Abstract
Growth factor receptor-binding protein-2 (Grb2) plays a key role in signal transduction initiated by Bcr/Abl oncoproteins and growth factors, functioning as an adaptor protein through its Src homology 2 and 3 (SH2 and SH3) domains. We found that Grb2 was tyrosine-phosphorylated in cells expressing BCR/ABL and in A431 cells stimulated with epidermal growth factor (EGF). Phosphorylation of Grb2 by Bcr/Abl or EGF receptor reduced its SH3-dependent binding to Sos in vivo, but not its SH2-dependent binding to Bcr/Abl. Tyr209 within the C-terminal SH3 domain of Grb2 was identified as one of the tyrosine phosphorylation sites, and phosphorylation of Tyr209 abolished the binding of the SH3 domain to a proline-rich Sos peptide in vitro. In vivo expression of a Grb2 mutant where Tyr209 was changed to phenylalanine enhanced BCR/ABL-induced ERK activation and fibroblast transformation, and potentiated and prolonged Grb2-mediated activation of Ras, mitogen-activated protein kinase and c-Jun N-terminal kinase in response to EGF stimulation. These results suggest that tyrosine phosphorylation of Grb2 is a novel mechanism of down-regulation of tyrosine kinase signaling.
Keywords: Abelson/chronic myeloid leukemia/CML/ SH3
Introduction
Growth factor receptor-binding protein-2 (Grb2) functions as an adaptor protein in signal transduction pathways in which protein-tyrosine kinases are involved (Pawson and Scott, 1997). Grb2 is a 25 kDa protein and contains a single Src homology 2 (SH2) domain flanked by two SH3 domains (Lowenstein et al., 1992). The SH2 domain of Grb2 recognizes specific phosphotyrosine-containing motifs on receptor tyrosine kinases such as epidermal growth factor (EGF) receptor and platelet-derived growth factor (PDGF) receptor (Lowenstein et al., 1992), on insulin receptor substrate (IRS)-1 (Skolnik et al., 1993b), on docking proteins such as Shc, FRS-2 and Gab1 (Rozakis-Adcook et al., 1992; Holgodo-Madruga et al., 1996; Kouhara et al., 1997) and on non-receptor tyrosine kinases such as Bcr/Abl and FAK (Pendergast et al., 1993; Puil et al., 1994; Schlaepfer et al., 1994). The SH3 domains of Grb2 bind to proline-rich motifs on the guanine nucleotide releasing factor son-of-sevenless (Sos) (Buday and Downward, 1993; Egan et al., 1993; Li et al., 1993; Rozakis-Adcock et al., 1993), which stimulates GTP binding to Ras, leading to the activation of mitogen-activated protein kinase (MAPK) and other signaling pathways (Baltensperger et al., 1993; Gale et al., 1993; Skolnik et al., 1993a).
The product of the Philadelphia chromosome in human chronic myeloid leukemia (CML), Bcr/Abl, is an oncogenic tyrosine kinase that can transform fibroblasts and hematopoietic cells in vitro (Sawyers et al., 1995) and induce CML-like disease in mice (Li et al., 1999). Tyrosine Y177 of Bcr is phosphorylated in the Bcr/Abl fusion protein and provides a binding site for the SH2 domain of Grb2. Mutation of Y177 to phenylalanine abolishes Grb2 binding to Bcr/Abl (Pendergast et al., 1993; Puil et al., 1994), and a Bcr/Abl Y177F mutant is defective for transformation of fibroblasts (Pendergast et al., 1993; Goga et al., 1995) and induction of CML-like leukemia in mice (Million and Van Etten, 2000). EGF receptor is activated through ligand binding, followed by dimerization and autophosphorylation (Schlessinger and Bar-Sagi, 1994), and Grb2 specifically binds a phosphorylation site in the activated receptor and mediates EGF-stimulated signal transduction (Lowenstein et al., 1992). Dominant-negative forms of Grb2 block transformation by Bcr/Abl and signaling by receptor tyrosine kinases (Clark et al., 1992; Gishizky et al., 1995), demonstrating that Grb2 plays an essential role in both Bcr/Abl- and EGF-stimulated signaling pathways.
Like other adaptor proteins such as Shc and CRK, Grb2 has no catalytic function but serves to mediate the formation of signaling protein complexes. While phosphorylation plays a central role in regulating signaling by Shc and Crk (Feller et al., 1994; van der Geep and Pawson, 1995), Grb2 phosphorylation has not been as well characterized. In the original description of Grb2, phosphorylation of Grb2 was not detected in EGF-stimulated fibroblasts but was observed upon transient overexpression of EGF receptor (Lowenstein et al., 1992). However, it has been recognized subsequently that tyrosine phosphorylation of Grb2 can be detected under physiological conditions, including PDGF treatment of vascular smooth muscle cells (Benjamin et al., 1994) and anti-CD3 stimulation of primary murine T lymphocytes (Ghosh and Miller, 1995). However, the effect of Grb2 phosphorylation on Grb2 function and signaling is not known. In this study, we show that Grb2 can be phosphorylated in vivo by the Bcr/Abl oncoprotein and EGF receptor, and Grb2 tyrosine residue 209 was identified as one of the phosphorylation sites. We also studied the physiological role of Grb2 phosphorylation, and provide evidence that Grb2 phosphorylation plays a negative regulatory role in its signaling pathways.
Results
Grb2 is tyrosine phosphorylated in Bcr/Abl-expressing cells
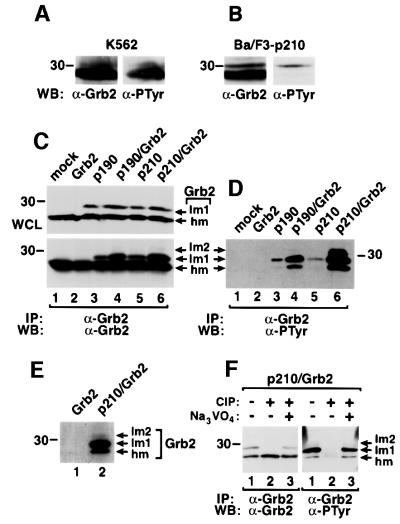
Grb2 protein immunoprecipitated from the human CML cell line K562 consisted of a major species migrating at the expected molecular mass of 25 kDa, and a minor Grb2 species of slightly lower mobility, both of which were reactive with anti-phosphotyrosine (anti-PTyr) antibody (Figure 1A). The same was true of Grb2 in murine Ba/F3 lymphoid cells transformed to interleukin-3 independence by p210 BCR/ABL (Figure 1B), although only the lower mobility species was prominently immunoreactive with anti-PTyr in these cells. To extend these observations, we expressed two forms of Bcr/Abl, p210 or p190, alone or with Grb2 in 293T cells. Three Grb2 species were observed in anti-Grb2 immunoprecipitates under these conditions. The most abundant Grb2 species of apparent molecular mass 25 kDa had the highest mobility (hm) and was present ubiquitously, while a Grb2 species of lower mobility (lm1) was only detected in lysates from Bcr/Abl-expressing cells (Figure 1C). A third Grb2 species (lm2) with even lower mobility was evident in lysates from cells transfected with both BCR/ABL and Grb2 (Figure 1C), but with longer exposure also detected in lysates from cells transfected with p190 or p210 alone (data not shown). When the same membrane was blotted with anti-PTyr antibody, all three Grb2 species were detected in lysates from cells expressing Bcr/Abl (Figure 1D), indicating that Grb2 is tyrosine phosphorylated upon co-expression of Bcr/Abl.
Fig. 1. Phosphorylation of Grb2 in Bcr/Abl-expressing cells. Lysates from K562 leukemia cells (A) or Ba/F3 cells transformed by p210 BCR/ABL (B) were immunoprecipitated with anti-Grb2 antibodies followed by blotting with anti-Grb2 or anti-PTyr antibodies. (C and D) Lysates of 293T cells mock transfected (lane 1) or transfected with Grb2 (lane 2), p190 BCR/ABL (lane 3), p190 BCR/ABL/Grb2 (lane 4), p210 BCR/ABL (lane 5) and p210 BCR/ABL/Grb2 (lane 6) in pMINV were analyzed directly (whole-cell lysates, WCL) by western blot with anti-Grb2 antibodies (C, top panel) or immunoprecipitated with anti-Grb2 antibodies, followed by blotting with anti-Grb2 (C, bottom panel) and anti-PTyr (D) antibodies. Three Grb2 species were observed: the highest mobility species (hm), lower mobility species (lm1) and a minor species of the lowest mobility (lm2). (E) 293T cells transfected with Grb2 (lane 1) or with p210 BCR/ABL/Grb2 (lane 2) were labeled with [32P]orthophosphate, and immunoprecipitated Grb2 proteins analyzed by autoradiography. (F) 293T cells were transfected with p210 BCR/ABL/Grb2, Grb2 proteins were immunoprecipitated with anti-Grb2 antibody, and treated without (lane 1) or with (lane 2) calf intestinal phosphatase (CIP) or with CIP and the phosphatase inhibitor sodium vanadate (1 µM Na3VO4, lane 3), then analyzed by western blot using anti-Grb2 (left panel) and anti-PTyr (right panel) antibodies.
To confirm this observation, we carried out two experiments. First, 293T cells transfected with wild-type Grb2 alone or with both p210 and wild-type Grb2 were labeled in vivo with [32P]orthophosphate, and cell lysates were immunoprecipitated with anti-Grb2 antibody and subjected to SDS–PAGE. Consistent with the anti-PTyr western blotting, [32P]orthophosphate was incorporated into all three species of Grb2 proteins in cells expressing Bcr/Abl (Figure 1E). Secondly, lysate from 293T cells transfected with both p210 and wild-type Grb2 was immunoprecipitated with anti-Grb2 antibody, and the immune complexes were incubated with or without calf intestinal phosphatase (CIP), then analyzed by western blot with anti-Grb2 and anti-PTyr antibodies. The mobility shift and anti-PTyr immunoreactivity of Grb2 were abolished by CIP treatment (Figure 1F).
Direct binding of Grb2 to Bcr/Abl facilitates Grb2 phosphorylation
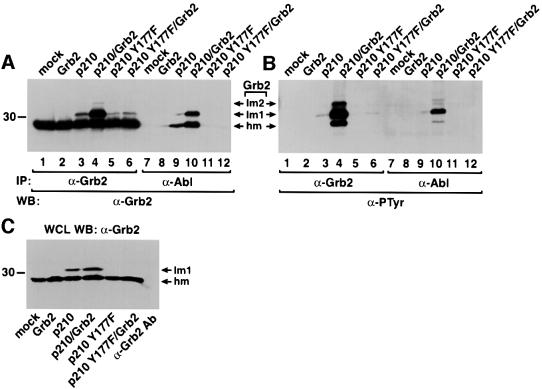
Phosphorylation of Bcr/Abl at Tyr177 provides a binding site for the SH2 domain of Grb2, and this binding is abolished when Tyr177 is changed to phenylalanine. To test whether direct binding between Grb2 and Bcr/Abl is required for Grb2 phosphorylation, we compared the mobility shift and phosphorylation level of Grb2 induced by wild-type p210 with those induced by p210 with the Y177F mutation (p210 Y177F). The amount of Grb2 protein with lower mobility and with tyrosine phosphorylation was significantly decreased in cells transfected with p210 Y177F alone or with both p210 Y177F and wild-type Grb2 (Figure 2), indicating that direct binding of Grb2 to Bcr/Abl greatly stimulates Grb2 phosphorylation, and suggesting that Grb2 is phosphorylated directly by Bcr/Abl. Grb2 failed to co-immunoprecipitate with p210 Y177F (Figure 2), in agreement with previous findings (Pendergast et al., 1993; Puil et al., 1994). Phosphoryl ation of Grb2 did not affect its SH2-dependent binding to Bcr/Abl, because the amount and phosphorylation level of Grb2 proteins that co-immunoprecipitated with p210 wild type were proportional to those immunoprecipitated with anti-Grb2 antibody (Figure 2).
Fig. 2. Direct binding of Grb2 to Bcr/Abl facilitates Grb2 phosphorylation. (A and B) Lysates of 293T cells mock transfected (lanes 1 and 7), or transfected with Grb2 (lanes 2 and 8), p210 BCR/ABL (lanes 3 and 9), p210 BCR/ABL/Grb2 (lanes 4 and 10), p210 BCR/ABL Y177F (lanes 5 and 11) and p210 BCR/ABL Y177F/Grb2 (lanes 6 and 12) were immunoprecipitated with anti-Grb2 (lanes 1–6) and anti-Abl (lanes 7–12) antibodies, and analyzed by western blot using anti-Grb2 (A) and anti-PTyr (B) antibodies. (C) Whole-cell lysates (WCL) from the samples in (A) were analyzed directly by western blotting with anti-Grb2 antibodies, demonstrating an ∼50% increase in total Grb2 levels upon Grb2 overexpression. To exclude the possibility that some or most of the Grb2 signal in the immunoprecipitation/western blots in (A) resulted from detection of light chains of the precipitating antibodies by the secondary antibody, the same amount of anti-Grb2 antibody was added to lysis buffer alone (C, right lane) and processed as in (A), yielding very low signal in the region of Grb2.
Phosphorylation of Grb2 affects its SH3 domain function
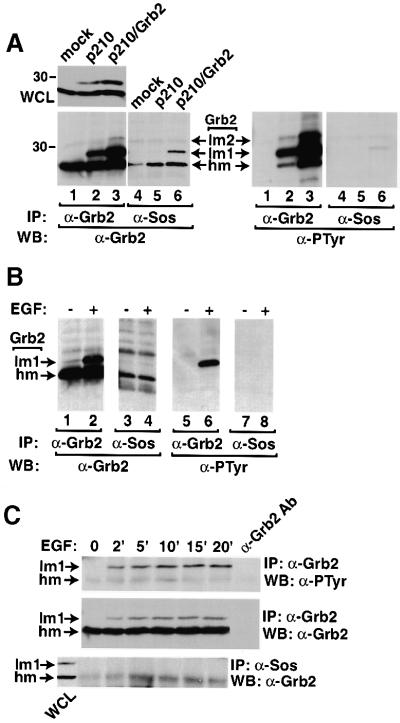
Grb2 has no catalytic activity, but functions as an adaptor through its SH2 and SH3 domains. Because phosphorylation of Grb2 did not affect its SH2 domain binding to Bcr/Abl (Figure 2), we investigated the SH3 domain function of phosphorylated Grb2. As Grb2 forms a complex with Sos through its SH3 domains, we examined the binding between phosphorylated Grb2 and Sos. Grb2 was detected readily in anti-Sos immunoprecipitates from Bcr/Abl-expressing cells, but proportionally less of the lower mobility Grb2 species was observed, and phosphotyrosine was barely detectable on the Grb2 population that co-precipitated with Sos (Figure 3A). These results suggest that tyrosine phosphorylation of Grb2 by Bcr/Abl reduces its binding to effector proteins such as Sos.
Fig. 3. Phosphorylation of Grb2 reduces its binding to Sos in vivo. (A) Lysates of 293T cells mock transfected (lanes 1 and 4), or transfected with p210 BCR/ABL (lanes 2 and 5) or p210 BCR/ABL/Grb2 (lanes 3 and 6) were analyzed directly (WCL) by anti-Grb2 western blot (upper left panel) or immunoprecipitated with anti-Grb2 (lanes 1–3) or anti-Sos (lanes 4–6) antibodies, and analyzed by western blot using anti-Grb2 (left lower panel) and anti-PTyr (right lower panel) antibodies. Densitometric quantitation of the relative amount of hm and lm1 Grb2 species in the left panel, lanes 2 and 3, versus those in lanes 5 and 6 demonstrated that 40–50% of the total Grb2 but only 25% of the Sos-associated Grb2 is found as the lm1 species (data not shown). (B) A431 cells were serum starved (lanes 1, 3, 5 and 7) then stimulated with EGF (200 ng/ml) (lanes 2, 4, 6 and 8) for 10 min. Cell lysates were immunoprecipitated with anti-Grb2 (lanes 1, 2, 5 and 6) or anti-Sos (lanes 3, 4, 7 and 8) antibodies, and analyzed by western blot using anti-Grb2 (left two panels) and anti-PTyr (right two panels) antibodies. (C) A431 cells were starved then stimulated with EGF for the indicated times. Cell lysates were immunoprecipitated with anti-Grb2 (top and middle panels) or anti-Sos (bottom panel) antibodies and blotted with anti-PTyr (top panel) or anti-Grb2 (middle and bottom panels) antibodies. A sample of lysis buffer with anti-Grb2 antibodies was processed (right lane) to demonstrate that very little of the Grb2 signal is attributable to immunoglobulin light chains. A lane containing whole-cell lysate (WCL) from cells stimulated for 10 min was run adjacent to the anti-Sos IPs (left lane, lower panel) to confirm specific co-precipitation of the Grb2 hm species with Sos.
Tyrosine phosphorylation of Grb2 by EGF receptor also inhibits binding to Sos
To extend these observations to growth factor receptor tyrosine kinases, we observed that EGF stimulation of A431 cells induced a change in the mobility and tyrosine phosphorylation of Grb2 (Figure 3B), but phosphorylated Grb2 was virtually undetectable in anti-Sos immunoprecipitates (Figure 3B). Analysis of the kinetics of Grb2 phosphorylation in A431 cells following EGF stimulation demonstrated that tyrosine phosphorylation of Grb2 reached a maximum between 10 to 15 min post-stimulation (Figure 3C), and was associated with a decrease in the amount of Sos-associated Grb2. These results are consistent with those obtained for Bcr/Abl-induced Grb2 phosphorylation, and suggest that both Bcr/Abl and EGF receptor induce Grb2 tyrosine phosphorylation that inhibits the SH3-dependent binding of downstream effector proteins.
Tyr209 is a phosphorylation site on Grb2
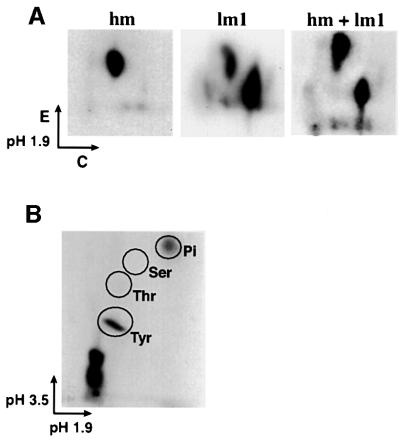
To characterize further the nature of Grb2 phosphorylation, wild-type Grb2 was co-expressed with p210 Bcr/Abl in 293T cells and labeled in vivo with [32P]orthophosphate. The highest mobility Grb2 species (hm) and the major lower mobility species (lm1) (lower and middle arrows in Figure 1C) were isolated by SDS–PAGE and analyzed by two-dimensional tryptic phosphopeptide mapping (Figure 4A) and phosphoamino acid analysis (Figure 4B). The high mobility Grb2 species contained one major tryptic phosphopeptide, whereas the lower mobility species (lm1) contained two, one of which co-migrated with the phosphopeptide from the high mobility form of Grb2 (Figure 4A). Phosphotyrosine was found in both the high (data not shown) and the lower (Figure 4B) mobility species of Grb2, but no phosphothreonine or phosphoserine was detected, suggesting that Grb2 is phosphorylated principally on tyrosine in Bcr/Abl-expressing cells.
Fig. 4. Characterization of Grb2 phosphorylation. (A) Tryptic phosphopeptide mapping of phosphorylated Grb2. 293T cells were transfected with p210 BCR/ABL/Grb2, and 48 h later were incubated with 1 mCi of [32P]orthophosphate in 2 ml of phosphate-free DMEM for 3 h. Cell lysate was immunoprecipitated with anti-Grb2 antibody, separated by SDS–PAGE and blotted onto a nitrocellulose membrane. The highest mobility (hm) and lower mobility (lm1) species of Grb2 proteins containing phosphorylated Grb2 labeled with [32P]ortho phosphate were cut out of the membrane, digested with TPCK-treated trypsin and subjected to two-dimensional peptide mapping by electrophoresis in pH 1.9 buffer for the first dimension, followed by thin-layer chromotography for the second dimension, as described (Boyle et al., 1991). (B) Phosphoamino acid analysis of phosphorylated Grb2. The lower mobility species (lm1) of Grb2 proteins labeled with [32P]orthophosphate (A) was treated with 6 M HCl, and subjected to electrophoresis in pH 1.9 buffer for the first dimension, and in pH 3.5 buffer for the second dimension. Tyr, tyrosine; Thr, threonine; Ser, serine; Pi, free phosphate.
To identify the phosphotyrosines in Grb2, tryptic peptides from phosphorylated Grb2 proteins were analyzed by HPLC and mass spectrometry. The peptide NYVTPVNR, containing Tyr209 within the C-terminal SH3 domain, was found to be phosphorylated based on the change of its molecular mass from 962.5 to 1042.5, and this phosphopeptide was present in tryptic digests from both the high (hm) and lower (lm1) mobility Grb2 species. Therefore, it is likely that NpYVTPVNR is the common phosphopeptide identified by two-dimensional tryptic phosphopeptide mapping of the two Grb2 species (Figure 4A), but this phosphorylation event alone does not account for the reduced mobility of Grb2. Mass spectrometry of phosphorylated Grb2 also suggested tyrosine phosphorylation of Grb2 at Tyr7 and Tyr52 (data not shown), but definitive identification was not possible due to low stoichiometry or low ionization efficiency of the phosphorylated peptides.
Mutational analysis of Grb2 tyrosine phosphorylation
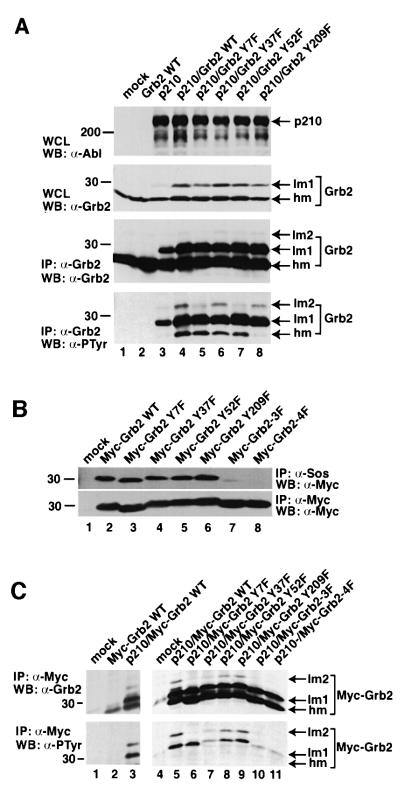
Human Grb2 has a total of seven tyrosine residues: three in the N-terminal SH3 domain (Tyr7, Tyr37 and Tyr52), two in the SH2 domain (Tyr118 and Tyr134), one between SH2 and the C-terminal SH3 domain (Tyr160) and one in the C-terminal SH3 domain (Tyr209). To characterize Grb2 phosphorylation further, we mutated each of the four tyrosines in the SH3 domains of Grb2 to phenylalanine, and co-expressed each Grb2 mutant (Y7F, Y37F, Y52F or Y209F) with p210 Bcr/Abl in 293T cells. When co-expressed with Bcr/Abl, these four Grb2 mutants exhibited a similar alteration in electrophoretic mobility and similar levels of tyrosine phosphorylation, except that the tyrosine phosphorylation of the highest mobility Grb2 species was dramatically lower for Grb2 Y209F, similar to the phosphorylation level of endogenous Grb2 in cells transfected with p210 Bcr/Abl alone (Figure 5A). In addition, tyrosine phosphorylation of the lowest mobility species was reduced for both the Grb2 Y7F and Y52F mutants. These results confirm that Tyr209 is the only site of phosphorylation of the high mobility Grb2 species.
Fig. 5. Multiple tyrosine phosphorylation sites on Grb2. (A) Lysates of 293T cells mock transfected (lane 1) or transfected with wild-type Grb2 (lane 2), p210 BCR/ABL (lane 3), p210 BCR/ABL/Grb2 WT (lane 4), p210 BCR/ABL/Grb2 Y7F (lane 5), p210 BCR/ABL/Grb2 Y37F (lane 6), p210 BCR/ABL/Grb2 Y52F (lane 7) and p210 BCR/ABL/Grb2 Y209F (lane 8) were loaded directly (top panel) or immunoprecipitated with anti-Grb2 antibody (middle and bottom panels), and analyzed by SDS–PAGE and western blotting using anti-Abl (top panel), anti-Grb2 (middle panel) and anti-PTyr (bottom panel) antibodies. (B) Effect of tyrosine to phenylalanine mutations of Grb2 on binding to Sos in vivo. Lysates of 293T cells mock transfected (lane 1) or transfected with wild-type Myc-Grb2 (lane 2), Myc-Grb2 Y7F (lane 3), Myc-Grb2 Y37F (lane 4), Myc-Grb2 Y52F (lane 5), Myc-Grb2 Y209F (lane 6), Myc-Grb2 Y7F/Y52F/Y209F (3F) (lane 7) and Myc-Grb2 Y7F/Y37F/Y52F/Y209F (4F) were immunoprecipitated with anti-Sos (top panel) or anti-Myc (bottom panel) antibodies, and analyzed by western blot using anti-Myc antibody. (C) Lysates of 293T cells mock transfected (lanes 1 and 4), transfected with wild-type Myc-Grb2 alone (lane 2) or co-transfected with p210 BCR/ABL/Myc-Grb2 WT (lane 3 and 5), p210 BCR/ABL/Myc-Grb2 Y7F (lane 6), p210 BCR/ABL/Myc-Grb2 Y37F (lane 7), p210 BCR/ABL/Myc-Grb2 Y52F (lane 8), p210 BCR/ABL/Myc-Grb2 Y209F (lane 9), p210 BCR/ABL/Myc-Grb2 Y7F/Y52F/Y209F (3F) (lane 10) and p210 BCR/ABL/Myc-Grb2 Y7F/Y37F/Y52F/Y209F (4F) (lane 11) in pcDNA were immunoprecipitated with anti-Myc (9E10) antibody, and analyzed by western blot using anti-Grb2 (top panel) and anti-PTyr (bottom panel) antibodies.
To analyze the Grb2 tyrosine mutants in vivo without detecting endogenous Grb2, we added a Myc epitope tag to the N-terminus of each Grb2 Y-to-F mutant, and also made Myc-tagged Grb2 Y7F/Y52F/Y209F triple (3F) and Grb2 Y7F/Y37F/Y52F/Y209F quadruple (4F) mutants. Myc-tagged wild-type Grb2 was immunoprecipitated specifically with anti-Myc monoclonal antibody and efficiently bound to Sos in vivo (Figure 5B). Similar results were obtained for Grb2 proteins with single Y-to-F substitutions (Y7F, Y37F, Y52F or Y209F), indicating that these mutations individually do not affect SH3-dependent ligand binding by Grb2 in the absence of an activated tyrosine kinase. However, Sos binding by the Grb2 3F and 4F mutants (Figure 5B) and by a Grb2 Y7F/Y209F double mutant (data not shown) was significantly impaired, demonstrating that multiple tyrosine to phenylalanine mutations may compromise the ability of the mutant Grb2 SH3 domains to bind proline-rich ligands. Co-expression of Bcr/Abl induced tyrosine phosphorylation and a mobility shift of wild-type Myc-tagged Grb2 (Figure 5C). When the Myc-tagged Grb2 single Y-to-F mutants were co-expressed with Bcr/Abl, there was no significant change in the resulting mobility shift and tyrosine phosphorylation, except that the lowest mobility species (lm2) was not observed with the Grb2 Y7F mutant (Figure 5C), suggesting that Y7 is also a site of in vivo tyrosine phosphorylation of Grb2 in this co-expression assay. The Grb2 triple and quadruple Y to F mutants exhibited greatly reduced tyrosine phosphorylation by Bcr/Abl, indicating that the majority of the phosphorylation of Grb2 by Bcr/Abl occurs at sites within the SH3 domains. Somewhat surprisingly, the lm1 species was still observed upon co-expression of the Grb2 4F mutant and Bcr/Abl, suggesting that SH3 domain tyrosine phosphorylation is not responsible for this alteration in electrophoretic mobility.
Phosphorylation of Tyr209 in the C-terminal SH3 domain of Grb2 reduces its binding to Sos
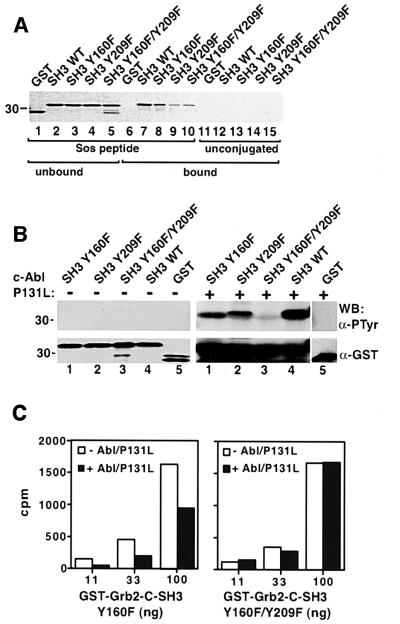
Tyr209 of Grb2 is a highly conserved residue among different SH3 domains, and forms part of the ligand-binding surface (Feng et al., 1994). Because phosphorylation of Grb2 reduced its binding to Sos in vivo (Figure 3) and Tyr209 was identified as a phosphorylation site, we examined whether phosphorylation of Tyr209 contributes to the reduced binding of Grb2 to Sos in an in vitro bind ing assay. We made fusion proteins of GST with the C-terminal SH3 domain of Grb2, and with SH3 mutants where Tyr160, Tyr209 or both were mutated to phenylalanine. A synthetic peptide containing the proline-rich PPVPPR motif of Sos was covalently coupled to agarose beads and employed in an in vitro binding assay with four different GST–Grb2-C-SH3 proteins (GST–Grb2-C-SH3 WT, Y160F, Y209F and Y160F/Y209F). All four fusion proteins bound to Sos peptide beads (Figure 6A, lanes 7–10) but not to unconjugated control beads (Figure 6A, lanes 12–15), while GST alone did not bind to either (Figure 6A, lanes 6 and 11). When incubated with puri fied, activated c-Abl kinase, the GST–Grb2-C-SH3 WT fusion protein was tyrosine phosphorylated efficiently (Figure 6B). SH3 fusion proteins with either Y160F or Y209F were also tyrosine phosphorylated by c-Abl but to a lesser extent, while phosphorylation of the Y160F/Y209F double mutant SH3 fusion protein was minimal, similar to the GST control (Figure 6B). The stoichiometry of phosphorylation of the GST–Grb2-C-SH3 Y160F fusion protein, judged from experiments where trace amounts of [γ-32P]ATP were included, was between 30 and 50% (data not shown).
Fig. 6. Grb2 phosphorylation at Tyr209 abolishes its binding to Sos. (A) Unphosphorylated GST–Grb2-C-SH3 WT or mutant fusion proteins bind Sos peptide beads. GST proteins were mixed with Affigel agarose beads coupled with Sos peptide (lanes 1–10) or unconjugated (lanes 11–15). Unbound (lanes 1–5) and bound (lanes 6–15) GST proteins were subjected to SDS–PAGE, and visualized by Coomassie Blue staining. (B) GST fusion proteins containing wild-type Grb2 C-terminal SH3 domain (Grb2-C-SH3) (lane 4), Y160F (lane 1), Y209F (lane 2), Y160F/Y209F (lane 3) or GST alone (lane 5) either untreated (left panels) or after phosphorylation by purified c-Abl P131L kinase (right panels) were analyzed by western blot using anti-PTyr (top panels) and anti-GST (bottom panels) antibodies. (C) GST–Grb2-C-SH3 Y160F (left panel) and Y160F/Y209F (right panel) were labeled with 32P using heart muscle kinase, then treated with or without purified c-Abl P131L kinase in the presence of non-radioactive ATP. Increasing amounts of each GST–Grb2 fusion protein were mixed with the same amount of Sos peptide beads, and total radioactivity (c.p.m.) associated with the beads in each binding reaction was measured. The binding of the phosphorylated GST–Grb2 Y160F single mutant to Sos beads was not tested because this residue is outside the SH3 domain, does not participate in ligand binding and is not phosphorylated significantly in vivo (Figure 5C).
To assess the effect of phosphorylation at Tyr209 on SH3 ligand binding, the GST–Grb2-C-SH3 Y160F and Y160F/Y209F fusion proteins were labeled with [γ-32P] ATP using heart muscle kinase (HMK) at a serine residue within the GST–SH3 linker region (see Materials and methods), followed by incubation in the presence or absence of purified activated c-Abl kinase and non-radioactive ATP. Phosphorylation of Tyr209 by c-Abl reduced binding of the GST–Grb2-C-SH3 Y160F fusion protein to Sos peptide beads by up to 50%, while incubation with c-Abl had no effect on the binding of the Y160F/Y209F SH3 mutant (Figure 6C). These results demonstrate that phosphorylation of Grb2 at Tyr209 directly inhibits the binding of Sos.
Expression of Grb2 Y209F enhances transformation of NIH-3T3 fibroblasts and ERK activation by p210 Bcr/Abl
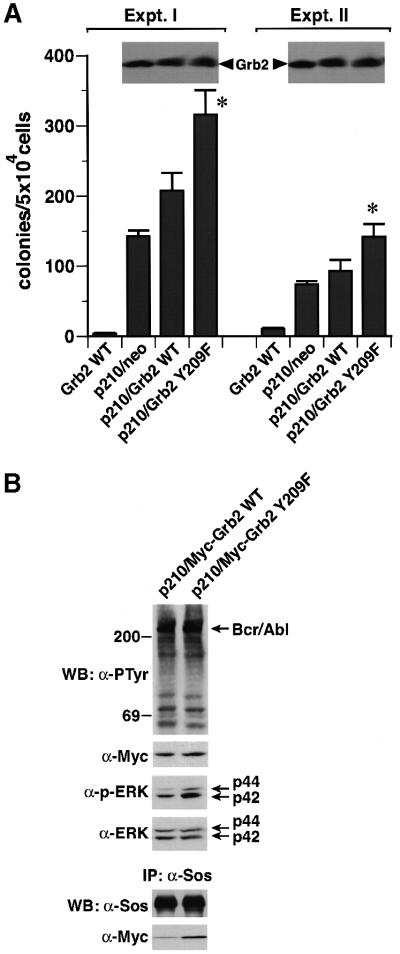
Direct binding of Grb2 to Bcr/Abl, through a Grb2 SH2–Bcr Y177 interaction, is required for Bcr/Abl-induced fibroblast transformation (Pendergast et al., 1993; Goga et al., 1995). If Grb2 phosphorylation plays a negative regulatory role in its signaling pathways, phosphorylation at Tyr209 should diminish Bcr/Abl-induced cell transformation. To test this hypothesis, we transduced NIH-3T3 fibroblasts with retroviruses expressing p210 Bcr/Abl alone, or co-expressing p210 and either wild-type Grb2 or the Grb2 Y209F mutant, and assessed anchorage-independent growth in soft agar. p210 Bcr/Abl alone efficiently transformed NIH-3T3 cells, while co-expression of wild-type Grb2 resulted in significantly increased soft agar colonies, as previously reported (Gishizky et al., 1995). Importantly, co-expression of Grb2 Y209F further increased p210 Bcr/Abl-induced transformation by ∼40–50% (Figure 7A). Co-transduction of Myc-tagged Grb2 Y209F with Bcr/Abl also increased Sos-associated Myc-Grb2 and activation of ERK, compared with co-transduction of wild-type Myc-Grb2 (Figure 7B). These results suggest that phosphorylation of Grb2 at Tyr209 plays a negative regulatory role in cell signaling and transformation induced by p210 Bcr/Abl.
Fig. 7. Mutation of Grb2 Tyr209 potentiates Bcr/Abl-induced transformation and ERK activation. (A) NIH-3T3 fibroblasts transduced with retrovirus expressing wild-type Grb2 alone or co-expressing p210 and either a neomycin resistance gene (neo), wild-type Grb2 or Grb2 Y209F were plated in soft agar and transformed colonies counted 3 weeks later (mean ± SE from triplicate plates). Two independent experiments with different retroviral stocks were performed. The increase in soft agar colonies with co-transduction of Grb2 Y209F relative to wild-type Grb2 in both experiments was statistically significant (asterisks, P <0.01, unpaired t-test). Lysates from the p210-transduced populations were blotted with anti-Grb2 antibodies (inserts) to demonstrate equivalent levels of Grb2 overexpression. (B) Cells co-transduced with p210 Bcr/Abl and either Myc-tagged wild-type Grb2 or the Y209F mutant were analyzed by direct western blotting of lysates with anti-PTyr, anti-Myc, anti-phospho-ERK and anti-pan-ERK antibodies (top four panels), or by immunoprecipitation with anti-Sos antibodies and blotting with anti-Sos and anti-Myc antibodies (bottom panels).
Phosphorylation of Grb2 at Tyr209 down-regulates EGF-mediated Ras activation
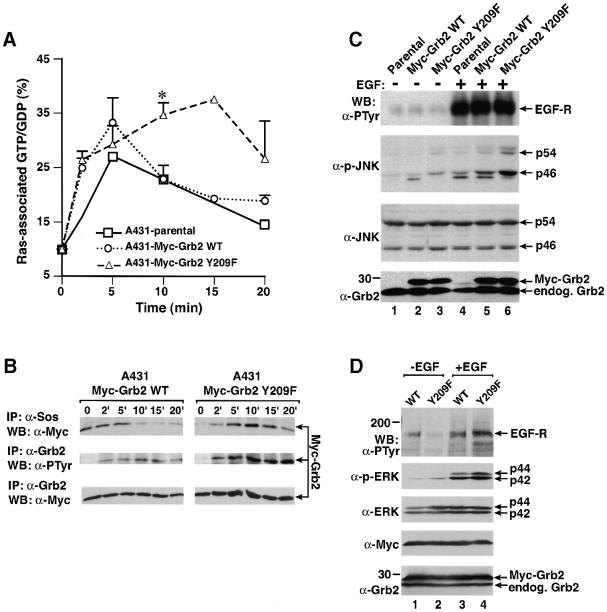
Because Grb2 mediates growth factor-stimulated Ras activation through Sos (Gale et al., 1993) and phosphorylation of Grb2 at Tyr209 reduces its binding to Sos (Figure 6C), it is plausible that phosphorylation of Grb2 might act to limit or reverse the activation of Ras following growth factor stimulation. To test this, we stably expressed Myc-tagged wild-type Grb2 or Myc-tagged Grb2 Y209F in A431 cells at levels equivalent to endogenous Grb2, and analyzed GTP loading of Ras upon treatment with EGF. The GTP fraction of Ras-bound guanine nucleotide in parental A431 cells increased rapidly after EGF stimulation, reached a maximum level by 5 min and declined by 10 min (Figure 8A). Stable expression of Myc-tagged wild-type Grb2 accentuated the maximal level of GTP loading but did not alter the kinetics of activation, in agreement with previous observations (Gale et al., 1993). In cells stably expressing the Myc-Grb2 Y209F mutant, the initial rate of Ras activation was unchanged, but the duration of Ras activation was significantly prolonged, as GTP-Ras remained at maximal levels at 10 and 15 min post-EGF stimulation (Figure 8A). Tyrosine phosphorylation of Myc-tagged Grb2 in these cells reached a maximum at 10–15 min post-EGF stimulation (Figure 8B). In the A431-Myc-Grb2 wild-type cells, this coincided with decreased association of wild-type Myc-Grb2 with Sos (Figure 8B) and down-regulation of Ras GTP levels (Figure 8A). In contrast, the level of Sos-associated Myc-Grb2 Y209F remained high at 10 and 15 min (Figure 8B). These results suggest that phosphorylation of Grb2 at Tyr209 down-regulates Ras activation after EGF stimulation by interfering with Sos binding.
Fig. 8. Mutation of Grb2 Tyr209 potentiates and prolongs EGF signaling pathways. (A) Ras activation. A431 parental cells and populations stably expressing wild-type Myc-Grb2 and Myc-Grb2 Y209F were starved, labeled with [32P]orthophosphate and stimulated with EGF (200 ng/ml) for 0, 2, 5, 10, 15 or 20 min, and Ras-associated guanine nucleotides analyzed as described in Materials and methods. Bars indicate positive SE for the A431-Myc-Grb2 WT and A431-Myc-Grb2 Y209F cells; the 15 min time point was only done once. The difference in Ras GTP levels at 10 min between wild-type Grb2 and Grb2 Y209F cells (asterisk) is statistically significant (P ≤0.05, unpaired t-test). (B) Kinetic analysis of Grb2 tyrosine phosphorylation and Sos association from the A431-Myc-Grb2 WT- and A431-Myc-Grb2 Y209F-expressing cells in (A). Cells were starved and stimulated with EGF for the indicated times and lysates analyzed by immunoprecipitation with anti-Sos (top panel) or anti-Grb2 (middle and bottom panels) antibodies and blotting with anti-Myc (top and bottom panels) or anti-PTyr (middle panel) antibodies. (C) JNK activation. Parental (lanes 1 and 4), Myc-Grb2 WT- (lane 2 and 5) and Myc-Grb2 Y209F-expressing (lanes 3 and 6) A431 cells were starved (lanes 1–3) and stimulated with EGF (200 ng/ml, lanes 4–6), and lysates analyzed by western blot using anti-PTyr antibody for detecting EGF-stimulated phosphorylation of EGF receptor (top panel), anti-phospho-JNK antibody (second panel from the top), anti-JNK antibody (third panel from the top) or anti-Grb2 antibody (bottom panel). (D) MAPK/ERK activation. 293T cells transfected with EGF receptor and either Myc-tagged wild-type Grb2 (lanes 1 and 3) or Myc-tagged Grb2 Y209F (lanes 2 and 4) were starved (lanes 1 and 2) or stimulated with EGF for 10 min (200 ng/ml, lanes 3 and 4), and lysates were analyzed by western blot using anti-PTyr antibody for detection of phosphorylated EGF receptor (top panel), anti-phospho-ERK antibody (second panel from the top), anti-ERK antibody (third panel from the top), anti-Myc antibody (fourth panel from the top) and anti-Grb2 antibody (bottom panel).
Expression of Grb2 Y209F increases and prolongs EGF-stimulated JNK and MAPK/ERK activation
To explore further the biochemical effects of Tyr209 phosphorylation of Grb2, we examined EGF-induced JNK activation (Logan et al., 1997) using an activation-specific anti-phospho-JNK antibody. Stimulation of parental A431 cells resulted in activation of the p46 and, to a lesser extent, the p54 isoforms of JNK (Figure 8C). EGF-induced JNK activation was potentiated slightly by stable expression of wild-type Grb2, but increased further by >50% in cells expressing the Grb2 Y209F mutant (Figure 8C). The enhanced activation of JNK persisted with prolonged EGF stimulation, and was still prominent after 40 min (data not shown). Similar results were obtained in a murine EGF receptor-expressing cell line, B82L (data not shown).
We found that A431 cells had unacceptably high levels of phosphorylated MAPK even after serum and growth factor starvation (data not shown), and therefore studied the effect of the Grb2 Y209F mutant on MAPK activation in transfected 293T cells. The EGF-induced MAPK activation of the p42 and p44 isoforms of MAPK was significantly enhanced in the presence of the Myc-Grb2 Y209F mutant, >3-fold relative to wild-type Grb2-expressing cells (Figure 8D). Collectively, these studies suggest that phosphorylation of Grb2 at Tyr209 acts to limit the duration and extent of activation of JNK and MAPK, two signaling pathways downstream of Ras, upon EGF stimulation.
Discussion
The intracellular signals induced by binding of growth factors to their receptors must be tightly controlled in order to prevent inappropriate responses. In particular, the cell must have efficient mechanisms for limiting the duration of a signaling response in time. In the signaling cascade that leads from growth factor receptors to MAPKs such as JNK and ERK, there are several known mechanisms for terminating a growth factor-induced signal. These include the intrinsic GTPase activity of Ras for hydrolysis of bound GTP to GDP, and Ras GTPase-activating proteins (GAPs) that stimulate this activity. Some growth factors such as insulin induce serine/threonine phosphorylation of Sos that coincides with dissociation of Grb2–Sos complexes and inactivation of Ras (Cherniack et al., 1995). Lastly, specific phosphatases such as MKP-1 (Sun et al., 1993) can deactivate MAPK members directly.
Here, we provide evidence for an additional and novel mechanism of regulation of tyrosine kinase signaling, phosphorylation of Grb2 on tyrosine. We found that Grb2 is highly tyrosine phosphorylated in leukemia cells expressing the Bcr/Abl fusion protein. We also detected tyrosine phosphorylation of Grb2 in response to EGF stimulation in A431 fibroblasts. A previous report did not observe significant tyrosine phosphorylation of Grb2 in EGF-stimulated HER14 fibroblasts (Lowenstein et al., 1992), which express ∼5-fold fewer EGF receptors. Although it is likely that Grb2 is not an efficient or major substrate for EGF receptor, the steady-state level of Grb2 tyrosine phosphorylation in EGF-stimulated fibroblasts may also reflect rapid dephosphorylation or even degradation of the protein. It is also possible that Grb2 tyrosine phosphorylation may be more prominent in different tissues in response to various stimuli. In support of this, tyrosine phosphorylation of Grb2 has also been observed in response to PDGF stimulation in vascular smooth muscle cells (Benjamin et al., 1994), and upon activation of CD3 in T cells (Ghosh and Miller, 1995).
Tyrosine phosphorylation might regulate the function of an adaptor protein such as Grb2 through several different mechanisms. Phosphorylation of Tyr225 in the c-Crk adaptor protein, principally by the c-Abl kinase in vivo (Feller et al., 1994), creates a site for intramolecular binding of the Crk SH2 domain and results in inactivation of the adaptor functions of Crk. Tyrosine phosphorylation can also directly inhibit the phosphotyrosine-binding function of an SH2 domain (Couture et al., 1996). However, Grb2 lacks the C-terminal extension of Crk and has a centrally located SH2 domain, making it implausible that the Grb2 SH2 domain could bind intramolecularly to a phosphorylated tyrosine. Furthermore, tyrosine-phosphorylated Grb2 bound efficiently to Bcr/Abl at the Tyr177 site in Bcr (Figure 2), demonstrating that the SH2 domain of phosphorylated Grb2 is accessible and capable of binding phosphotyrosine ligands. Rather, our data suggest that the principal effect of tyrosine phosphorylation of Grb2 is to interfere with the ligand-binding function of the SH3 domains, because the SH3-dependent binding of Sos to Grb2 in vivo was greatly diminished by tyrosine phosphorylation.
The crystal structure of Grb2 has been solved (Maignan et al., 1995), and the N- and C-terminal SH3 domains form β-barrels, as described for other SH3 domains. The PxxP ligand-binding surface of SH3 domains is formed by three pockets whose walls are formed by hydrophobic amino acids that are highly conserved among different SH3 domains. Mass spectrometric data confirm that Tyr209 in the C-terminal SH3 domain of Grb2 is an in vivo site of tyrosine phosphorylation by Bcr/Abl. This residue is extremely highly conserved, is a tyrosine or rarely phenylalanine in nearly all sequenced SH3 domains, and forms part of the wall between pockets I and II that directly contact the +1 and +4 prolines of type I and II PxxP ligands (Feng et al., 1994). Interestingly, the homologous tyrosine (Tyr138) of the c-Src SH3 domain is a site of phosphorylation by PDGF receptor, and phosphorylation at this site reduces binding of peptide ligands by Src SH3 in vitro (Broome and Hunter, 1997), although the in vivo consequences of this event are unknown. Similarly, we demonstrated that phosphorylation of Tyr209 directly inhibits the binding of a proline-rich Sos peptide by the Grb2 C-terminal SH3 domain. We also found evidence of phosphorylation of additional tyrosines in the N-terminal SH3 domain of Grb2, particularly Tyr7. This tyrosine is also highly conserved among SH3 domains and forms part of the ligand-binding surface. The homologous tyrosine (Tyr223) in the SH3 domain of Btk is autophosphorylated during activation of Btk, which may negatively regulate ligand binding and Btk function (Park et al., 1996).
The Bcr/Abl tyrosine kinase has been shown to activate many of the same signaling pathways stimulated by growth factors and cytokines (Sawyers, 1997). Ras is required for transformation of fibroblasts and bone marrow by Bcr/Abl (Sawyers et al., 1995), while dominant-negative forms of Grb2 inhibit transformation by Bcr/Abl (Gishizky et al., 1995) and mutation of the binding site for the SH2 domain of Grb2 on Bcr/Abl impairs transformation (Goga et al., 1995) and leukemogenesis (Million and Van Etten, 2000) by Bcr/Abl. These observations demonstrate that Grb2 plays a predominantly positive role in signaling and transformation by Bcr/Abl. However, direct binding of Grb2 to Bcr/Abl also facilitates its tyrosine phosphorylation, which we propose reflects activation of a physiological negative regulatory mechanism by this oncogenic tyrosine kinase. Because only a fraction of Grb2 is phosphorylated, signaling by Grb2 is not blocked completely but merely attenuated, as revealed by the increased ERK activation and transformation mediated by the phosphorylation-resistant Grb2 Y209F mutant. Pharmacological inhibition of Bcr/Abl tyrosine kinase activity is now being tested as a therapeutic strategy in human CML (Druker et al., 2001). Because some Bcr/Abl phosphorylation events, such as Grb2 phosphorylation, are inhibitory for cell transformation, it is possible that untoward effects might be observed in vivo if Bcr/Abl kinase inhibitors affected the phosphorylation of some substrates more than others. Mutations in Grb2 Tyr209 might also play a role in progression of CML to the more aggressive blast crisis phase.
The biochemical consequences of Grb2 tyrosine phosphorylation were investigated by examining the effect of expression of Grb2 proteins with mutations in potential tyrosine phosphorylation sites on EGF-induced Ras, JNK and MAPK activation. In resting fibroblasts, a significant fraction of Grb2 protein is constitutively complexed with Sos, and EGF-induced dimerization and autophosphorylation of the EGF receptor generate binding sites for the Grb2 SH2 domain, resulting in recruitment of Grb2–Sos complexes to the membrane, where they stimulate GTP loading of Ras (Schlessinger and Bar-Sagi, 1994), followed by activation of the Raf–MAPK cascade. The precise mechanism of JNK activation in response to EGF stimulation is not as clear. JNK may be activated through a phosphatidylinositol 3-kinase-dependent pathway (Logan et al., 1997), which may be downstream of Grb2–Sos and Ras (Downward et al., 1984), or Grb2 may interact with and directly activate MEKK1, a Jun kinase kinase (Pomerance et al., 1998). We observed that EGF-induced activation of both Ras and JNK was accentuated and prolonged in fibroblasts stably expressing a Grb2 Y209F mutant, while EGF-induced MAPK activation was enhanced similarly in 293T cells transiently expressing Grb2 Y209F and EGF receptor.
It is likely that both SH3 domains of Grb2 are required for efficient binding to the C-terminal proline-rich domain of Sos (Egan et al., 1993; Li et al., 1993), suggesting that monophosphorylation of the C-terminal Grb2 SH3 domain at Tyr209 would be sufficient to block binding of Sos. We also tested the Myc-Grb2 Y7F, Y37F and Y52F N-terminal SH3 domain mutants and found that expression of Myc-Grb2 Y7F, but not Y37F or Y52F, significantly potentiated EGF-stimulated JNK activation relative to wild-type Myc-Grb2 (data not shown), suggesting that Tyr7 may also be phosphorylated and impair Grb2 function in response to EGF. It might be expected that prevention of EGF-induced tyrosine phosphorylation of both the N- and C-terminal SH3 domains of Grb2 would accentuate further the signaling response to EGF. However, we observed a decrease rather than an enhancement of JNK activation in cells expressing a Grb2 Y7F/Y209F double mutant, but this mutant exhibited very low baseline binding to Sos, suggesting that it might be functioning as a dominant-negative in this assay (data not shown). Although an alteration in the electrophoretic mobility of Grb2 first alerted us to the fact that Grb2 is tyrosine phosphorylated in Bcr/Abl-expressing cells, a Grb2 mutant with phenylalanine substitutions at all four SH3 tyrosine residues still exhibited a slower mobility lm1 species when co-expressed with Bcr/Abl. The loss of this species upon treatment of Grb2 with CIP (Figure 1F) suggests that tyrosine phosphorylation at other sites or serine/threonine phosphorylation (despite the low phosphoserine/threonine content detected in Figure 4B) is responsible. Although understanding the precise mechanism of the change in mobility of Grb2 in Bcr/Abl-expressing cells will require further study, this is not central to the conclusions of this study.
Collectively, our results strongly support the hypothesis that phosphorylation of Grb2 at Tyr209 and perhaps Tyr7 inhibits the binding of ligands such as Sos or MEKK1, limiting or reversing the activation of downstream signaling pathways. Because Ras activation is still reversible in cells expressing the Grb2 Y209F mutant, there are certainly other mechanisms for terminating EGF-induced signals. While the data here clearly establish a role for Grb2 tyrosine phosphorylation in Bcr/Abl transformation, the relative importance of Grb2 phosphorylation as a physiologically negative regulatory mechanism in receptor tyrosine kinase signaling is more difficult to assess. Grb2 deficiency causes embryonic lethality in mice shortly after implantation due to lack of endoderm differentiation (Cheng et al., 1998) and, although Grb2–/– embryonic stem cells exist, they are not amenable to biochemical analysis of EGF signaling. The lack of cell lines that do not express Grb2 required us to express the Grb2 mutants in vivo at similar levels to endogenous Grb2, which may decrease the impact of the mutant Grb2 on signaling events. Introduction of the Grb2 phosphorylation site mutants into the mouse germline by homologous recombination (Saxton et al., 2001) may be one approach to addressing the role of Grb2 tyrosine phosphorylation in physiology and development.
In conclusion, we have demonstrated here that the SH3 domains of the Grb2 adaptor protein are phosphorylated by the Bcr/Abl oncoprotein of human CML and by the EGF receptor. Tyrosine phosphorylation of the Grb2 SH3 domains impairs the binding of proline-rich ligands such as Sos, and negatively regulates the activation of downstream signaling pathways, including Ras, MAPK/ERK and JNK. Inhibition of SH3 function by tyrosine phosphorylation thus represents a novel regulatory mechanism that may be broadly utilized in tyrosine kinase signaling.
Materials and methods
DNA constructs
EcoRI fragments of BCR/ABL and BCR/ABL Y177F from pMSCVneo (Million and Van Etten, 2000) were cloned into EcoRI–MunI sites of the retroviral expression vector pMINV (Hawley et al., 1996) containing an internal ribosomal entry site (IRES). For cloning of both BCR/ABL and Grb2 into the same vector, pMINV was digested with NcoI, fitted with BamHI linkers, and wild-type or mutant Grb2 was introduced into the 3′ position as a BamHI fragment. All Grb2 tyrosine to phenylalanine point mutations were made by enzymatic inverse PCR (Hughes and Andrews, 1996) and were confirmed by DNA sequencing.
Cells and cell culture
A431 cells (kind gift of Dr Tom Kirchhausen, Center for Blood Research, Harvard Medical School, Boston, MA) were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% normal bovine serum. A431 cells that stably express Myc-tagged wild-type Grb2 (Myc-Grb2) or Grb2 with mutation of Tyr209 to phenylalanine (Myc-Grb2 Y209F) were made by transduction with amphitrophic MINV retrovirus stock co-expressing Myc-Grb2 or Myc-Grb2 Y209F and a neomycin resistance gene. Polyclonal populations of G418-resistant cells were selected and used for analysis.
Transfection, immunoprecipitation and western blotting
293T cells were plated at 2.5 × 106 cells per 10 cm plate, and transfected by the calcium phosphate method using 10 µg of plasmid DNA for pMINV-IRES-Grb2 and pMINV-BCR/ABL-IRES-Grb2. For co-transfection using pcDNA3, 10 µg of pcDNA3-EGF receptor and 2 µg of pcDNA-Myc-Grb2 were used. Preparation of cell lysates, immunoprecipitation and western blotting were as previously described (Million and Van Etten, 2000). Rabbit anti-Grb2, rabbit anti-Sos and mouse anti-Myc (9E10) antibodies were obtained from Santa Cruz Biotechnology, mouse anti-phosphotyrosine (4G10) antibody was obtained from Upstate Biotechnology, and mouse anti-Abl monoclonal antibody 3F12 was the gift of Dr Ravi Salgia, Dana-Farber Cancer Institute, Boston.
Tryptic phosphopeptide mapping, phosphoamino acid assay and mass spectrometry
293T cells transfected with pMINV-p210 BCR/ABL-IRES-Grb2 were labeled 48 h later with [32P]orthophosphate (0.5 mCi/ml) in serum-free and phosphate-free DMEM (Gibco) for 3 h, and Grb2 proteins in cell lysates immunoprecipitated with rabbit anti-Grb2 antibody, fractionated on a 15% SDS–polyacrylamide gel, transferred to a nitrocellulose membrane, excised, and used for tryptic phosphopeptide mapping and phosphoamino acid assay as described previously (Boyle et al., 1991).
For mass spectrometric analysis, Grb2 protein from 293T cells transfected with pMINV-p210 BCR/ABL-IRES-Grb2 was immunoprecipitated with anti-Grb2 antibody and separated on a 15% SDS– polyacrylamide gel. The gel was stained with Coomassie Blue R-250 and Grb2 protein bands were excised. The gel slices were de-stained, washed in 50% acetonitrile and then treated with trypsin. The tryptic peptides were purified by HPLC, and analyzed by mass spectrometry by the Harvard University Microchemistry Facility (Cambridge, MA).
In vitro kinase assay and Sos binding assay
Catalytically active murine type IV c-Abl with an activating SH3 domain (P131L) was purified from transfected 293T cells as described (Brasher and Van Etten, 2000). GST fusion proteins containing the C-terminal domain of Grb2 were purified from Escherichia coli and labeled with 32P using HMK as previously described (Bedford et al., 1997). For phosphorylation by c-Abl, 5 µg of GST proteins were incubated with or without 50 ng of purified Abl in the presence of 1 mM ATP and 0.1 mM Na3VO4, in a total volume of 20 µl at 30°C for 2 h.
For Sos binding, a synthetic Sos peptide (NH2-KDEVPVPPPVPPRRRPESA-COOH; Research Genetics) (Li et al., 1993) dissolved in dimethylsulfoxide (DMSO) was covalently coupled to Affigel 10 agarose beads (Bio-Rad), and the kinase reaction containing Abl-phosphorylated GST fusion proteins was applied directly to Sos peptide beads. For each binding reaction, 5 µg of 32P-labeled GST fusion proteins treated with Abl kinase were mixed with 5 µl (packed volume) of Sos peptide beads and 25 µl (packed volume) of G75 Sephadex beads (to minimize the loss of Sos peptide beads during the washes) in the presence of 60 µl of 1× binding solution (50 mM Tris–HCl pH 7.5, 50 mM NaCl and 0.01% Brij 35). The binding reaction was kept at 4°C overnight, and the beads were washed twice with Tris-buffered saline–Tween 20 (TBST), once with high-salt buffer (20 mM Tris–HCl pH 7.4, 500 mM NaCl, 1% Triton X-100) and once with 20 mM PIPES pH 7.2 in the presence of 1 mM Na3VO4. Radioactivity (c.p.m.) from total 32P-labeled GST fusion proteins bound to the beads was measured by liquid scintillation counting.
BCR/ABL fibroblast transformation assay
NIH-3T3 cells were plated at 5 × 105 cells per 100 cm tissue culture dish, and transduced 16 h later with ecotropic retroviral stock containing pMINV-p210 BCR/ABL-IRESneo, pMINV-p210 BCR/ABL-IRES-Grb2 WT or pMINV-p210 BCR/ABL-IRES-Grb2 Y209F in the presence of polybrene (8 µg/ml) for 4 h; polybrene-containing medium was then removed and replaced with tissue culture medium. Stocks were matched for titer by determination of proviral copy number by Southern blotting. After 18 h, cells were trypsinized and plated in 0.4% agar-containing medium at 5 × 104 per 3.5 cm dish containing 1 ml of 0.6% base agar. Anchorage-independent colonies were counted 3 weeks later.
Ras activation assay
Parental and Myc-Grb2-expressing A431 cells were starved in serum-free DMEM containing 0.5% bovine serum albumin (BSA) for 48 h, labeled with 2 ml of phosphate-free DMEM containing 0.3–0.4 mCi/ml [32P]orthophosphate for 3 h, and Ras-associated guanine nucleotides isolated by immunoprecipitation of cell lysates with anti-Ras monoclonal antibody Y13-259 (Santa Cruz) and thin-layer chromatography, as previously (Downward et al., 1990). The ratio of GTP/GDP was determined by phosphoimager analysis (Molecular Dynamics).
JNK and MAPK activation assays
For JNK assays, parental and Myc-Grb2-expressing A431 cells were plated at 2.5 × 105 cells per 6 cm tissue culture dish. For MAPK assays, 293T cells were transfected with expression constructs for Myc-Grb2 and EGF receptor as described above. Cells were washed twice in phosphate-buffered saline (PBS) lacking calcium and magnesium chloride and incubated in DMEM containing 0.5% BSA for 24 h (for 293T cells) or 48 h (for A431 cells). Following stimulation by EGF (2–40 min) in DMEM, cells were washed once with PBS, collected by trypsinization, and lysed in cold lysis buffer. Samples were factionated on a 5–20% SDS–polyacrylamide gel, transferred to nitrocellulose membranes, and antibody-bound proteins visualized using activation-specific and pan-reactive antibodies directed against SAPK/JNK and ERK/MAPK (New England Biolabs) according to the manufacturer’s instructions.
Acknowledgments
Acknowledgements
We thank Dr William Lane of the Harvard Microchemistry Facility for invaluable assistance with mass spectrometry, and Dr Mark Showers for helpful comments and critically reading the manuscript. This work was supported in part by NIH grants CA90576 and CA72465 (R.A.V.). R A.V. is a scholar of The Leukemia and Lymphoma Society and the Carl and Margaret Walter Scholar in Blood Research at Harvard Medical School.
References
- Baltensperger K., Kozma,L.M., Cherniack,A.D., Klarlund,J., Chawla,A., Banerjee,U. and Czech,M.P. (1993) Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science, 260, 1950–1952. [DOI] [PubMed] [Google Scholar]
- Bedford M.T., Chan,D.C. and Leder,P. (1997) FBP WW domains and the Abl SH3 domain bind to a specific class of proline-rich ligands. EMBO J., 16, 2376–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin C.W., Linseman,D.A. and Jones,D.A. (1994) Platelet-derived growth factor stimulates phosphorylation of growth factor receptor-binding protein-2 in vascular smooth muscle cells. J. Biol. Chem., 269, 31346–31349. [PubMed] [Google Scholar]
- Boyle W.J., van Der Greer,P. and Hunter,T. (1991) Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol., 201, 110–149. [DOI] [PubMed] [Google Scholar]
- Brasher B.B. and Van Etten,R.A. (2000) c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J. Biol. Chem., 275, 35631–35637. [DOI] [PubMed] [Google Scholar]
- Broome M.A. and Hunter,T. (1997) The PDGF receptor phosphorylated Tyr138 in the c-Src SH3 domain in vivo reducing peptide ligand binding. Oncogene, 14, 17–34. [DOI] [PubMed] [Google Scholar]
- Buday L. and Downward,J. (1993) Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein and Sos nucleotide exchange factor. Cell, 73, 611–620. [DOI] [PubMed] [Google Scholar]
- Cheng A.M. et al. (1998) Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell, 95, 793–803. [DOI] [PubMed] [Google Scholar]
- Cherniack A.D., Klarlund,J.K., Conway,B.R. and Czech,M.P. (1995) Disassembly of son of sevenless proteins from Grb2 during p21ras desensitization by insulin. J. Biol. Chem., 270, 1485–1488. [PubMed] [Google Scholar]
- Clark S.G., Stern,M.J. and Horvitz,H.R. (1992) C.elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature, 356, 340–344. [DOI] [PubMed] [Google Scholar]
- Couture C., Songyang,Z., Jascur,T., Williams,S., Tailor,P., Cantley,L.C. and Mustelin,T. (1996) Regulation of the Lck SH2 domain by tyrosine phosphorylation. J. Biol. Chem., 271, 24880–24884. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden,Y., Mayes,E., Scrace,G., Totty,N., Stockwell,P., Ullrich,A., Schlessinger,J. and Waterfield,M.D. (1984) Close similarity of epidermal growth factor receptor and v-erbB oncogene protein sequences. Nature, 307, 521–527. [DOI] [PubMed] [Google Scholar]
- Downward J., Graves,J.D., Warne,P.H., Rayter,S. and Cantrell,D.A. (1990) Stimulation of p21ras upon T-cell activation. Nature, 346, 719–723. [DOI] [PubMed] [Google Scholar]
- Druker B.J. et al. (2001) Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med., 344, 1084–1086. [DOI] [PubMed] [Google Scholar]
- Egan S.E., Giddings,B.W., Brooks,M.W., Buday,L., Sizeland,A.M. and Weinberg,R.A. (1993) Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature, 363, 45–51. [DOI] [PubMed] [Google Scholar]
- Feller S.M., Knudsen,B. and Hanafusa,H. (1994) c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J., 13, 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Chen,J.K., Yu,H., Simon,J.A. and Schreiber,S.L. (1994) Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3–ligand interactions. Science, 266, 1241–1247. [DOI] [PubMed] [Google Scholar]
- Gale N.W., Kaplan,S., Lowenstein,E.J., Schlessinger,J. and Bar-Sagi,D. (1993) Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature, 363, 88–92. [DOI] [PubMed] [Google Scholar]
- Ghosh J. and Miller,R.A. (1995) Rapid tyrosine phosphorylation of Grb2 and Shc in T cells exposed to anti-CD3, anti-CD4 and anti-CD45 stimuli: differential effect of aging. Mech. Ageing Dev., 80, 171–87. [DOI] [PubMed] [Google Scholar]
- Gishizky M.L., Cortez,D. and Pendergast,A.M. (1995) Mutant forms of growth factor-binding protein-2 reverse BCR-ABL-induced trans formation. Proc. Natl Acad. Sci. USA, 92, 10889–10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goga A., McLaughlin,J., Afar,D.E., Saffran,D.C. and Witte,O.N. (1995) Alternative signals to RAS for hematopoietic transformation by the BCR-ABL oncogene. Cell, 82, 981–988. [DOI] [PubMed] [Google Scholar]
- Hawley R.G., Lieu,F.H.L., Fong,A.Z.C., Goldman,S.J., Leonard,J.P. and Hawley,T.S. (1996) Retroviral vectors for production of interleukin-12 in the bone marrow to induce a graft-versus leukemia effect. Ann. N. Y. Acad. Sci., 795, 341–345. [DOI] [PubMed] [Google Scholar]
- Holgodo-Madruga M., Emlet,D.R., Moscatello,D.R., Godwin,A.K. and Wong,A.J. (1996) A Grb2-associated docking protein in EGF-receptor signaling. Nature, 379, 560–564. [DOI] [PubMed] [Google Scholar]
- Hughes M.J.G. and Andrews,D.W. (1996) Creation of deletion, insertion and substitution mutations using a single pair of primers and PCR. Biotechniques, 20, 188–196. [DOI] [PubMed] [Google Scholar]
- Kouhara H., Hadari,Y.R., Spivak-Kroizman,T., Schilling,J., Bar-Sagi,D., Lax,I. and Schlessinger,J. (1997) A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell, 89, 693–702. [DOI] [PubMed] [Google Scholar]
- Li N., Batzer,A., Daly,R., Yajnik,V., Skolnik,E., Chardin,P., Bar-Sagi,D., Margolis,B. and Schlessinger,J. (1993) Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to ras signaling. Nature, 363, 85–88. [DOI] [PubMed] [Google Scholar]
- Li S., Ilaria,R.L., Million,R.P., Daley,G.Q. and Van Etten,R.A. (1999) The P190, P210 and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J. Exp. Med., 189, 1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S.K., Falasca,M., Hu,P. and Schlessinger,J. (1997) Phosphatidyl inositol 3-kinase mediates epidermal growth factor-induced activation of the c-Jun N-terminal kinase signaling pathway. Mol. Cell. Biol., 17, 5784–5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein E.J. et al. (1992) The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell, 70, 431–442. [DOI] [PubMed] [Google Scholar]
- Maignan S., Guilloteau,J.P., Fromage,N., Arnoux,B., Becquart,J. and Ducruix,A. (1995) Crystal structure of the mammalian Grb2 adaptor. Science, 268, 291–293. [DOI] [PubMed] [Google Scholar]
- Million R.P. and Van Etten,R.A. (2000) The Grb2 binding site is required for induction of chronic myeloid leukemia-like disease in mice by the Bcr/Abl tyrosine kinase. Blood, 96, 664–670. [PubMed] [Google Scholar]
- Park H., Wahl,M.I., Afar,D.E.H., Turck,C.W., Rawlings,D.J., Tam,C., Scharenberg,A.M., Kinet,J.P. and Witte,O.N. (1996) Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity, 4, 515–525. [DOI] [PubMed] [Google Scholar]
- Pawson T. and Scott,J.D. (1997) Signaling through scaffold, anchoring and adoptor proteins. Science, 278, 2075–2080. [DOI] [PubMed] [Google Scholar]
- Pendergast A.M. et al. (1993) BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell, 75, 175–185. [PubMed] [Google Scholar]
- Pomerance M., Multon,M.C., Parker,F., Venot,C., Blondeau,J.P., Tocque,B. and Schweighoffer,F. (1998) Grb2 interaction with MEK-kinase 1 is involved in regulation of Jun-kinase avtivities in response to epidermal growth factor. J. Biol. Chem., 273, 24301–24304. [DOI] [PubMed] [Google Scholar]
- Puil L., Liu,J., Gish,G., Mbamalu,G., Bowtell,D., Pelicci,P.G., Arlinghaus,R. and Pawson,T. (1994) Bcr-Abl oncoproteins bind directly to activators of the Ras signalling pathway. EMBO J., 13, 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcook M. et al. (1992) Association of the Shc and Grb2/Sem-5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinase. Nature, 360, 689–692. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley,R., Wade,J., Pawson,T. and Bowtell,D. (1993) The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature, 363, 83–85. [DOI] [PubMed] [Google Scholar]
- Sawyers C.L. (1997) Signal transduction pathways involved in BCR-ABL transformation. Baillieres Clin. Haematol., 10, 223–231. [DOI] [PubMed] [Google Scholar]
- Sawyers C.L., McLaughlin,J. and Witte,O.N. (1995) Genetic require ment for Ras in the transformation of fibroblasts and hematopoietic cells by the Bcr-Abl oncogene. J. Exp. Med., 181, 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton T.M., Cheng,A.M., Ong,S.H., Lu,Y., Sakai,R., Cross,J.C. and Pawson,T. (2001) Gene dosage-dependent functions for phospho tyrosine–Grb2 signaling during mammalian tissue morphogenesis. Curr. Biol., 11, 662–670. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D.D., Hanks,S.K., Hunter,T. and van der Geep,P. (1994) Integrin-mediated signal transduction linked to Ras pathway by Grb2 binding to focal adhesion kinase. Nature, 372, 786–791. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. and Bar-Sagi,D. (1994) Activation of Ras and other signaling pathways by receptor tyrosine kinases. Cold Spring Harb. Symp. Quant. Biol., 59, 173–179. [DOI] [PubMed] [Google Scholar]
- Skolnik E.Y., Batzer,A., Li,N., Lee,C.H., Lowenstein,E., Mohammadi,M., Margolis,B. and Schlessinger,J. (1993a) The function of Grb2 in linking the insulin receptor to Ras signaling pathway. Science, 260, 1953–1955. [DOI] [PubMed] [Google Scholar]
- Skolnik E.Y. et al. (1993b) The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated RIS1 and Shc: implications for insulin control of ras signalling. EMBO J., 12, 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Charles,C.H., Lau,L.F. and Tonks,N.K. (1993) MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell, 75, 487–493. [DOI] [PubMed] [Google Scholar]
- van der Geep P. and Pawson,T. (1995) The PTB domain: a new protein module implicated in signaling transduction. Trends Biochem. Sci., 20, 277–280. [DOI] [PubMed] [Google Scholar]