. Relative survival was calculated by dividing the absolute survival by the survival of cells of the same strain not exposed to DNA-damaging agents. For MMS survival, cells were plated in duplicate directly on MMS-containing plates made up the previous day. For HU sensitivity, a spot test was performed: cells were spotted in different dilutions on plates containing different concentrations of HU and grown for 3 days at 30°C. Experiments were repeated at least three times and gave similar results.
Determination of doubling time and percentage of dead cells in liquid cultures
For studying the growth kinetics of different S.pombe strains (5-163, EH6, EH65 and EH68; see Table I for genotype), single cells were grown to colonies and pre-cultured in YEL. After inoculating the appropriate amount of cells, cultures of the different strains were grown overnight until they simultaneously had reached a cell density of ∼0.5 × 106/ml the next morning. Samples for counting and viability determination were sonicated to disrupt cell clumps. Cell density was determined in triplicate for each culture with a Coulter counter. Appropriate dilutions of each culture were plated in triplicate on supplemented YEA plates. Viability of the cells was determined in triplicate in a microscope the next morning by counting the number of microcolonies (resulting from a living cell) and single cells (which were considered to be dead). For each single determination, at least 500 cells and microcolonies were counted. Determination of cell density and viability as described above was repeated after ∼7 h of logarithmic growth, taking care that cell density was below 5 × 106/ml. From the determination of total cell density and viability, the doubling time and percentage of dead cells was calculated for the different strains. A repetition of the experiment gave highly similar results.
Tetrad analysis
Strains 89-3531 and EH65 were crossed and tetrad analysis was performed using standard procedures. Plates were allowed to grow for 5 days and were replica plated on minimal media without uracil (to test for the presence of the rad2::ura4 deletion) and on YEA + G418 (to test for the presence of the rad50::kanMX6 deletion).
Synchronization and FACS analysis
To study DNA replication, rad50+ (EH45) and rad50Δ (EH46) strains were nitrogen starved for 20 h in EMM2 without nitrogen at 25°C. To get rid of the high percentage of dead cells in the rad50Δ culture, which remain elongated after nitrogen starvation, both cultures were centrifuged on a 7–30% lactose gradient, and the small cells on top of the gradient were harvested. The resulting cultures were virtually free of elongated (dead) cells. FACS analysis was performed according to Alfa et al. (1993). A repetition of the experiment gave similar results.
Telomere length
Telomeres were detected using the 1.9 kb ApaI fragment of plasmid pEN42 (Nimmo et al., 1994). Genomic DNA from rad50+ and rad50Δ strains (EH68 and EH65, respectively; see Table I) was isolated as described in Moreno et al. (1991), digested with ApaI and separated on a 1.2% agarose gel. DNA was blotted on Bio-Rad Zeta Probe membranes and hybridized with the standard protocol as supplied by the manufacturer. Several repetitions of this experiment showed a similar reduction of telomere length in rad50Δ.
Chromosome loss/inter-homologue recombination assay and sister chromatid recombination assay
Non-sporulating diploids of strains EH44/EH45 and EH42/EH46 (see Table I) were made freshly by protoplast fusion (Alfa et al., 1993) and re-streaked on MMA plates supplemented with uracil. For each diploid, a minimum of 10 independent colonies were inoculated in supplemented YEL medium and grown until stationary phase. Appropriate dilutions were plated on supplemented YEA plates to determine the viable cell number and on YEA + FOA + uracil (without adenine) to select for colonies that lost the ura4+ gene. Haploid FOA-resistant colonies that lose ura4+ due to chromosome loss turn red on this medium, whereas diploid colonies that lose the ura4+ marker because of recombination between the centromere and ura4 remain white due to intragenic complementation of ade6-M210 and ade6-M216 (explained in Figure 5). White and red colonies were counted after 5 days at 30°C and the frequencies of chromosome loss and inter-homologue recombination per division were determined with the method of the median (Lea and Coulson, 1949).
For the sister chromatid recombination assay, a minimum of 10 independent single colonies from strains EH118 and EH119 (see Table I for genotype; constructed from PS3; Schuchert and Kohli, 1988) were inoculated in 10 ml of YEL + supplements and grown to stationary phase. Appropriate dilutions were plated on supplemented YEA for determination of viable cell numbers. To select for ade+ recombinants, appropriate dilutions were plated on MMA + uracil and colonies were counted after 5 days at 30°C. The frequency of sister chromatid recombination events per division was determined with the method of the median (Lea and Coulson, 1949).
Statistically significant differences in the chromosome loss and recombination assays described above were detected using the non-parametric Mann–Whitney U-test. Repetitions of these experiments gave similar results.
Acknowledgments
Acknowledgements
Special thanks go to Oliver Fleck for help during the initial stages of the project, Peter Munz for help with the genetics and viability assays, and Thomas Caspari and Alan Lehmann for reading the manuscript and helpful discussions. This work was supported in part by grants from the Swiss National Science Foundation (J.K.) and the Human Frontiers Science Programme (A.M.C.). E.H. was supported by fellowships from the Swiss National Science Foundation and a Marie Curie Fellowship from the European Union (contract No. MCF1-1999-01425).
References
- Alfa C., Fantes,P., Hyams,J. and McLeod,M. (1993) Experiments with the Fission Yeast: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Alleva J.L. and Doetsch,P.W. (1998) Characterization of Schizo saccharomyces pombe Rad2 protein, a FEN-1 homolog. Nucleic Acids Res., 26, 3645–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangioli B. (1998) A site- and strand-specific DNA break confers asymmetric switching potential in fission yeast. EMBO J., 17, 4503–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangioli B. and de Lahondes,R. (2000) Fission yeast switches mating type by a replication–recombination coupled process. EMBO J., 19, 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J., Wu,J.Q., Longtine,M.S., Shah,N.G., McKenzie,A.,III, Steever,A.B., Wach,A., Philippsen,P. and Pringle,J.R. (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast, 14, 943–951 [DOI] [PubMed] [Google Scholar]
- Bambara R.A., Murante,R.S. and Henricksen,L.A. (1997) Enzymes and reactions at the eukaryotic DNA replication fork. J. Biol. Chem., 272, 4647–4650 [DOI] [PubMed] [Google Scholar]
- Birkenbihl R.P. and Subramani,S. (1992) Cloning and characterization of rad21, an essential gene of Schizosaccharomyces pombe involved in DNA double-strand-break repair. Nucleic Acids Res., 20, 6605–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenbihl R.P. and Subramani,S. (1995) The rad21 gene product of Schizosaccharomyces pombe is a nuclear, cell cycle-regulated phosphoprotein. J. Biol. Chem., 270, 7703–7711. [DOI] [PubMed] [Google Scholar]
- Boulton S.J. and Jackson,S.P. (1998) Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J., 17, 1819–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan D.A., Baxter,B.K. and Petrini,H.J. (1999) The Mre11– Rad50–Xrs2 protein complex facilitates homologous recombination-based double strand break repair in Saccharomyces cerevisiae. Mol. Cell. Biol., 19, 7681–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney J.P. et al. (1998) The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell, 93, 477–486 [DOI] [PubMed] [Google Scholar]
- Cha R.S., Weiner,B.M., Keeney,S., Dekker,J. and Kleckner,N. (2000) Progression of meiotic DNA replication is modulated by interchromosomal interaction proteins, negatively by Spo11p and positively by Rec8p. Genes Dev., 14, 493–503. [PMC free article] [PubMed] [Google Scholar]
- Chlebowicz E. and Jachymczyk,W.J. (1979) Repair of MMS-induced DNA double-strand breaks in haploid cells of Saccharomyces cerevisiae, which requires the presence of a duplicate genome. Mol. Gen. Genet., 167, 279–286 [DOI] [PubMed] [Google Scholar]
- Dahle D.B., Griffiths,T.D. and Carpenter,J.G. (1978) Inhibition of deoxyribonucleic acid synthesis and replicon elongation in mammalian cells exposed to methyl methanesulfonate. Mol. Pharmacol., 14, 278–289 [PubMed] [Google Scholar]
- Dalgaard J.Z. and Klar,A.J. (1999) Orientation of DNA replication establishes mating-type switching pattern in S.pombe. Nature, 400, 181–184. [DOI] [PubMed] [Google Scholar]
- D’Amours D. and Jackson,S.P. (2001) The yeast Xrs2 complex functions in S phase checkpoint regulation. Genes Dev., 15, 2238–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolganov G.M., Maser,R.S., Novikov,A., Tosto,L., Chong,S., Bressan,D.A. and Petrini,J.H.J. (1996) Human Rad50 is physically associated with human Mre11: identification of a conserved multiprotein complex implicated in recombinational DNA repair. Mol. Cell. Biol., 16, 4832–4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke U., Grabowski,L., Gutz,H., Heim,L. and Schmidt,H. (1987) Molecular characterisation of h– mutants of Schizosaccharomyces pombe. Curr. Genet., 12, 535–542 [Google Scholar]
- Game J.C. (1993) DNA double-strand breaks and the RAD50–RAD57 genes in Saccharomyces. Semin. Cancer Biol., 4, 73–83. [PubMed] [Google Scholar]
- Goedecke W., Eijpe,M., Offenberg,H.H., van Aalderen,M. and Heyting,C. (1999) Mre11 and Ku70 interact in somatic cells, but are differentially expressed in early meiosis. Nature Genet., 23, 194–198. [DOI] [PubMed] [Google Scholar]
- Griffith J.D., Comeau,L., Rosenfield,S., Stansel,R.M., Bianchi,A., Moss,H. and de Lange,T. (1999) Mammalian telomeres end in a large duplex loop. Cell, 97, 503–514. [DOI] [PubMed] [Google Scholar]
- Gutz H., Leslot,H., Leupold,U. and Loprieno,N. (1974) Schizo saccharomyces pombe. In King,R.C. (ed.), Handbook of Genetics. Plenum Press, New York, NY, Vol. 1, pp. 395–446
- Haber J.E. (1998) The many interfaces of Mre11. Cell, 95, 583–586. [DOI] [PubMed] [Google Scholar]
- Heemst D. van and Heyting,C. (2000) Sister chromatid cohesion and recombination in meiosis. Chromosoma, 109, 10–26 [DOI] [PubMed] [Google Scholar]
- Hobohm U. and Sander,C. (1995) A sequence property approach to searching protein databases. J. Mol. Biol., 251, 390–399 [DOI] [PubMed] [Google Scholar]
- Hoheisel J.D., Maier,E., Mott,R., McCarthy,L., Grigoriev,A.V., Schalkwyk,L.C., Nizetic,D., Francis,F. and Lehrach,H. (1993) High resolution cosmid and P1 maps spanning the 14 Mb genome of the fission yeast S.pombe. Cell, 73, 109–120 [DOI] [PubMed] [Google Scholar]
- Hopfner K.P., Karcher,A., Shin,D.S., Craig,L., Arthur,L.M., Carney,J.P. and Tainer,J.A. (2000) Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell, 101, 789–800. [DOI] [PubMed] [Google Scholar]
- Ivanov E.L., Sugawara,N., White,C.I., Fabre,F.and Haber,J.E. (1994) Mutations in XRS2 and RAD50 delay but do not prevent mating type switching in Saccharomyces cerevisiae. Mol. Cell. Biol., 14, 3414–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass-Eisler A. and Greider,C.W. (2000) Recombination in telomere-length maintenance. Trends Biochem. Sci., 25, 200–4. [DOI] [PubMed] [Google Scholar]
- Khasanov F.K., Savchenko,G.V., Bashkirova,E.V., Korolev,V.G., Heyer,W.D. and Bashkirov,V.I. (1999) A new recombinational DNA repair gene from Schizosaccharomyces pombe with homology to Escherichia coli RecA. Genetics, 152, 1557–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kironmai K.M. and Muniyappa,K. (1997) Alteration of telomeric sequences and senescence caused by mutations in RAD50 of Saccharomyces cerevisiae. Genes Cells, 2, 443–455 [DOI] [PubMed] [Google Scholar]
- Kowalczykowski S,C. (2000) Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci., 25, 156–165. [DOI] [PubMed] [Google Scholar]
- Laval J. (1977) Two enzymes are required for strand incision in repair of alkylated DNA. Nature, 269, 829–832 [DOI] [PubMed] [Google Scholar]
- Lea D.E. and Coulson,C.A. (1949) The distribution of the number of mutants in bacterial populations. J. Genet., 49, 264–285 [DOI] [PubMed] [Google Scholar]
- Lehmann A.R., Walicka,M., Griffiths,D.J.F., Murray,J.M., Watts,F.Z., McCready,S. and Carr,A.M. (1995) The rad18 gene of Schizo saccharomyces pombe defines a new subgroup of the SMC superfamily involved in DNA repair. Mol. Cell. Biol., 15, 7067–7080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L.K. and Resnick,M.A. (2000) Tying up loose ends: nonhomologous end-joining in Saccharomyces cerevisiae. Mutat. Res., 451, 71–89. [DOI] [PubMed] [Google Scholar]
- Luo G., Yao,M.S., Bender,C.F., Mills,M., Bladl,A.R., Bradley,A. and Petrini,J.H. (1999) Disruption of mRad50 causes embryonic stem cell lethality, abnormal embryonic development and sensitivity to ionizing radiation. Proc. Natl Acad. Sci. USA, 96, 7376–7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R.E., Ward,T., Lin,S. and Waring,J. (1990) The RAD50 gene, a member of the double strand break repair epistasis group, is not required for spontaneous mitotic recombination in yeast. Curr. Genet., 18, 111–116 [DOI] [PubMed] [Google Scholar]
- Manolis K.G., Nimmo,E.R., Hartsuiker,E., Carr,A.M., Jeggo,P.A. and Allshire,R.C. (2001) Novel functional requirements for non-homologous DNA end joining in Schizosaccharomyces pombe. EMBO J., 20, 210–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill B.J. and Holm,C. (1998) The RAD52 recombinational repair pathway is essential in pol30 (PCNA) mutants that accumulate small single-stranded DNA fragments during DNA synthesis. Genetics, 148, 611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar,A. and Nurse,P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol., 194, 795–823 [DOI] [PubMed] [Google Scholar]
- Muris D.F.R., Vreeken,K., Carr,A.M., Broughton,B.C., Lehmann,A.R., Lohmann,P.H.M. and Pastink,A. (1993) Cloning the RAD51 homologue of Schizosaccharomyces pombe. Nucleic Acids Res., 21, 4586–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris D.F.R., Vreeken,K., Carr,A.M.,Murray,J.M., Smit,C., Lohmann,P.H.M. and Pastink,A. (1996) Isolation of the Schizosaccharomyces pombe rad54 homologue, rhp54, a gene involved in the repair of radiation damage and replication fidelity. J. Cell Sci., 109, 73–81 [DOI] [PubMed] [Google Scholar]
- Muris D.F., Vreeken,K., Schmidt,H., Ostermann,K., Clever,B., Lohman,P.H. and Pastink,A. (1997) Homologous recombination in the fission yeast Schizosaccharomyces pombe: different requirements for the rhp51+, rhp54+ and rad22+ genes. Curr. Genet., 31, 248–254 [DOI] [PubMed] [Google Scholar]
- Murray J.M., Tavassoli,M., al-Harithy,R., Sheldrick,K.S., Lehmann,A.R., Carr,A.M. and Watts,F.Z. (1994) Structural and functional conservation of the human homolog of the Schizosaccharomyces pombe rad2 gene, which is required for chromosome segregation and recovery from DNA damage. Mol. Cell. Biol., 14, 4878–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T.M., Cooper,J.P. and Cech,T.R. (1998) Two modes of survival of fission yeast without telomerase. Science, 282, 493–496 [DOI] [PubMed] [Google Scholar]
- Nimmo E.R., Cranston,G. and Allshire,R.C. (1994) Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J., 13, 3801–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent C.I., Bosco,G., Ross,L.O., Evans,S.K., Salinger,A.P., Moore,J.K., Haber,J.E. and Lundblad,V. (1998) Telomere maintenance is dependent on activities required for end repair of double-strand breaks. Curr. Biol., 8, 657–660 [DOI] [PubMed] [Google Scholar]
- Ohta K. Nicolas,A. Furuse,M. Nabetani,A. Ogawa,H. and Shibata,S. (1998) Mutations in the MRE11, RAD50, XRS2 and MRE2 genes alter chromatin configuration at meiotic DNA double-stranded break sites in premeiotic and meiotic cells. Proc. Natl Acad. Sci. USA, 95, 646–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann K., Lorentz,A. and Schmidt,H. (1993) The fission yeast rad22 gene, having a function in mating-type switching and repair of DNA damages, encodes a protein homolog to Rad52 of Saccharomyces cerevisiae. Nucleic Acids Res., 21, 5940–5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pâques F. and Haber,J.E. (1999) Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev., 63, 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini J.H. (1999) The mammalian Mre11–Rad50–Nbs1 protein complex: integration of functions in the cellular DNA-damage response. Am. J. Hum. Genet., 64, 1264–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini J.H. (2000) The Mre11 complex and ATM: collaborating to navigate S phase. Curr. Opin. Cell Biol., 12, 293–296. [DOI] [PubMed] [Google Scholar]
- Petrini J.H., Bressan,D.A. and Yao,M.S. (1997) The RAD52 epistasis group in mammalian double strand break repair. Semin. Immunol., 9, 181–188. [DOI] [PubMed] [Google Scholar]
- Prabhala G., Rosenberg,G.J. and Käufer,N.F. (1992) Architectural features of pre-mRNA introns in the fission yeast Schizo saccharomyces pombe. Yeast, 8, 171–182 [DOI] [PubMed] [Google Scholar]
- Ranganathan V. et al. (2001) Rescue of a telomere length defect of Nijmegen breakage syndrome cells requires NBS and telomerase catalytic subunit. Curr. Biol., 11, 962–966 [DOI] [PubMed] [Google Scholar]
- Schuchert P. and Kohli,J. (1988) The ade6-M26 mutation of Schizosaccharomyces pombe increases the frequency of crossing over. Genetics, 119, 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples G.J. and Leach,D.R.F. (1995) Structural and functional similarities between the SbcCD proteins of Escherichia coli and the RAD50 and MRE11 (RAD32) recombination and repair proteins of yeast. Mol. Microbiol., 17, 1215–1220 [DOI] [PubMed] [Google Scholar]
- Strunnikov A.V. and Jessberger,R. (1999) Structural maintenance of chromosomes (SMC) proteins. Conserved molecular properties for multiple biological functions Eur. J. Biochem., 263, 6–13 [DOI] [PubMed] [Google Scholar]
- Styrkársdóttir U., Egel,R. and Nielsen,O. (1993) The smt-0 mutation, which abolishes mating-type switching in fission yeast is a deletion. Curr. Genet., 23, 184–186 [DOI] [PubMed] [Google Scholar]
- Tavassoli M., Shayeghi,M., Nasim,A. and Watts,F.Z. (1995) Cloning and characterization of the Schizosaccharomyces pombe rad32 gene: a gene required for repair of double strand breaks and recombination. Nucleic Acids Res., 23, 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y., Taggart,A.K. and Zakian,V.A. (2001) The role of the Mre11–Rad50–Xrs2 complex in telomerase-mediated lengthening of Saccharomyces cerevisiae telomeres. Curr. Biol., 11, 1328–1335 [DOI] [PubMed] [Google Scholar]
- Uhlmann F. and Nasmyth,K. (1998) Cohesion between sister chromatids must be established during DNA replication. Curr. Biol., 8, 1095–101. [DOI] [PubMed] [Google Scholar]
- Usui T., Ogawa,H. and Petrini,J.H. (2001) A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol. Cell, 7, 1255–1266. [DOI] [PubMed] [Google Scholar]
- Varon R. et al. (1998) Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell, 93, 467–476 [DOI] [PubMed] [Google Scholar]
- Walker J.E., Saraste,M., Runswick,M.J. and Gay,N.J. (1982) Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J., 1, 945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y. and Weaver,D.T. (1997) Conditional gene targeted deletion by Cre recombinase demonstrates the requirement for the double-strand break repair Mre11 protein in murine embryonic stem cells. Nucleic Acids Res., 25, 2985–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.D., Kuster,B., Mann,M., Petrini,J.H. and de Lange,T. (2000) Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nature Genet., 25, 347–352. [DOI] [PubMed] [Google Scholar]